Effects of Exogenous Abscisic Acid on Bioactive Components and Antioxidant Capacity of Postharvest Tomato during Ripening
Abstract
1. Introduction
2. Results
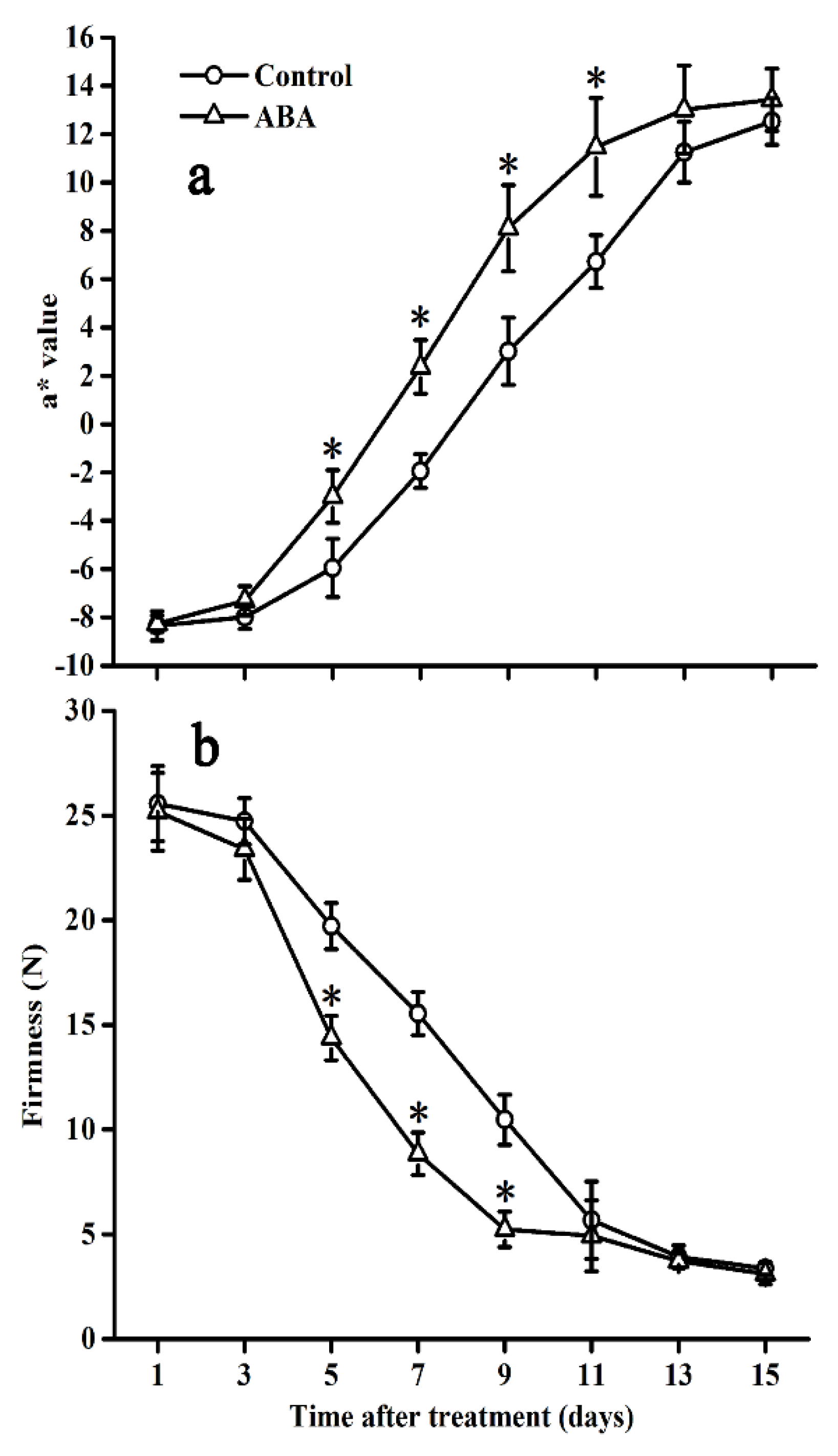
2.1. Effect of ABA on Colour and Firmness
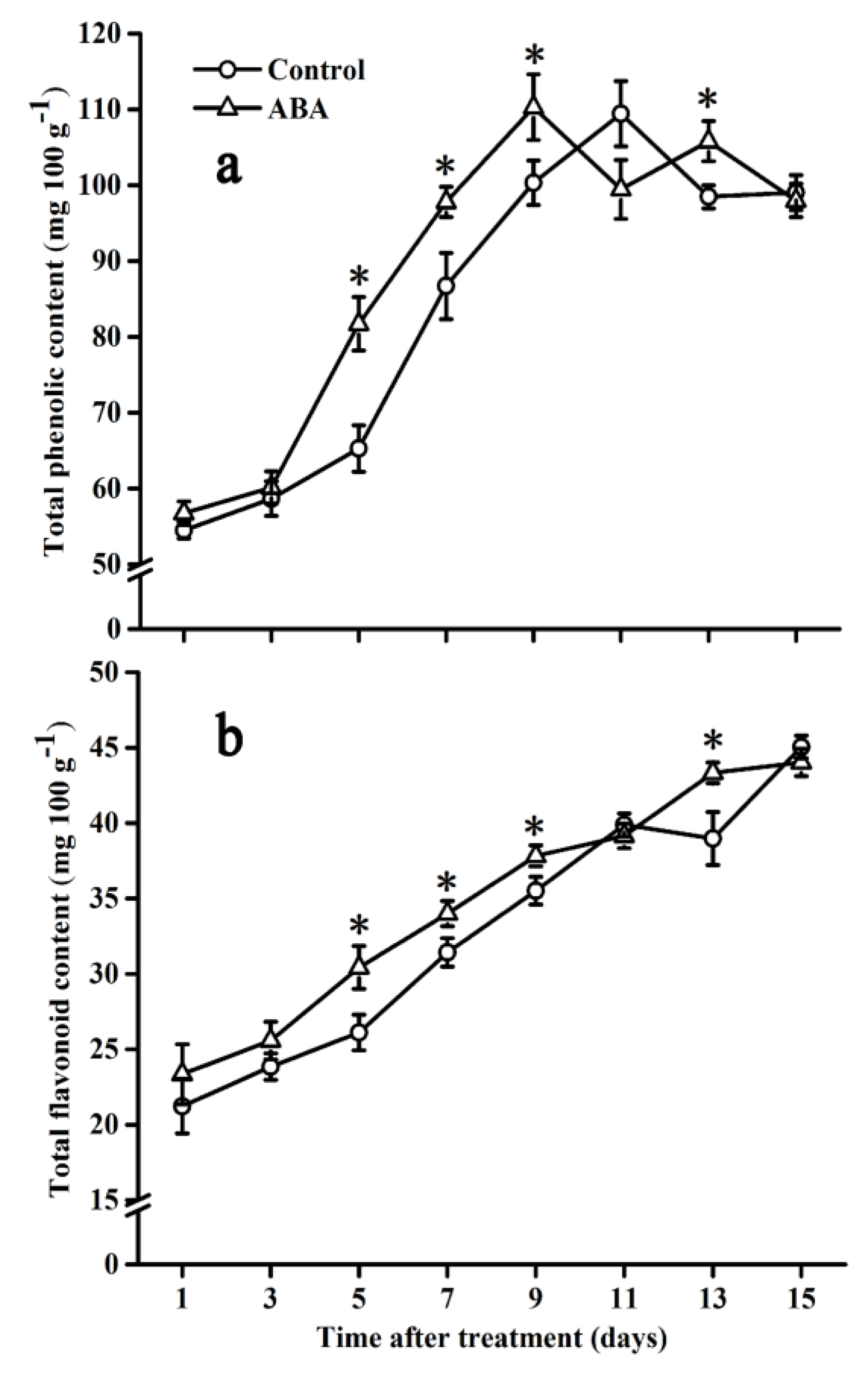
2.2. Effect of ABA on Total Phenolic and Flavonoid Contents
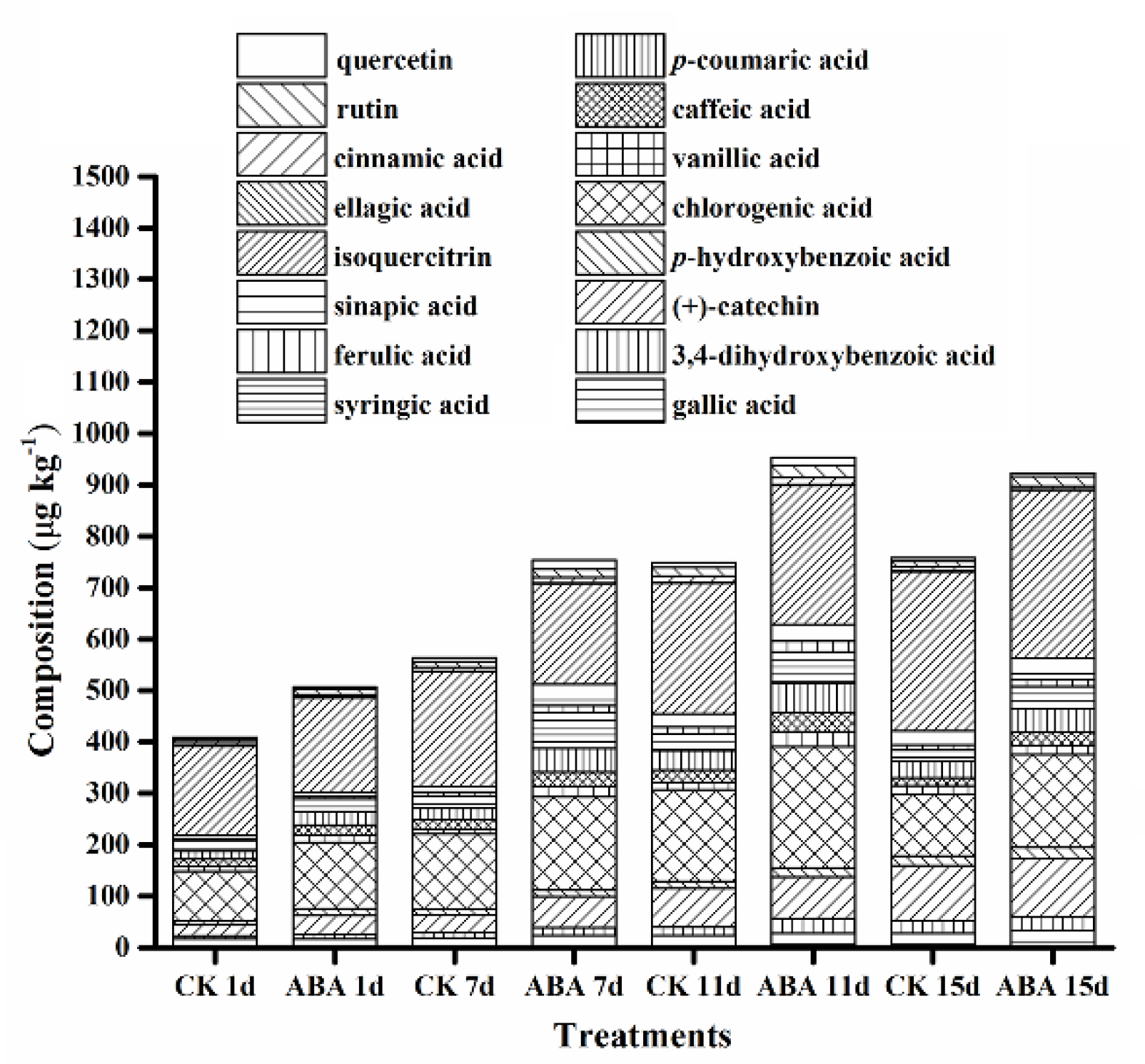
2.3. Effect of ABA on Phenolic Compounds
2.4. Effect of ABA on Lycopene and Ascorbic Acid Contents
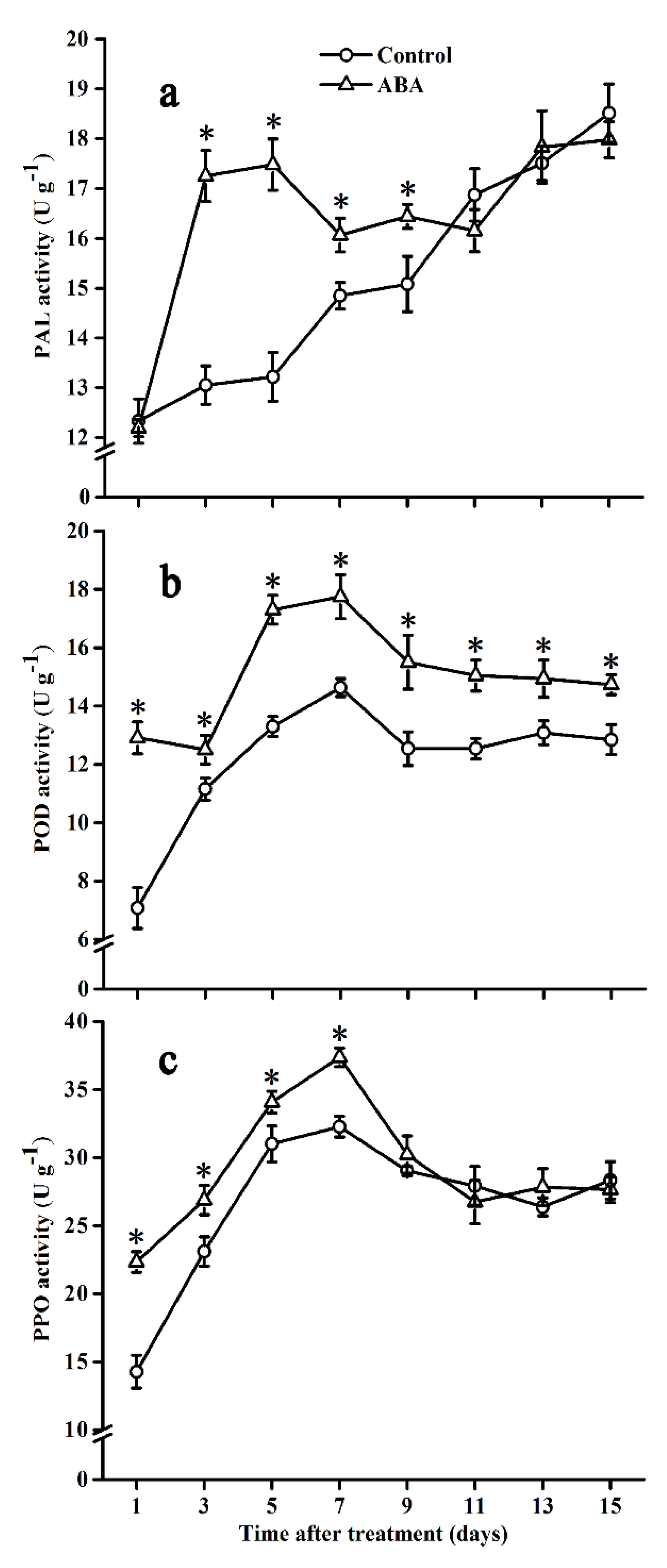
2.5. Effect of ABA on PAL, POD, and PPO Activities
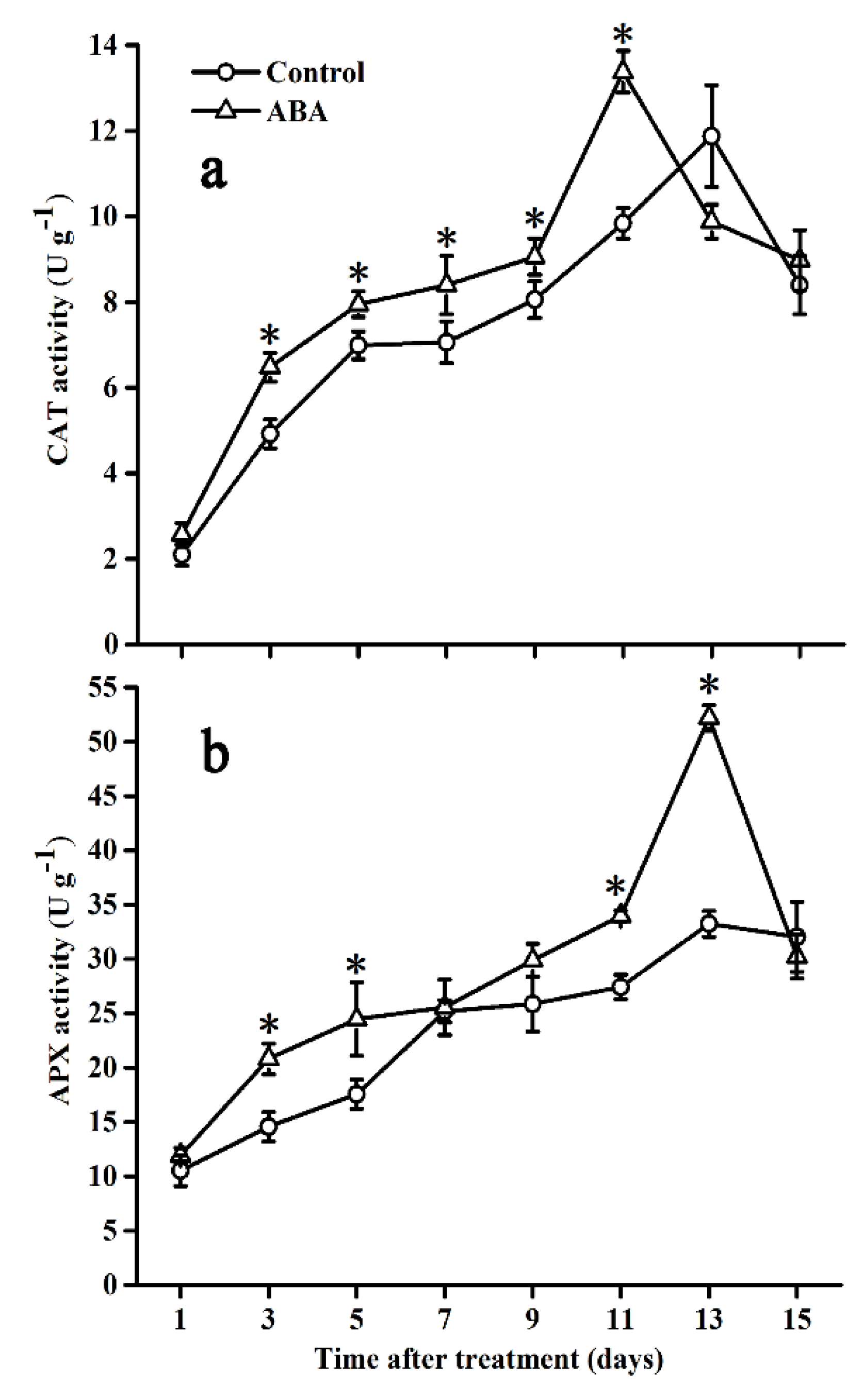
2.6. Effect of ABA on CAT and APX Activities
2.7. Effect of ABA on Antioxidant Capacity
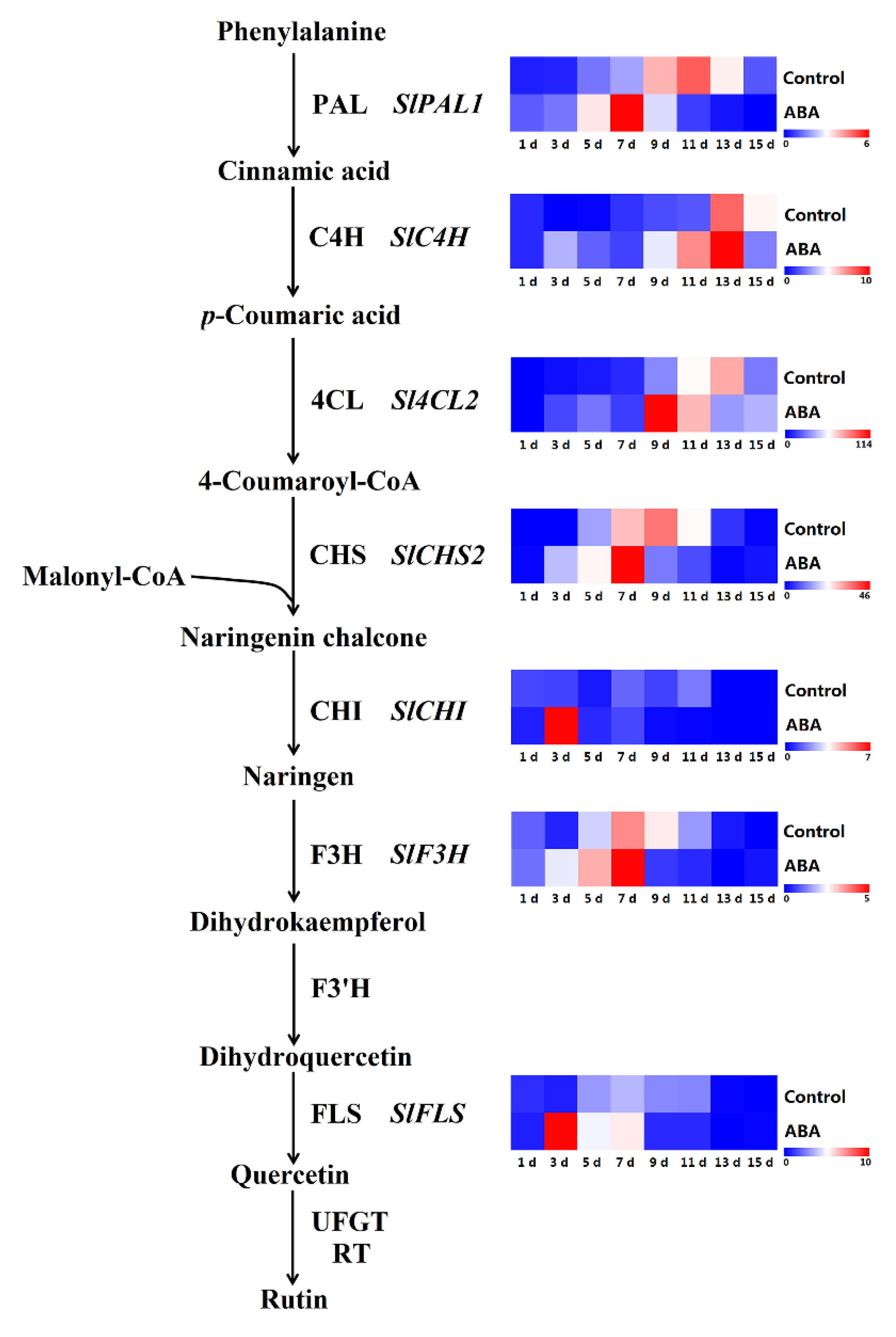
2.8. Effect of ABA on Gene Expression
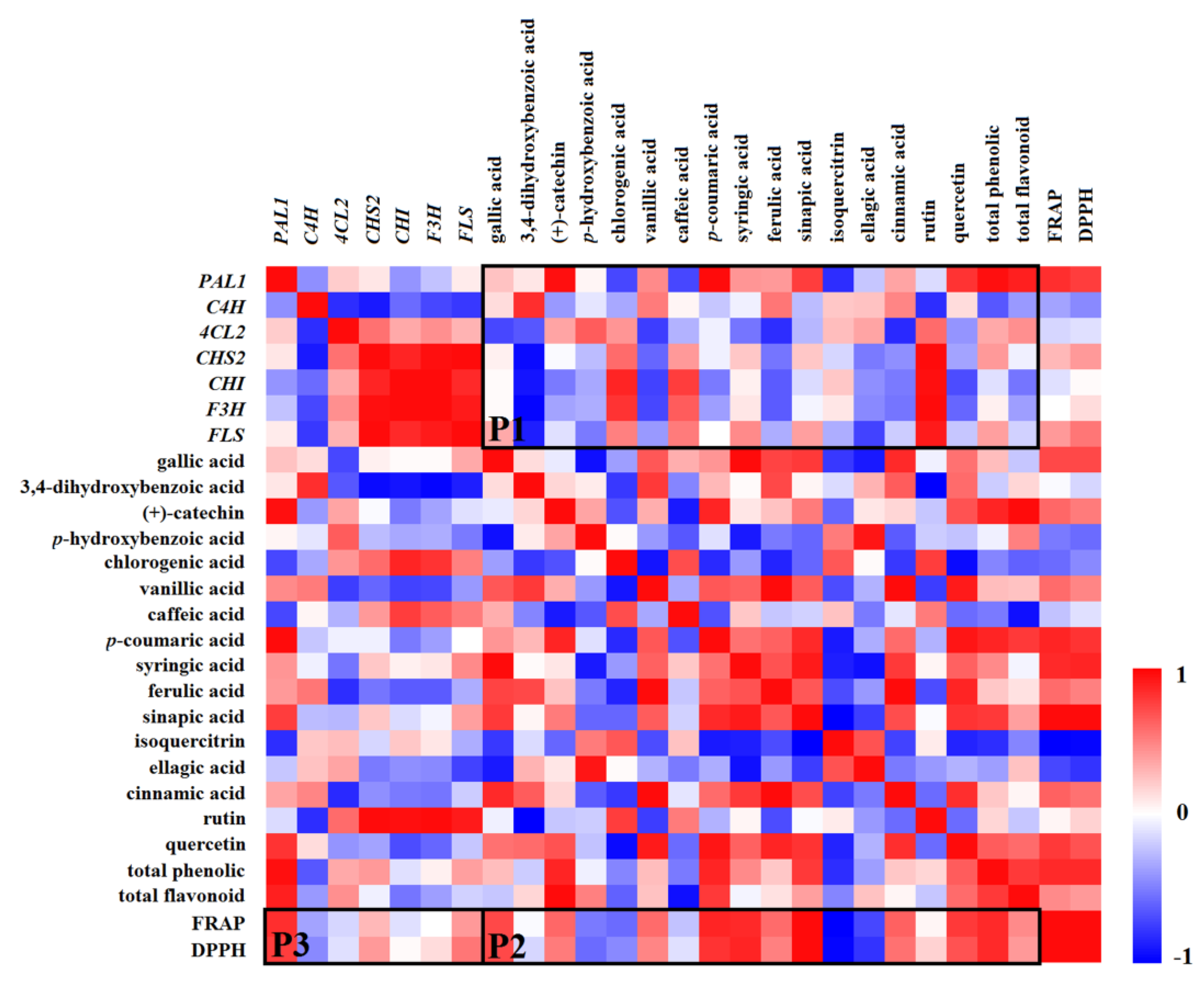
2.9. Correlation Between Gene Expression, Phenolic Compounds, and Antioxidant Capacities
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. ExogenousABA Treatment
4.3. Determination of Fruit Colour and Firmness
4.4. Analysis of Total Phenolic and Flavonoid Contents
4.5. Analysis of Phenolic Compounds
4.6. Analysis of Lycopene and Ascorbic Acid
4.7. Analysis of Enzymatic Activities
4.8. Analysis of Antioxidant Capacities
4.9. Analysis of Gene Expression
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dorais, M.; Ehret, D.L.; Papadopoulos, A.P. Tomato (Solanum lycopersicum) health components: From the seed to the consumer. Phytochem. Rev. 2008, 7, 231–250. [Google Scholar] [CrossRef]
- Mordente, A.; Guantario, B.; Meucci, E.; Silvestrini, A.; Lombardi, E.; Martorana, E.G.; Giardina, B.; Bohm, V. Lycopene and cardiovascular diseases: An update. Curr. Med. Chem. 2011, 18, 1146–1163. [Google Scholar] [CrossRef]
- Vallverdu-Queralt, A.; Medina-Remon, A.; Andres-Lacueva, C.; Lamuela-Raventos, R.M. Changes in phenolic profile and antioxidant activity during production of diced tomatoes. Food Chem. 2011, 126, 1700–1707. [Google Scholar] [CrossRef]
- El-Gaied, L.F.; Abu El-Heba, G.A.; El-Sherif, N.A. Effect of growth hormones on some antioxidant parameters and gene expression in tomato. GM Crops Food 2013, 4, 67–73. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Sacco, A.; Di Matteo, A.; Lombardi, N.; Trotta, N.; Punzo, B.; Mari, A.; Barone, A. Quantitative trait loci pyramiding for fruit quality traits in tomato. Mol. Breed. 2013, 31, 217–222. [Google Scholar] [CrossRef]
