Development of New Canned Chub Mackerel Products Incorporating Edible Seaweeds—Influence on the Minerals and Trace Elements Composition
Abstract
1. Introduction
2. Results and Discussion
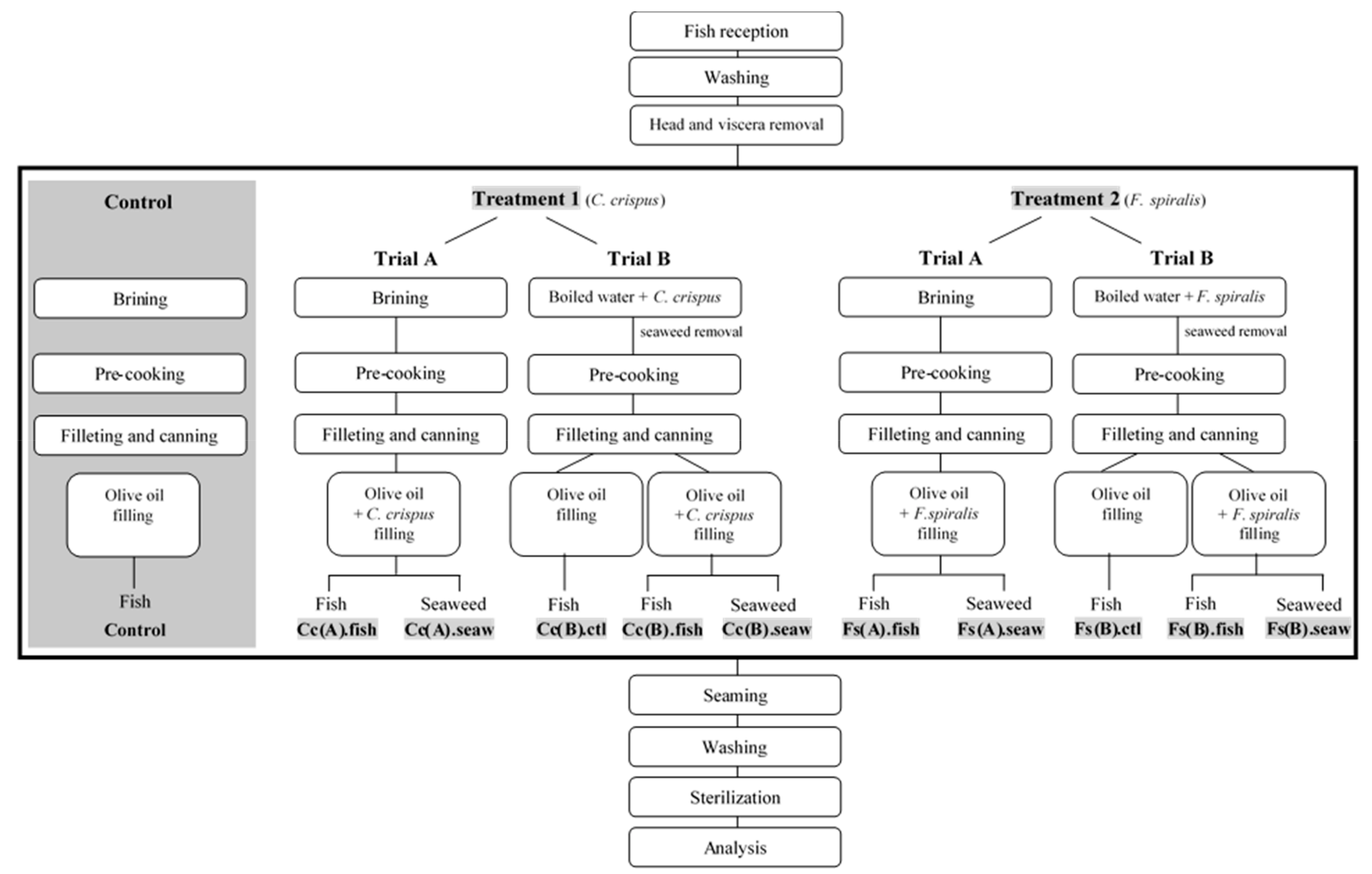
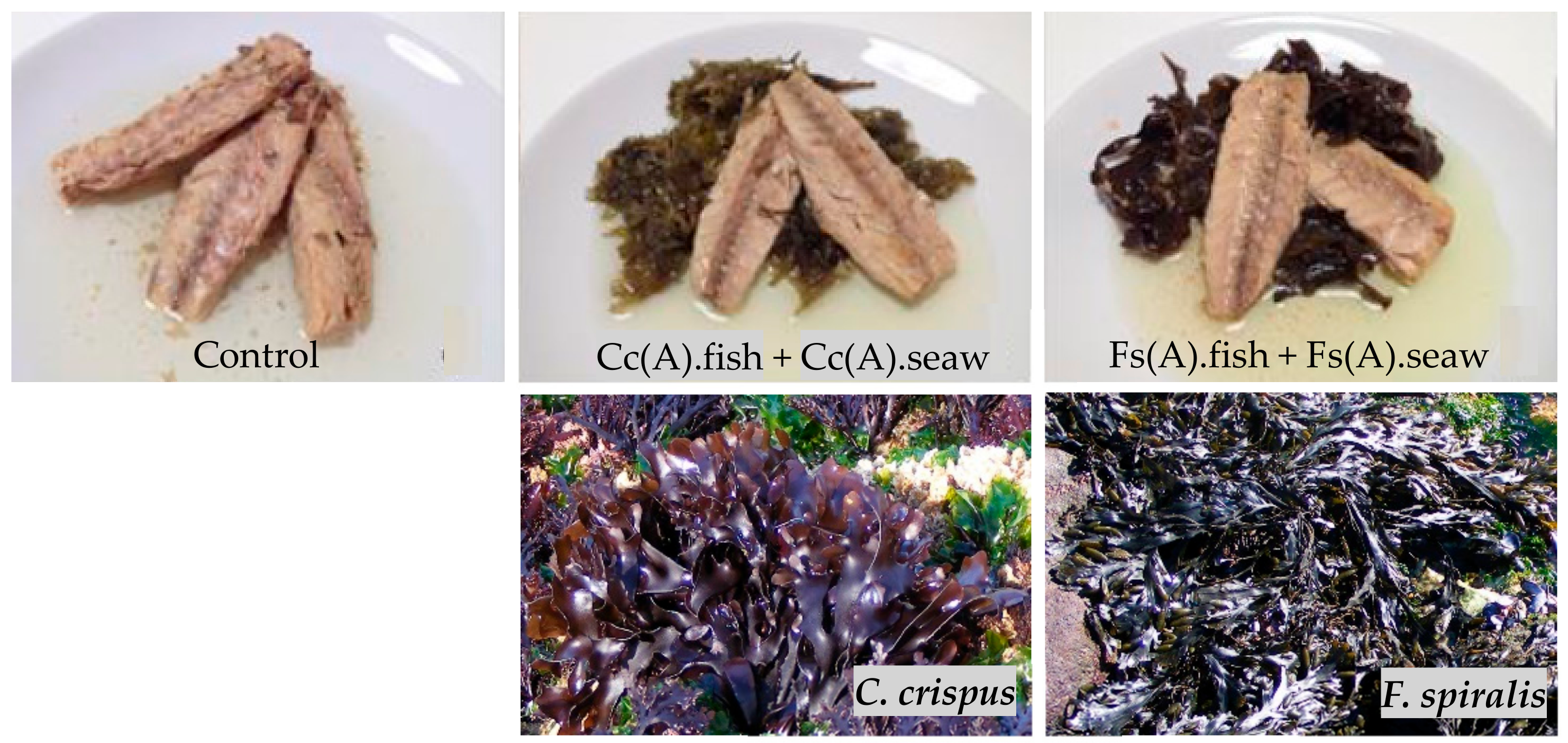
2.1. Optimization of the Canned Chub Mackerel Products Incorporating Seaweeds
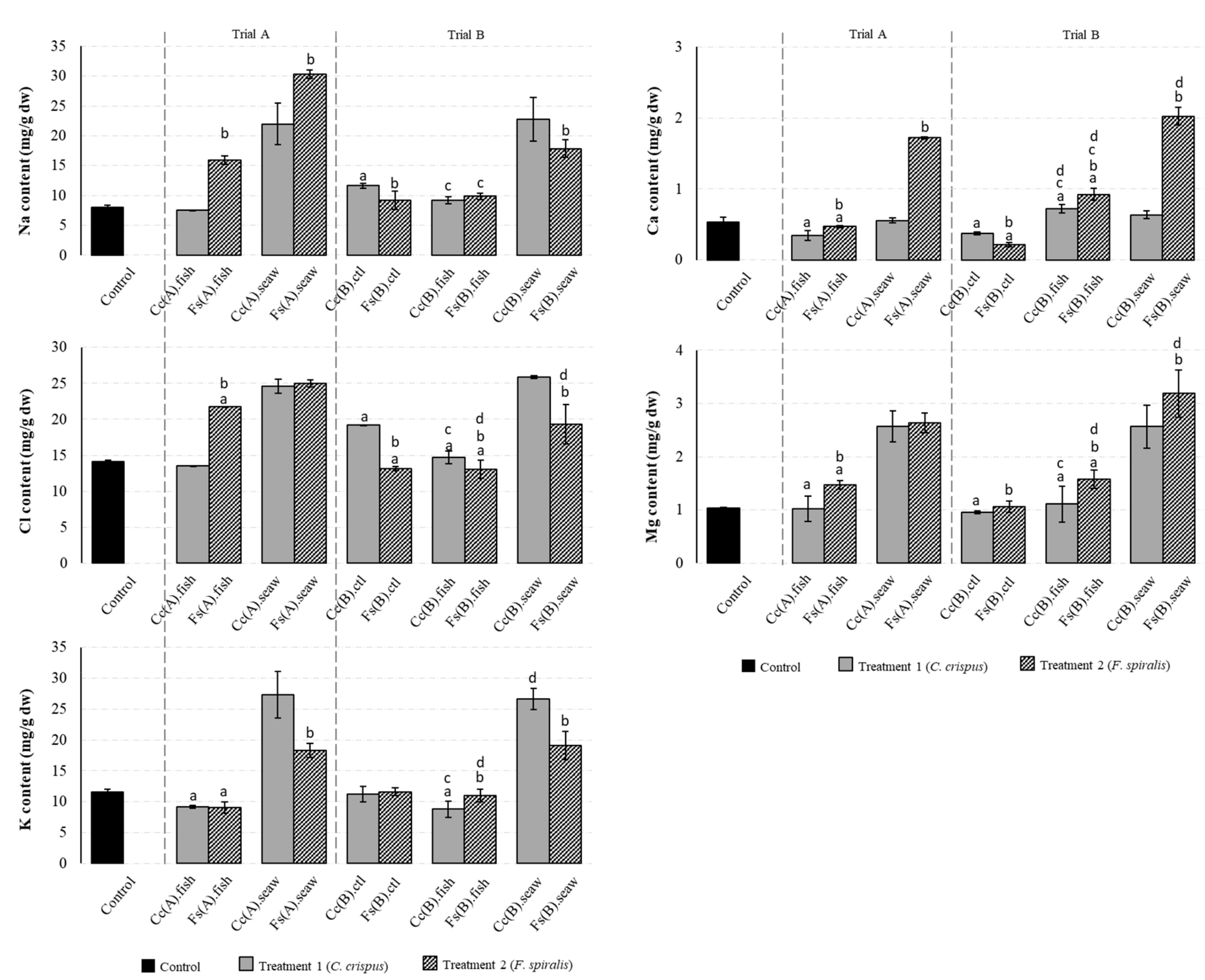
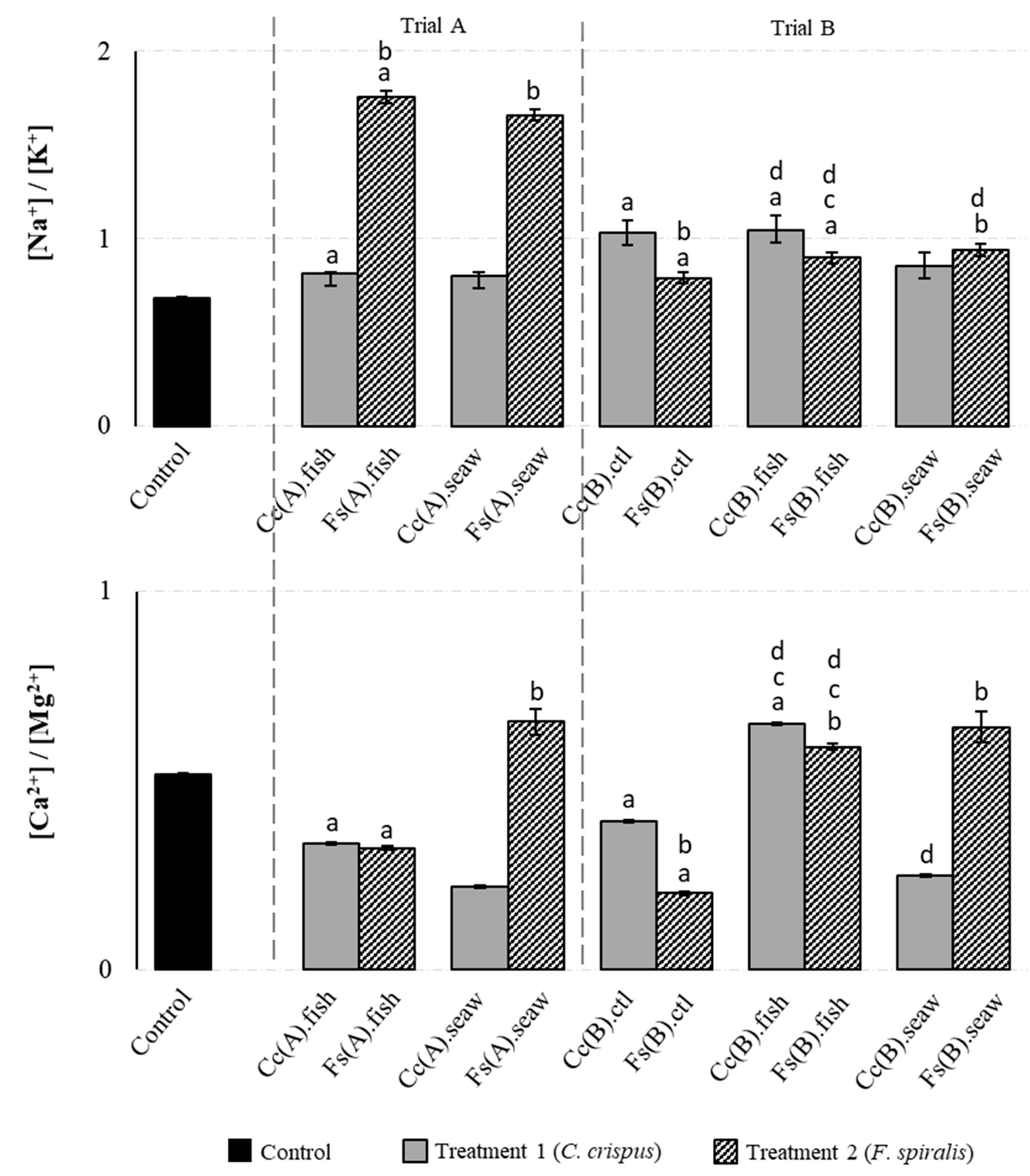
2.2. Macromineral Composition of the Canned Chub Mackerel Products Incorporating Seaweeds
2.3. Trace Elements Composition of the Canned Chub Mackerel Products Incorporating Seaweeds
2.4. Contribution of Canned Samples to Mineral RDA/AI
3. Materials and Methods
3.1. Reagents and Analytical Solutions
3.2. Preparation of Canned Chub Mackerel Incorporating Seaweeds
3.2.1. Selection and Preparation of Seaweeds
3.2.2. Canned Products Conception and Optimization
3.3. Chemical Characterization of Canned Samples
3.3.1. Moisture and Ash Contents
3.3.2. Microwave Digestion
3.3.3. Elemental Composition
3.3.4. Quality Control
3.4. Assessment of Daily Intake
