Characterization, Quantification and Quality Assessment of Avocado (Persea americana Mill.) Oils
Abstract
1. Introduction
2. Results and Discussion
2.1. Supercritical Fluid Extraction of Avocado Oil
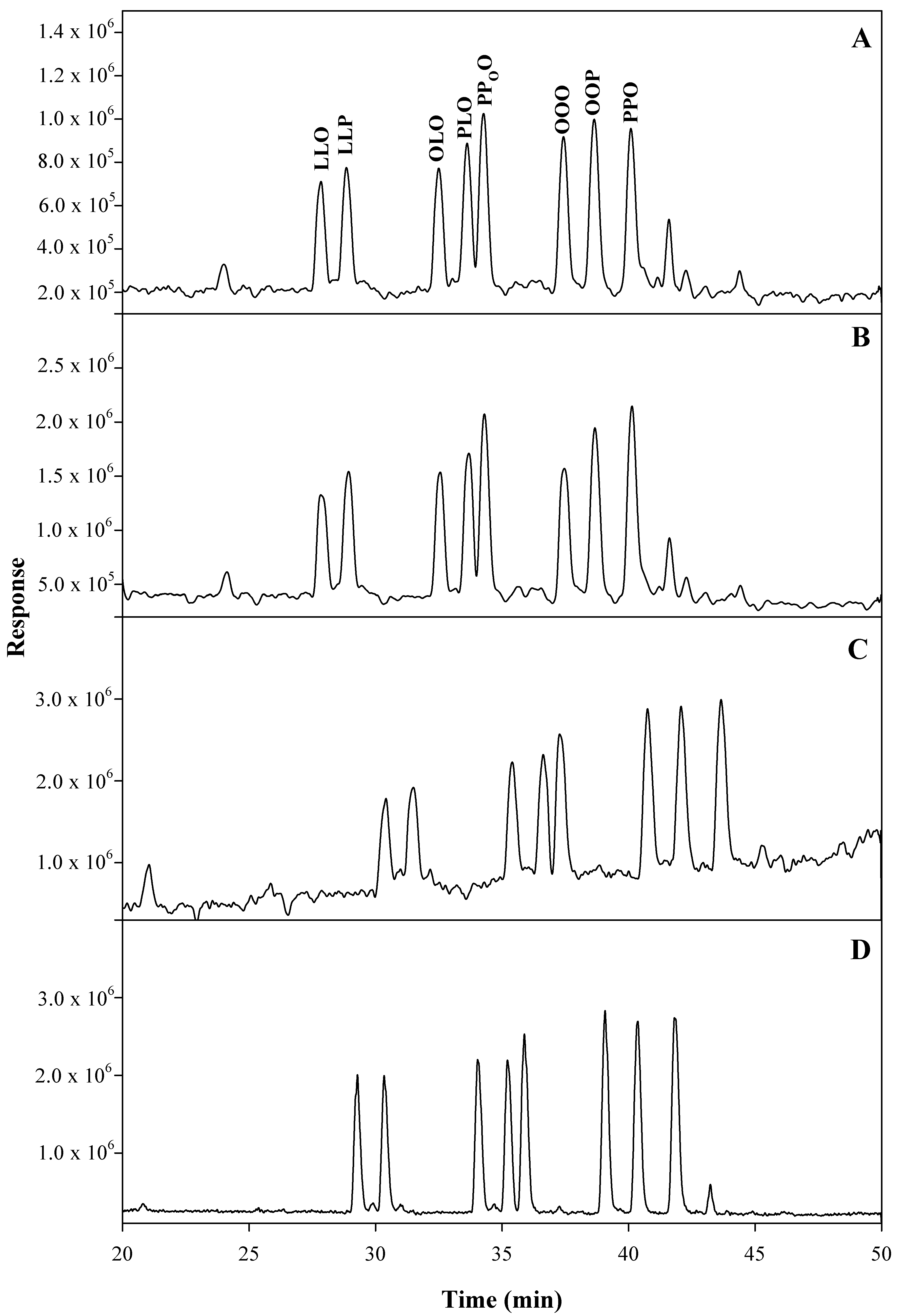
2.2. TGs Profile of Avocado Oils
2.2.1. Method Development and Optimization
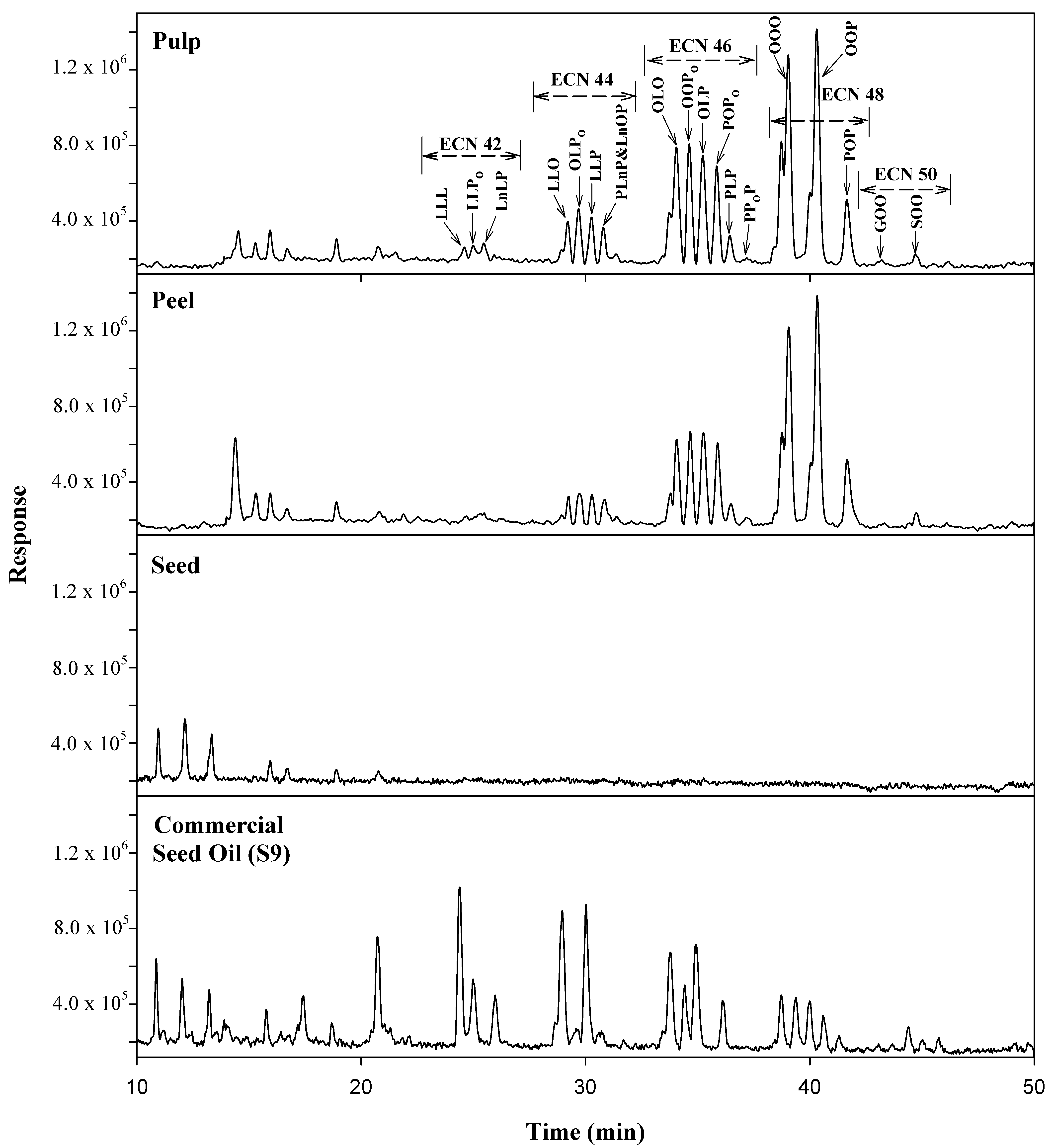
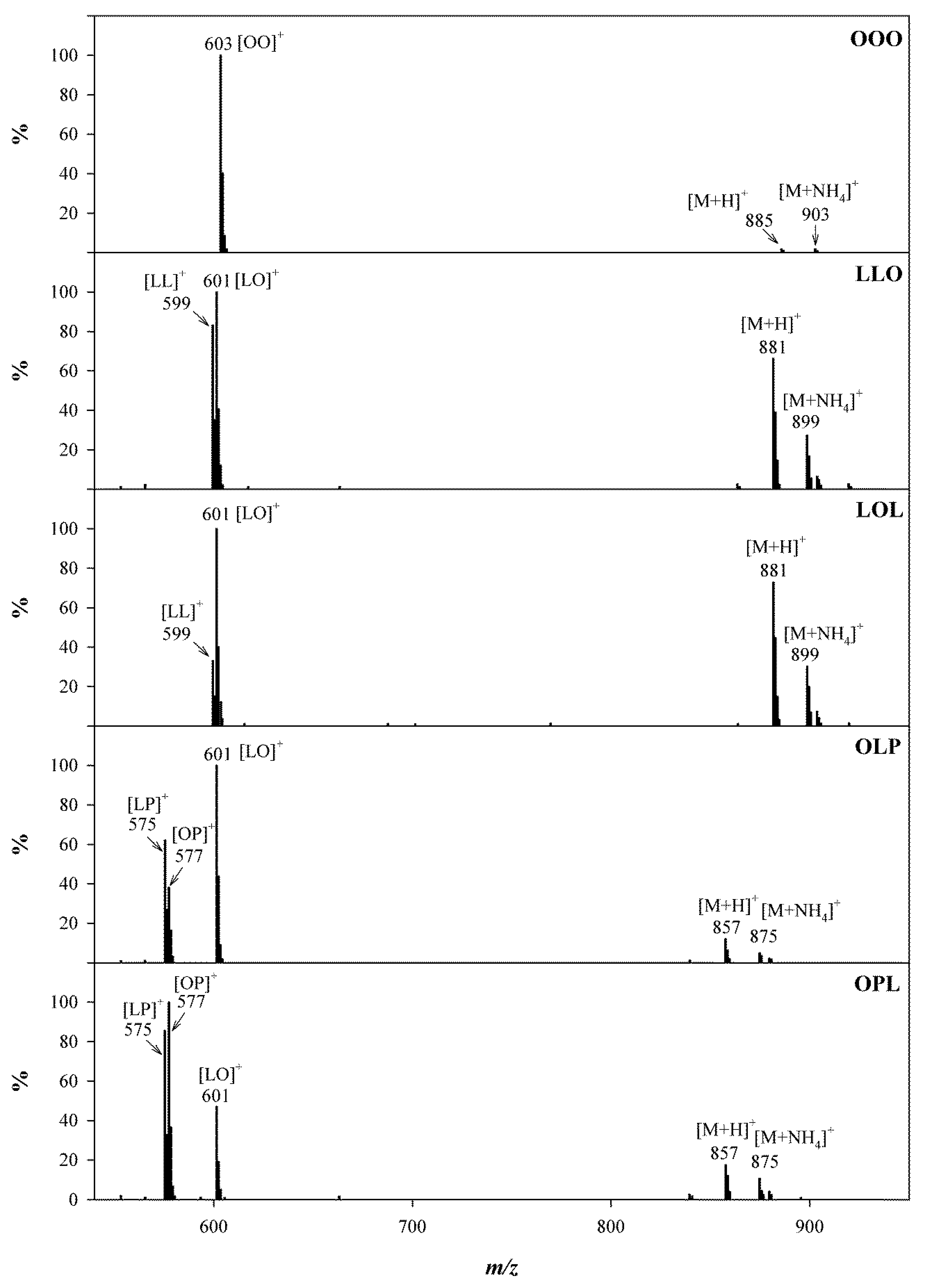
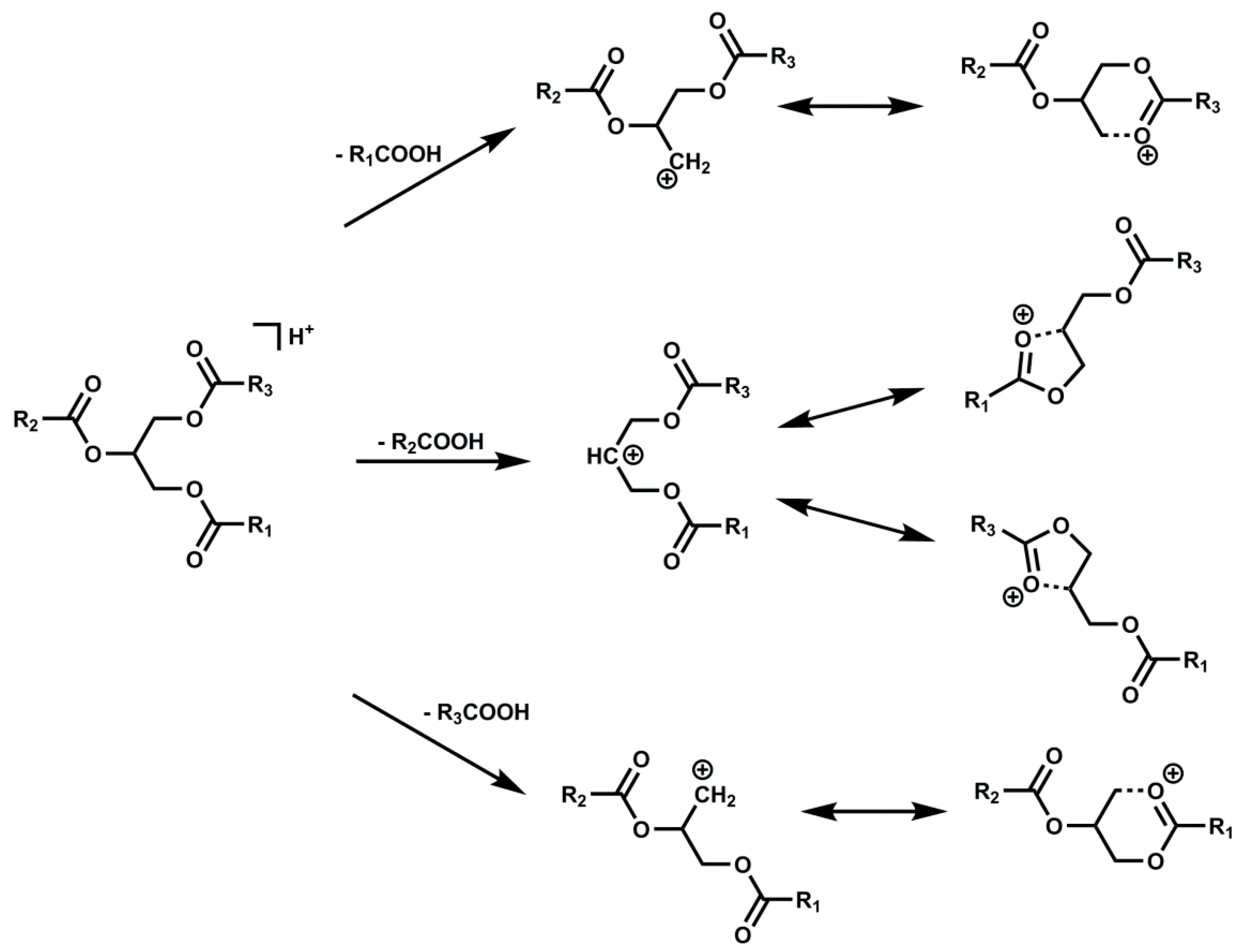
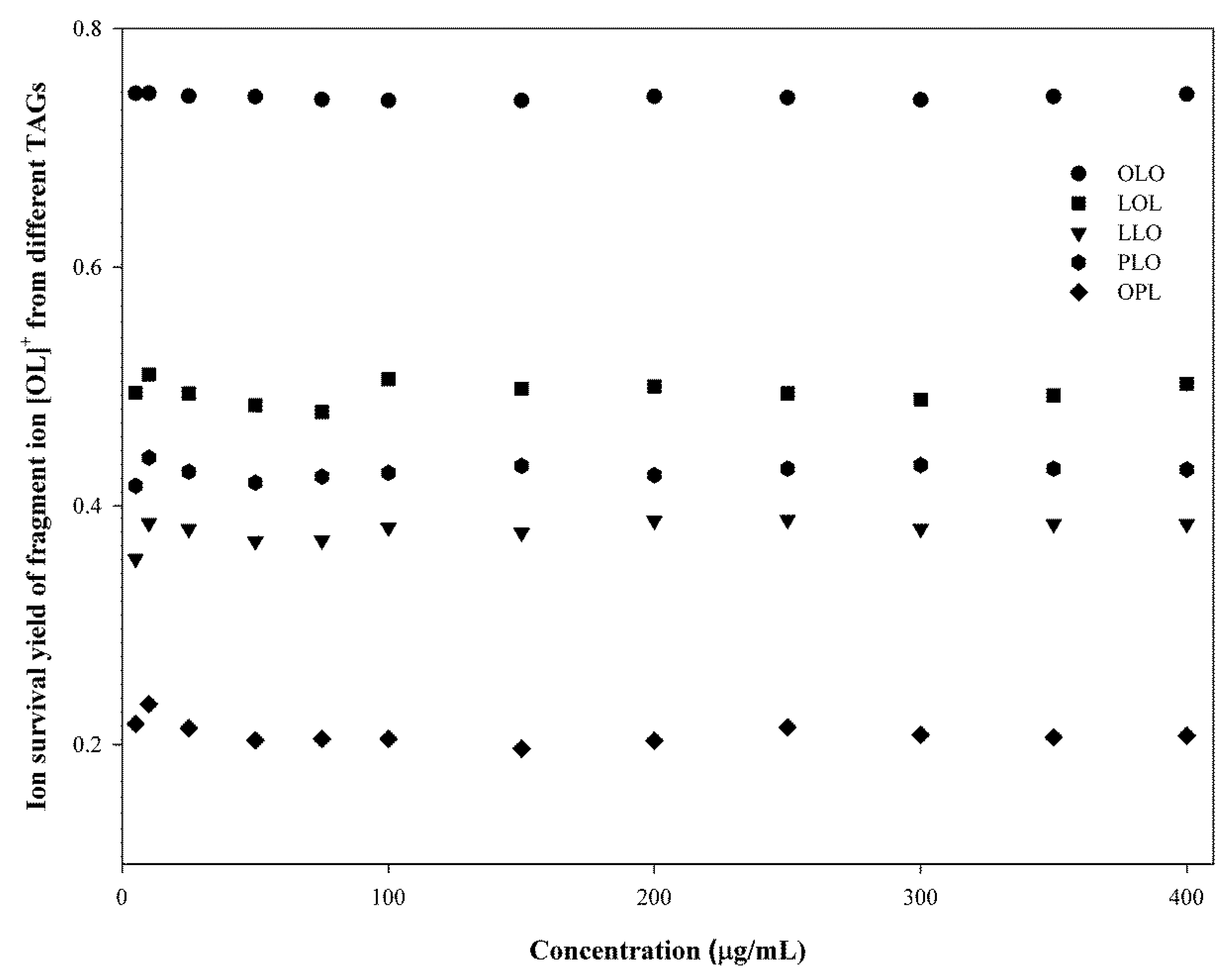
2.2.2. Identification of TGs
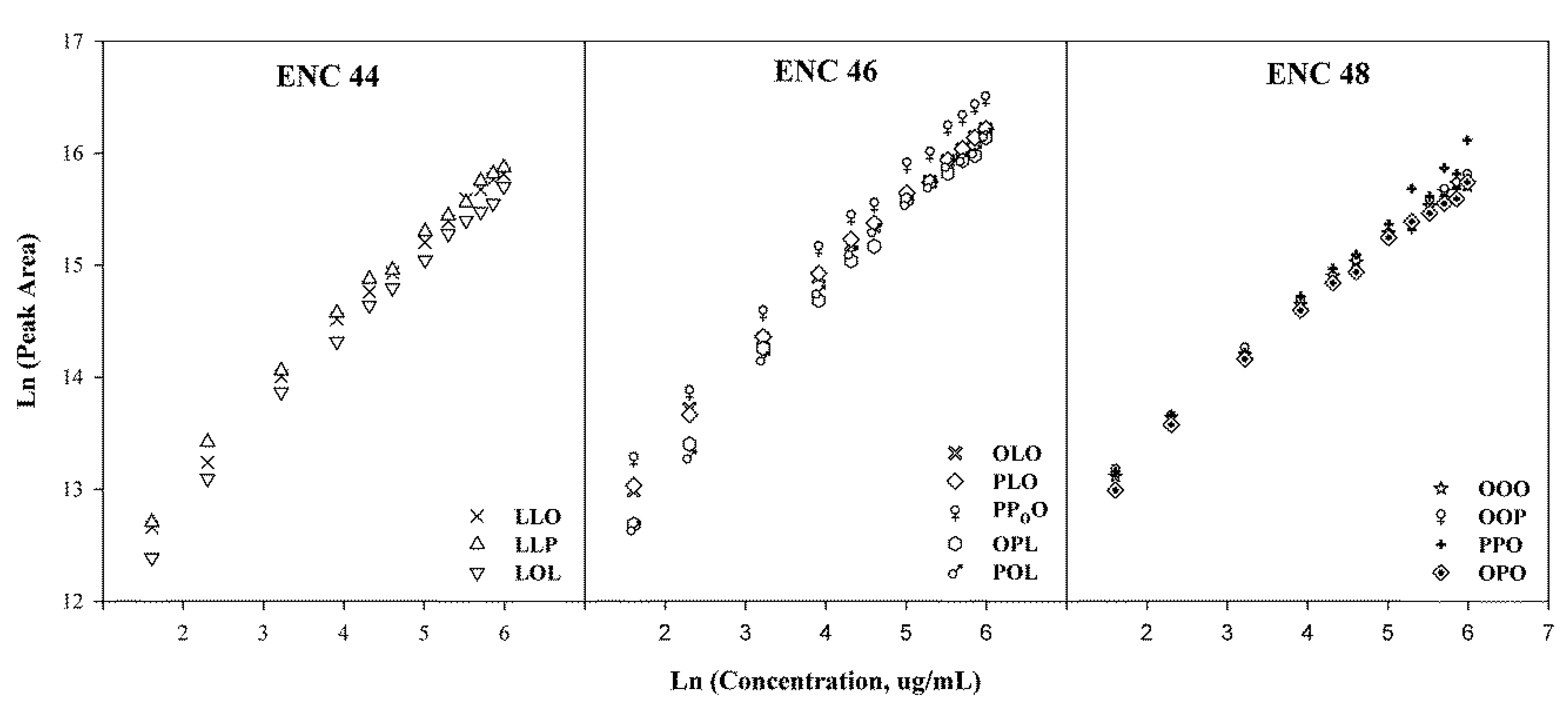
2.2.3. Quantification of TGs
2.3. FAs Profile of Avocado Oils
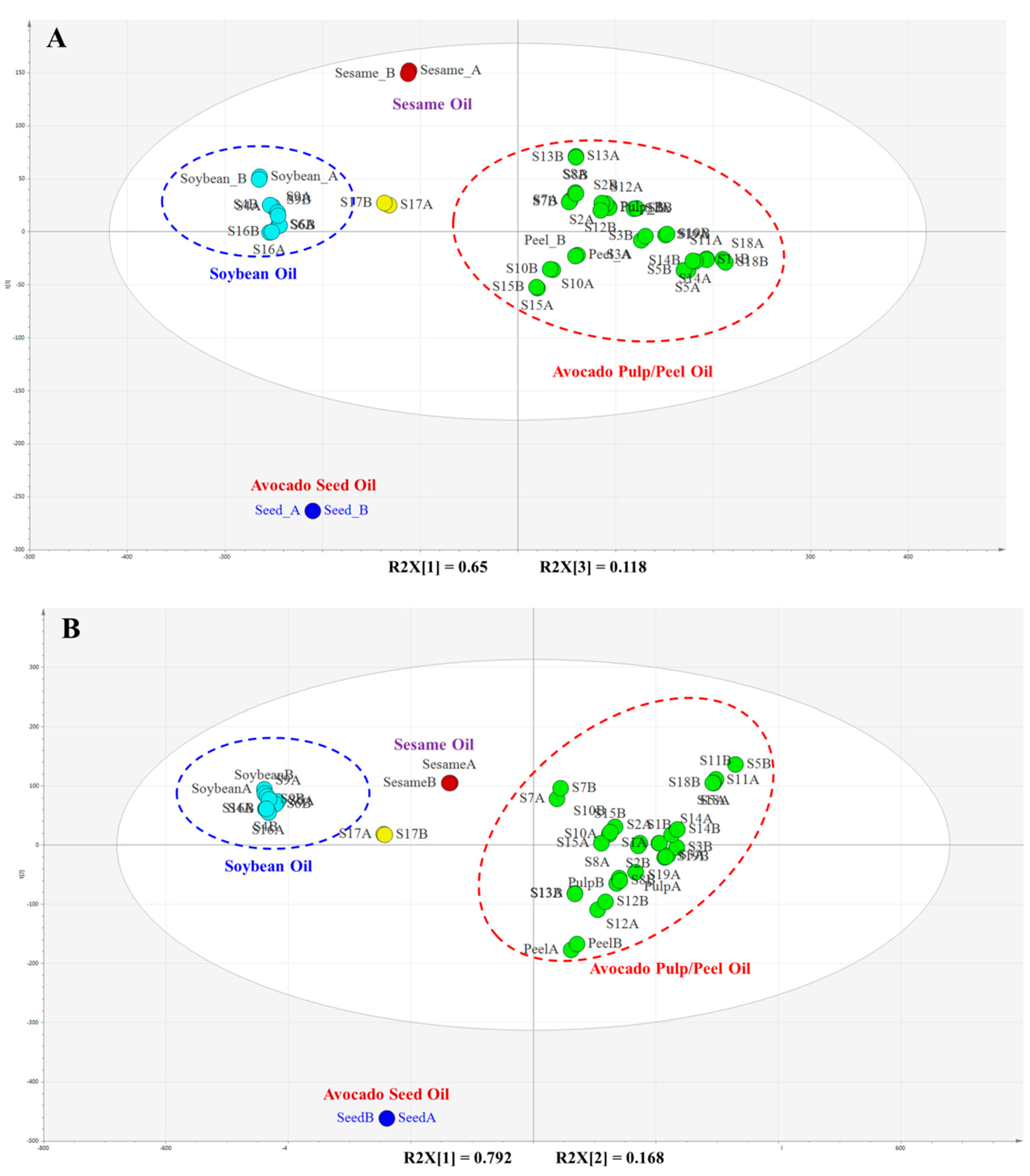
2.4. Identification of Adulteration Using Chemometric Method
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Sample Preparation
3.3. Determination of TGs Using UHPLC/ESI-MS
3.4. Determination of FAs Using GC/MS
