Effect of Coffee Cascara Dietary Fiber on the Physicochemical, Nutritional and Sensory Properties of a Gluten-Free Bread Formulation
Abstract
1. Introduction
2. Results and Discussion
2.1. Food Safety of Isolated Coffee Cascara Dietary Fibre and Breads
2.2. Nutritional Analysis of CC, ICCDF and Gluten-Free Breads
2.2.1. Dietary Fiber and Starch Content.
2.2.2. Total Proteins and Amino Acid Profile
2.2.3. Total Fat and Fatty Acid Profile
2.3. Physical Properties of Breads
Water Holding Capacity (WHC) and Oil Holding Capacity (OHC)
2.4. Instrumental Analyses
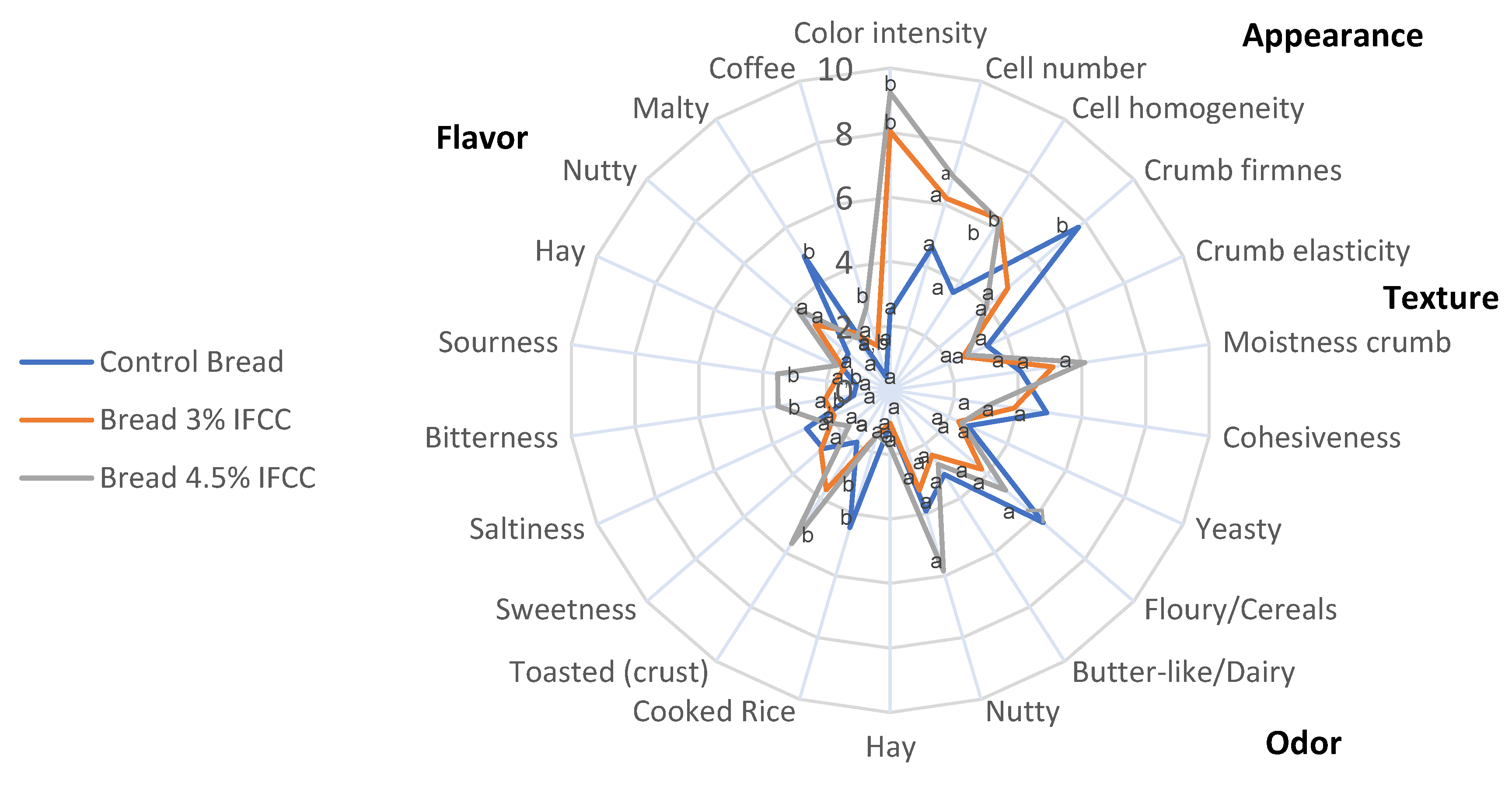
2.5. Sensory Analysis of Breads
3. Conclusions
4. Materials and Methods
4.1. Samples
4.1.1. Isolated Coffee Cascara Dietary Fiber (ICCDF)
4.1.2. Breads
4.2. Food Safety of ICCDF
4.2.1. Analysis of Mycotoxins
4.2.2. Caffeine
4.2.3. Gluten-Free Certification
4.3. Nutritional Analysis of CC, ICCDF and Breads
4.3.1. Dietary Fiber
4.3.2. Starch
4.3.3. Total Protein
4.3.4. Total Amino Acids
4.3.5. Total Fat
4.3.6. Fatty Acids
4.4. Physical Properties
4.4.1. Moisture
4.4.2. Water Holding Capacity and Oil Holding Capacity
4.5. Instrumental Analysis of Gluten-Free Breads
4.5.1. Bread Volume
4.5.2. Crumb Density
4.5.3. Crumb Moisture
4.5.4. Texture Parameters
4.5.5. Crumb Color
4.6. Sensory Analysis of Gluten-Free Breads
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wieben, E. Save Food For A Better Climate: Converting the Food Loss and Waste Challenge into Climate Action; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017. [Google Scholar]
- International Coffee Organization. Total Production by All Exporting Countries. In Trade Statistics Tables. 2019. Available online: http://www.ico.org/trade_statistics.asp (accessed on 10 December 2019).
- de Mendes Ferrão, J. O Café, A Bebida Negra Dos Sonhos Claros; Chaves Ferreira-Publicações S. A.: Lisbon, Portugal, 2009. [Google Scholar]
