Paper-Based Oil Barrier Packaging using Lignin-Containing Cellulose Nanofibrils
Abstract
1. Introduction
2. Results
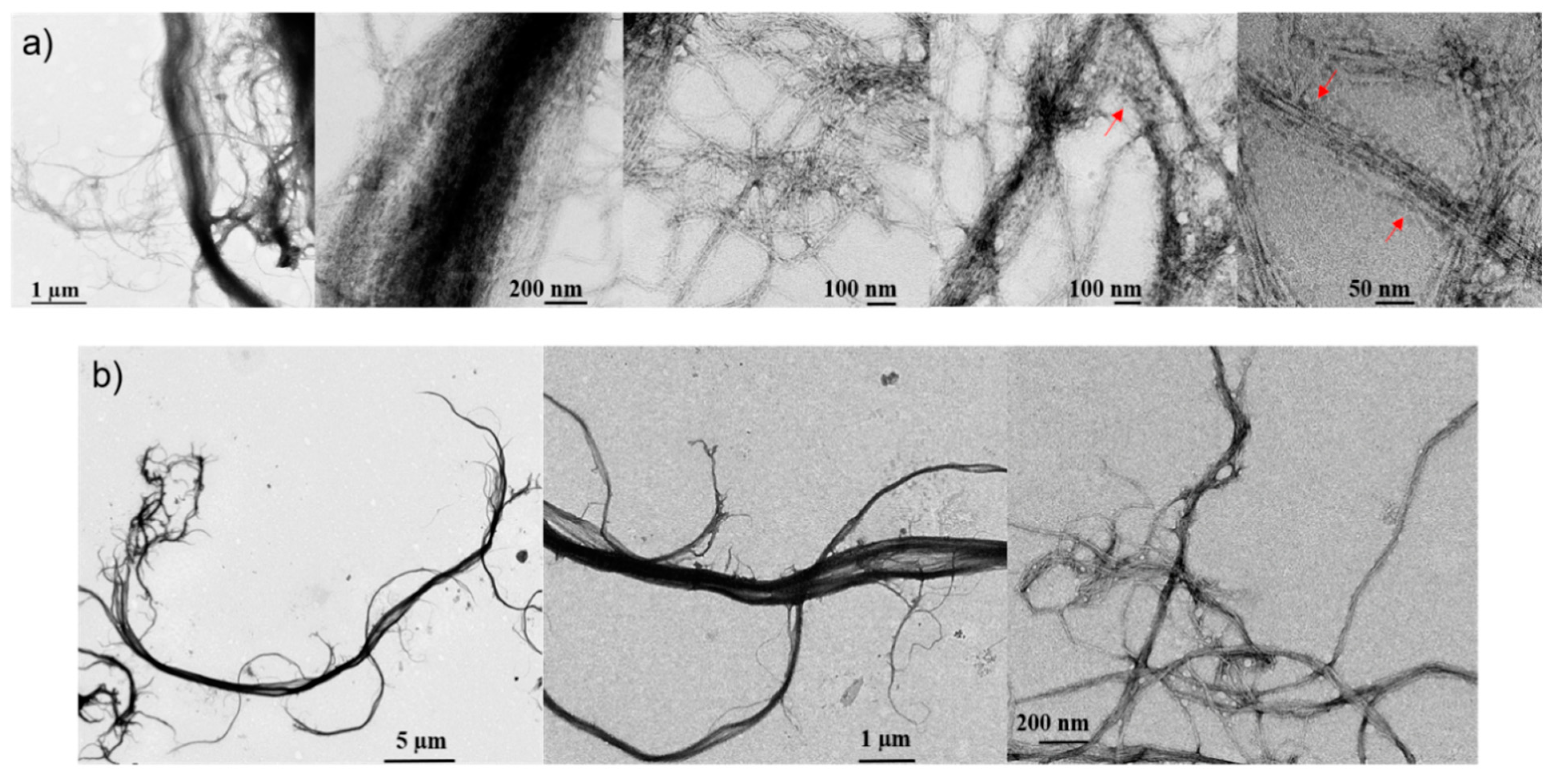
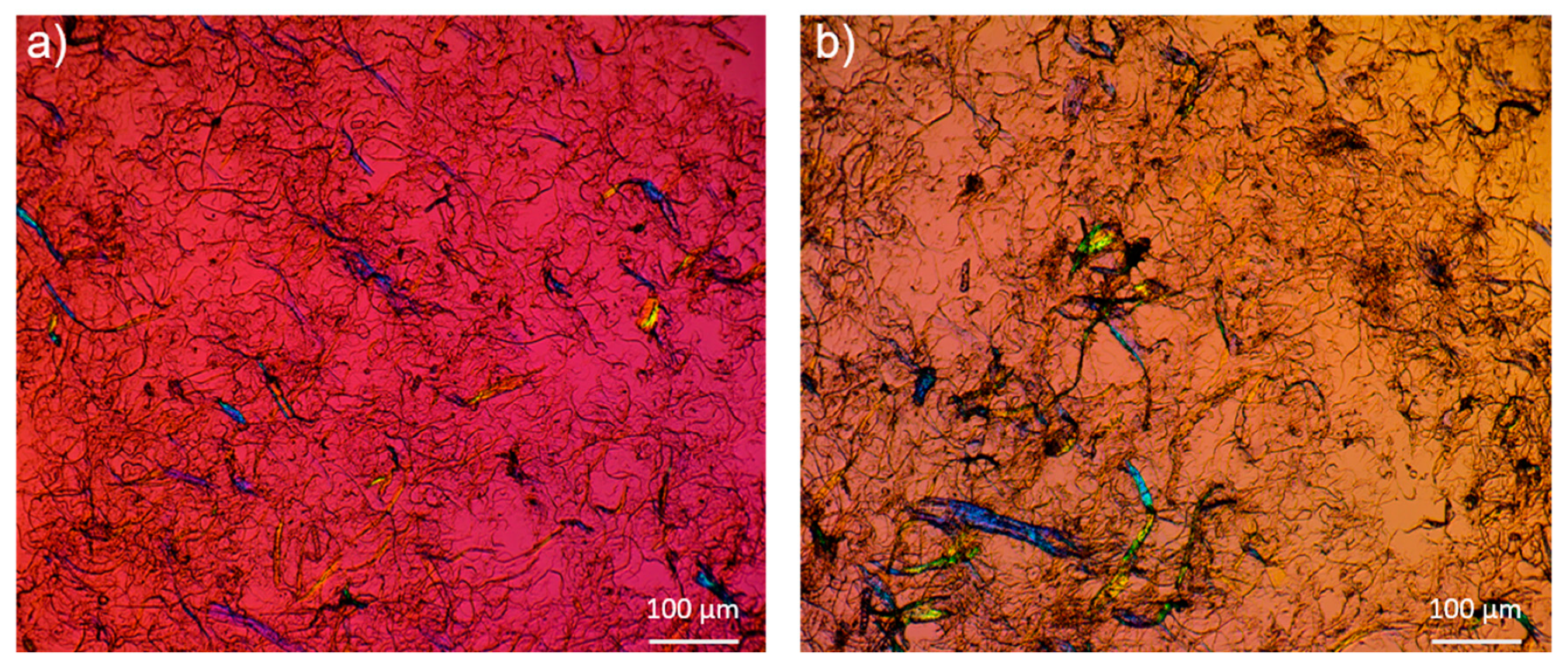