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Costagli, G.; Betti, M. Avocado oil extraction processes: Method for cold-pressed high-quality edible oil production versus traditional production. J. Agric. Eng. 2015, 46, 115–122. [Google Scholar] [CrossRef]
- Dabas, D.; Shegog, R.M.; Ziegler, G.R.; Lambert, J.D. Avocado (Persea americana) seed as a source of bioactive phytochemicals. Curr. Pharm. Des. 2013, 19, 6133–6140. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, M.A.Z.; Alicieo, T.V.R.; Pereira, C.M.P.; Ramis-Ramos, G.; Mendonca, C.R.B. Profile of bioactive compounds in avocado pulp oil: Influence of the drying processes and extraction methods. J. Am. Oil Chem. Soc. 2014, 91, 19–27. [Google Scholar] [CrossRef]
- Barros, H.D.F.Q.; Coutinho, J.P.; Grimaldi, R.; Godoy, H.T.; Cabral, F.A. Simultaneous extraction of edible oil from avocado and capsanthin from red bell pepper using supercritical carbon dioxide as solvent. J. Supercrit. Fluids 2016, 107, 315–320. [Google Scholar] [CrossRef]
- Woolf, A.; Wong, M.; Eyres, L.; McGhie, T.K. Avocado Oil; AOCS Press: Urbana, IL, USA, 2008. [Google Scholar]
- Qin, X.; Zhong, J. A review of extraction techniques for avocado oil. J. Oleo Sci. 2016, 65, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Fernández, E.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Avocado fruit—Persea americana. In Exotic Fruits; Rodrigues, S., de Oliveira Silva, E., de Brito, E.S., Eds.; Academic Press: London, UK, 2018. [Google Scholar]
- Tan, C.X.; Chong, G.H.; Hamzah, H.; Ghazali, H.M. Effect of virgin avocado oil on diet-induced hypercholesterolemia in rats via 1H NMR-based metabolomics approach. Phytother. Res. 2018, 32, 2264–2274. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.X.; Chong, G.H.; Hamzah, H.; Ghazali, H.M. Hypocholesterolaemic and hepatoprotective effects of virgin avocado oil in diet-induced hypercholesterolaemia rats. Int. J. Food Sci. Technol. 2018, 53, 2706–2713. [Google Scholar] [CrossRef]