- De Bruyn, F.; Zhang, S.J.; Pothakos, V.; Torres, J.; Lambot, C.; Moroni, A.V.; Callanan, M.; Sybesma, W.; Weckx, S.; De Vuyst, L. Exploring the Impacts of Postharvest Processing on the Microbiota and Metabolite Profiles during Green Coffee Bean Production. Appl. Environ. Microbiol. 2016, 83, AEM.02398–16. [Google Scholar]
- Gouvea, B.M.; Torres, C.; Franca, A.S.; Oliveira, L.S.; Oliveira, E.S. Feasibility of ethanol production from coffee husks. Biotechnol. Lett. 2009, 31, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.S.; Naidu, M.M.; Srinivas, P. Production of α-amylase under solid-state fermentation utilizing coffee waste. J. Chem. Technol. Biotechnol. 2009, 84, 1246–1249. [Google Scholar] [CrossRef]
- Oliveira, W.E.; Franca, A.S.; Oliveira, L.S.; Rocha, S.D. Untreated coffee husks as biosorbents for the removal of heavy metals from aqueous solutions. J. Hazard. Mater. 2008, 152, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Bekalo, S.A.; Reinhardt, H. Fibers of coffee husk and hulls for the production of particleboard. Mater. Struct. 2009, 43, 1049–1060. [Google Scholar] [CrossRef]
- Mazzafera, P. Degradation of caffeine by microorganisms and potential use of decaffeinated coffee husk and pulp in animal feeding. Sci. Agricola 2002, 59, 815–821. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M. Recovery of Phenolic Antioxidants and Functional Compounds from Coffee Industry By-Products. Food Bioprocess Technol. 2010, 5, 897–903. [Google Scholar] [CrossRef]
- The Coffee Cherry Co. Available online: https://coffeecherryco.com/ (accessed on 15 December 2019).
- Pectcof B.V. Available online: https://pectcof.com/ (accessed on 15 December 2019).
- European Comission Novel Food Catalogue. Available online: https://ec.europa.eu/food/safety/novel_food/catalogue_en (accessed on 15 December 2019).