- Leibowitz, A.; Faltin, Z.; Perl, A.; Eshdat, Y.; Hagay, Y.; Peleg, E.; Grossman, E. Red grape berry-cultured cells reduce blood pressure in rats with metabolic-like syndrome. Eur. J. Nutr. 2014, 53, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Pennycooke, J.C.; Cox, S.; Stushnoff, C. Relationship of cold acclimation, total phenolic content and antioxidant capacity with chilling tolerance in petunia (Petunia x hybrida). Environ. Exp. Bot. 2005, 53, 225–232. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, S.H.; Kim, H.J.; Lee, I.S.; Kozukue, N.; Levin, C.E.; Friedman, M. Changes in free amino acid, phenolic, chlorophyll, carotenoid, and glycoalkaloid contents in tomatoes during 11 stages of growth and inhibition of cervical and lung human cancer cells by green tomato extracts. J. Agric. Food Chem. 2010, 58, 7547–7556. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Valverde, V.; Navarro-Gonzalez, I.; Garcia-Alonso, J.; Periago, M.J. Antioxidant Bioactive Compounds in Selected Industrial Processing and Fresh Consumption Tomato Cultivars. Food Bioprocess Technol. 2013, 6, 391–402. [Google Scholar] [CrossRef]
- Robards, K.; Prenzler, P.D.; Tucker, G.; Swatsitang, P.; Glover, W. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999, 66, 401–436. [Google Scholar] [CrossRef]
- Karakaya, S.; El, S.N.; Tas, A.A. Antioxidant activity of some foods containing phenolic compounds. Int. J. Food Sci. Nutr. 2001, 52, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Story, E.N.; Kopec, R.E.; Schwartz, S.J.; Harris, G.K. An Update on the Health Effects of Tomato Lycopene. Annu. Rev. Food Sci. Technol. 2010, 1, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Raiola, A.; Rigano, M.M.; Calafiore, R.; Frusciante, L.; Barone, A. Enhancing the health-promoting effects of tomato fruit for biofortified food. Mediat. Inflamm. 2014. [Google Scholar] [CrossRef] [PubMed]
- Biddle, M.J.; Moser, D.; Song, E.K.; Heo, S.; Payne-Emerson, H.; Dunbar, S.B.; Pressler, S.; Lennie, T. Higher dietary lycopene intake is associated with longer cardiac event-free survival in patients with heart failure. Eur. J. Cardiovasc. Nurs. 2012, 12, 377–384. [Google Scholar] [CrossRef][Green Version]
