Electrical and Optical Properties of Silicon Oxide Lignin Polylactide (SiO2-L-PLA)
Abstract
1. Introduction
2. Results and Discussion
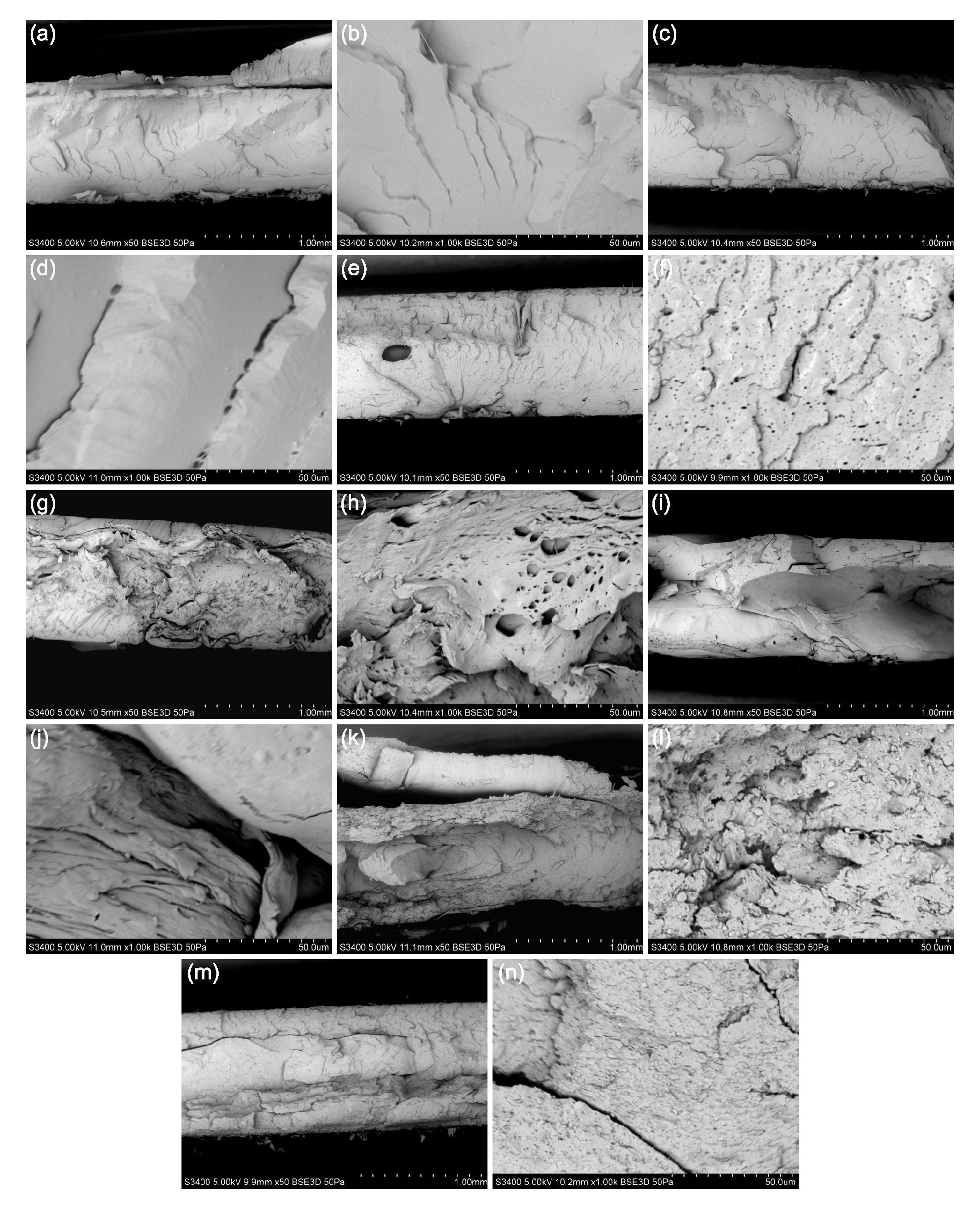
2.1. SEM Investigation
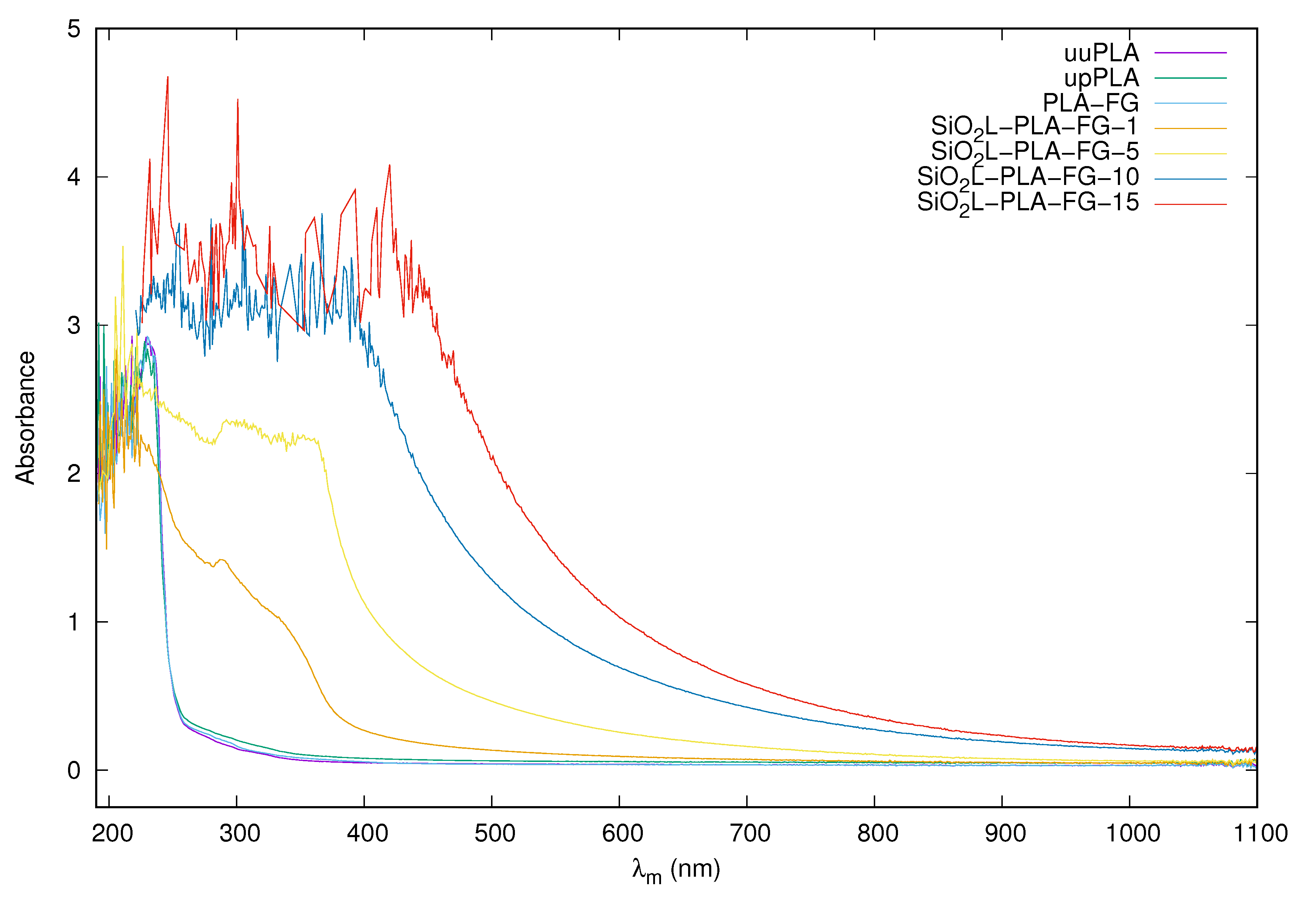
2.2. Visual Appearance and Absorbance
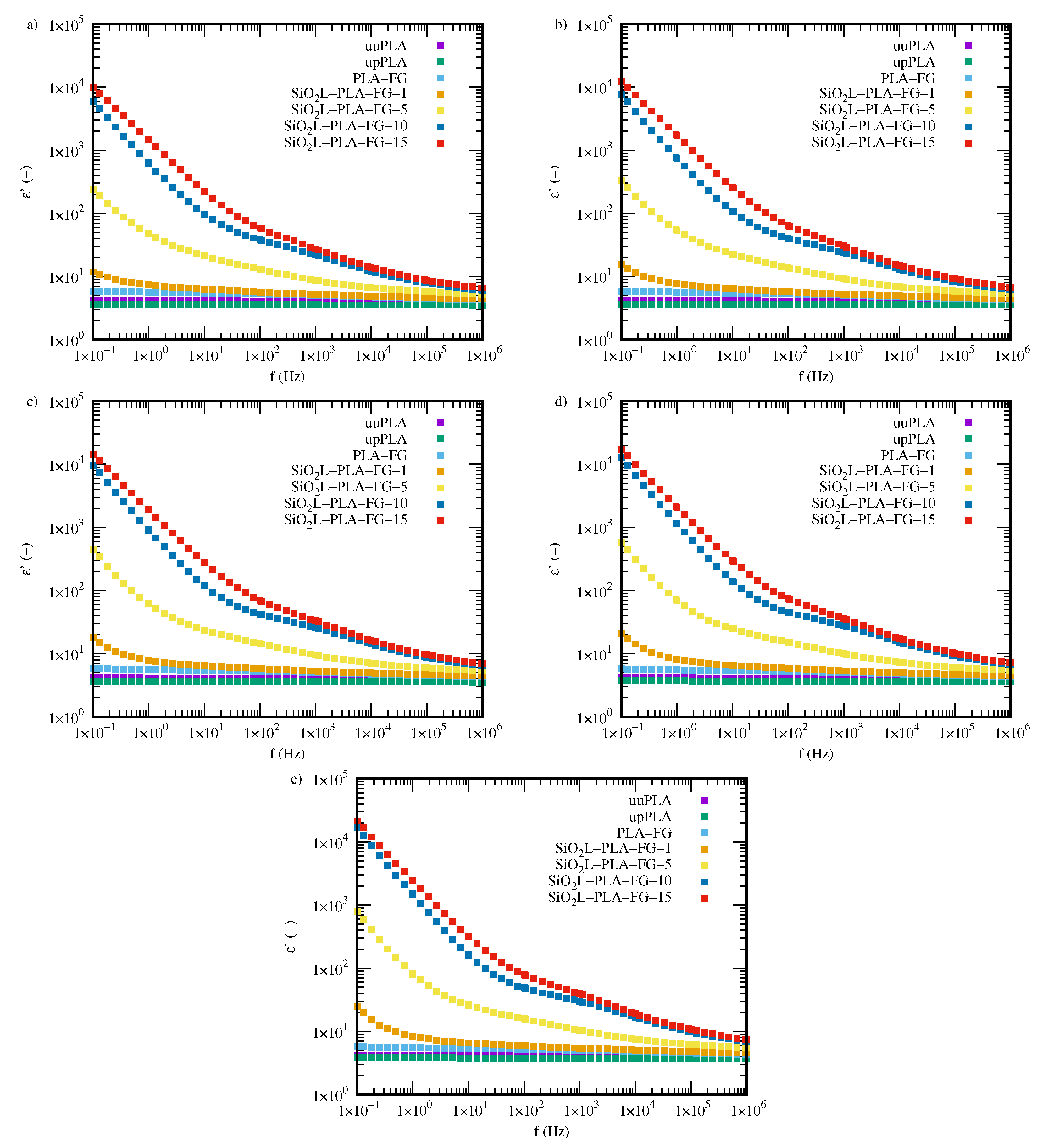
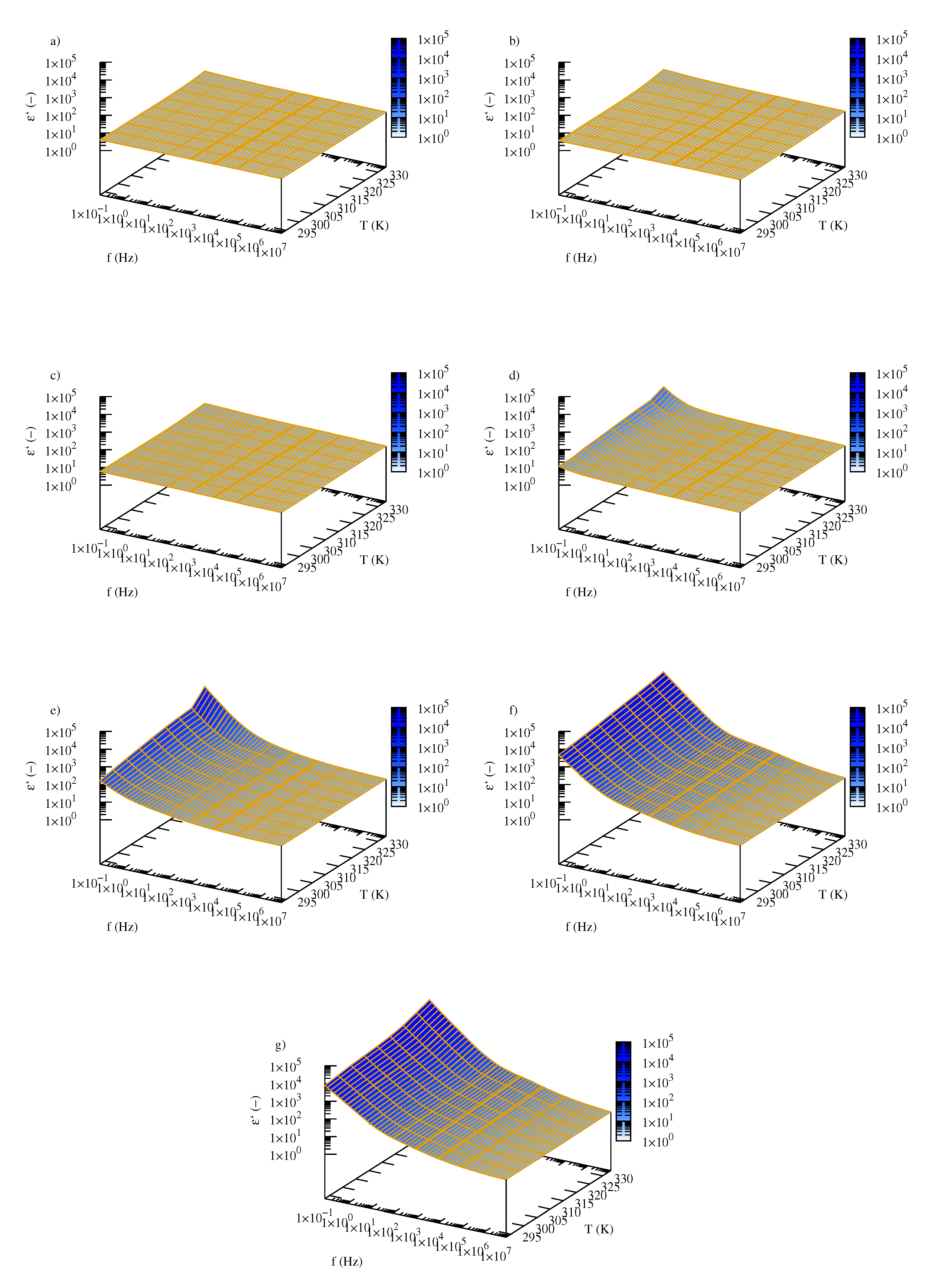
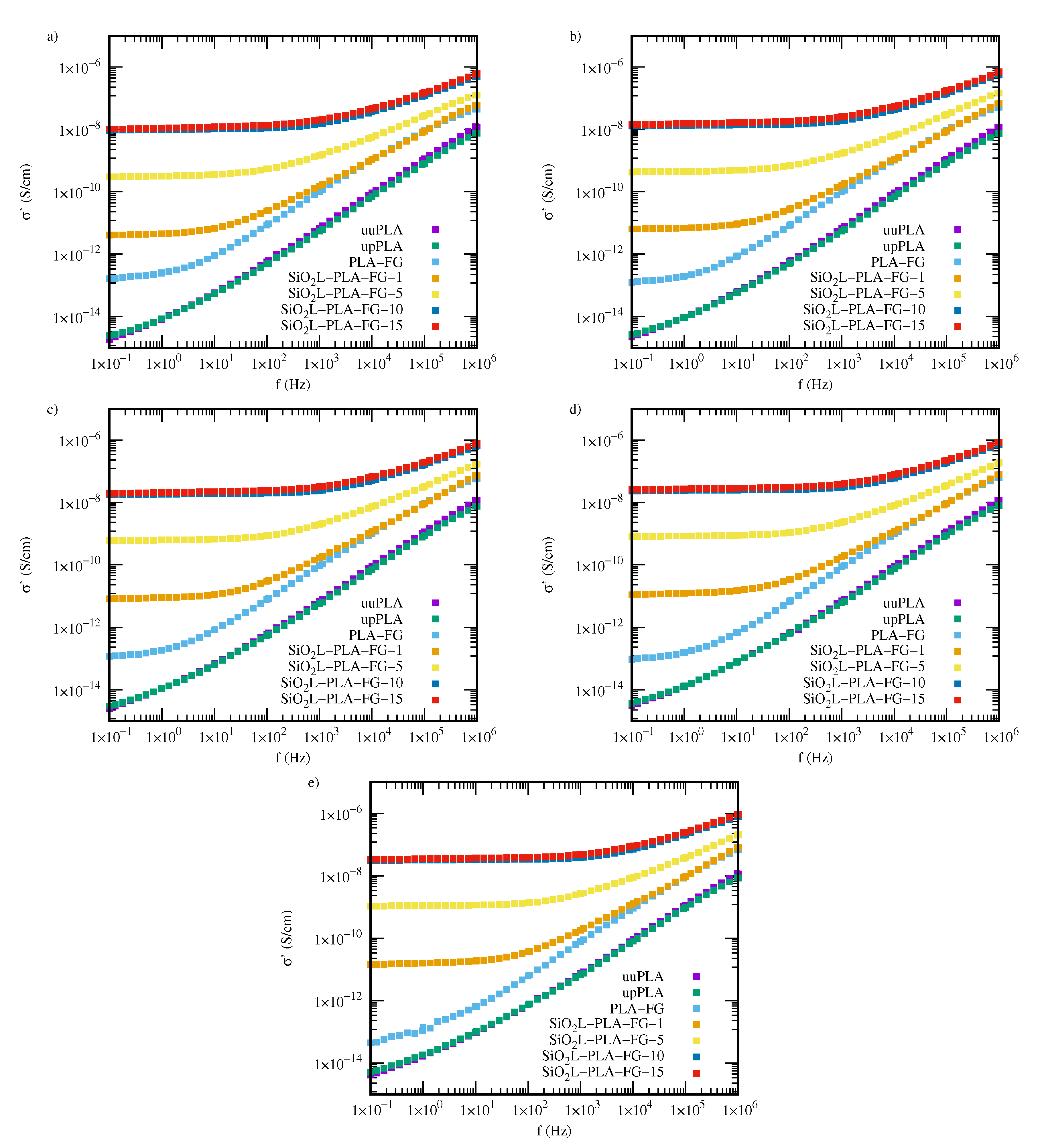
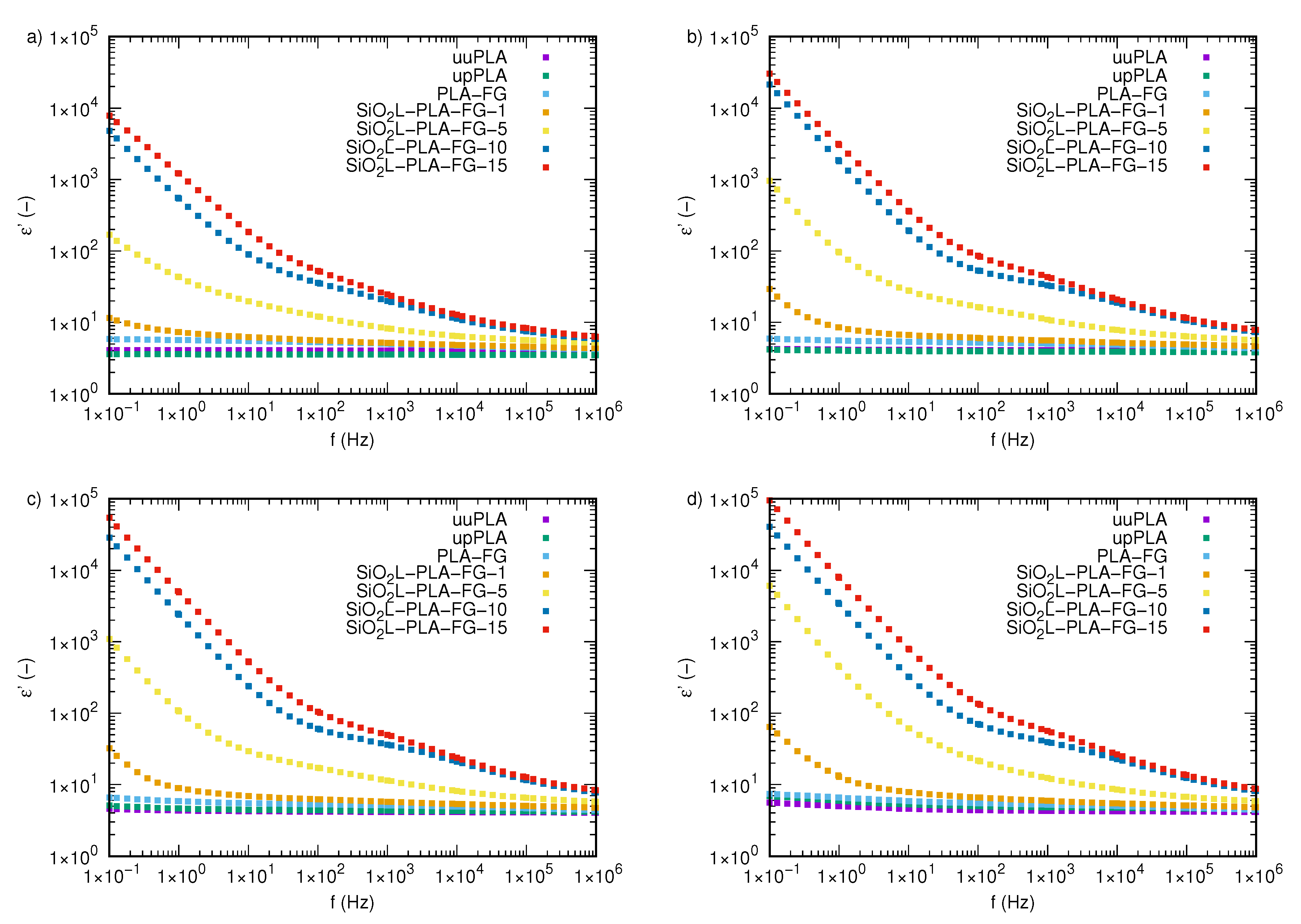
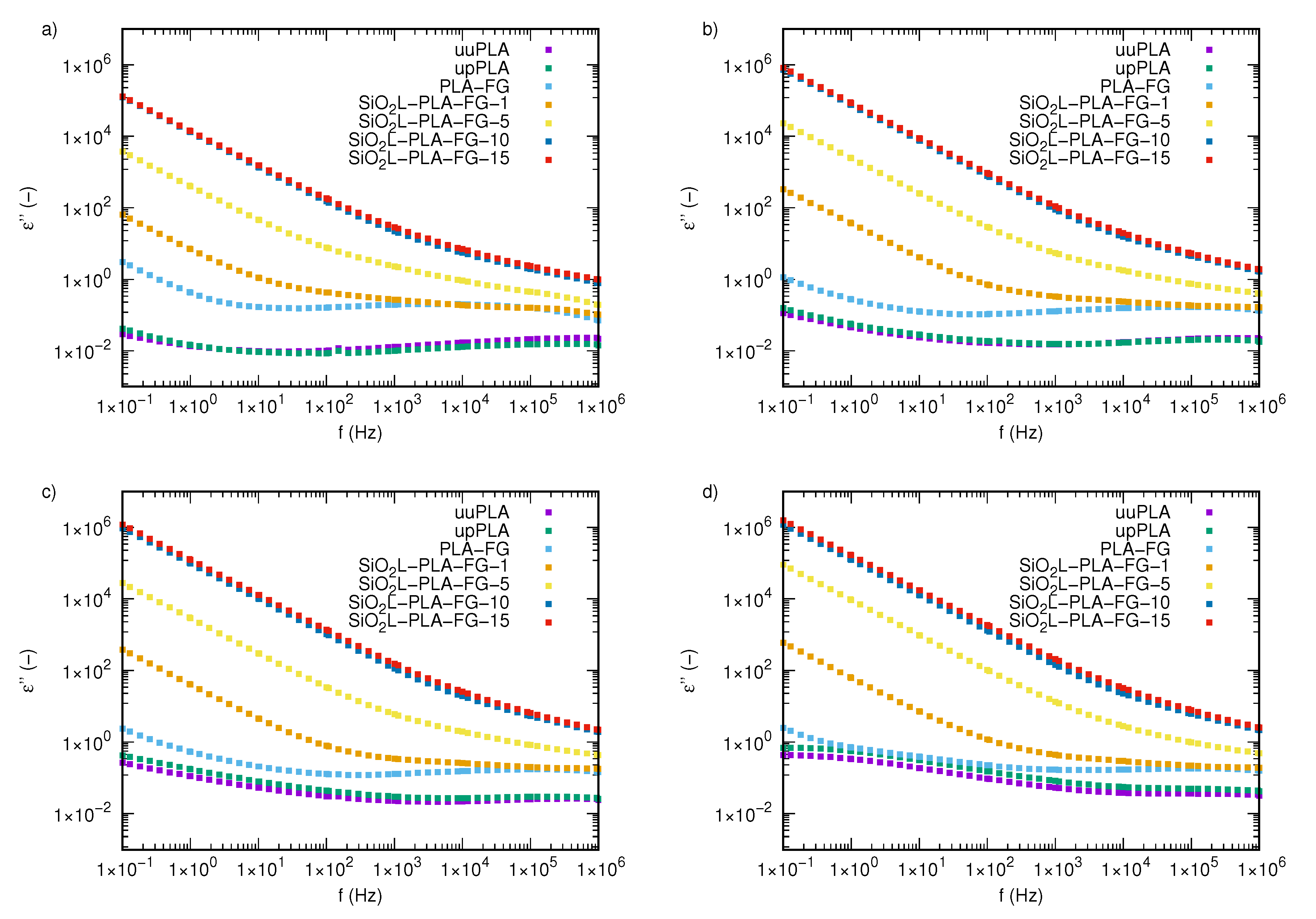
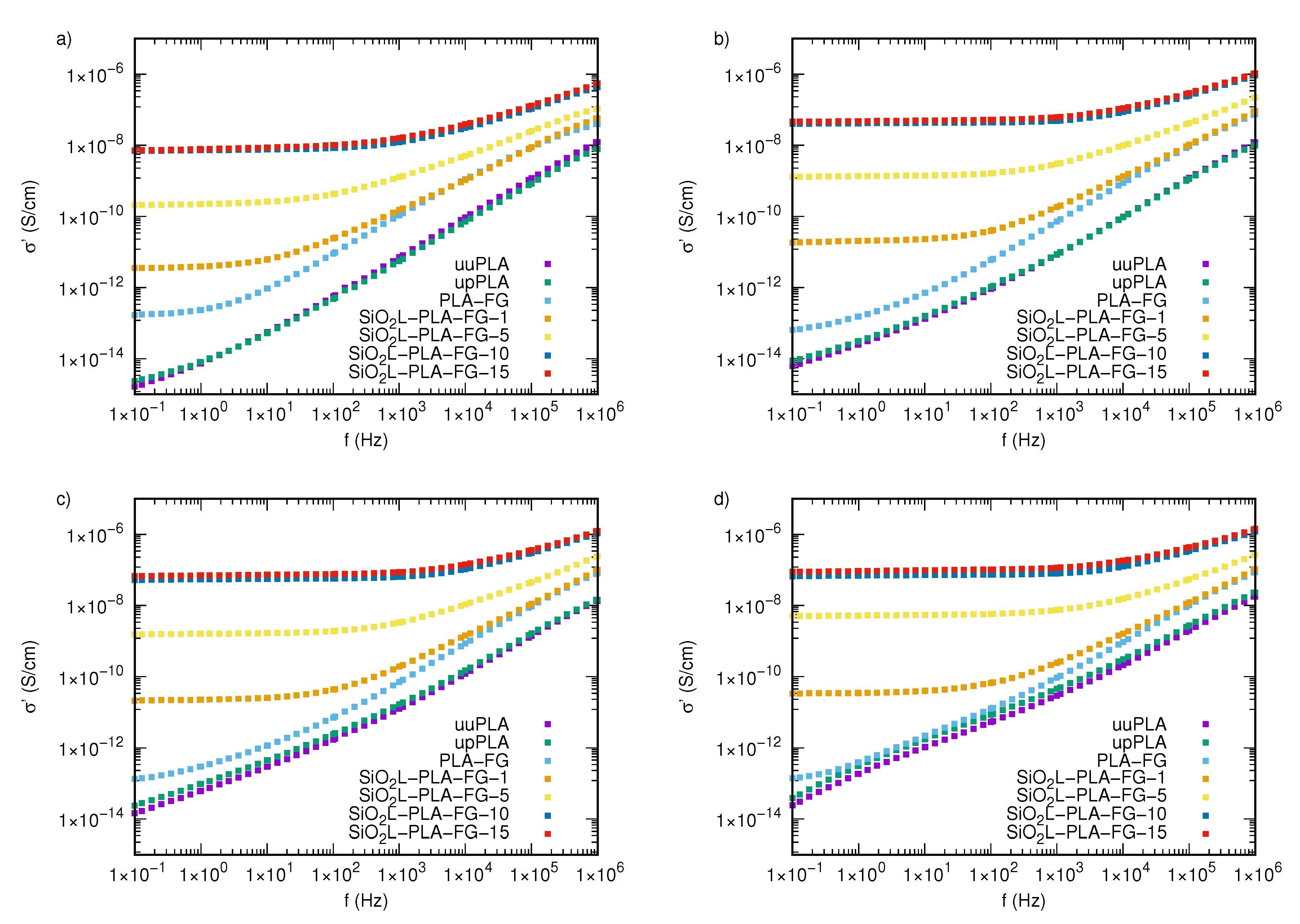
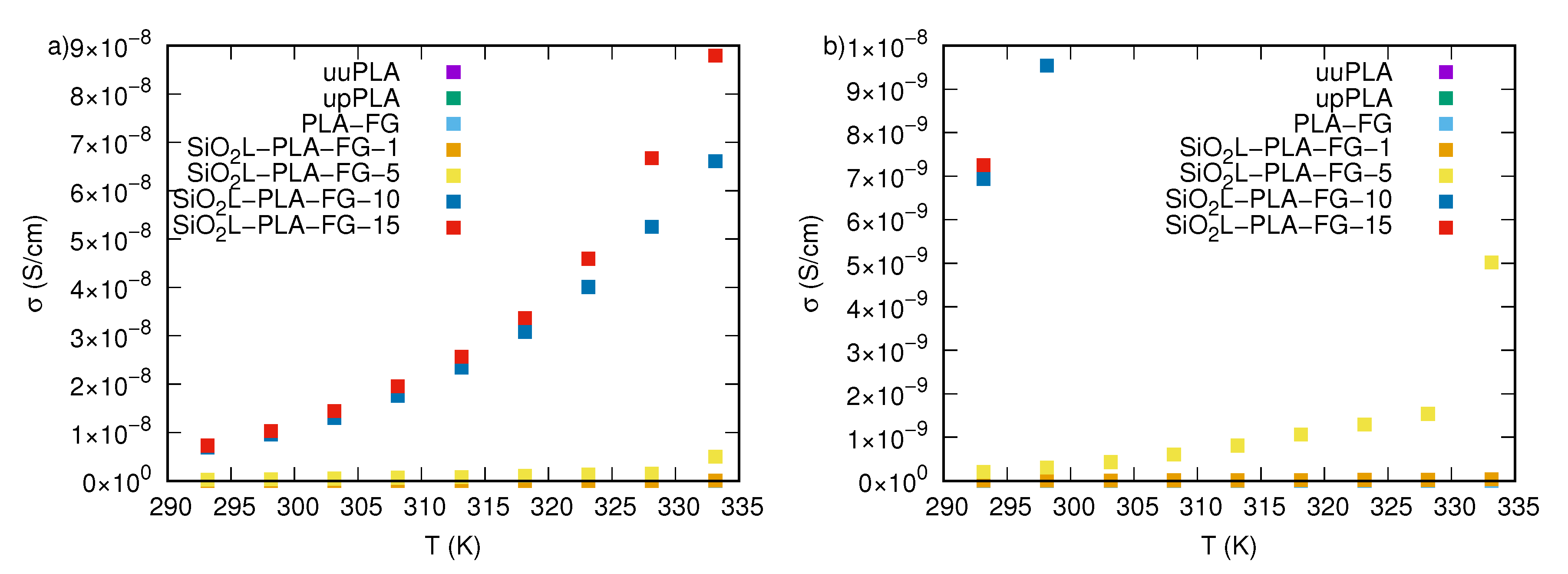
2.3. Electrical Properties
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.3. Characterization Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

References
- Patel, M.; Marscheider-Weidemann, F.; Schleich, J.; Hüsing, B.; Angerer, G.; Wolf, O.; Crank, M.; Patel, M. Techno-Economic Feasibility of Large-Scale Production of Bio-Based Polymers in Europe; Techncial Report EUR; Publications Office of the European Union: Luxembourg, 2005; Volume 22103. [Google Scholar]
- Shen, L.; Haufe, J.; Patel, M.K. Product overview and market projection of emerging bio-based plastics PRO-BIP 2009. In Report for European Polysaccharide Network of Excellence (EPNOE) and European Bioplastics; Copernicus Institute for Sustainable Development and Innovation, Utrecht University: Utrecht, The Netherlands, 2009; Volume 243. [Google Scholar]