- Lebwohl, B.; Sanders, D.S.; Green, P.H.R. Coeliac disease. Lancet 2017, 391, 70–81. [Google Scholar] [CrossRef]
- Bardella, M.; Fredella, C.; Prampolini, L.; Molteni, N.; Giunta, A.M.; A Bianchi, P. Body composition and dietary intakes in adult celiac disease patients consuming a strict gluten-free diet. Am. J. Clin. Nutr. 2000, 72, 937–939. [Google Scholar] [CrossRef]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef]
- Rybicka, I.; Doba, K.; Bińczak, O. Improving the sensory and nutritional value of gluten-free bread. Int. J. Food Sci. Technol. 2019, 54, 2661–2667. [Google Scholar] [CrossRef]
- Scazzina, F.; Dall’Asta, M.; Pellegrini, N.; Brighenti, F. Glycaemic index of some commercial gluten-free foods. Eur. J. Nutr. 2014, 54, 1021–1026. [Google Scholar] [CrossRef]
- Kylökäs, A.; Kaukinen, K.; Huhtala, H.; Collin, P.; Mäki, M.; Kurppa, K. Type 1 and type 2 diabetes in celiac disease: prevalence and effect on clinical and histological presentation. BMC Gastroenterol. 2016, 16, 76. [Google Scholar] [CrossRef]
- Guglielmetti, A.; Fernandez-Gomez, B.; Zeppa, G.; del Castillo, M.D. Nutritional Quality, Potential Health Promoting Properties and Sensory Perception of an Improved Gluten-Free Bread Formulation Containing Inulin, Rice Protein and Bioactive Compounds Extracted from Coffee Byproducts. Pol. J. Food Nutr. Sci. 2019, 69, 157–166. [Google Scholar] [CrossRef]
- Cho, S.S. Handbook of Dietary Fiber, 1st ed.; Marcel Dekker, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Thebaudin, J.; Lefebvre, A.; Harrington, M.; Bourgeois, C. Dietary fibres: Nutritional and technological interest. Trends Food Sci. Technol. 1997, 8, 41–48. [Google Scholar] [CrossRef]
- Sciarini, L.; Ribotta, P.; León, A.; Pérez, G.T. Incorporation of several additives into gluten free breads: Effect on dough properties and bread quality. J. Food Eng. 2012, 111, 590–597. [Google Scholar] [CrossRef]
- Anton, A.A.; Artfield, S.D. Hydrocolloids in gluten-free breads: A review. Int. J. Food Sci. Nutr. 2008, 59, 11–23. [Google Scholar] [CrossRef] [PubMed]
- European Commission Whole Grain. Available online: https://ec.europa.eu/jrc/en/health-knowledge-gateway/promotion-prevention/nutrition/whole-grain (accessed on 9 January 2020).
- BOE Real Decreto 308/2019, de 26 de abril, por el que se aprueba la norma de calidad para el pan. Boletín Of. Del Estado 2019, 113, 50168–50175.
- Remypro: A Gluten-Free Rice Protein. Available online: https://www.beneo.com/ingredients/human-nutrition/specialty-rice-ingredients/rice-protein (accessed on 9 March 2020).
- The Commission of the European Communities Regulation (EC) 123/2005. OchratoxinA 2005, 2005, 2004–2006.
- Batista, L.R.; Chalfoun, S.M.; Silva, C.F.; Cirillo, M.; Varga, E.A.; Schwan, R.F. Ochratoxin A in coffee beans (Coffea arabica L.) processed by dry and wet methods. Food Control. 2009, 20, 784–790. [Google Scholar] [CrossRef]
- Atanda, S.A.; Pessu, P.O.; Agoda, S.; Isong, I.U.; Adekalu, O.A.; Echendu, M.A.; Falade, T.C. Fungi and mycotoxins in stored foods. Afr. J. Microbiol. Res. 2011, 5, 5. [Google Scholar]
- Iriondo-DeHond, A.; García, N.A.; Fernandez-Gomez, B.; Guisantes-Batán, E.; Escobar, F.V.; Blanch, G.P.; Andres, M.S.; Rodríguez, S.S.-F.; del Castillo, M.D. Validation of coffee by-products as novel food ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef]
- Heeger, A.; Kosińska-Cagnazzo, A.; Cantergiani, E.; Andlauer, W. Bioactives of coffee cherry pulp and its utilisation for production of Cascara beverage. Food Chem. 2017, 221, 969–975. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific Opinion on the safety of caffeine. EFSA J. 2015, 13, 4102. [Google Scholar]
- Barriga, X.; Callejo, M.J. Pan y Salud: De los Granos Ancestrales al pan de hoy; Grijalbo: Barcelona, Spain, 2017. [Google Scholar]
- World Health Organization (WHO) Food Based Dietary Guidelines in the WHO European Region. Copenhagen, Denmark, 2003. Available online: https://apps.who.int/iris/handle/10665/107490 (accessed on 10 January 2020).