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cofrades, S.; Benedí, J.; Garcimartin, A.; Sánchez-Muniz, F.J.; Jimenez-Colmenero, F. A comprehensive approach to formulation of seaweed-enriched meat products: From technological development to assessment of healthy properties. Food Res. Int. 2017, 99, 1084–1094. [Google Scholar] [CrossRef]
- Mišurcová, L.; Machů, L.; Orsavová, J. Seaweed minerals as nutraceuticals. In Marine Medicinal Foods: Implications and Applications, Macro and Microalgae; Kim, S.-K., Ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 371–390. [Google Scholar]
- Astorga-España, M.S.; Rodríguez Galdón, B.; Rodríguez Rodríguez, E.M.; Díaz Romero, C. Mineral and trace element concentrations in seaweeds from the sub-Antarctic ecoregion of Magallanes (Chile). J. Food Compos. Anal. 2015, 39, 69–76. [Google Scholar] [CrossRef]
- Circuncisão, A.; Catarino, M.; Cardoso, S.; Silva, A. Minerals from macroalgae origin: Health benefits and risks for consumers. Mar. Drugs 2018, 16, 400. [Google Scholar] [CrossRef]
- Cabrita, A.R.; Maia, M.R.; Oliveira, H.M.; Sousa-Pinto, I.; Almeida, A.A.; Pinto, E.; Fonseca, A.J. Tracing seaweeds as mineral sources for farm-animals. J. Appl. Phycol. 2016, 28, 3135–3150. