Underutilized Agricultural Co-Product as a Sustainable Biofiller for Polyamide 6,6: Effect of Carbonization Temperature
Abstract
1. Introduction
2. Results and Discussion
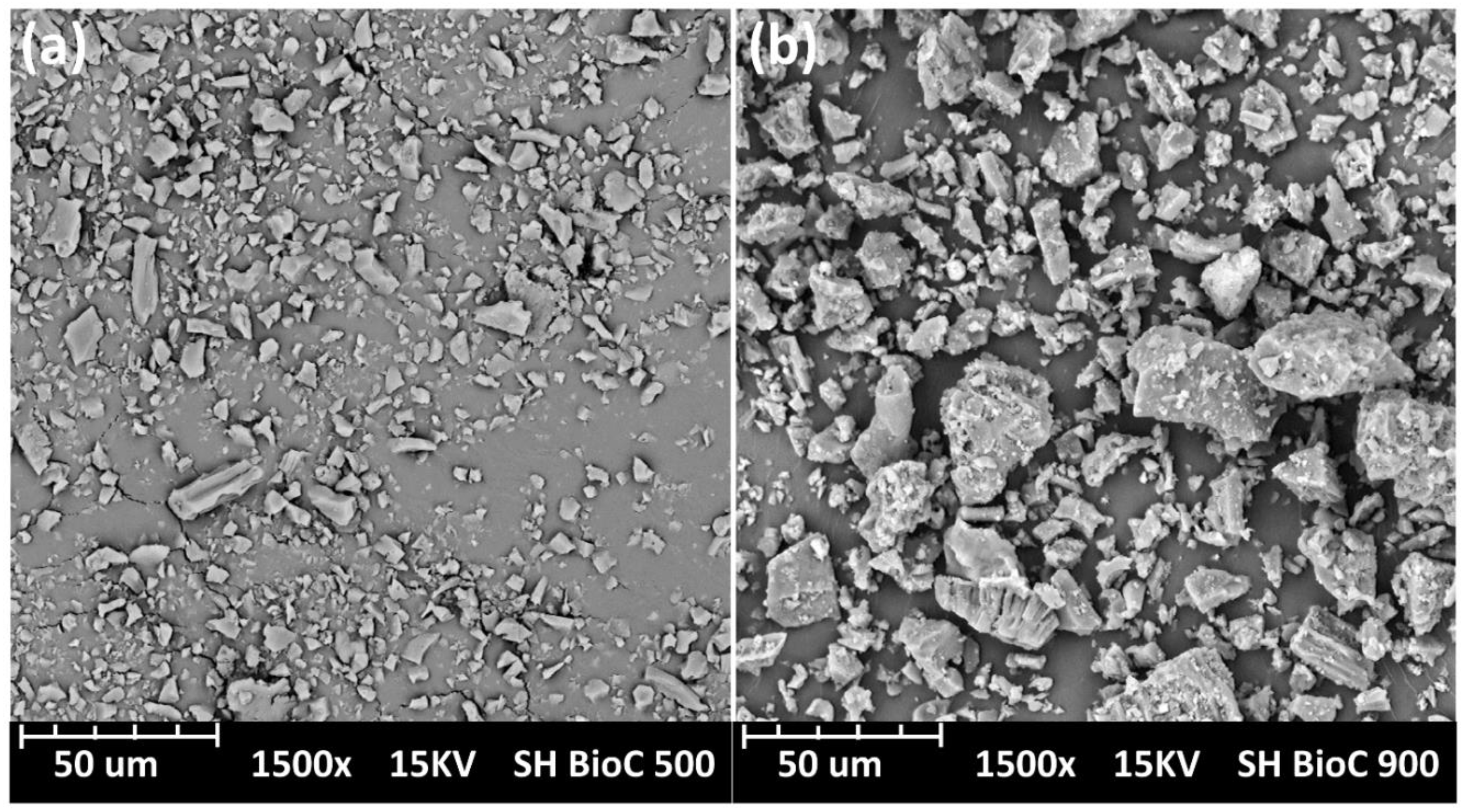
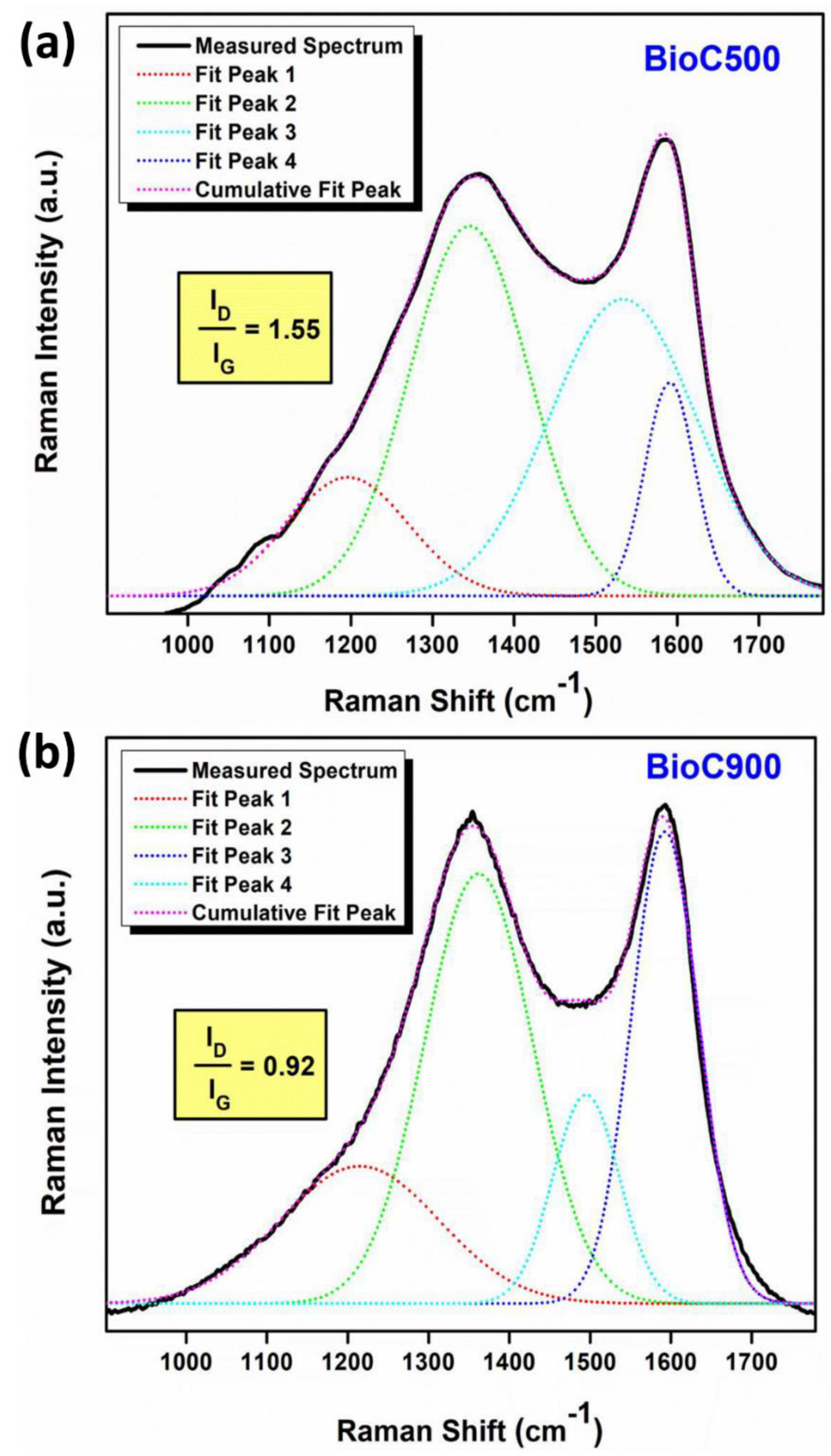
2.1. Biocarbon Morphology and Characterization
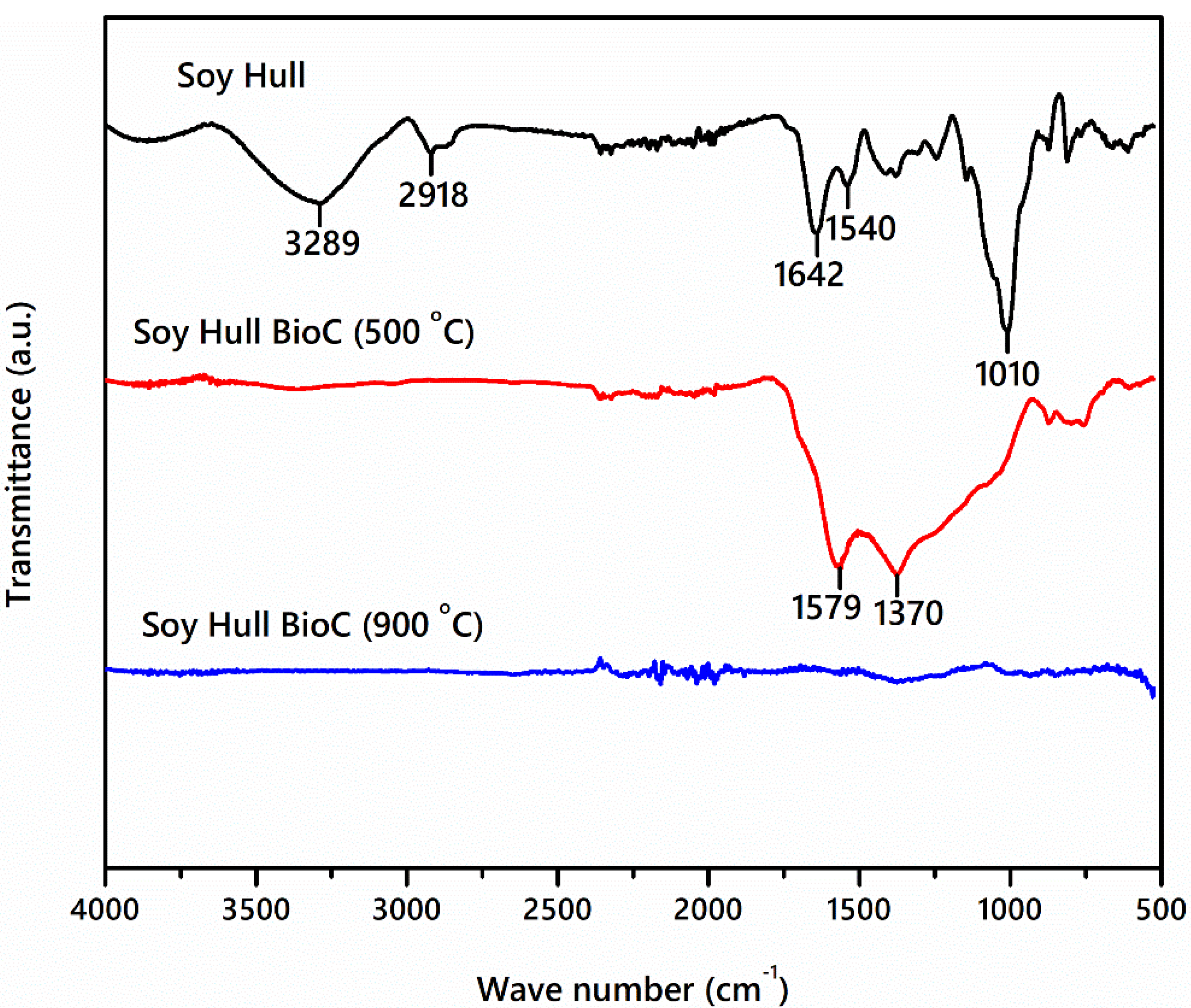
2.2. Fourier Transform Infrared Spectroscopy (FTIR)
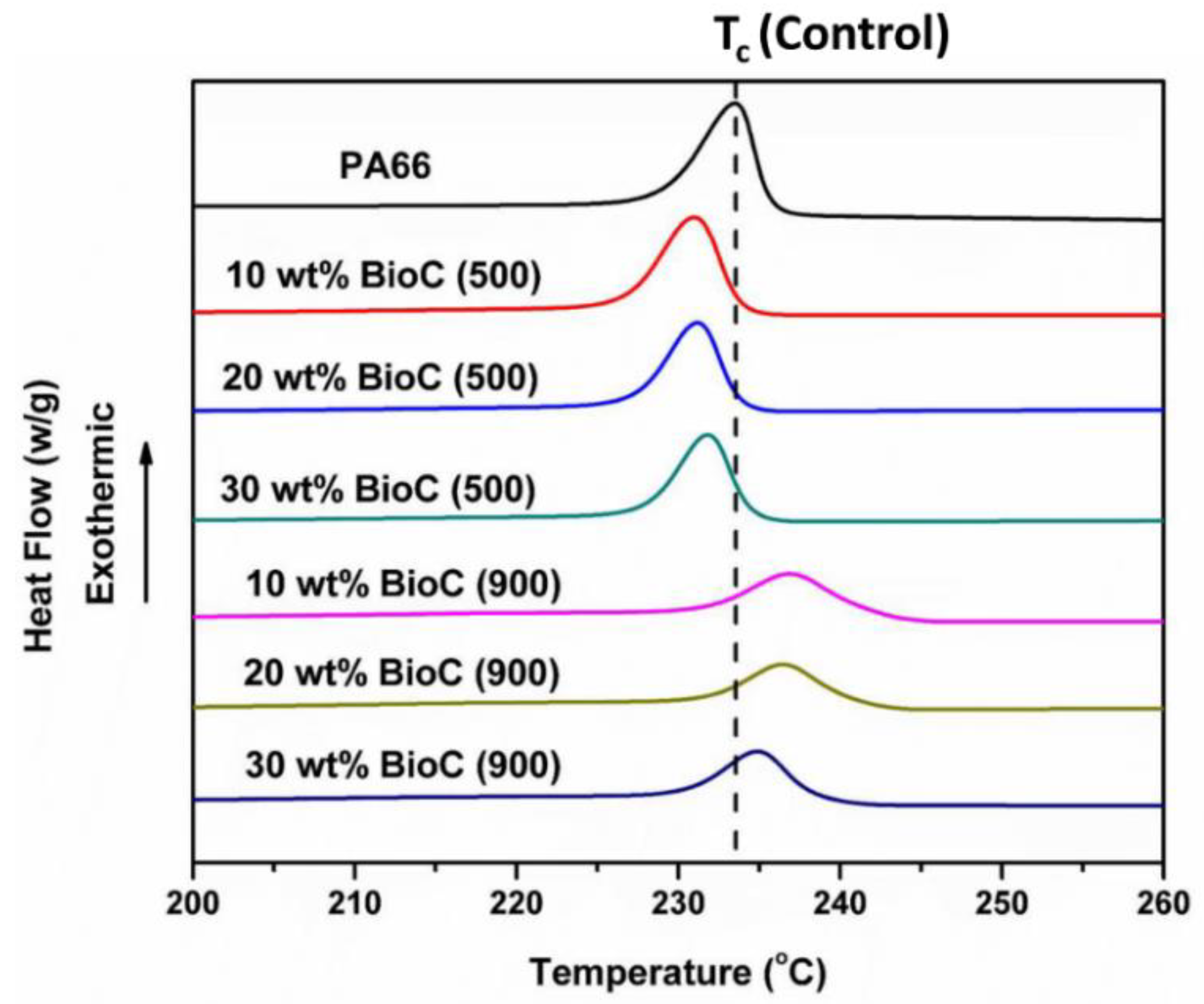
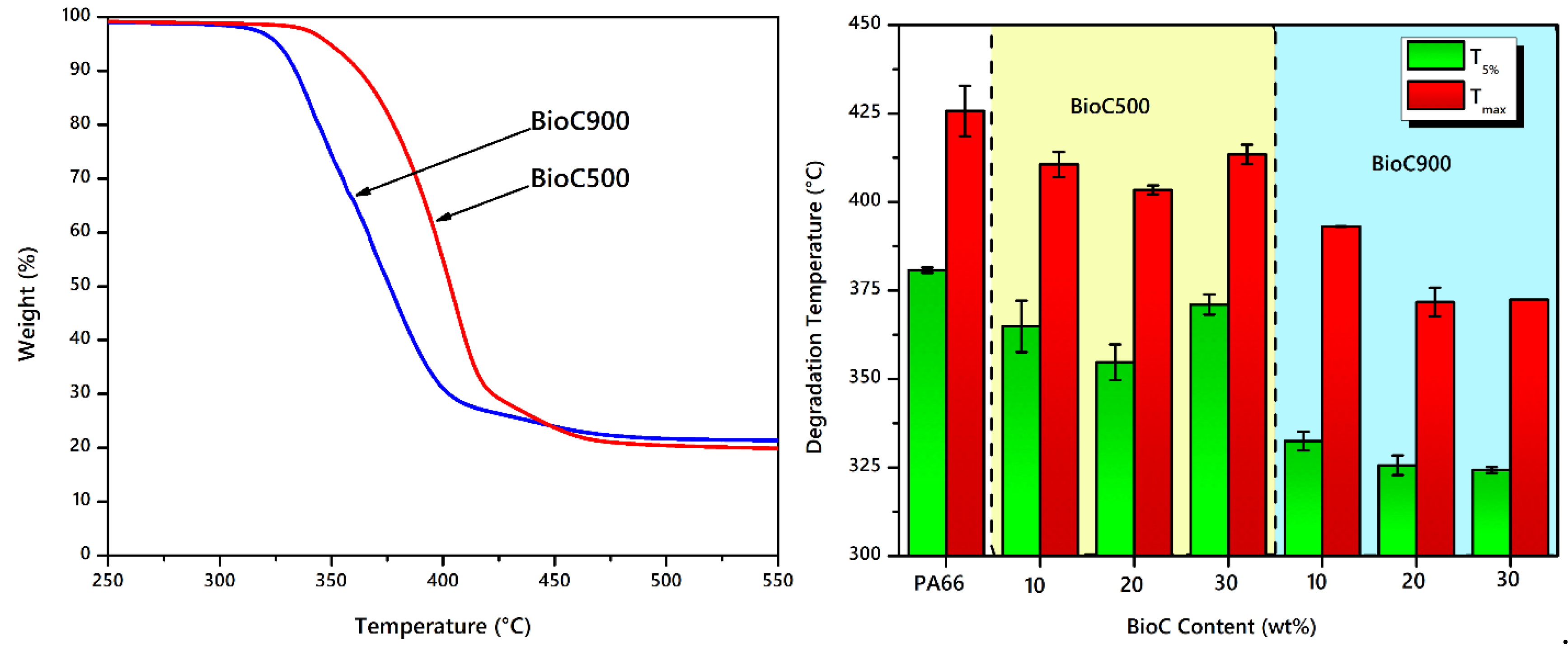
2.3. Thermal Analysis and Characteristics
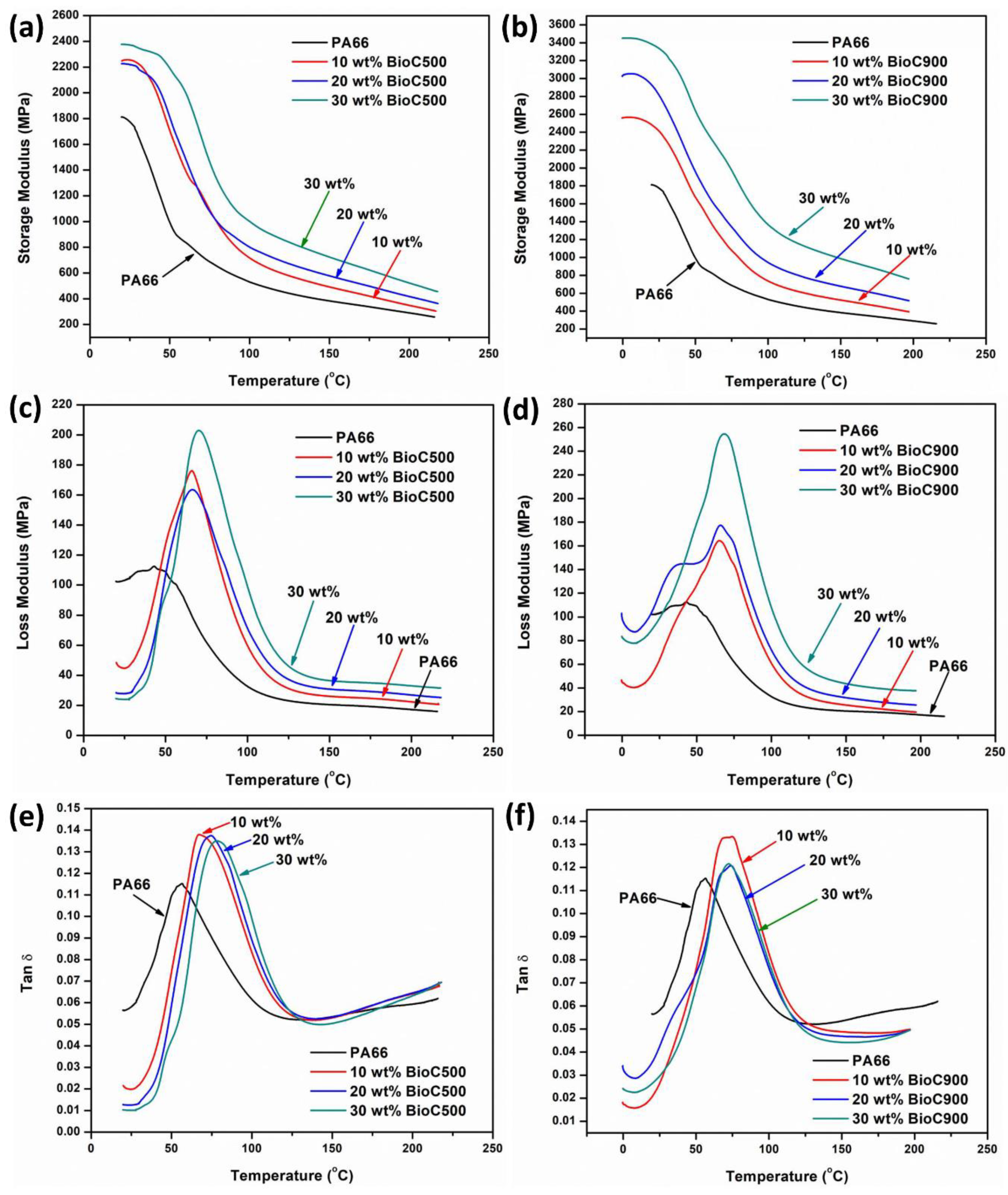
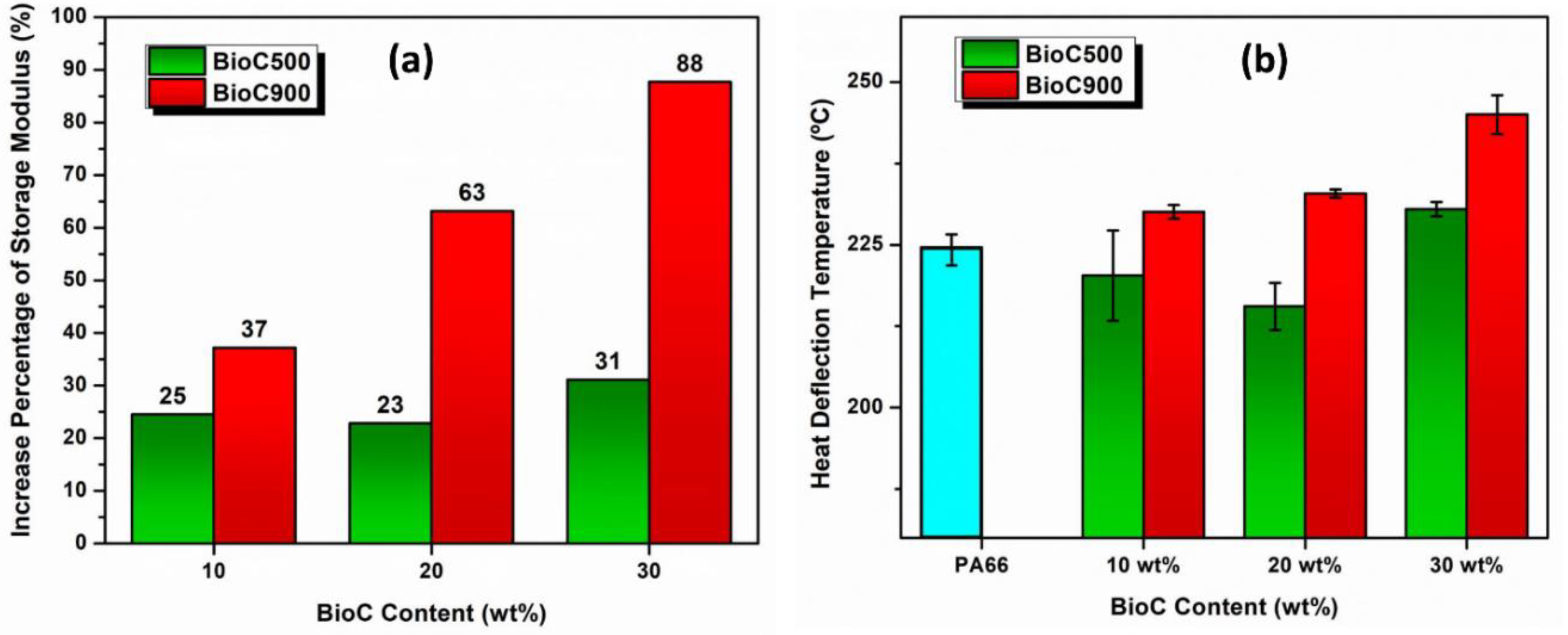
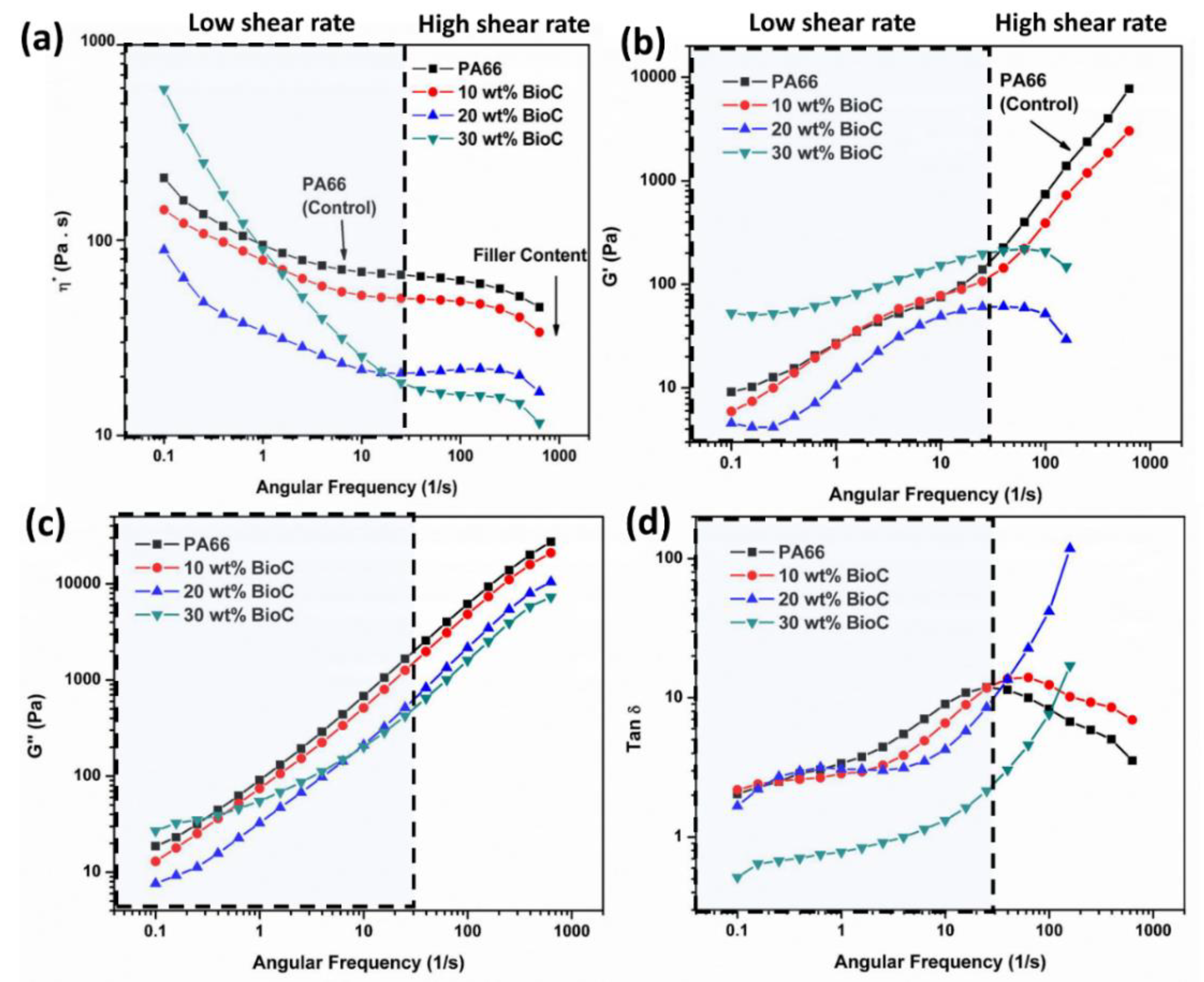
2.4. Dynamic Mechanical Analysis (DMA)
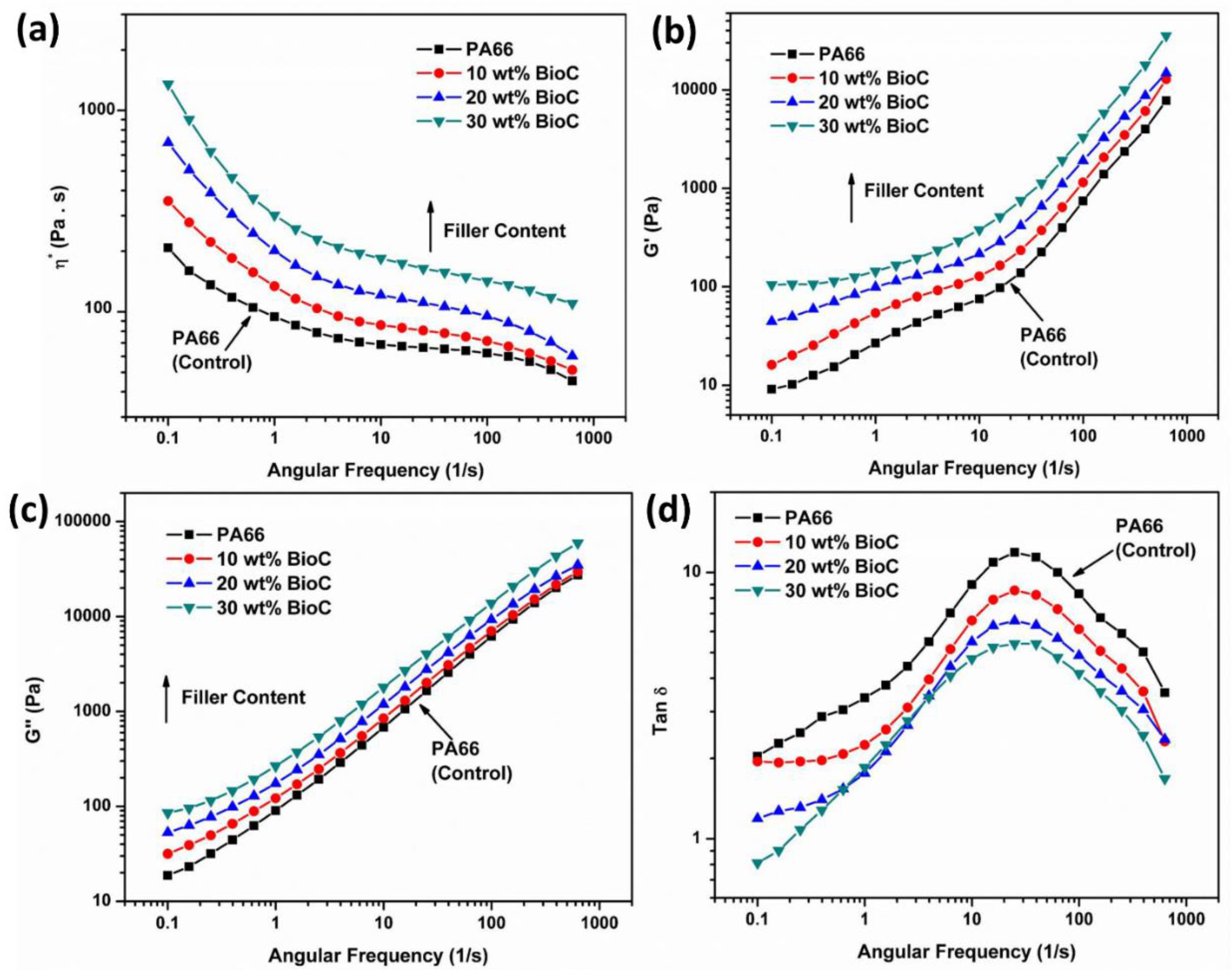
2.5. Rheological Behavior
3. Experimental Details
3.1. Materials
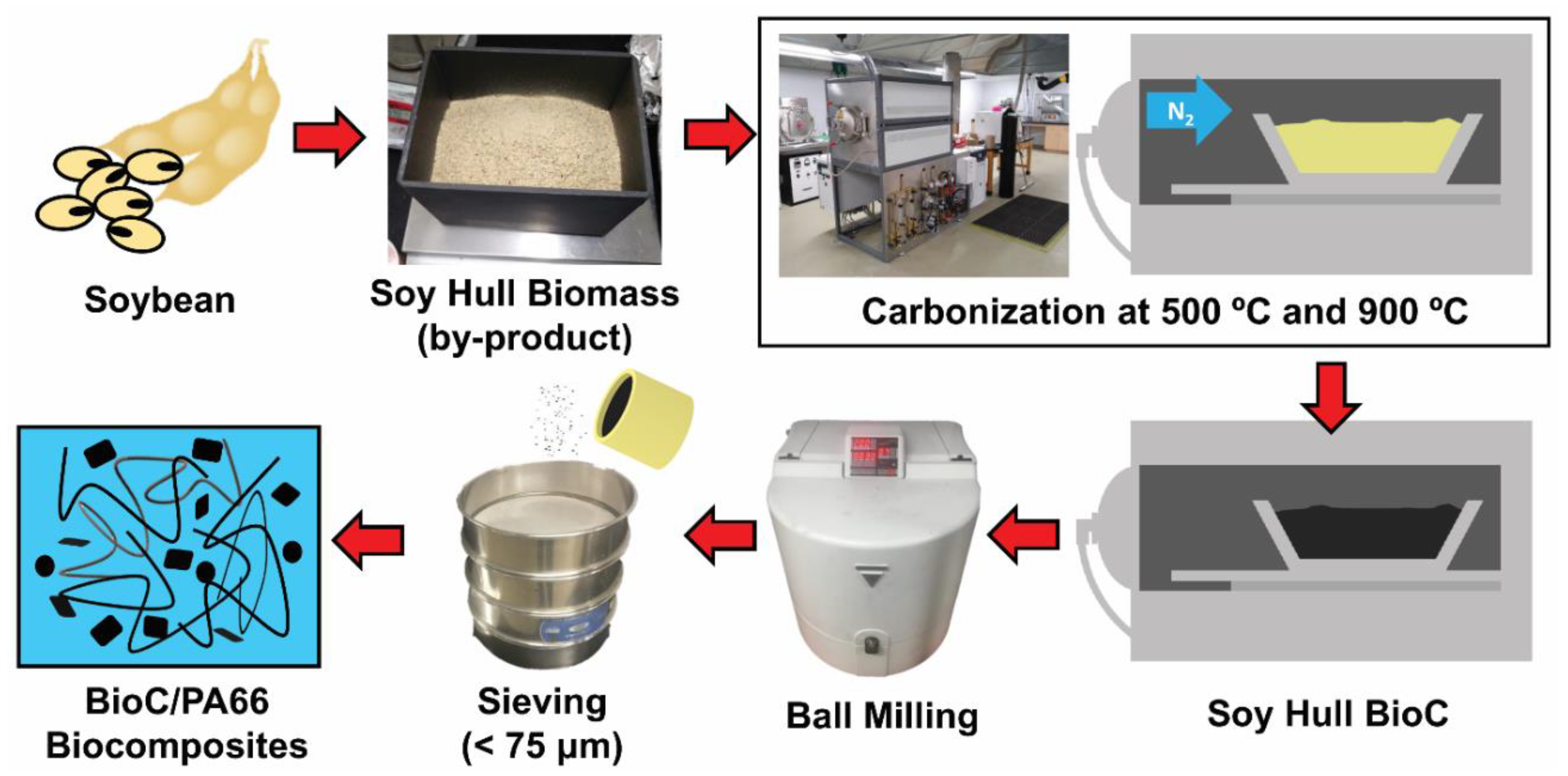
3.2. Biocarbon Preparation
3.3. Biocomposites Preparation
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, S.; Di, Y.; Liang, X. Effects of SEBS-g-MA on the Tribological Behavior of PA66/Montmorillonite Nanocomposites. Int. J. Polymeric Mater. Polym. Biomater. 2013, 62, 172–174. [Google Scholar] [CrossRef]
- Krause, B.; Kroschwald, L.; Pötschke, P. The Influence of the Blend Ratio in PA6/PA66/MWCNT Blend Composites on the Electrical and Thermal Properties. Polymers 2019, 11, 122. [Google Scholar] [CrossRef]
- Esposito, F. Plastics Resin Pricing—Engineering Thermoplastics. Available online: https://www.plasticsnews.com/resin/currentPricing/engineering-thermoplastics (accessed on 11 March 2020).
- Ha, J.-H.; Hong, S.-K.; Ryu, J.-K.; Bae, J.; Park, S.-H. Development of Multi-Functional Graphene Polymer Composites Having Electromagnetic Interference Shielding and De-Icing Properties. Polymers 2019, 11, 2101. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-M.; Behera, K.; Yadav, M.; Chiu, F.-C. Polyamide 6/Poly(vinylidene fluoride) Blend-Based Nanocomposites with Enhanced Rigidity: Selective Localization of Carbon Nanotube and Organoclay. Polymers 2020, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Geng, L.