- Alvarado, A.; Pacheco-Delahaye, E.; Hevia, P. Value of a tomato byproduct as a source of dietary fiber in rats. Plant Foods Hum. Nutr. 2001, 56, 335–348. [Google Scholar] [CrossRef]
- Martínez-Castaño, M.; Lopera-Idarraga, J.; Pazmiño-Arteaga, J.; Gallardo-Cabrera, C. Evaluation of the behaviour of unripe banana flour with non-conventional flours in the production of gluten-free bread. Food Sci. Technol. Int. 2019, 26, 160–172. [Google Scholar] [CrossRef]
- Southey, F. Upcycling Coffee Cherries for Food: “Every Major Chocolate Company Is Looking at This”. Available online: https://www.foodnavigator.com/Article/2019/05/20/Upcycling-coffee-cherries-for-food-Every-major-chocolate-company-is-looking-at-this (accessed on 10 January 2020).
- Regulation EC 1924 European Community (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Off. J. Eur. Union 2006, 9–25.
- Arendt Status of Carbohydrates and Dietary Fiber in Gluten-free Diets. Cereal Foods World 2011, 56, 109–114.
- Calvo-Lerma, J.; Crespo-Escobar, P.; Martínez-Barona, S.; Fornés, V.; Donat, E.; Ribes-Koninckx, C. Differences in the macronutrient and dietary fibre profile of gluten-free products as compared to their gluten-containing counterparts. Eur. J. Clin. Nutr. 2019, 73, 930–936. [Google Scholar] [CrossRef]
- Naqash, F.; Gani, A.; Gani, A.; Masoodi, F. Gluten-free baking: Combating the challenges - A review. Trends Food Sci. Technol. 2017, 66, 98–107. [Google Scholar] [CrossRef]
- Chawla, R.; Patil, G. Soluble Dietary Fiber. Compr. Rev. Food Sci. Food Saf. 2010, 9, 178–196. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Position of the American Dietetic Association: Health Implications of Dietary Fiber. J. Am. Diet. Assoc. 2008, 108, 1716–1731. [CrossRef] [PubMed]
- Veronese, N.; Solmi, M.; Caruso, M.G.; Giannelli, G.; Osella, A.R.; Evangelou, E.; Maggi, S.; Fontana, L.; Stubbs, B.; Tzoulaki, I. Dietary fiber and health outcomes: an umbrella review of systematic reviews and meta-analyses. Am. J. Clin. Nutr. 2018, 107, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Roman, L.; Belorio, M.; Gómez, M. Gluten-Free Breads: The Gap Between Research and Commercial Reality. Compr. Rev. Food Sci. Food Saf. 2019, 18, 690–702. [Google Scholar] [CrossRef]
- Elías, L.G. Chemical Composition of Coffee-Berry By-Products. Coffee Pulp Compos. Technol. Util. 1979, 11–16. [Google Scholar]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Saturni, L.; Ferretti, G.; Bacchetti, T. The Gluten-Free Diet: Safety and Nutritional Quality. Nutrients 2010, 2, 16–34. [Google Scholar] [CrossRef]
- Homem, R.V.; Joaquim, A.D.S.; Da Silva, H.P.; Evangelista, S.M.; Komeroski, M.R.; Doneda, D.; Rockett, F.C.; Schmidt, H.D.O.; Rios, A.D.O.; Schäfer, L.; et al. Effect of Teff (Eragrostis tef) on Chemical and Technological Quality of Gluten-free Breads. J. Culin. Sci. Technol. 2019, 1–14. [Google Scholar] [CrossRef]
- Reis, N.; Franca, A.S.; Oliveira, L.S. Discrimination between roasted coffee, roasted corn and coffee husks by Diffuse Reflectance Infrared Fourier Transform Spectroscopy. LWT 2013, 50, 715–722. [Google Scholar] [CrossRef]
- Diet, nutrition and the prevention of chronic diseases. TRS 931 2003, 916, 149.