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Patarra, R.F.; Neto, A.I.; Baptista, J. Edible Azorean macroalgae as source of rich nutrients with impact on human health. Food Chem. 2014, 164, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Costa Leite, J.; Keating, E.; Pestana, D.; Cruz Fernandes, V.; Maia, M.L.; Norberto, S.; Pinto, E.; Moreira-Rosário, A.; Sintra, D.; Moreira, B.; et al. Iodine status and iodised salt consumption in Portuguese school-aged children: The Iogeneration Study. Nutrients 2017, 9, 458. [Google Scholar] [CrossRef] [PubMed]
- Roohinejad, S.; Koubaa, M.; Barba, F.J.; Saljoughian, S.; Amid, M.; Greiner, R. Application of seaweeds to develop new food products with enhanced shelf-life, quality and health-related beneficial properties. Food Res. Int. 2017, 99, 1066–1083. [Google Scholar] [CrossRef] [PubMed]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K.; Cheng, H.W. Antioxidant activities and phenolics content of eight species of seaweeds from north Borneo. J. Appl. Phycol. 2008, 20, 367–373. [Google Scholar] [CrossRef]
- Rupérez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- WHO. Guideline: Potassium intake for adults and children; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the debate for a regulatory framework. Br. J. Clin. Pharm. 2018, 84, 659–672. [Google Scholar] [CrossRef]
- European Nutraceutical Association (ENA). Science behind Nutraceuticals; European Nutraceutical Association: Basel, Switzerland, 2016. [Google Scholar]
- Durazzo, A.; Lisciani, S.; Camilli, E.; Gabrielli, P.; Marconi, S.; Gambelli, L.; Aguzzi, A.; Lucarini, M.; Maiani, G.; Casale, G.; et al. Nutritional composition and antioxidant properties of traditional Italian dishes. Food Chem. 2017, 218, 70–77. [Google Scholar] [CrossRef] [PubMed]
- López-López, I.; Bastida, S.; Ruiz-Capillas, C.; Bravo, L.; Larrea, M.T.; Sánchez-Muniz, F.; Cofrades, S.; Jiménez-Colmenero, F. Composition and antioxidant capacity of low-salt meat emulsion model systems containing edible seaweeds. Meat Sci. 2009, 83, 492–498. [Google Scholar] [CrossRef] [PubMed]
- López-López, I.; Cofrades, S.; Ruiz-Capillas, C.; Jiménez-Colmenero, F. Design and nutritional properties of potential functional frankfurters based on lipid formulation, added seaweed and low salt content. Meat Sci. 2009, 83, 255–262. [Google Scholar] [CrossRef] [PubMed]