-H.; Amiralian, N.; Martin, D.J.; Hsiao, B.S. Nanocellulose from Spinifex as an Effective Adsorbent to Remove Cadmium(II) from Water. ACS Sustain. Chem. Eng. 2018, 6, 3279–3290. [Google Scholar] [CrossRef]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Hsiao, B.S. Efficient Removal of UO22+ from Water Using Carboxycellulose Nanofibers Prepared by the Nitro-Oxidation Method. Ind. Eng. Chem. Res. 2017, 56, 13885–13893. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Antoine, R.; Hsiao, B.S. Efficient Removal of Arsenic Using Zinc Oxide Nanocrystal-Decorated Regenerated Microfibrillated Cellulose Scaffolds. ACS Sustain. Chem. Eng. 2019, 7, 6140–6151. [Google Scholar] [CrossRef]
- Mohammed, N.; Grishkewich, N.; Tam, K.; Grishkewich, N. Cellulose nanomaterials: Promising sustainable nanomaterials for application in water/wastewater treatment processes. Environ. Sci. Nano 2018, 5, 623–658. [Google Scholar] [CrossRef]
- Sharma, P.R.; Varma, A. Functional nanoparticles obtained from cellulose: Engineering the shape and size of 6-carboxycellulose. Chem. Commun. 2013, 49, 8818. [Google Scholar] [CrossRef]
- Geng, L.; Peng, X.; Zhan, C.; Naderi, A.; Sharma, P.R.; Mao, Y.; Hsiao, B.S. Structure characterization of cellulose nanofiber hydrogel as functions of concentration and ionic strength. Cellulose 2017, 24, 5417–5429. [Google Scholar] [CrossRef]
- Rajinipriya, M.; Nagalakshmaiah, M.; Robert, M.; Elkoun, S.; Malladi, R. Importance of Agricultural and Industrial Waste in the Field of Nanocellulose and Recent Industrial Developments of Wood Based Nanocellulose: A Review. ACS Sustain. Chem. Eng. 2018, 6, 2807–2828. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; B, A.K.; H, R.M.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Goldsmith, P.D. World soybean production: Area harvested, yield, and long-term projections. Int. Food Agribus. Manag. Rev. 2009, 12, 1–20. [Google Scholar]