- Rozite, L.; Varna, J.; Joffe, R.; Pupurs, A. Nonlinear behavior of PLA and lignin-based flax composites subjected to tensile loading. J. Thermoplast. Compos. Mat. 2013, 26, 476–496. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Yamada, K.; Okamoto, M.; Ueda, K. Polylactide-layered silicate nanocomposite: A novel biodegradable material. Nano Lett. 2002, 2, 1093–1096. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly (lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Cohn, D.; Salomon, A.H. Designing biodegradable multiblock PCL/PLA thermoplastic elastomers. Biomaterials 2005, 26, 2297–2305. [Google Scholar] [CrossRef]
- Park, C.W.; Youe, W.J.; Kim, S.J.; Han, S.Y.; Park, J.S.; Lee, E.A.; Kwon, G.J.; Kim, Y.S.; Kim, N.H.; Lee, S.H. Effect of lignin plasticization on physico-mechanical properties of lignin/poly (lactic acid) composites. Polymers 2019, 11, 2089. [Google Scholar] [CrossRef]
- Oksman, K.; Skrifvars, M.; Selin, J.F. Natural fibres as reinforcement in polylactic acid (PLA) composites. Compos. Sci. Technol. 2003, 63, 1317–1324. [Google Scholar] [CrossRef]
- Meinander, K.; Niemi, M.; Hakola, J.S.; Selin, J.F. Polylactides-degradable polymers for fibres and films. In Macromolecular Symposia; Hüthig & Wepf: Basel, Switzerland, 1997; Volume 123, pp. 147–153. [Google Scholar]
- Armentano, I.; Bitinis, N.; Fortunati, E.; Mattioli, S.; Rescignano, N.; Verdejo, R.; López-Manchado, M.A.; Kenny, J.M. Multifunctional nanostructured PLA materials for packaging and tissue engineering. Prog. Polym. Sci. 2013, 38, 1720–1747. [Google Scholar] [CrossRef]
- Nampoothiri, K.M.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Biores. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Adhikari, R. Biodegradable synthetic polymers for tissue engineering. Eur. Cell Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef]