- Kurek, M.A.; Wyrwisz, J.; Karp, S.; Wierzbicka, A. Effect of fiber sources on fatty acids profile, glycemic index, and phenolic compound content of in vitro digested fortified wheat bread. J. Food Sci. Technol. 2018, 55, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, L.; Tan, B.; Zhang, W. Dietary fibre extracted from different types of whole grains and beans: a comparative study. Int. J. Food Sci. Technol. 2020, 14472. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Coimbra, M.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- McRorie, J.W., Jr.; McKeown, N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef]
- Kuntz, L.A. Fiber: from frustation to funtionality. Food Prod. Des. 1994, 2, 108. [Google Scholar]
- Ghoshal, G.; Shivhare, U.; Banerjee, U. Effect of Xylanase on Quality Attributes of Whole?wheat bread. J. Food Qual. 2013, 36, 172–180. [Google Scholar] [CrossRef]
- Sudha, M.L.; Baskaran, V.; Leelavathi, K. Apple pomace as a source of dietary fiber and polyphenols and its effect on the rheological characteristics and cake making. Food Chem. 2007, 104, 686–692. [Google Scholar] [CrossRef]
- Louis, L.; Fairchild, R.; Setarehnejad, A. Effects of ingredients on sensory attributes of gluten-free breads available in the UK. Br. Food J. 2019, 121, 926–936. [Google Scholar] [CrossRef]
- Oldways Whole Grains Council. Whole Grains Statistics. Available online: https://wholegrainscouncil.org/newsroom/whole-grain-statistics (accessed on 9 March 2020).
- del Castillo, M.D.; Ibañez, M.E.; Amigo, M.; Herrero, M.; Plaza del Moral, M.; Ullate, M. Application of products of coffee silverskin in anti-ageing cosmetics and functional food. Patent WO 2013/004873, 10 January 2013. [Google Scholar]
- del Castillo, M.D.; Ames, J.M.; Gordon, M.H. Effect of Roasting on the Antioxidant Activity of Coffee Brews. J. Agric. Food Chem. 2002, 50, 3698–3703. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; García, A.T.; Pérez, I.D.; Rebollo-Hernanz, M.; Mesias, M.; Morales, F.J.; Martin-Cabrejas, M.A.; del Castillo, M.D. Use of spent coffee grounds as food ingredient in bakery products. Food Chem. 2017, 216, 114–122. [Google Scholar] [CrossRef]
- Toschi, T.G.; Cardenia, V.; Bonaga, G.; Mandrioli, M.; Rodriguez-Estrada, M. Coffee Silverskin: Characterization, Possible Uses, and Safety Aspects. J. Agric. Food Chem. 2014, 62, 10836–10844. [Google Scholar] [CrossRef]
- CIE Technical Committee. Available online: http://cie.co.at/publications/colorimetry-3rd-edition (accessed on 10 January 2020).
- Callejo, M.J. Present Situation On The Descriptive Sensory Analysis Of Bread. J. Sens. Stud. 2011, 26, 255–268. [Google Scholar] [CrossRef]