- López-López, I.; Cofrades, S.; Cañeque, V.; Díaz, M.T.; López, O.; Jiménez-Colmenero, F. Effect of cooking on the chemical composition of low-salt, low-fat Wakame/olive oil added beef patties with special reference to fatty acid content. Meat Sci. 2011, 89, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.; Vivanco, J.P.; Aubourg, S.P. Lipid and sensory quality of canned Atlantic salmon (Salmo salar): Effect of the use of different seaweed extracts as covering liquids. Eur. J. Lipid Sci. Technol. 2014, 116, 596–605. [Google Scholar] [CrossRef]
- Hanjabam, M.D.; Zynudheen, A.A.; Ninan, G.; Panda, S. Seaweed as an ingredient for nutritional improvement of fish jerky. J. Food Process. Preserv. 2017, 41, e12845. [Google Scholar] [CrossRef]
- European Commission. Facts and figures on the common fisheries policy-basic statistical data; Commission DG Maritime Affairs and Fisheries: Brussels, Belgium, 2018. [Google Scholar]
- Edible Seaweed-French. European Regulation. Available online: http://www.cybercolloids.net/sites (accessed on 15 February 2019).
- Pereira, L. (2019). MACOI-Portuguese Seaweeds Website. Available online: http://macoi.ci.uc.pt (accessed on 13 February 2019).