- Gordobil, O.; Egüés, I.; Llano-Ponte, R.; Labidi, J. Physicochemical properties of PLA lignin blends. Polym. Degrad. Stab. 2014, 108, 330–338. [Google Scholar] [CrossRef]
- Gordobil, O.; Delucis, R.; Egüés, I.; Labidi, J. Kraft lignin as filler in PLA to improve ductility and thermal properties. Ind. Crops Prod. 2015, 72, 46–53. [Google Scholar] [CrossRef]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Misra, M.; Mohanty, A.K. Enhanced properties of lignin-based biodegradable polymer composites using injection moulding process. Compos. Part A Appl. Sci. Manuf. 2011, 42, 1710–1718. [Google Scholar] [CrossRef]
- Kumar, S.; Mohanty, A.; Erickson, L.; Misra, M. Lignin and its applications with polymers. J. Biob. Mater. Bioenergy 2009, 3, 1–24. [Google Scholar] [CrossRef]
- Glasser, W.G.; Barnett, C.A.; Muller, P.C.; Sarkanen, K.V. The chemistry of several novel bioconversion lignins. J. Agric. Food Chem. 1983, 31, 921–930. [Google Scholar] [CrossRef]
- Wen, J.L.; Xue, B.L.; Xu, F.; Sun, R.C.; Pinkert, A. Unmasking the structural features and property of lignin from bamboo. Ind. Crops Prod. 2013, 42, 332–343. [Google Scholar] [CrossRef]
- Buranov, A.U.; Mazza, G. Lignin in straw of herbaceous crops. Ind. Crops Prod. 2008, 28, 237–259. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, J.Y.; Youn, H.J.; Choi, J.W. Utilization of lignin fractions in UV resistant lignin-PLA biocomposites via lignin-lactide grafting. Int. J. Biol. Macromol. 