- Marquez-Ramirez, C.A.; Hernandez de la Paz, J.L.; Ortiz-Avila, O.; Raya-Farias, A.; Gonzalez-Hernandez, J.C.; Rodriguez-Orozco, A.R.; Salgado-Garciglia, R.; Saavedra-Molina, A.; Godinez-Hernandez, D.; Cortes-Rojo, C. Comparative effects of avocado oil and losartan on blood pressure, renal vascular function, and mitochondrial oxidative stress in hypertensive rats. Nutrition 2018, 54, 60–67. [Google Scholar] [CrossRef]
- Ortiz-Avila, O.; Gallegos-Corona, M.A.; Sanchez-Briones, L.A.; Calderon-Cortes, E.; Montoya-Perez, R.; Rodriguez-Orozco, A.R.; Campos-Garcia, J.; Saavedra-Molina, A.; Mejia-Zepeda, R.; Cortes-Rojo, C. Protective effects of dietary avocado oil on impaired electron transport chain function and exacerbated oxidative stress in liver mitochondria from diabetic rats. J. Bioenerg. Biomembr. 2015, 47, 337–353. [Google Scholar] [CrossRef]
- Furlan, C.P.B.; Valle, S.C.; Ostman, E.; Marostica, M.R., Jr.; Tovar, J. Inclusion of Hass avocado-oil improves postprandial metabolic responses to a hypercaloric-hyperlipidic meal in overweight subjects. J. Funct. Foods 2017, 38, 349–354. [Google Scholar] [CrossRef]
- Lu, Q.-Y.; Arteaga, J.R.; Zhang, Q.; Huerta, S.; Go, V.L.W.; Heber, D. Inhibition of prostate cancer cell growth by an avocado extract: Role of lipid-soluble bioactive substances. J. Nutr. Biochem. 2005, 16, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M.L.; Davenport, A.J. Hass avocado composition and potential health effects. Crit. Rev. Food Sci. Nutr. 2013, 53, 738–750. [Google Scholar] [CrossRef] [PubMed]
- FAO (Food and Agriculture Organisation). FAO-STAT. 2014. Available online: http://faostat3.fao.org/home/index.html (accessed on 25 January 2020).
- FAO (ORGANIZAÇÃO DAS UNIDAS PARA ALIMENTAÇÃOE AGRICULTURA). Available online: http://www.fao.org (accessed on 16 January 2020).