- Sun, Y.; Chen, P.; Duan, C.; Tao, P.; Wang, Y.; Ji, K.; Hu, Y.; Li, Q.; Dai, S.; Wu, Y.; et al. Transcriptional Regulation of Genes Encoding Key Enzymes of Abscisic Acid Metabolism During Melon (Cucumis melo L.) Fruit Development and Ripening. J. Plant Growth Regul. 2012, 32, 233–244. [Google Scholar] [CrossRef]
- Luo, H.; Dai, S.J.; Ren, J.; Zhang, C.X.; Ding, Y.; Li, Z.; Sun, Y.; Ji, K.; Wang, Y.; Li, Q. The Role of ABA in the Maturation and Postharvest Life of a Nonclimacteric Sweet Cherry Fruit. J. Plant Growth Regul. 2014, 33, 373–383. [Google Scholar] [CrossRef]
- Mou, W.; Li, D.; Bu, J.; Jiang, Y.; Khan, Z.U.; Luo, Z.; Mao, L.; Ying, T. Comprehensive Analysis of ABA Effects on Ethylene Biosynthesis and Signaling during Tomato Fruit Ripening. PLoS ONE 2016. [Google Scholar] [CrossRef]
- Chen, J.; Mao, L.; Lu, W.; Ying, T.; Luo, Z. Transcriptome profiling of postharvest strawberry fruit in response to exogenous auxin and abscisic acid. Planta 2016, 243, 183–197. [Google Scholar] [CrossRef]
- Sandhu, A.K.; Gray, D.J.; Lu, J.; Gu, L. Effects of exogenous abscisic acid on antioxidant capacities, anthocyanins, and flavonol contents of muscadine grape (Vitis rotundifolia) skins. Food Chem. 2011, 126, 982–988. [Google Scholar] [CrossRef]
- Tian, L.-X.; Li, J. The effects of exogenous ABA applied to maize (Zea mays L.) roots on plant responses to chilling stress. Acta Physiol. Plant. 2018. [Google Scholar] [CrossRef]
- Wang, Y.L.; Ma, F.W.; Li, M.J.; Liang, D.; Zou, J. Physiological responses of kiwifruit plants to exogenous ABA under drought conditions. Plant Growth Regul. 2011, 64, 63–74. [Google Scholar] [CrossRef]
- Hu, X.; Jiang, M.; Zhang, A.; Lu, J. Abscisic acid-induced apoplastic H2O2 accumulation up-regulates the activities of chloroplastic and cytosolic antioxidant enzymes in maize leaves. Planta 2005, 223, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Karimi, R.; Ershadi, A.; Nejad, A.R.; Khanizadeh, S. Abscisic acid alleviates the deleterious effects of cold stress on ‘Sultana’ grapevine (Vitis vinifera L.) plants by improving the anti-oxidant activity and photosynthetic capacity of leaves. J. Hortic. Sci. Biotechnol. 2016, 91, 386–395. [Google Scholar] [CrossRef]
- Jeong, S.T.; Goto-Yamamoto, N.; Kobayashi, S.; Esaka, M. Effects of plant hormones and shading on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in grape berry skins. Plant Sci. 2004, 167, 247–252. [Google Scholar] [CrossRef]