2019, 138, 1029–1034. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- El Mansouri, N.E.; Salvadó, J. Structural characterization of technical lignins for the production of adhesives: Application to lignosulfonate, kraft, soda-anthraquinone, organosolv and ethanol process lignins. Ind. Crops Prod. 2006, 24, 8–16. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Hatakeyama, T. Lignin structure, properties, and applications. In Biopolymers; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–63. [Google Scholar]
- Lora, J.H.; Glasser, W.G. Recent industrial applications of lignin: A sustainable alternative to nonrenewable materials. J. Polym. Environ. 2002, 10, 39–48. [Google Scholar] [CrossRef]
- Collins, M.N.; Nechifor, M.; Tanasă, F.; Zănoagă, M.; McLoughlin, A.; Stróżyk, M.A.; Culebras, M.; Teacă, C.A. Valorization of lignin in polymer and composite systems for advanced engineering applications—A review. Int. J. Biol. Macromol. 2019, 131, 828–849. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Ben, H.; Ragauskas, A.; Deng, Y. Lignin pyrolysis components and upgrading—Technology review. Bioenergy Res. 2013, 6, 1183–1204. [Google Scholar] [CrossRef]
- Li, J.; He, Y.; Inoue, Y. Thermal and mechanical properties of biodegradable blends of poly (L-lactic acid) and lignin. Polym. Int. 2003, 52, 949–955. [Google Scholar] [CrossRef]
- Kadla, J.F.; Kubo, S. Lignin-based polymer blends: Analysis of intermolecular interactions in lignin–synthetic polymer blends. Compos. Part A Appl. Sci. Manuf. 2004, 35, 395–400. [Google Scholar] [CrossRef]