- Quinones-Islas, N.; Meza-Marquez, O.G.; Osorio-Revilla, G.; Gallardo-Velazquez, T. Detection of adulterants in avocado oil by Mid-FTIR spectroscopy and multivariate analysis. Food Res. Int. 2013, 51, 148–154. [Google Scholar] [CrossRef]
- Jimenez-Sotelo, P.; Hernandez-Martinez, M.; Osorio-Revilla, G.; Meza-Marquez, O.G.; Garcia-Ochoa, F.; Gallardo-Velazquez, T. Use of ATR-FTIR spectroscopy coupled with chemometrics for the authentication of avocado oil in ternary mixtures with sunflower and soybean oils. Food Addit. Contam. Part A 2016, 33, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Merchak, N.; Rizk, T.; Silvestre, V.; Remaud, G.S.; Bejjani, J.; Akoka, S. Olive oil characterization and classification by 13C NMR with a polarization transfer technique: A comparison with gas chromatography and 1H NMR. Food Chem. 2018, 245, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Ji, S.-X.; Hu, N.; Lv, X.; Dong, X.-Y.; Feng, Y.-Q.; Chen, H. Online profiling of triacylglycerols in plant oils by two-dimensional liquid chromatography using a single column coupled with atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. A 2013, 1312, 69–79. [Google Scholar] [CrossRef]
- Hou, J.-J.; Cao, C.-M.; Xu, Y.-W.; Yao, S.; Cai, L.-Y.; Long, H.-L.; Bi, Q.-R.; Zhen, Y.-Y.; Wu, W.-Y.; Guo, D.-A. Exploring lipid markers of the quality of coix seeds with different geographical origins using supercritical fluid chromatography mass spectrometry and chemometrics. Phytomedicine 2018, 45, 1–7. [Google Scholar] [CrossRef]
- Lisa, M.; Holcapek, M.; Rezanka, T.; Kabatova, N. High-performance liquid chromatography-atmospheric pressure chemical ionization mass spectrometry and gas chromatography-flame ionization detection characterization of Δ5-polyenoic fatty acids in triacylglycerols from conifer seed oils. J. Chromatogr. A 2007, 1146, 67–77. [Google Scholar] [CrossRef]
- Lisa, M.; Netusilova, K.; Franek, L.; Dvorakova, H.; Vrkoslav, V.; Holcapek, M. Characterization of fatty acid and triacylglycerol composition in animal fats using silver-ion and non-aqueous reversed-phase high-performance liquid chromatography/mass spectrometry and gas chromatography/flame ionization detection. J. Chromatogr. A 2011, 1218, 7499–7510. [Google Scholar] [CrossRef]
- Byrdwell, W.C.; Neff, W.E. Dual parallel electrospray ionization and atmospheric pressure chemical ionization mass spectrometry (MS), MS/MS and MS/MS/MS for the analysis of triacylglycerols and triacylglycerol oxidation products. Rapid Commun. Mass Spectrom. 2002, 16, 300–319. [Google Scholar] [CrossRef]
- Yoshida, H.; Tomiyama, Y.; Yoshida, N.; Mizushina, Y. Characteristics of lipid components, fatty acid distributions and triacylglycerol molecular species of adzuki beans (Vigna angularis). Food Chem. 2009, 115, 1424–1429. [Google Scholar] [CrossRef]
- Krumreich, F.D.; Borges, C.D.; Mendonca, C.R.B.; Jansen-Alves, C.; Zambiazi, R.C. Bioactive compounds and quality parameters of avocado oil obtained by different processes. Food Chem. 2018, 257, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Official and Tentative Methods of the American Oils Chemists’ Society; American Oil Chemists’ Society: Champaign, IL, USA, 1992.
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Horwitz, W., Latimer, G., Eds.; AOAC International: Maryland, MD, USA, 2007. [Google Scholar]
- Wei, F.; Hu, N.; Lv, X.; Dong, X.-Y.; Chen, H. Quantitation of triacylglycerols in edible oils by off-line comprehensive two-dimensional liquid chromatography-atmospheric pressure chemical ionization mass spectrometry using a single column. J. Chromatogr. A 2015, 1404, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Lisa, M.; Holcapek, M. Triacylglycerols profiling in plant oils important in food industry, dietetics and cosmetics using high-performance liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. A 2008, 1198–1199, 115–130. [Google Scholar] [CrossRef]
- Holcapek, M.; Lisa, M.; Jandera, P.; Kabatova, N. Quantitation of triacylglycerols in plant oils using HPLC with APCI-MS, evaporative light-scattering, and UV detection. J. Sep. Sci. 2005, 28, 1315–1333. [Google Scholar] [CrossRef]
- Lee, K.W.Y.; Porter, C.J.H.; Boyd, B.J. A simple quantitative approach for the determination of long and medium chain lipids in bio-relevant matrices by high performance liquid chromatography with refractive index detection. AAPS PharmSciTech 2013, 14, 927–934. [Google Scholar] [CrossRef]
- Holcapek, M.; Jandera, P.; Zderadicka, P.; Hruba, L. Characterization of triacylglycerol and diacylglycerol composition of plant oils using high-performance liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. A 2003, 1010, 195–215. [Google Scholar] [CrossRef]
- Parcher, J.F.; Wang, M.; Chittiboyina, A.G.; Khan, I.A. In-source collision-induced dissociation (IS-CID): Applications, issues and structure elucidation with single-stage mass analyzers. Drug Test. Anal. 2018, 10, 28–36. [Google Scholar] [CrossRef]