- Quosai, P.; Anstey, A.; Mohanty, A.K.; Misra, M. Characterization of biocarbon generated by high- and low-temperature pyrolysis of soy hulls and coffee chaff: For polymer composite applications. R. Soc. Open Sci. 2018, 5, 171970. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-M.; Li, H.-Y. Application and Conversion of Soybean Hulls. In Soybean—The Basis of Yield, Biomass and Productivity; IntechOpen: Rijeka, Croatia, 2017; pp. 111–132. [Google Scholar]
- Agyei, D.; Shanbhag, B.; He, L. Enzymes for food waste remediation and valorisation. In Improving and Tailoring Enzymes for Food Quality and Functionality; Elsevier BV, Woodhead Publishing: Cambridge, UK; Cambridge, MA, USA; Kidlington, UK, 2015; pp. 123–145. [Google Scholar]
- Mielenz, J.R.; Bardsley, J.S.; Wyman, C.E. Fermentation of soybean hulls to ethanol while preserving protein value. Bioresour. Technol. 2009, 100, 3532–3539. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Siqueira, P.F.; Miranda, L.C.; Tardioli, P.W.; Giordano, R.L. Sequential proteolysis and cellulolytic hydrolysis of soybean hulls for oligopeptides and ethanol production. Ind. Crop. Prod. 2014, 61, 202–210. [Google Scholar] [CrossRef]
- Nanda, M.R.; Misra, M.; Mohanty, A.K. Mechanical Performance of Soy-Hull-Reinforced Bioplastic Green Composites: A Comparison with Polypropylene Composites. Macromol. Mater. Eng. 2011, 297, 184–194. [Google Scholar] [CrossRef]
- Nanda, M.R.; Misra, M.; Mohanty, A.K. Performance Evaluation of Biofibers and Their Hybrids as Reinforcements in Bioplastic Composites. Macromol. Mater. Eng. 2012, 298, 779–788. [Google Scholar] [CrossRef]
- Nyambo, C.; Mohanty, A.K.; Misra, M. Polylactide-Based Renewable Green Composites from Agricultural Residues and Their Hybrids. Biomacromolecules 2010, 11, 1654–1660. [Google Scholar] [CrossRef]
- Quirino, R.L.; LaRock, R.C. Synthesis and properties of soy hull-reinforced biocomposites from conjugated soybean oil. J. Appl. Polym. Sci. 2009, 112, 2033–2043. [Google Scholar] [CrossRef]