- INSA. Tabela da composição de alimentos, 1st ed.; Instituto Nacional de Saúde Dr. Ricardo Jorge (INSA): Lisboa, Portugal, 2006. [Google Scholar]
- Rosanoff, A. Rising Ca: Mg intake ratio from food in USA Adults: A concern? Magnes. Res. 2010, 23, 181–193. [Google Scholar]
- Campbell, L.M.; Fisk, A.T.; Wang, X.; Köck, G.; Muir, D.C. Evidence for biomagnification of rubidium in freshwater and marine food webs. Can. J. Fish Aquat. Sci. 2005, 62, 1161–1167. [Google Scholar] [CrossRef]
- Badmaev, V.; Prakash, S.; Majeed, M. Vanadium: A review of its potential role in the fight against diabetes. J. Altern Complement. Med. 1999, 5, 273–291. [Google Scholar] [CrossRef]
- WHO. Guideline: Sodium intake for adults and children; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Mendel, R.R. Molybdenum: Biological activity and metabolism. Dalton Trans. 2005, 21, 3404–3409. [Google Scholar] [CrossRef]
- Chen, P.; Bornhorst, J.; Aschner, M. Manganese metabolism in humans. Front. Biosci. (Landmark Ed.) 2018, 23, 1655–1679. [Google Scholar] [CrossRef] [PubMed]
- FNIC. Dietary Reference Intakes (DRIs): Recommended dietary allowances and adequate intakes, elements; Food and Nutrition Board, Institute of Medicine, National Academy of Sciences: Washington, DC, USA, 2010. [Google Scholar]
- Vieira, E.F.; Soares, C.; Machado, S.; Correia, M.; Ramalhosa, M.J.; Oliva-Teles, M.T.; Carvalho, A.P.; Domingues, V.F.; Antunes, F.; Oliveira, T.A.C.; et al. Seaweeds from the Portuguese coast as a source of proteinaceous material: Total and free amino acid composition profile. Food Chem. 2018, 269, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Lewicki, P.P. Some remarks on rehydration of dried foods. J. Food Eng. 1998, 36, 81–87. [Google Scholar] [CrossRef]
- Kemp, S.E.; Hollowood, T.; Hort, J. Sensory evaluation: A practical handbook; John Wiley Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2005. [Google Scholar]
- Torrinha, A.; Gomes, F.; Oliveira, M.; Cruz, R.; Mendes, E.; Delerue-Matos, C.; Casal, S.; Morais, S. Commercial squids: Characterization, assessment of potential health benefits/risks and discrimination based on mineral, lipid and vitamin E concentrations. Food Chem. Toxicol. 2014, 67, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Pacquette, L.H.; Levenson, A.M.; Thompson, J.J.; Dowell, D. Total iodine in infant formula and nutritional products by inductively coupled plasma/mass spectrometry: First Action 2012.14. J. Aoac. Int. 2013, 96, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Ramos, S.; Delerue-Matos, C.; Morais, S. Espresso beverages of pure origin coffee: Mineral characterization, contribution for mineral intake and geographical discrimination. Food Chem. 2015, 177, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Linsinger, T.P.J. Comparison of measurement result with the certified value. Available online: http://www.erm-crm.org (accessed on 3 March 2020).
- ISO/IEC Guide. Uncertainty of measurement—Part. 3: Guide to the expression of uncertainty in measurement (GUM:1995); International Organization for Standardization: Geneva, Switzerland, 1995.