- Larraneta, E.; Imízcoz, M.; Toh, J.X.; Irwin, N.J.; Ripolin, A.; Perminova, A.; Domínguez-Robles, J.; Rodríguez, A.; Donnelly, R.F. Synthesis and characterization of lignin hydrogels for potential applications as drug eluting antimicrobial coatings for medical materials. ACS Sustain. Chem. Eng. 2018, 6, 9037–9046. [Google Scholar] [CrossRef]
- Liu, D.; Li, Y.; Qian, Y.; Xiao, Y.; Du, S.; Qiu, X. Synergistic antioxidant performance of lignin and quercetin mixtures. ACS Sustain. Chem. Eng. 2017, 5, 8424–8428. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.; Kenny, J.; Puglia, D. Effect of cellulose and lignin on disintegration, antimicrobial and antioxidant properties of PLA active films. Int. J. Biol. Macromol. 2016, 89, 360–368. [Google Scholar] [CrossRef]
- Ouyang, W.; Huang, Y.; Luo, H.; Wang, D. Poly (lactic acid) blended with cellulolytic enzyme lignin: Mechanical and thermal properties and morphology evaluation. J. Polym. Environ. 2012, 20, 1–9. [Google Scholar] [CrossRef]
- Spiridon, I.; Leluk, K.; Resmerita, A.M.; Darie, R.N. Evaluation of PLA–lignin bioplastics properties before and after accelerated weathering. Compos Part B Eng. 2015, 69, 342–349. [Google Scholar] [CrossRef]
- Sahoo, S.; Misra, M.; Mohanty, A.K. Effect of compatibilizer and fillers on the properties of injection molded lignin-based hybrid green composites. J. Appl. Polym. Sci. 2013, 127, 4110–4121. [Google Scholar] [CrossRef]