- Creamer, A.E.; Gao, F.; Wang, S. Carbon dioxide capture using various metal oxyhydroxide–biochar composites. Chem. Eng. J. 2016, 283, 826–832. [Google Scholar] [CrossRef]
- Mohd, A.; Ghani, W.A.W.A.K.; Resitanim, N.Z.; Sanyang, L. A Review: Carbon Dioxide Capture: Biomass-Derived-Biochar and Its Applications. J. Dispers. Sci. Technol. 2013, 34, 974–984. [Google Scholar] [CrossRef]
- Vold, J.L.; Ulven, C.; Chisholm, B.J. Torrefied biomass filled polyamide biocomposites: Mechanical and physical property analysis. J. Mater. Sci. 2014, 50, 725–732. [Google Scholar] [CrossRef]
- Zhang, Q.; Yi, W.; Li, Z.; Wang, L.; Cai, H. Mechanical Properties of Rice Husk Biochar Reinforced High Density Polyethylene Composites. Polymers 2018, 10, 286. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.P.; Mohanty, A.K.; Misra, M. Sustainable biocarbon as an alternative of traditional fillers for poly(butylene terephthalate)-based composites: Thermo-oxidative aging and durability. J. Appl. Polym. Sci. 2019, 136, 47722. [Google Scholar] [CrossRef]
- Codou, A.; Misra, M.; Mohanty, A.K. Sustainable biocomposites from Nylon 6 and polypropylene blends and biocarbon—Studies on tailored morphologies and complex composite structures. Compos. Part A Appl. Sci. Manuf. 2020, 129, 105680. [Google Scholar] [CrossRef]
- Abdelwahab, M.; Codou, A.; Anstey, A.; Mohanty, A.K.; Misra, M. Studies on the dimensional stability and mechanical properties of nanobiocomposites from polyamide 6-filled with biocarbon and nanoclay hybrid systems. Compos. Part A Appl. Sci. Manuf. 2020, 129, 105695. [Google Scholar] [CrossRef]
- Codou, A.; Misra, M.; Andrzejewski, J. Sustainable biocarbon reinforced nylon 6/polypropylene compatibilized blends: Effect of particle size and morphology on performance of the biocomposites. Compos. Part A Appl. Sci. Manuf. 2018, 112, 1–10. [Google Scholar] [CrossRef]