- Koyama, K.; Sadamatsu, K.; Goto-Yamamoto, N. Abscisic acid stimulated ripening and gene expression in berry skins of the Cabernet Sauvignon grape. Funct. Integr. Genom. 2010, 10, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Perin, E.C.; da Silva Messias, R.; Borowski, J.M.; Crizel, R.L.; Schott, I.B.; Carvalho, I.R.; Rombaldi, C.V.; Galli, V. ABA-dependent salt and drought stress improve strawberry fruit quality. Food Chem. 2019, 271, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Borghesi, E.; Ferrante, A.; Gordillo, B.; Rodríguez-Pulido, F.J.; Cocetta, G.; Trivellini, A.; Mensuali-Sodi, A.; Malorgio, F.; Heredia, F.J. Comparative physiology during ripening in tomato rich-anthocyanins fruits. Plant Growth Regul. 2016, 80, 207–214. [Google Scholar] [CrossRef]
- Zhang, M.; Yuan, B.; Leng, P. The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J. Exp. Bot. 2009, 60, 1579–1588. [Google Scholar] [CrossRef]
- Kumar, R.; Khurana, A.; Sharma, A.K. Role of plant hormones and their interplay in development and ripening of fleshy fruits. J. Exp. Bot. 2014, 65, 4561–4575. [Google Scholar] [CrossRef]
- Mou, W.; Li, D.; Luo, Z.; Mao, L.; Ying, T. Transcriptomic Analysis Reveals Possible Influences of ABA on Secondary Metabolism of Pigments, Flavonoids and Antioxidants in Tomato Fruit during Ripening. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Perez-Balibrea, S.; Moreno, D.A.; Garcia-Viguera, C. Improving the phytochemical composition of broccoli sprouts by elicitation. Food Chem. 2011, 129, 35–44. [Google Scholar] [CrossRef]
- Martinez-Valverde, I.; Periago, M.J.; Provan, G.; Chesson, A. Phenolic compounds, lycopene and antioxidant activity in commercial varieties of tomato (Lycopersicum esculentum). J. Sci. Food Agric. 2002, 82, 323–330. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Ghasemzadeh, N. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plants Res. 2011, 5, 6697–6703. [Google Scholar] [CrossRef]
- Kaur, L.; Zhawar, V.K. Phenolic parameters under exogenous ABA, water stress, salt stress in two wheat cultivars varying in drought tolerance. Indian J. Plant Physiol. 2015, 20, 151–156. [Google Scholar] [CrossRef]
- Tiecher, A.; de Paula, L.A.; Chaves, F.C.; Rombaldi, C.V. UV-C effect on ethylene, polyamines and the regulation of tomato fruit ripening. Postharvest Biol. Technol. 2013, 86, 230–239. [Google Scholar] [CrossRef]