- Crellin, K.C.; Sible, E.; Van Antwerp, J. Quantification and confirmation of identity of analytes in various matrices with in-source collision-induced dissociation on a single quadrupole mass spectrometer. Int. J. Mass Spectrom. 2003, 222, 281–311. [Google Scholar] [CrossRef]
- Hou, J.-J.; Guo, J.-L.; Cao, C.-M.; Yao, S.; Long, H.-L.; Cai, L.-Y.; Da, J.; Wu, W.-Y.; Guo, D.-A. Green quantification strategy combined with chemometric analysis for triglycerides in seeds used in traditional chinese medicine. Planta Med. 2018, 84, 457–464. [Google Scholar] [CrossRef]
- Yang, Y.; Ferro, M.D.; Cavaco, I.; Liang, Y. Detection and Identification of Extra Virgin Olive Oil Adulteration by GC-MS Combined with Chemometrics. J. Agric. Food Chem. 2013, 61, 3693–3702. [Google Scholar] [CrossRef] [PubMed]
- Ai, F.-F.; Bin, J.; Zhang, Z.-M.; Huang, J.-H.; Wang, J.-B.; Liang, Y.-Z.; Yu, L.; Yang, Z.-Y. Application of random forests to select premium quality vegetable oils by their fatty acid composition. Food Chem. 2014, 143, 472–478. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| Extraction Method | SFE | Solvent | |||||
|---|---|---|---|---|---|---|---|
| Extraction Conditions | Solvent | CO2 | n-Hexane | ||||
| Time (min) | 40 | 480 | |||||
| Temperature (°C) | 50 | 70 | |||||
| Pressure (bar) | 250 | atmospheric | |||||
| Sample | Peel | Pulp | Seed *,b | Peel | Pulp | Seed *,b | |
| Oil Yield *,a (%) | 16.89 ± 0.56 | 56.10 ± 0.66 | 1.61 ± 0.31 | 17.32 ± 0.30 | 58.35 ± 0.27 | 1.66 ± 0.61 | |
| Physicochemical Properties | Acid Value (g/oleic acid 100 g) | 1.60 ± 0.13 | 0.94 ± 0.03 | N/A | 0.69 ± 0.03 | 0.38 ± 0.00 | N/A |
| Saponification Value (mg KOH/g) | 186.90 ± 0.74 | 193.71 ± 1.05 | N/A | 182.15 ± 0.11 | 186.15 ± 0.88 | N/A | |
| Iodine Value (g I2/100 g) | 80.62 ± 0.38 | 90.76 ± 0.46 | N/A | 72.38 ± 0.41 | 88.40 ± 0.91 | N/A | |
| Peroxide Value | 2.36 ± 0.13 | 1.18 ± 0.02 | N/A | 3.53 ± 0.09 | 2.41 ± 0.13 | N/A | |
| No. | Compound | Abbr. | ECN | MW | Formula | CAS # |
|---|---|---|---|---|---|---|
| 1 | 1,3-linolein-2-olein | LOL | 44 | 881.40 | C57H100O6 | 2190-22-9 |
| 2 | 1,2-linolein-3-olein | LLO | 44 | 881.40 | C57H100O6 | 2190-21-8 |
| 3 | 1,2- linolein-3-palmitin | LLP | 44 | 855.36 | C55H98O6 | 2190-15-0 |
| 4 | 1,3-olein-2-linolein | OLO | 46 | 883.42 | C57H102O6 | 2190-19-4 |
| 5 | 1-olein-2-palmitin-3-linolein | OPL | 46 | 857.38 | C55H100O6 | 2534-97-6 |
| 6 | 1-palmitin-2-linolein-3-olein | PLO | 46 | 857.38 | C55H100O6 | 2680-59-3 |
| 7 | 1-palmitin-2-palmitolein-3-olein | PPoO | 46 | 831.34 | C53H98O6 | 81637-60-7 |
| 8 | 1-palmitin-2-olein-3-linolein | POL | 46 | 857.38 | C55H100O6 | 2680-59-3 |
| 9 | 1,2-olein-3-palmitin | OOP | 48 | 859.39 | C55H102O6 | 2190-30-9 |
| 10 | 1,2-palmitin-3-olein | PPO | 48 | 833.36 | C53H100O6 | 1867-91-0 |
| 11 | 1,3-olein-2-palmitin | OPO | 48 | 859.39 | C55H102O6 | 1716-07-0 |
| 12 | triolein | OOO | 48 | 885.43 | C57H104O6 | 122-32-7 |
| Sample | LLO | OLPo | LLP | PLnP | OLO | OOPo | OLP | POPo | PLP | OOO | OOP | POP | SOO | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ENC 44 | ENC 46 | ENC 48 | ENC 50 | ||||||||||||
| Authenticated Sample | |||||||||||||||
| Avocado | Peel | 6.11 | 9.88 | 8.90 | 3.03 | 114.56 | 48.36 | 46.24 | 45.04 | 4.87 | 184.01 | 168.52 | 32.22 | 2.54 | 674.27 |
| Pulp | 12.13 | 23.00 | 17.71 | 5.36 | 163.65 | 64.59 | 57.27 | 53.46 | 7.14 | 215.97 | 213.03 | 26.88 | 2.37 | 862.55 | |
| Seed | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Sesame | 153.08 | ND | 53.07 | ND | 360.28 | ND | 96.16 | ND | 8.04 | 87.04 | 51.14 | 6.97 | 39.92 | 855.69 | |
| Soybean | 105.65 | ND | 100.94 | ND | 130.00 | ND | 65.01 | ND | 18.16 | 11.16 | 14.07 | 2.10 | 4.57 | 451.67 | |
| Commercial Sample | |||||||||||||||
| S1 | 20.08 | 10.19 | 14.80 | 3.52 | 196.21 | 38.00 | 51.55 | 36.95 | 6.57 | 286.11 | 179.71 | 27.78 | 17.39 | 888.87 | |
| S2 | 27.56 | 8.12 | 18.09 | 2.88 | 172.84 | 41.11 | 44.14 | 33.82 | 3.78 | 246.88 | 159.06 | 22.50 | 23.77 | 804.52 | |
| S3 | 13.27 | 8.47 | 11.56 | 4.12 | 137.46 | 38.66 | 40.19 | 39.87 | 6.87 | 265.76 | 179.46 | 29.03 | 22.59 | 797.32 | |
| S4 | 24.77 | ND | ND | 0.34 | 96.29 | ND | ND | ND | ND | ND | 20.10 | 3.27 | 18.80 | 163.56 | |
| S5 | 44.51 | ND | 13.41 | ND | 165.75 | 6.64 | 27.82 | ND | 1.45 | 377.75 | 97.94 | 4.35 | 51.84 | 791.44 | |
| S6 | 107.34 | ND | 93.88 | 0.58 | 137.12 | ND | 61.63 | ND | 14.79 | 14.30 | 13.24 | 1.96 | 6.40 | 451.24 | |
| S7 | 96.36 | ND | 55.88 | ND | 190.11 | ND | 53.50 | ND | 5.76 | 250.13 | 57.13 | 4.27 | 33.76 | 746.91 | |
| S8 | 8.66 | 22.47 | 18.53 | 8.29 | 120.27 | 55.97 | 64.96 | 68.08 | 12.79 | 155.42 | 214.73 | 46.91 | 3.49 | 800.57 | |
| S9 | 106.36 | ND | 104.00 | 0.68 | 134.13 | ND | 60.83 | ND | 15.26 | 15.09 | 13.43 | 2.05 | 5.30 | 457.14 | |