- Das, O.; Kim, N.K.; Hedenqvist, M.S.; Lin, R.J.T.; Sarmah, A.K.; Bhattacharyya, D. An Attempt to Find a Suitable Biomass for Biochar-Based Polypropylene Biocomposites. Environ. Manag. 2018, 62, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Dahal, R.K.; Acharya, B.; Saha, G.; Bissessur, R.; Dutta, A.; Farooque, A. Biochar as a filler in glassfiber reinforced composites: Experimental study of thermal and mechanical properties. Compos. Part B Eng. 2019, 175, 107169. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Jadhav, A.; Qureshi, S.S.; Baloch, H.A.; Siddiqui, M.T.H.; Mubarak, N.M.; Griffin, G.; Madapusi, S.; Tanksale, A.; Ahamed, M.I. Synthesis and characterization of polylactide/rice husk hydrochar composite. Sci. Rep. 2019, 9, 5445. [Google Scholar] [CrossRef]
- Behazin, E.; Mohanty, A.K.; Misra, M. Sustainable lightweight biocomposite from toughenned polypropylene and biocarbon for automobile applications. In Proceedings of the 21st International Conference on Composite Materials, Xi’an, China, 20–25 August 2017; pp. 20–25. [Google Scholar]
- Mohanty, A.; Misra, M.; Ogunsona, E.O.; Anstey, A.J.; Torres-Galves, S.; Codou, A.M.F.M.-S.; Jubinville, D.F. Biocarbon and Nylon Based Hybrid Carbonaceous Biocomposites and Methods of Making Those and Using Thereof. Google Patents US20180022, 2018. [Google Scholar]
- Fang, Q.; Chen, B.; Lin, Y.; Guan, Y. Aromatic and Hydrophobic Surfaces of Wood-derived Biochar Enhance Perchlorate Adsorption via Hydrogen Bonding to Oxygen-containing Organic Groups. Environ. Sci. Technol. 2013, 48, 279–288. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, D.; Zhu, L. Transitional Adsorption and Partition of Nonpolar and Polar Aromatic Contaminants by Biochars of Pine Needles with Different Pyrolytic Temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, B.; Chiou, C.T. Fast and Slow Rates of Naphthalene Sorption to Biochars Produced at Different Temperatures. Environ. Sci. Technol. 2012, 46, 11104–11111. [Google Scholar] [CrossRef]