- Park, C.W.; Youe, W.J.; Han, S.Y.; Park, J.S.; Lee, E.A.; Park, J.Y.; Kwon, G.J.; Kim, S.J.; Lee, S.H. Influence of lignin and polymeric diphenylmethane diisocyante addition on the properties of poly (butylene succinate)/wood flour composite. Polymers 2019, 11, 1161. [Google Scholar] [CrossRef] [PubMed]
- Agüero, A.; Morcillo, M.d.C.; Quiles-Carrillo, L.; Balart, R.; Boronat, T.; Lascano, D.; Torres-Giner, S.; Fenollar, O. Study of the influence of the reprocessing cycles on the final properties of polylactide pieces obtained by injection molding. Polymers 2019, 11, 1908. [Google Scholar] [CrossRef] [PubMed]
- Herrera, N.; Mathew, A.P.; Oksman, K. Plasticized polylactic acid/cellulose nanocomposites prepared using melt-extrusion and liquid feeding: Mechanical, thermal and optical properties. Compos. Sci. Technol. 2015, 106, 149–155. [Google Scholar] [CrossRef]
- Pradhan, D.K.; Choudhary, R.; Samantaray, B. Studies of dielectric relaxation and AC conductivity behavior of plasticized polymer nanocomposite electrolytes. Int. J. Electrochem. Sci. 2008, 3, 597–608. [Google Scholar]
- Wongtimnoi, K.; Guiffard, B.; Bogner-Van de Moortele, A.; Seveyrat, L.; Cavaillé, J.Y. Electrostrictive thermoplastic polyurethane-based nanocomposites filled with carboxyl-functionalized multi-walled carbon nanotubes (MWCNT-COOH): Properties and improvement of electromechanical activity. Compos. Sci. Technol. 2013, 85, 23–28. [Google Scholar] [CrossRef]
- Barick, A.K.; Tripathy, D.K. Preparation, characterization and properties of acid functionalized multi-walled carbon nanotube reinforced thermoplastic polyurethane nanocomposites. Mater. Sci. Eng. B 2011, 176, 1435–1447. [Google Scholar] [CrossRef]