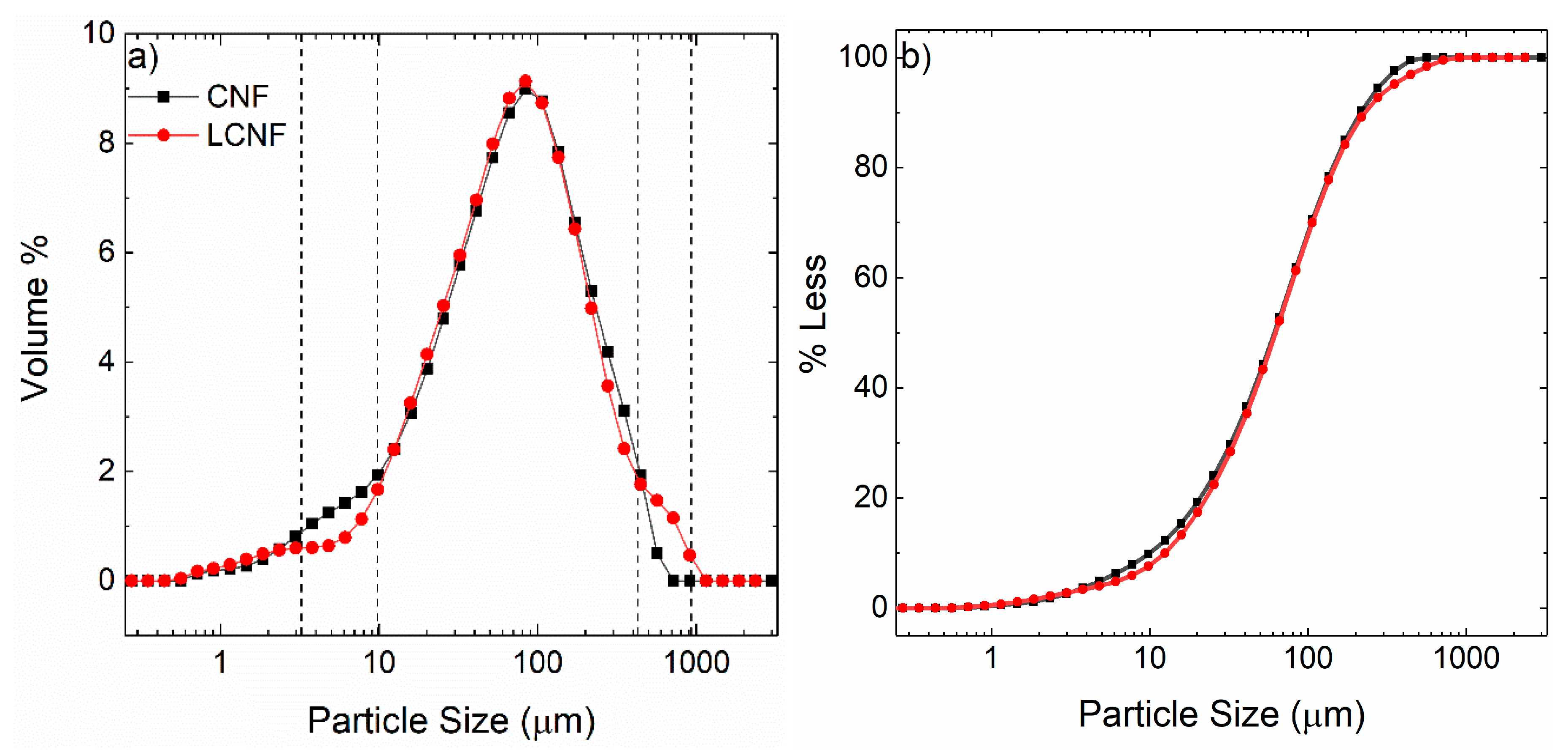
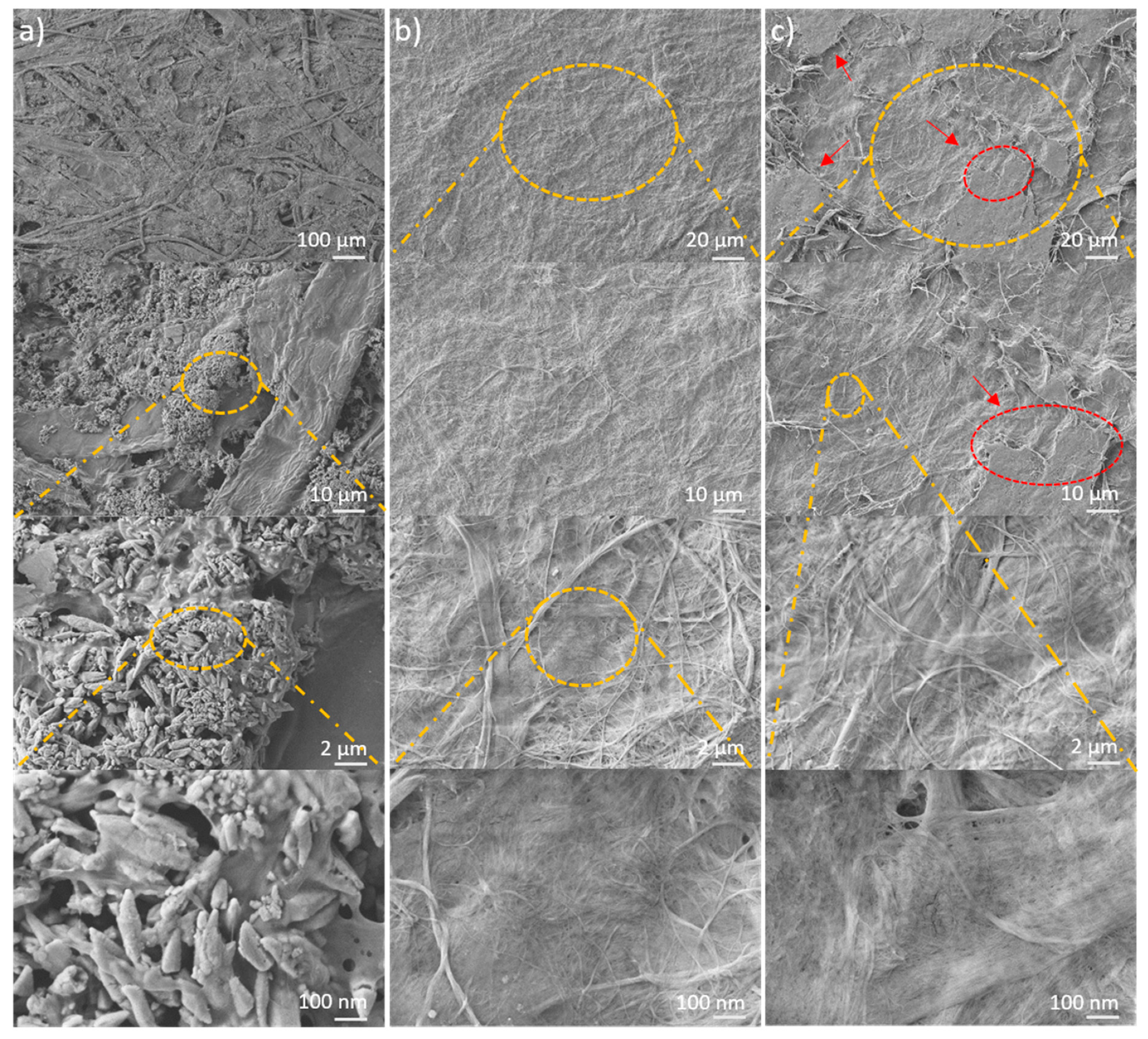