- Chandane, V.; Singh, V. Adsorption of safranin dye from aqueous solutions using a low-cost agro-waste material soybean hull. Desalination Water Treat. 2014, 57, 1–13. [Google Scholar] [CrossRef]
- Gnanasambandam, R.; Proctor, A. Preparation of soy hull pectin. Food Chem. 1999, 65, 461–467. [Google Scholar] [CrossRef]
- Ogunsona, E.O.; Misra, M.; Mohanty, A.K. Impact of interfacial adhesion on the microstructure and property variations of biocarbons reinforced nylon 6 biocomposites. Compos. Part A Appl. Sci. Manuf. 2017, 98, 32–44. [Google Scholar] [CrossRef]
- Behazin, E.; Misra, M.; Mohanty, A.K. Sustainable biocarbon from pyrolyzed perennial grasses and their effects on impact modified polypropylene biocomposites. Compos. Part B Eng. 2017, 118, 116–124. [Google Scholar] [CrossRef]
- Navarro-Pardo, F.; Martínez-Barrera, G.; Martinez-Hernandez, A.L.; Castaño, V.M.; Armenta, J.L.R.; Medellín-Rodríguez, F.; Velasco-Santos, C. Effects on the Thermo-Mechanical and Crystallinity Properties of Nylon 6,6 Electrospun Fibres Reinforced with One Dimensional (1D) and Two Dimensional (2D) Carbon. Materials 2013, 6, 3494–3513. [Google Scholar] [CrossRef]
- Sengupta, R.; Tikku, V.; Somani, A.K.; Chaki, T.K.; Bhowmick, A.K. Electron beam irradiated polyamide-6,6 films—I: Characterization by wide angle X-ray scattering and infrared spectroscopy. Radiat. Phys. Chem. 2005, 72, 625–633. [Google Scholar] [CrossRef]
- Ghadami Jadval Ghadam, A.; Karimi, H. Synthesis and characterization of polyamide-66/calcium carbonate composites. J. Chem. Pet. Eng. 2015, 49, 63–78. [Google Scholar]
- Wu, Y.; Xu, Y.; Wang, D.; Zhao, Y.; Weng, S.; Xu, D.; Wu, J. FT-IR spectroscopic investigation on the interaction between nylon 66 and lithium salts. J. Appl. Polym. Sci. 2004, 91, 2869–2875. [Google Scholar] [CrossRef]