- Vallverdu-Queralt, A.; Oms-Oliu, G.; Odriozola-Serrano, I.; Lamuela-Raventós, R.M.; Martín-Belloso, O.; Elez-Martínez, P. Metabolite profiling of phenolic and carotenoid contents in tomatoes after moderate-intensity pulsed electric field treatments. Food Chem. 2013, 136, 199–205. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, H.; Sheng, K.; Liu, W.; Zheng, L. Effects of postharvest UV-C irradiation on phenolic acids, flavonoids, and key phenylpropanoid pathway genes in tomato fruit. Sci. Hortic. 2018, 241, 107–114. [Google Scholar] [CrossRef]
- Pérez-Conesa, D.; García-Alonso, J.; García-Valverde, V.; Iniesta, M.D.; Jacob, K.; Sánchez-Siles, L.M.; Ros, G.; Periago, M.J. Changes in bioactive compounds and antioxidant activity during homogenization and thermal processing of tomato puree. Innov. Food Sci. Emerg. Technol. 2009, 10, 179–188. [Google Scholar] [CrossRef]
- Vallverdu-Queralt, A.; Medina-Remon, A.; Casals-Ribes, I.; Andres-Lacueva, C.; Waterhouse, A.L.; Lamuela-Raventos, R.M. Effect of tomato industrial processing on phenolic profile and hydrophilic antioxidant capacity. LWT-Food Sci. Technol. 2012, 47, 154–160. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Kim, S.; Chung, I.M. Exogenous phytohormones increase the accumulation of health-promoting metabolites, and influence the expression patterns of biosynthesis related genes and biological activity in Chinese cabbage (Brassica rapa spp. pekinensis). Sci. Hortic. 2015, 193, 136–146. [Google Scholar] [CrossRef]
- Sun, B.; Yan, H.; Zhang, F.; Wang, Q. Effects of plant hormones on main health-promoting compounds and antioxidant capacity of Chinese kale. Food Res. Int. 2012, 48, 359–366. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, X.; Sandhu, A.K.; Gu, L. Effects of exogenous abscisic acid on yield, antioxidant capacities, and phytochemical contents of greenhouse grown lettuces. J. Agric. Food Chem. 2010, 58, 6503–6509. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-Gonzalez, L.; Pena-Neira, A.; Ibanez, F.; Pastenes, C. Long-term effects of abscisic acid (ABA) on the grape berry phenylpropanoid pathway: Gene expression and metabolite content. Plant Physiol. Biochem. 2016, 105, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Medina-Puche, L.; Cumplido-Laso, G.; Amil-Ruiz, F.; Hoffmann, T.; Ring, L.; Rodriguez-Franco, A.; Caballero, J.L.; Schwab, W.; Munoz-Blanco, J.; Blanco-Portales, R. MYB10 plays a major role in the regulation of flavonoid/phenylpropanoid metabolism during ripening of Fragaria x ananassa fruits. J. Exp. Bot. 2014, 65, 401–417. [Google Scholar] [CrossRef]