2.1. Morphology Differences between CNF and LCNF
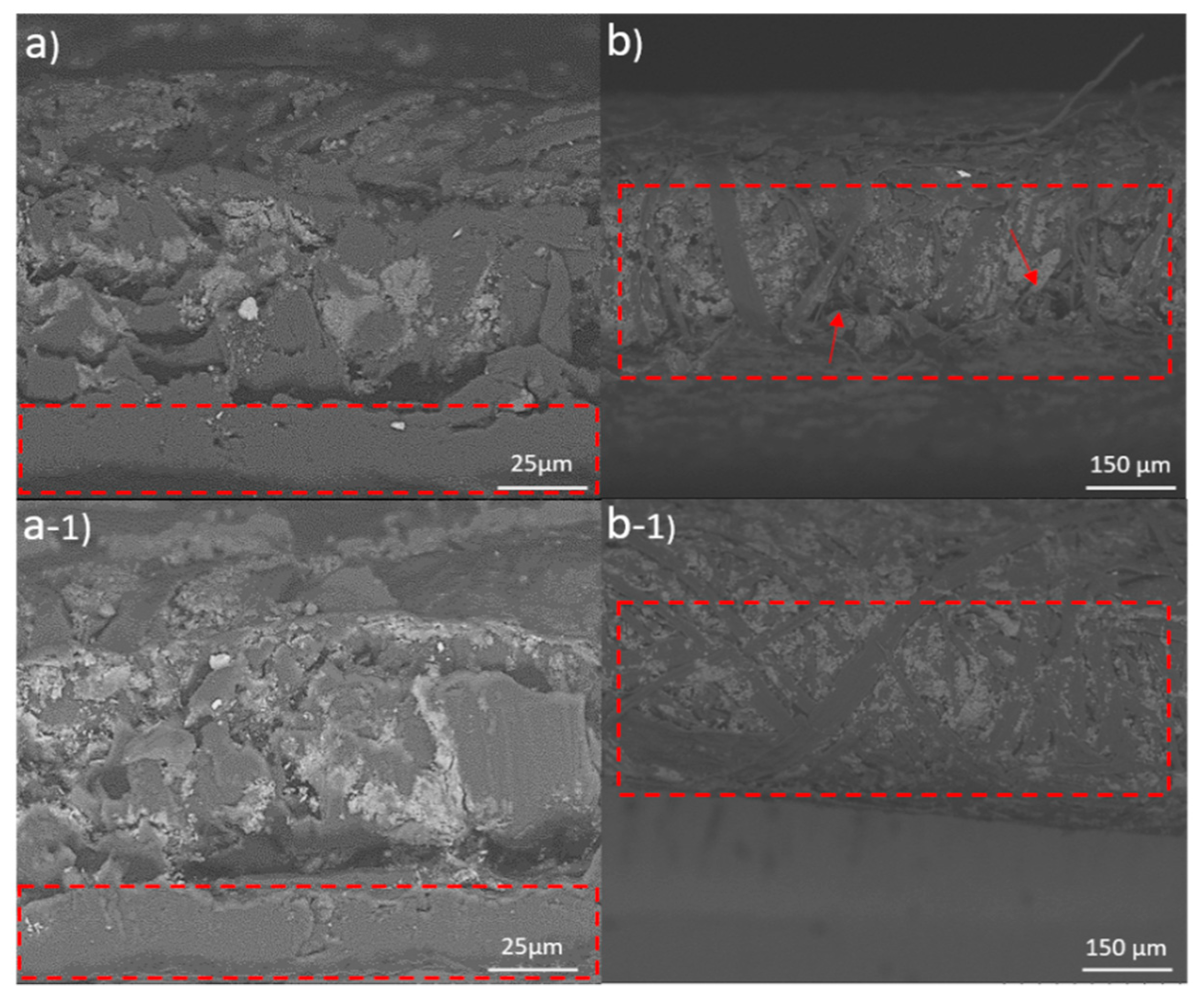