- Xu, X.; Li, B.; Lu, H.; Zhang, Z.; Wang, H. The interface structure of nano-SiO2/PA66 composites and its influence on material’s mechanical and thermal properties. Appl. Surf. Sci. 2007, 254, 1456–1462. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Q.; Berglund, L.A. Polymorphism in polyamide 66/clay nanocomposites. Polymer 2002, 43, 4967–4972. [Google Scholar] [CrossRef]
- Kuo, M.; Huang, J.; Chen, M. Non-isothermal crystallization kinetic behavior of alumina nanoparticle filled poly(ether ether ketone). Mater. Chem. Phys. 2006, 99, 258–268. [Google Scholar] [CrossRef]
- Xu, X.; Li, B.; Lu, H.; Zhang, Z.; Wang, H. The effect of the interface structure of different surface-modified nano-SiO2 on the mechanical properties of nylon 66 composites. J. Appl. Polym. Sci. 2007, 107, 2007–2014. [Google Scholar] [CrossRef]
- Andrzejewski, J.; Misra, M.; Mohanty, A.K. Polycarbonate biocomposites reinforced with a hybrid filler system of recycled carbon fiber and biocarbon: Preparation and thermomechanical characterization. J. Appl. Polym. Sci. 2018, 135, 46449. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Biocomposites with Size-Fractionated Biocarbon: Influence of the Microstructure on Macroscopic Properties. ACS Omega 2016, 1, 636–647. [Google Scholar] [CrossRef]
- Azizi, H.; Ghasemi, I. Investigation on the dynamic melt rheological properties of polypropylene/wood flour composites. Polym. Compos. 2009, 30, 429–435. [Google Scholar] [CrossRef]
- Shenoy, A.V. Rheology of Filled Polymer Systems; Kluwer Academic: New Dehli, India, 1999; pp. 339–423. [Google Scholar]
Sample Availability: Samples of the Carbonized soy hull/PA66 are available from the authors. |


| Samples | Density (g/cm3) |
|---|---|
| PA 6,6 | 1.137 ± 0.002 |
| 10 wt% 500 BioC | 1.162 ± 0.012 |
| 20 wt% 500 BioC | 1.194 ± 0.003 |
| 30 wt% 500 BioC | 1.227 ± 0.001 |
| 10 wt% 900 BioC | 1.173 ± 0.003 |
| 20 wt% 900 BioC | 1.206 ± 0.002 |
| 30 wt% 900 BioC | 1.232 ± 0.004 |
| First Heating | Second Heating | ||||||
|---|---|---|---|---|---|---|---|
| Sample | Tm (°C) | ∆Hm (J g−1) | Xc (%) | Tc (°C) | Tm (°C) | ∆Hm (J g−1) | Xc (%) |