- Ma, F.; Cheng, L. The sun-exposed peel of apple fruit has higher xanthophyll cycle-dependent thermal dissipation and antioxidants of the ascorbate–glutathione pathway than the shaded peel. Plant Sci. 2003, 165, 819–827. [Google Scholar] [CrossRef]
- Mellidou, I.; Keulemans, J.; Kanellis, A.K.; Davey, M.W. Regulation of fruit ascorbic acid concentrations during ripening in high and low vitamin C tomato cultivars. BMC Plant Biol. 2012. [Google Scholar] [CrossRef]
- Wang, J.; Xia, H.; Lin, L.J.; He, H.; Liang, D.; Lv, X.L. Exogenous abscisic acid increases resistances against abiotic stress and improve fruit quality of grape. J. Anim. Plant Sci. 2016, 26, 1326–1333. [Google Scholar]
- Ghassemian, M.; Lutes, J.; Chang, H.S.; Lange, I.; Chen, W.; Zhu, T.; Wang, X.; Lange, B.M. Abscisic acid-induced modulation of metabolic and redox control pathways in Arabidopsis thaliana. Phytochemistry 2008, 69, 2899–2911. [Google Scholar] [CrossRef]
- Pandey, P.; Singh, J.; Achary, V.; Reddy, M.K. Redox homeostasis via gene families of ascorbate-glutathione pathway. Front. Environ. Sci. 2015. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Involvement of plasma-membrane NADPH oxidase in abscisic acid- and water stress-induced antioxidant defense in leaves of maize seedlings. Planta 2002, 215, 1022–1030. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Cross-talk between calcium and reactive oxygen species originated from NADPH oxidase in abscisic acid-induced antioxidant defence in leaves of maize seedlings. Plant Cell. Environ. 2003, 26, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.S.; Li, X.W.; Zhang, L.X.; Li, B.; Han, M.; Liu, F.; Zheng, P.; Alva, A.K. Role of abscisic acid (ABA) in modulating the responses of two apple rootstocks to drought stress. Pak. J. Bot. 2014, 46, 117–126. [Google Scholar]
- Thiruvengadam, M.; Baskar, V.; Kim, S.H.; Chung, I.-M. Effects of abscisic acid, jasmonic acid and salicylic acid on the content of phytochemicals and their gene expression profiles and biological activity in turnip (Brassica rapa ssp rapa). Plant Growth Regul. 2016, 80, 377–390. [Google Scholar] [CrossRef]