- Al-Saleh, M.H.; Jawad, S.A.; El Ghanem, H.M. Electrical and dielectric behaviors of dry-mixed CNT/UHMWPE nanocomposites. High Perform. Polym. 2014, 26, 205–211. [Google Scholar] [CrossRef]
- Shehzad, K.; Dang, Z.M.; Ahmad, M.N.; Sagar, R.U.R.; Butt, S.; Farooq, M.U.; Wang, T.B. Effects of carbon nanotubes aspect ratio on the qualitative and quantitative aspects of frequency response of electrical conductivity and dielectric permittivity in the carbon nanotube/polymer composites. Carbon 2013, 54, 105–112. [Google Scholar] [CrossRef]
- Bula, K.; Klapiszewski, Ł; Jesionowski, T. A novel functional silica/lignin hybrid material as a potential bio-based polypropylene filler. Polym. Compos. 2015, 36, 913–922. [Google Scholar] [CrossRef]
- Klapiszewski, Ł; Bula, K.; Sobczak, M.; Jesionowski, T. Influence of processing conditions on the thermal stability and mechanical properties of PP/silica-lignin composites. Int. J. Polym. Sci. 2016, 2016. [Google Scholar]
- Fal, J.; Wanic, M.; Budzik, G.; Oleksy, M.; Żyła, G. Electrical conductivity and dielectric properties of ethylene glycol-based nanofluids containing silicon oxide–lignin hybrid particles. Nanomaterials 2019, 9, 1008. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
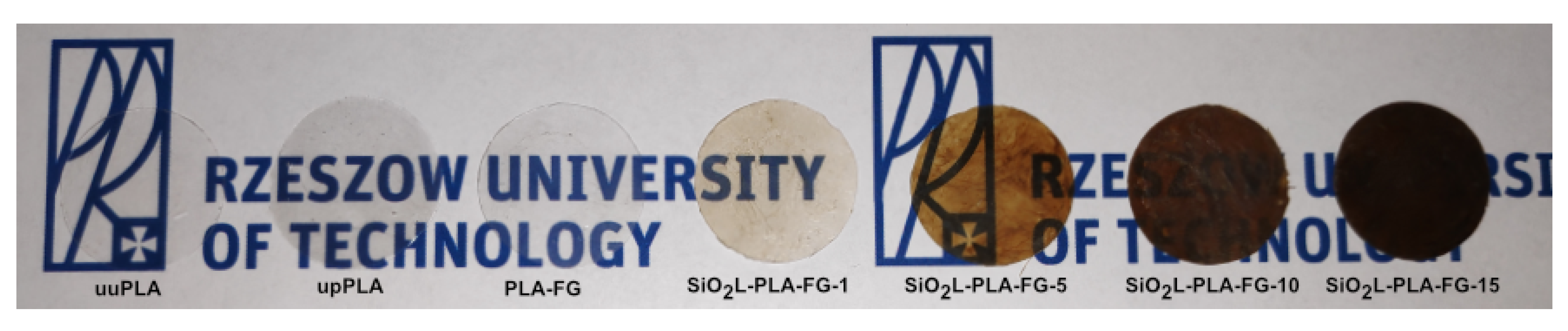


| Full Name of Ingredients | Label | NP | Fusabond | Glycerine | PLA |
|---|---|---|---|---|---|
| unfilled (u) and unprocessed (u) PLA | uuPLA | - | - | - | 1 |
| unfilled (u) and processed (p) PLA | upPLA | - | - | - | 1 |