Sample Availability: Samples of the isolated coffee cascara dietary fiber and novel bread formulations are available from the authors. |


| Analysis | By-Products | Gluten-Free Breads | |||
|---|---|---|---|---|---|
| CC | ICCDF | B1 | B2 | B3 | |
| Starch | |||||
| TS (%) | 2.26 ± 0.06 | nd | 25.02 ± 0.71 b | 20.01 ± 1.74 ab | 19.04 ± 0.93 a |
| RS (%) | nd | nd | 0.85 ± 0.01 a | 0.84 ± 0.16 a | 0.68 ± 0.06 a |
| NRS (%) | 2.19 ± 0.05 | nd | 24.17 ± 1.28 b | 19.17 ± 0.38 a | 18.36 ± 0.09 a |
| Dietary fiber | |||||
| TDF (%) | 47.44 ± 1.85 a | 61.25 ± 0.63 b | 1.11 ± 0.53 a | 6.12 ± 0.26 b | 11.36 ± 0.59 c |
| IDF (%) | 31.32 ± 1.51 a | 55.10 ± 0.30 b | 1.11 ± 0.53 a | 5.17 ± 0.16 b | 9.84 ± 0.06 c |
| SDF (%) | 16.12 ± 1.15 a | 6.20 ± 0.33 b | 0.00 ± 0.00 a | 0.95 ± 0.10 b | 1.52 ± 0.65 c |
| Analysis | By-Products | Breads | |||
|---|---|---|---|---|---|
| CC | ICCDF | B1 | B2 | B3 | |
| Total proteins (%) | 9.55 ± 0.11 b | 10.96 ± 0.40 d | 7.25 ± 0.08 a | 10.02 ± 0.24 bc | 10.58 ± 0.14 cd |
| Amino acids (nmol) | |||||
| Asparagine (Asn) | 2.84 ± 0.14 a | 6.99 ± 3.33 b | 1.51 ± 0.25 a | 2.25 ± 0.21 a | 2.53 ± 0.49 a |
| Threonine (Thr) | 0.96 ± 0.03 a | 2.76 ± 1.73 b | 0.34 ± 0.09 a | 0.51 ± 0.05 a | 0.65 ± 0.14 a |
| Serine (Ser) | 1.91 ± 0.02 a | 5.46 ± 2.69 b | 0.98 ± 0.17 a | 1.63 ± 0.16 a | 1.88 ± 0.36 a |
| Glutamic acid (Glu) | 2.13 ± 0.04 a | 6.24 ± 3.58 b | 2.27 ± 0.59 a | 3.27 ± 0.28 ab | 3.67 ± 0.73 ab |
| Proline (Pro) | 1.60 ± 0.01 a | 4.10 ± 2.12 b | 0.82 ± 0.25 a | 1.24 ± 0.12 a | 1.50 ± 0.35 a |
| Glycine (Gly) | 2.71 ±0.17 a | 7.91 ± 3.39 b | 1.75 ± 0.20 a | 2.88 ± 0.34 a | 3.45 ± 0.68 a |
| Alanine (Ala) | 2.06 ± 0.11 a | 5.63 ± 2.52 b | 1.91 ± 0.32 a | 2.90 ± 0.32 a | 3.20 ± 0.65 a |
| Cysteine (Cys) | 0.18 ± 0.00 a | 0.33 ± 0.15 b | 0.22 ± 0.01 ab | 0.26 ± 0.02 ab | 0.22 ± 0.03 ab |
| Valine (Val) | 1.27 ± 0.02 a | 3.21 ± 1.64 b | 0.96 ± 0.15 a | 1.28 ± 0.10 a | 1.45 ± 0.27 a |
| Methionine (Met) | 0.25 ± 0.02 a | 0.62 ± 0.30 b | 0.17 ± 0.04 a | 0.20 ± 0.03 a | 0.18 ± 0.03 a |
| Isoleucine (Ile) | 0.63 ± 0.05 a | 1.91 ± 1.22 b | 0.40 ± 0.06 a | 0.49 ± 0.04 a | 0.60 ± 0.11 a |
| Leucine (Leu) | 1.17 ± 0.03 a | 3.33 ± 1.98 b | 2.66 ± 0.23 a | 1.28 ± 0.10 a | 1.58 ± 0.32 a |
| Tyrosine (Tyr) | 0.63 ± 0.05 a | 1.56 ± 0.68 b | 0.33 ± 0.07 a | 0.41 ± 0.11 a | 0.46 ± 0.11 a |
| Phenylalanine (Phe) | 0.98 ± 0.10 a | 2.41 ± 0.94 b | 0.98 ± 0.15 a | 1.28 ± 0.12 a | 1.44 ± 0.20 a |
| Histidine (His) | 0.52 ± 0.01 a | 1.67 ± 0.79 b | 0.28 ± 0.05 a | 0.39 ± 0.04 a | 0.58 ± 0.09 a |
| Lysine (Lys) | 0.38 ± 0.01 a | 1.41 ± 0.63 b | 0.56 ± 0.11 a | 0.82 ± 0.13 ab | 1.01 ± 0.23 ab |