| S10 | 43.90 | ND | 8.32 | ND | 342.05 | ND | 24.27 | ND | 0.49 | 243.62 | 33.42 | 0.69 | 13.73 | 710.50 | |
| S11 | 47.24 | ND | 13.44 | ND | 166.03 | 5.03 | 29.59 | ND | 2.99 | 372.85 | 97.72 | 9.37 | 40.82 | 785.08 | |
| S12 | 9.16 | 20.91 | 20.09 | 8.69 | 139.01 | 54.32 | 53.33 | 68.14 | 11.85 | 204.35 | 213.48 | 35.05 | 4.10 | 842.47 | |
| S13 | 18.04 | 22.61 | 21.84 | 6.08 | 183.92 | 43.22 | 64.18 | 36.20 | 9.24 | 190.67 | 182.76 | 22.59 | 2.23 | 803.58 | |
| S14 | 26.41 | 2.20 | 12.33 | 0.67 | 121.38 | 15.54 | 28.78 | 10.28 | 2.16 | 330.82 | 98.37 | 10.09 | 36.80 | 695.84 | |
| S15 | 41.38 | ND | 8.15 | ND | 302.47 | ND | 21.06 | ND | 0.41 | 219.81 | 27.92 | 0.45 | 12.43 | 634.07 | |
| S16 | 97.55 | ND | 89.99 | ND | 120.85 | ND | 58.65 | ND | 13.95 | 9.48 | 11.62 | 1.62 | 4.53 | 408.24 | |
| S17 | 68.92 | 1.41 | 70.08 | 1.68 | 109.23 | 20.16 | 60.72 | 13.79 | 12.50 | 66.07 | 62.91 | 13.00 | 4.47 | 504.95 | |
| S18 | 44.27 | ND | 12.57 | ND | 145.54 | 5.11 | 23.75 | 0.75 | 1.39 | 364.13 | 82.06 | 4.46 | 43.27 | 727.28 | |
| S19 | 10.69 | ND | 10.13 | 1.41 | 72.82 | 23.30 | 40.16 | 17.31 | 16.65 | 278.46 | 129.25 | 37.57 | 26.84 | 664.59 | |
| Sample | Palmitic | Palmitoleic | Stearic | Oleic | Vaccenic | Linoleic | Linolenic | Total | |
|---|---|---|---|---|---|---|---|---|---|
| * C16:0 | C16:1 | C18:0 | C18:1 n9 | C18:1 n11 | C18:2 n9,12 | C18:3 n9,12,15 | |||
| Authenticated Sample | |||||||||
| Avocado | Peel | 101.76 | 37.26 | 1.79 | 416.79 | 39.82 | 55.09 | 7.24 | 659.76 |
| Pulp | 138.42 | 67.19 | 1.36 | 538.68 | 52.66 | 89.38 | 5.61 | 893.30 | |
| Seed | 5.72 | 1.46 | ND | 11.21 | 1.84 | 14.34 | 3.11 | 37.67 | |
| Sesame | 87.76 | 2.91 | 49.91 | 428.78 | 8.91 | 384.17 | 2.38 | 964.83 | |
| Soybean | 103.50 | ND | 36.05 | 192.76 | 12.07 | 551.96 | 71.20 | 967.54 | |
| Commercial Sample | |||||||||
| S1 | 140.54 | 45.56 | 9.75 | 628.24 | 35.28 | 104.96 | 5.12 | 969.44 | |
| S2 | 135.90 | 44.55 | 17.57 | 600.69 | 32.53 | 122.65 | 4.59 | 958.48 | |
| S3 | 149.64 | 52.67 | 11.02 | 636.63 | 32.51 | 81.85 | 3.86 | 968.19 | |
| S4 | 96.24 | ND | 36.00 | 176.21 | 11.83 | 529.57 | 68.28 | 918.14 | |
| S5 | 57.99 | 2.87 | 18.56 | 780.24 | 10.99 | 124.76 | 2.36 | 997.76 | |
| S6 | 99.11 | 1.46 | 45.46 | 195.88 | 12.15 | 524.70 | 75.54 | 954.29 | |
| S7 | 70.61 | 1.70 | 19.69 | 555.30 | 7.45 | 263.92 | 6.65 | 925.31 | |
| S8 | 216.42 | 68.96 | 4.18 | 540.09 | 39.63 | 100.33 | 7.17 | 976.78 | |
| S9 | 103.67 | 1.39 | 37.24 | 189.92 | 12.12 | 539.78 | 73.01 | 957.13 | |
| S10 | 31.11 | 2.36 | 12.81 | 584.51 | 24.07 | 157.89 | 58.09 | 870.84 | |
| S11 | 69.93 | 2.80 | 16.78 | 763.75 | 11.01 | 125.40 | 2.46 | 992.14 | |
| S12 | 176.64 | 86.07 | 2.61 | 492.78 | 46.63 | 86.01 | 5.51 | 896.25 | |
| S13 | 140.03 | 60.34 | 1.39 | 472.40 | 54.00 | 126.68 | 7.34 | 862.18 | |
| S14 | 75.76 | 16.95 | 18.62 | 659.98 | 20.40 | 100.36 | 3.29 | 895.36 | |
| S15 | 30.87 | 2.45 | 13.00 | 570.62 | 23.34 | 155.03 | 58.48 | 853.80 | |
| S16 | 101.52 | ND | 38.11 | 177.06 | 11.93 | 523.32 | 77.03 | 928.98 | |
| S17 | 128.68 | 22.63 | 28.12 | 296.92 | 24.61 | 381.71 | 51.09 | 933.77 | |
| S18 | 59.78 | 3.24 | 18.18 | 758.07 | 10.49 | 123.22 | 2.86 | 975.85 | |
| S19 | 144.53 | 31.56 | 19.40 | 621.16 | 24.22 | 77.97 | 6.96 | 925.79 | |
| Fatty Acid | Avocado Peel | Avocado Pulp | ||
|---|---|---|---|---|
| LC/MS | GC/MS | LC/MS | GC/MS | |
| Palmitic C16:0 | 16.31 | 15.42 | 15.35 | 15.59 |
| Palmitoleic C16:1 | 4.81 | 5.65 | 5.13 | 7.57 |
| Stearic C18:0 | 0.36 | 0.27 | 0.21 | 0.15 |
| Oleic & Vaccenic C18:1 | 68.15 | 69.21 | 66.98 | 66.21 |
| Linoleic C18:2 | 10.21 | 8.35 | 12.11 | 10.07 |
| Linolenic C18:3 | 0.16 | 1.10 | 0.22 | 0.63 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Yu, P.; Chittiboyina, A.G.; Chen, D.; Zhao, J.; Avula, B.; Wang, Y.-H.; Khan, I.A. Characterization, Quantification and Quality Assessment of Avocado (Persea americana Mill.) Oils. Molecules 2020, 25, 1453. https://doi.org/10.3390/molecules25061453
Wang M, Yu P, Chittiboyina AG, Chen D, Zhao J, Avula B, Wang Y-H, Khan IA. Characterization, Quantification and Quality Assessment of Avocado (Persea americana Mill.) Oils. Molecules. 2020; 25(6):1453. https://doi.org/10.3390/molecules25061453
Chicago/Turabian StyleWang, Mei, Ping Yu, Amar G. Chittiboyina, Dilu Chen, Jianping Zhao, Bharathi Avula, Yan-Hong Wang, and Ikhlas A. Khan. 2020. "Characterization, Quantification and Quality Assessment of Avocado (Persea americana Mill.) Oils" Molecules 25, no. 6: 1453. https://doi.org/10.3390/molecules25061453
APA StyleWang, M., Yu, P., Chittiboyina, A. G., Chen, D., Zhao, J., Avula, B., Wang, Y.-H., & Khan, I. A. (2020). Characterization, Quantification and Quality Assessment of Avocado (Persea americana Mill.) Oils. Molecules, 25(6), 1453. https://doi.org/10.3390/molecules25061453