- Machado, S.; Oliva-Teles, M.T.; Soares, C.; Antunes, F.; Carvalho, A.P.; Correia, M.; Ramalhosa, M.J.; Domingues, V.F.; Morais, S.; Oliveira, T.A.C.; et al. Chloride in edible seaweeds from North Atlantic Portuguese coast; Livro de Atas do XIII Encontro Química dos Alimentos: Porto, Portugal, 2016. [Google Scholar]
- Plácido, A.; Kupers, R.; Paíga, P.; Magalhães, J.; Nouws, H.P.A.; Delerue-Matos, C.; Oliveira, M.B.P.P. Salt content in bread and dough from northern Portugal: Method development and comparison. J. Food Compos. Anal. 2012, 27, 14–20. [Google Scholar] [CrossRef][Green Version]
Sample Availability: Not available. |

| Element | CRM (mg/kg dW) | UCRM (mg/kd dw) | MV (mg/kg dw) | UMV (mg/kg dw) | ΔC (mg/kg dw) | UΔ (mg/kg dw) |
|---|---|---|---|---|---|---|
| As | 12.7 | 0.7 | 13.6 | 0.2 | 0.861 | 1.49 |
| Cd | 0.0075 | 0.0018 | 0.0068 | 0.00072 | 0.000720 | 0.00388 |
| Cu | 1.67 | 0.16 | 1.88 | 0.055 | 0.212 | 0.339 |
| Fe | 9.4 | 1.4 | 10.2 | 0.8 | 0.783 | 3.26 |
| I | 1.4 | 0.4 | 1.6 | 0.2 | 0.250 | 0.87 |
| Mn | 0.368 | 0.028 | 0.356 | 0.028 | 0.0118 | 0.0792 |
| Se | 1.33 | 0.13 | 1.18 | 0.13 | 0.151 | 0.365 |
| Zn | 16.0 | 1.1 | 14.0 | 0.2 | 2.018 | 2.23 |
| Co | Cu | Fe | I | Li | Mn | Mo | Rb | Se | Sr | V | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0.02 ± 0.01 | 3.04 ± 0.25 | 31.1 ± 3.57 | 1.59 ± 0.18 | 0.11 ± 0.02 | 0.39 ± 0.03 | 0.13 ± 0.02 | 1.51 ± 0.07 | 2.29 ± 0.26 | 3.80 ± 1.04 | 0.15 ± 0.01 | 19.6 ± 0.26 |
| Trial A | ||||||||||||
| Treatment 1 | ||||||||||||
| Cc(A).fish | 0.09 ± 0.01 a | 3.63 ± 0.49 a | 41.4 ± 3.56 a | 5.26 ± 0.23 a | 0.26 ± 0.01 a | 4.10 ± 0.29 a | 0.36 ± 0.02 a | 4.55 ± 0.13 a | 2.68 ± 0.12 a | 13.2 ± 0.61 a | 0.39 ± 0.02 a | 11.4 ± 1.80 a |
| Cc(A).seaw | 0.03 ± 0.01 | 4.60 ± 0.10 | 37.0 ± 4.36 | 133 ± 13.3 | 0.13 ± 0.01 | 3.20 ± 0.24 | 0.24 ± 0.02 | 1.41 ± 0.03 | 1.82 ± 0.23 | 4.03 ± 0.36 | 0.10 ± 0.01 | 17.1 ± 0.73 |
| Treatment 2 | ||||||||||||
| Fs(A).fish | 0.21 ± 0.01 ab | 2.78 ± 0.14 b | 34.5 ±9.39 ab | 9.92 ± 1.07 ab | 0.34 ± 0.02 ab | 16.7 ± 0.44 ab | 0.12 ± 0.01 b | 2.89 ± 0.11 ab | 2.71 ± 0.19 a | 36.0 ± 3.0 ab | 0.49 ± 0.02 ab | 30.6 ± 2.79 ab |
| Fs(A).seaw | 0.04 ± 0.01 a | 2.82 ± 0.19 b | 26.3 ± 2.16 b | 47.9 ± 2.70 b | 0.15 ± 0.02 | 3.42 ± 0.15 | 0.17 ± 0.02 b | 1.35 ± 0.06 | 2.88 ± 0.22 b | 17.4 ± 1.71 b | 0.15 ± 0.01 b | 14.0 ± 1.28 b |
| Trial B | ||||||||||||
| Treatment 1 | ||||||||||||
| Cc(B).ctl | 0.04 ± 0.01 | 6.97 ± 0.60 a | 37.1 ± 3.38 a | 2.51 ± 0.16 a | 0.16 ± 0.01 | 0.42 ± 0.03 | 0.46 ± 0.04 a | 1.48 ± 0.08 | 2.98 ± 0.32 | 3.50 ± 0.22 | 0.04 ± 0.02 a | 20.1 ± 1.90 a |
| Cc(B).fish | 0.12 ± 0.01 ac | 5.69 ± 0.38 acd | 45.7 ± 2.95 acd | 3.18 ± 0.19 acd | 0.33 ± 0.02 acd | 5.79 ± 0.35 acd | 0.48 ± 0.03 ad | 4.57 ± 0.64 ac | 2.39 ± 0.23 a | 16.8 ± 1.21 ac | 0.27 ± 0.01 acd | 12.4 ± 0.33 acd |
| Cc(B).seaw | 0.04 ± 0.01 | 5.50 ± 0.17 d | 48.3 ± 3.29 d | 102 ± 8.44 d | 0.13 ± 0.01 | 4.82 ± 0.41 d | 0.34 ± 0.07 d | 1.27 ± 0.06 | 2.35 ± 0.11 d | 9.45 ± 0.62 d | 0.07 ± 0.01 d | 21.1 ± 2.16 d |
| Treatment 2 | ||||||||||||