| Arginine (Arg) | 0.48 ± 0.03 a | 1.43 ± 1.03 a | 0.38 ± 0.08 a | 0.43 ± 0.09 a | 0.75 ± 0.60 a |
| EAA (% total) | 33.69 ± 0.06 ab | 33.51 ± 0.78 ab | 35.27 ± 0.64 b | 32.24 ± 1.16 a | 32.66 ± 1.37 ab |
| BCAA (Val + Leu + Ile) (%total) | 14.83 ± 0.55 ab | 14.55 ± 1.17 ab | 15.78 ± 0.46 b | 14.19 ± 0.35 a | 14.44 ± 0.26 ab |
| AAA (Phe + Tyr) (% total) | 7.79 ± 0.70 a | 7.15 ± 0.76 a | 8.88 ± 1.07 a | 7.89 ± 1.02 a | 7.63 ± 0.70 a |
| Analysis | By-products | Breads | |||
|---|---|---|---|---|---|
| CC | ICCDF | B1 | B2 | B3 | |
| Total lipids (%) | 2.00 ± 0.5 a | 2.71 ± 0.80 a | 2.95 ± 0.35 a | 2.75 ± 0.52 a | 2.51 ± 0.22 a |
| Fatty acid profile (g/100 g) | |||||
| C12:0 | 0.10 ± 0.02 a | 0.08 ± 0.02 a | N.D. | N.D. | N.D. |
| C14:0 | 1.18 ± 0.02 c | 1.11 ± 0.02 d | 0.27 ± 0.01 a | 0.31 ± 0.01 b | 0.33 ± 0.01 b |
| C15:0 | 0.37 ± 0.01 b | 0.31 ± 0.00 a | N.D. | N.D. | N.D. |
| C16:0 | 36.02 ± 0.32 e | 33.96 ± 0.01 d | 12.21 ± 0.07 a | 13.02 ± 0.04 b | 13.42 ± 0.14 c |
| C16:1n7 | 3.04 ± 0.19 b | 3.46 ± 0.05 c | 0.37 ± 0.01 a | 0.47 ± 0.01 a | 0.43 ± 0.00 a |
| C17:0 | 0.56 ± 0.01 b | 0.49 ± 0.01 a | N.D. | N.D. | N.D. |
| C18:0 | 5.64 ± 0.22 a | 5.64 ± 0.02 a | 8.66 ± 0.35 d | 7.82 ± 0.04 c | 6.65 ± 0.02 b |
| C18:1n7c | 1.79 ± 0.05 b | 1.90 ± 0.01 c | 0.75 ± 0.03 a | 0.74 ± 0.02 a | 0.78 ± 0.01 a |
| C18:1n9c | 6.72 ± 0.37 a | 10.38 ± 0.05 b | 24.98 ± 0.11 c | 27.68 ± 0.01 d | 25.14 ± 0.09 c |
| C18:2n6c | 21.80 ± 0.34 a | 22.30 ± 0.09 a | 50.66 ± 0.29 d | 47.05 ± 0.07 b | 50.13 ± 0.10 c |
| C18:3n3 | 17.37 ± 0.24 e | 15.76 ± 0.06 d | 0.29 ± 0.01 a | 0.80 ± 0.01 b | 1.09 ± 0.05 c |
| C20:0 | 2.82 ± 0.07 d | 2.60 ± 0.01 c | 0.36 ± 0.01 a | 0.46 ± 0.01 b | 0.46 ± 0.01 b |
| C20:1n9 | 0.09 ± 0.00 a | 0.09 ± 0.00 a | 0.16 ± 0.01 b | 0.16 ± 0.00 b | 0.16 ± 0.00 b |
| C20:2n6 | 0.09 ± 0.00 a | 0.09 ± 0.00 a | N.D. | N.D. | N.D. |
| C20:3n3 | 0.19 ± 0.04 a | 0.18 ± 0.00 a | N.D. | N.D. | N.D. |
| C20:5n3 | N.D. | 0.09 ± 0.00 bc | 0.08 ± 0.01 b | 0.11 ± 0.01 d | 0.10 ± 0.01 cd |
| C21:0/C20:3n6 * | 0.10 ± 0.01 a | 0.10 ± 0.00 a | N.D. | N.D. | N.D. |
| C22:0 | 0.64 ± 0.04 b | 0.59 ± 0.01 a | 0.70 ± 0.00 c | 0.74 ± 0.01 d | 0.78 ± 0.01 a |
| C22:1n9 | N.D. | N.D. | 0.16 ± 0.01 a | 0.25 ± 0.00 b | 0.22 ± 0.02 b |
| C22:6n3 | 0.76 ± 0.60 b | 0.20 ± 0.01 a | N.D. | N.D. | N.D. |
| C23:0 | 0.16 ± 0.02 a | 0.17 ± 0.01 a | N.D. | N.D. | N.D. |
| C24:0/C22:5n3 * | 0.57 ± 0.00 c | 0.51 ± 0.01 b | 0.36 ± 0.01 a | 0.38 ± 0.00 a | 0.37 ± 0.01 a |
| SFA | 47.48 ± 0.10 d | 44.94 ± 0.01 c | 22.19 ± 0.41 ab | 22.35 ± 0.07 b | 21.57 ± 0.14 a |
| MUFA | 11.64 ± 0.12 a | 15.83 ± 0.11 b | 26.42 ± 0.12 c | 29.30 ± 0.02 e | 26.74 ± 0.09 d |