- Agarwal, S.; Sairam, R.K.; Srivastava, G.C.; Meena, R.C. Changes in antioxidant enzymes activity and oxidative stress by abscisic acid and salicylic acid in wheat genotypes. Biol. Plant. 2005, 49, 541–550. [Google Scholar] [CrossRef]
- de Souza, T.C.; Magalhaes, P.C.; de Castro, E.M.; Carneiro, N.P.; Padilha, F.A.; Júnior, C.C.G. ABA application to maize hybrids contrasting for drought tolerance: Changes in water parameters and in antioxidant enzyme activity. Plant Growth Regul. 2014, 73, 205–217. [Google Scholar] [CrossRef]
- Ye, N.; Zhu, G.; Liu, Y.; Zhang, J. ABA Controls H2O2 Accumulation Through the Induction of OsCATB in Rice Leaves Under Water Stress. Plant Cell Physiol. 2011, 52, 689–698. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, K.; Wang, Z.Q.; Yang, J. Abscisic acid, ethylene and antioxidative systems in rice grains in relation with grain filling subjected to postanthesis soil-drying. Plant Growth Regul. 2015, 76, 135–146. [Google Scholar] [CrossRef]
- Toor, R.K.; Savage, G.P. Antioxidant activity in different fractions of tomatoes. Food Res. Int. 2005, 38, 487–494. [Google Scholar] [CrossRef]
- Vallverdu-Queralt, A.; Jauregui, O.; Medina-Remon, A.; Lamuela-Raventos, R.M. Evaluation of a method to characterize the phenolic profile of organic and conventional tomatoes. J. Agric. Food Chem. 2012, 60, 3373–3380. [Google Scholar] [CrossRef]
- Serino, S.; Gomez, L.; Costagliola, G.; Gautier, H. HPLC assay of tomato carotenoids: Validation of a rapid microextraction technique. J. Agric. Food Chem. 2009, 57, 8753–8760. [Google Scholar] [CrossRef] [PubMed]
- Bu, J.W.; Ni, Z.D.; Aisikaer, G.; Jiang, Z.; Khan, Z.U.; Mou, W.; Ying, T. Postharvest ultraviolet-C irradiation suppressed Psy 1 and Lcy-beta-expression and altered color phenotype in tomato (Solanum lycopersicum) fruit. Postharvest Biol. Technol. 2014, 89, 1–6. [Google Scholar] [CrossRef]
- Jagadeesh, S.L.; Charles, M.T.; Gariepy, Y.; Goyette, B.; Raghavan, G.S.V.; Vigneault, C. Influence of Postharvest UV-C Hormesis on the Bioactive Components of Tomato during Post-treatment Handling. Food Bioprocess Technol. 2011, 4, 1463–1472. [Google Scholar] [CrossRef]