| Fs(B).ctl | 0.06 ± 0.01 | 2.87 ± 0.11 ab | 31.1 ± 3.16 b | 1.60 ± 0.18 b | 0.17 ± 0.17 ab | 0.33 ± 0.02 ab | 0.10 ± 0.03 b | 1.43 ± 0.04 | 2.72 ± 0.22 a | 3.44 ± 0.88 | 0.04 ± 0.01 a | 17.9 ± 0.75 a |
| Fs(B).fish | 0.14 ± 0.04 acd | 2.78 ± 0.47 ab | 35.6 ± 2.40 ab | 15.8 ± 1.74 abcd | 0.20 ± 0.09 abcd | 14.3 ± 1.36 abcd | 0.10 ± 0.02 b | 1.63 ± 0.78 ad | 1.97 ± 0.11 abcd | 27.8 ± 2.18 abcd | 0.15 ± 0.08 bcd | 10.5 ± 0.93 abcd |
| Fs(B).seaw | 0.05 ± 0.02 | 3.01 ± 0.33 bd | 25.5 ± 1.83 b | 54.1 ± 4.31 bd | 0.17 ± 0.05 b | 4.03 ± 0.36 bd | 0.06 ± 0.01 bd | 1.31 ± 0.39 | 2.63 ± 0.23 | 20.2 ± 4.76 b | 0.05 ± 0.03 d | 16.6 ± 1.37 bd |
| EDI (% of RDA or AI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment 1 | Treatment 2 | |||||||
| Element | RDA or AI• | Control | Cc(B).fish | Cc(B).seaw | serving | Fs(B).fish | Fs(B)seaw | serving |
| Ca | 1000 | 1.1 | 1.4 | 0.1 | 1.6 * | 1.8 | 0.4 | 2.3 * |
| Cl | 2300 | 12.3 | 12.8 | 2.3 | 15.1 * | 11.4 | 1.7 | 13.1 * |
| Cu | 0.9 | 6.8 | 12.6 * | 1.2 | 13.9 * | 6.2 | 0.7 | 6.8 * |
| Fe | 8 | 7.8 | 11.4 * | 1.2 | 12.6 * | 8.9 | 0.6 | 9.5 * |
| I | 0.15 | 21.2 | 42.4 * | 135 | 178 * | 211* | 72.1 | 283 * |
| Li | 1# | 0.2 | 0.7 | 0.0 | 0.7 * | 0.4 | 0.0 | 0.4 * |
| Mg | 420 | 4.9 | 5.3 | 1.2 | 6.5 * | 7.5* | 1.5 | 9.0 * |
| Mn | 2.3 | 0.4 | 6.4 | 0.5 | 7.0 * | 15.9 * | 0.4 | 16.4 * |
| Mo | 0.045 | 5.8 | 21.2 * | 1.5 | 22.7 * | 4.5 | 0.3 | 4.8 * |
| Na | 1500 | 10.6 | 12.3 * | 3.0 | 15.3 * | 13.1 * | 2.4 | 15.5 * |
| K | 4700 | 4.9 | 3.7 | 1.1 | 4.9 * | 4.7 | 0.8 | 5.5 * |
| Se | 0.055 | 83.3 | 86.9 * | 8.5 | 95.4 * | 71.5 * | 9.6 | 81.1 * |
| Zn | 11 | 4.9 | 3.1 | 0.5 | 3.6 * | 2.6 | 0.4 | 3.0 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, E.F.; Soares, C.; Machado, S.; Oliva-Teles, M.T.; Correia, M.; João Ramalhosa, M.; Carvalho, A.; Domingues, V.F.; Antunes, F.; Morais, S.; et al. Development of New Canned Chub Mackerel Products Incorporating Edible Seaweeds—Influence on the Minerals and Trace Elements Composition. Molecules 2020, 25, 1133. https://doi.org/10.3390/molecules25051133
Vieira EF, Soares C, Machado S, Oliva-Teles MT, Correia M, João Ramalhosa M, Carvalho A, Domingues VF, Antunes F, Morais S, et al. Development of New Canned Chub Mackerel Products Incorporating Edible Seaweeds—Influence on the Minerals and Trace Elements Composition. Molecules. 2020; 25(5):1133. https://doi.org/10.3390/molecules25051133
Chicago/Turabian StyleVieira, Elsa F., Cristina Soares, Susana Machado, M. Teresa Oliva-Teles, Manuela Correia, Maria João Ramalhosa, Ana Carvalho, Valentina F. Domingues, Filipa Antunes, Simone Morais, and et al. 2020. "Development of New Canned Chub Mackerel Products Incorporating Edible Seaweeds—Influence on the Minerals and Trace Elements Composition" Molecules 25, no. 5: 1133. https://doi.org/10.3390/molecules25051133
APA StyleVieira, E. F., Soares, C., Machado, S., Oliva-Teles, M. T., Correia, M., João Ramalhosa, M., Carvalho, A., Domingues, V. F., Antunes, F., Morais, S., & Delerue-Matos, C. (2020). Development of New Canned Chub Mackerel Products Incorporating Edible Seaweeds—Influence on the Minerals and Trace Elements Composition. Molecules, 25(5), 1133. https://doi.org/10.3390/molecules25051133