| PUFA | 40.21 ± 0.01 b | 38.62 ± 0.14 a | 50.74 ± 0.30 e | 47.17 ± 0.08 c | 50.22 ± 0.10 d |
| Parameters | Gluten-Free Breads | ||
|---|---|---|---|
| B1 | B2 | B3 | |
| Volume (mL) | 1527.50 ± 208.60 a | 1800.00 ± 169.71 a | 1715.00 ± 162.63 a |
| Crumb density (g/mL) | 0.65 ± 0.10 a | 0.55 ± 0.05 a | 0.57 ± 0.06 a |
| Crumb moisture (%) | 45.50 ± 1.22 a | 55.35 ± 0.06 b | 58.54 ± 0.42 c |
| Texture | |||
| Hardness (gf) | 5585.00 ± 726.69 b | 2046.63 ± 323.33 a | 1560.75 ± 202.87 a |
| Elasticity (%) | 16.00 ± 4.08 a | 13.00 ± 4.98 a | 26.50 ± 3.10 b |
| Color | |||
| L * | 80.32 ± 0.98 c | 39.95 ± 1.72 b | 36.22 ± 2.86 a |
| a * | 0.19 ± 0.34 a | 9.49 ± 0.27 b | 9.46 ± 0.30 b |
| b * | 16.77 ± 0.52 a | 20.80 ± 0.52 b | 19.70 ± 0.38 b |
| Ingredients (g) | Gluten-Free Breads | ||
|---|---|---|---|
| B1 | B2 | B3 | |
| Gluten-free baking pre-mix | 547 | 387 | 331 |
| ICCDF | 0 | 57 | 79 |
| Rice protein | 55 | 55 | 51 |
| Yeast | 5 | 5 | 5 |
| Sunflower oil | 19 | 19 | 18 |
| Water | 422 | 525 | 565 |
| Estimated calories (kcal/100 g bread) | 229 | 185 | 167 |
| Estimated protein energetic value (% on total kcal) | 8.6 | 11.7 ‡ | 12.8 ‡ |
| Estimated fiber content (% fiber/100 g bread) | 2.8 | 5.5 ɸ | 6.6 ɸ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rios, M.B.; Iriondo-DeHond, A.; Iriondo-DeHond, M.; Herrera, T.; Velasco, D.; Gómez-Alonso, S.; Callejo, M.J.; del Castillo, M.D. Effect of Coffee Cascara Dietary Fiber on the Physicochemical, Nutritional and Sensory Properties of a Gluten-Free Bread Formulation. Molecules 2020, 25, 1358. https://doi.org/10.3390/molecules25061358
Rios MB, Iriondo-DeHond A, Iriondo-DeHond M, Herrera T, Velasco D, Gómez-Alonso S, Callejo MJ, del Castillo MD. Effect of Coffee Cascara Dietary Fiber on the Physicochemical, Nutritional and Sensory Properties of a Gluten-Free Bread Formulation. Molecules. 2020; 25(6):1358. https://doi.org/10.3390/molecules25061358
Chicago/Turabian StyleRios, Maria Belen, Amaia Iriondo-DeHond, Maite Iriondo-DeHond, Teresa Herrera, Diego Velasco, Sergio Gómez-Alonso, María Jesús Callejo, and Maria Dolores del Castillo. 2020. "Effect of Coffee Cascara Dietary Fiber on the Physicochemical, Nutritional and Sensory Properties of a Gluten-Free Bread Formulation" Molecules 25, no. 6: 1358. https://doi.org/10.3390/molecules25061358
APA StyleRios, M. B., Iriondo-DeHond, A., Iriondo-DeHond, M., Herrera, T., Velasco, D., Gómez-Alonso, S., Callejo, M. J., & del Castillo, M. D. (2020). Effect of Coffee Cascara Dietary Fiber on the Physicochemical, Nutritional and Sensory Properties of a Gluten-Free Bread Formulation. Molecules, 25(6), 1358. https://doi.org/10.3390/molecules25061358