| PA 6,6 | 264.03 (0.82) | 76.91 (2.39) | 30.11 (0.94) | 233.04 (0.63) | 262.62 (0.04) | 74.27 (0.27) | 29.08 (0.11) |
| 10 wt% BioC (500) | 262.76 (0.03) | 65.23 (1.98) | 28.38 (0.86) | 230.97 (0.01) | 261.79 (0.39) | 65.91 (0.78) | 28.67 (0.34) |
| 20 wt% BioC (500) | 262.67 (0.17) | 58.21 (0.72) | 28.49 (0.35) | 231.25 (0.54) | 261.33 (0.17) | 60.01 (0.10) | 29.37 (0.05) |
| 30 wt% BioC (500) | 262.49 (0.95) | 51.76 (0.26) | 28.95 (0.15) | 231.94 (0.13) | 260.93 (0.10) | 51.43 (0.20) | 28.77 (0.11) |
| 10 wt% BioC (900) | 262.34 (0.25) | 63.51 (2.35) | 27.63 (1.02) | 237.01 (0.17) | 260.96 (0.25) | 68.35 (0.51) | 29.73 (0.22) |
| 20 wt% BioC (900) | 261.91 (0.57) | 55.08 (1.49) | 26.95 (0.73) | 236.52 (0.35) | 259.50 (0.18) | 59.84 (0.03) | 29.29 (0.01) |
| 30 wt% BioC (900) | 262.96 (0.29) | 50.22 (1.66) | 28.09 (0.93) | 235.02 (0.06) | 258.02 (0.14) | 54.44 (0.92) | 30.45 (0.51) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balint, T.; Chang, B.P.; Mohanty, A.K.; Misra, M. Underutilized Agricultural Co-Product as a Sustainable Biofiller for Polyamide 6,6: Effect of Carbonization Temperature. Molecules 2020, 25, 1455. https://doi.org/10.3390/molecules25061455
Balint T, Chang BP, Mohanty AK, Misra M. Underutilized Agricultural Co-Product as a Sustainable Biofiller for Polyamide 6,6: Effect of Carbonization Temperature. Molecules. 2020; 25(6):1455. https://doi.org/10.3390/molecules25061455
Chicago/Turabian StyleBalint, Thomas, Boon Peng Chang, Amar K. Mohanty, and Manjusri Misra. 2020. "Underutilized Agricultural Co-Product as a Sustainable Biofiller for Polyamide 6,6: Effect of Carbonization Temperature" Molecules 25, no. 6: 1455. https://doi.org/10.3390/molecules25061455
APA StyleBalint, T., Chang, B. P., Mohanty, A. K., & Misra, M. (2020). Underutilized Agricultural Co-Product as a Sustainable Biofiller for Polyamide 6,6: Effect of Carbonization Temperature. Molecules, 25(6), 1455. https://doi.org/10.3390/molecules25061455