- Yingsanga, P.; Srilaong, V.; Kanlayanarat, S.; Noichinda, S.; McGlasson, W.B. Relationship between browning and related enzymes (PAL, PPO and POD) in rambutan fruit (Nephelium lappaceum Linn.) cvs. Rongrien and See-Chompoo. Postharvest Biol. Technol. 2008, 50, 164–168. [Google Scholar] [CrossRef]
- Zhang, J.; Kirkham, M.B. Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytol. 1996, 132, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Nahakpam, S.; Shah, K. Expression of key antioxidant enzymes under combined effect of heat and cadmium toxicity in growing rice seedlings. Plant Growth Regul. 2011, 63, 23–35. [Google Scholar] [CrossRef]
- Alothman, M.; Bhat, R.; Karim, A.A. UV radiation-induced changes of antioxidant capacity of fresh-cut tropical fruits. Innov. Food Sci. Emerg. 2009, 10, 512–516. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Martin-Belloso, O. Antioxidant properties and shelf-life extension of fresh-cut tomatoes stored at different temperatures. J. Sci. Food Agric. 2008, 88, 2606–2614. [Google Scholar] [CrossRef]
- Bu, J.; Yu, Y.; Aisikaer, G.; Ying, T. Postharvest UV-C irradiation inhibits the production of ethylene and the activity of cell wall-degrading enzymes during softening of tomato (Lycopersicon esculentum L.) fruit. Postharvest Biol. Technol. 2013, 86, 337–345. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, X.; Wu, Q.; Aalim, H.; Li, L.; Mao, L.; Luo, Z.; Ying, T. Effects of Exogenous Abscisic Acid on Bioactive Components and Antioxidant Capacity of Postharvest Tomato during Ripening. Molecules 2020, 25, 1346. https://doi.org/10.3390/molecules25061346
Tao X, Wu Q, Aalim H, Li L, Mao L, Luo Z, Ying T. Effects of Exogenous Abscisic Acid on Bioactive Components and Antioxidant Capacity of Postharvest Tomato during Ripening. Molecules. 2020; 25(6):1346. https://doi.org/10.3390/molecules25061346
Chicago/Turabian StyleTao, Xiaoya, Qiong Wu, Halah Aalim, Li Li, Linchun Mao, Zisheng Luo, and Tiejin Ying. 2020. "Effects of Exogenous Abscisic Acid on Bioactive Components and Antioxidant Capacity of Postharvest Tomato during Ripening" Molecules 25, no. 6: 1346. https://doi.org/10.3390/molecules25061346
APA StyleTao, X., Wu, Q., Aalim, H., Li, L., Mao, L., Luo, Z., & Ying, T. (2020). Effects of Exogenous Abscisic Acid on Bioactive Components and Antioxidant Capacity of Postharvest Tomato during Ripening. Molecules, 25(6), 1346. https://doi.org/10.3390/molecules25061346