| mixture of PLA, Fusabond (F), glycerine (G) | PLA-FG | - | 0.005 | 0.2 | 0.795 |
| silicon-dioxide–lignin (1 wt %) PLA | SiOL-PLA-FG-1 | 0.01 | 0.005 | 0.2 | 0.785 |
| silicon-dioxide–lignin (5 wt %) PLA | SiOL-PLA-FG-5 | 0.05 | 0.005 | 0.2 | 0.745 |
| silicon-dioxide–lignin (10 wt %) PLA | SiOL-PLA-FG-10 | 0.10 | 0.005 | 0.2 | 0.695 |
| silicon-dioxide–lignin (15 wt %) PLA | SiOL-PLA-FG-15 | 0.15 | 0.005 | 0.2 | 0.645 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fal, J.; Bulanda, K.; Traciak, J.; Sobczak, J.; Kuzioła, R.; Grąz, K.M.; Budzik, G.; Oleksy, M.; Żyła, G. Electrical and Optical Properties of Silicon Oxide Lignin Polylactide (SiO2-L-PLA). Molecules 2020, 25, 1354. https://doi.org/10.3390/molecules25061354
Fal J, Bulanda K, Traciak J, Sobczak J, Kuzioła R, Grąz KM, Budzik G, Oleksy M, Żyła G. Electrical and Optical Properties of Silicon Oxide Lignin Polylactide (SiO2-L-PLA). Molecules. 2020; 25(6):1354. https://doi.org/10.3390/molecules25061354
Chicago/Turabian StyleFal, Jacek, Katarzyna Bulanda, Julian Traciak, Jolanta Sobczak, Rafał Kuzioła, Katarzyna Maria Grąz, Grzegorz Budzik, Mariusz Oleksy, and Gaweł Żyła. 2020. "Electrical and Optical Properties of Silicon Oxide Lignin Polylactide (SiO2-L-PLA)" Molecules 25, no. 6: 1354. https://doi.org/10.3390/molecules25061354
APA StyleFal, J., Bulanda, K., Traciak, J., Sobczak, J., Kuzioła, R., Grąz, K. M., Budzik, G., Oleksy, M., & Żyła, G. (2020). Electrical and Optical Properties of Silicon Oxide Lignin Polylactide (SiO2-L-PLA). Molecules, 25(6), 1354. https://doi.org/10.3390/molecules25061354