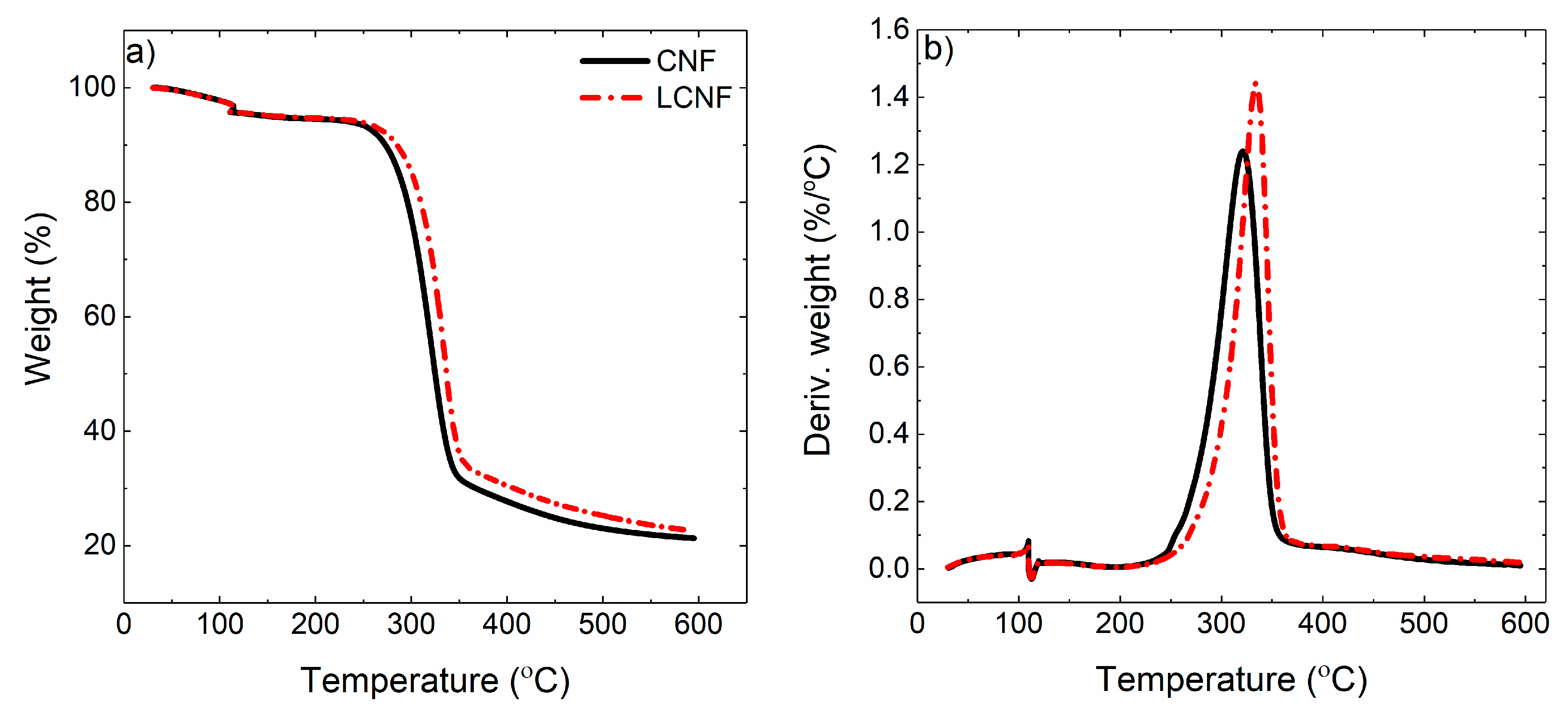
2.2. Film Properties
3. Conclusions
4. Experimental
4.1. Materials
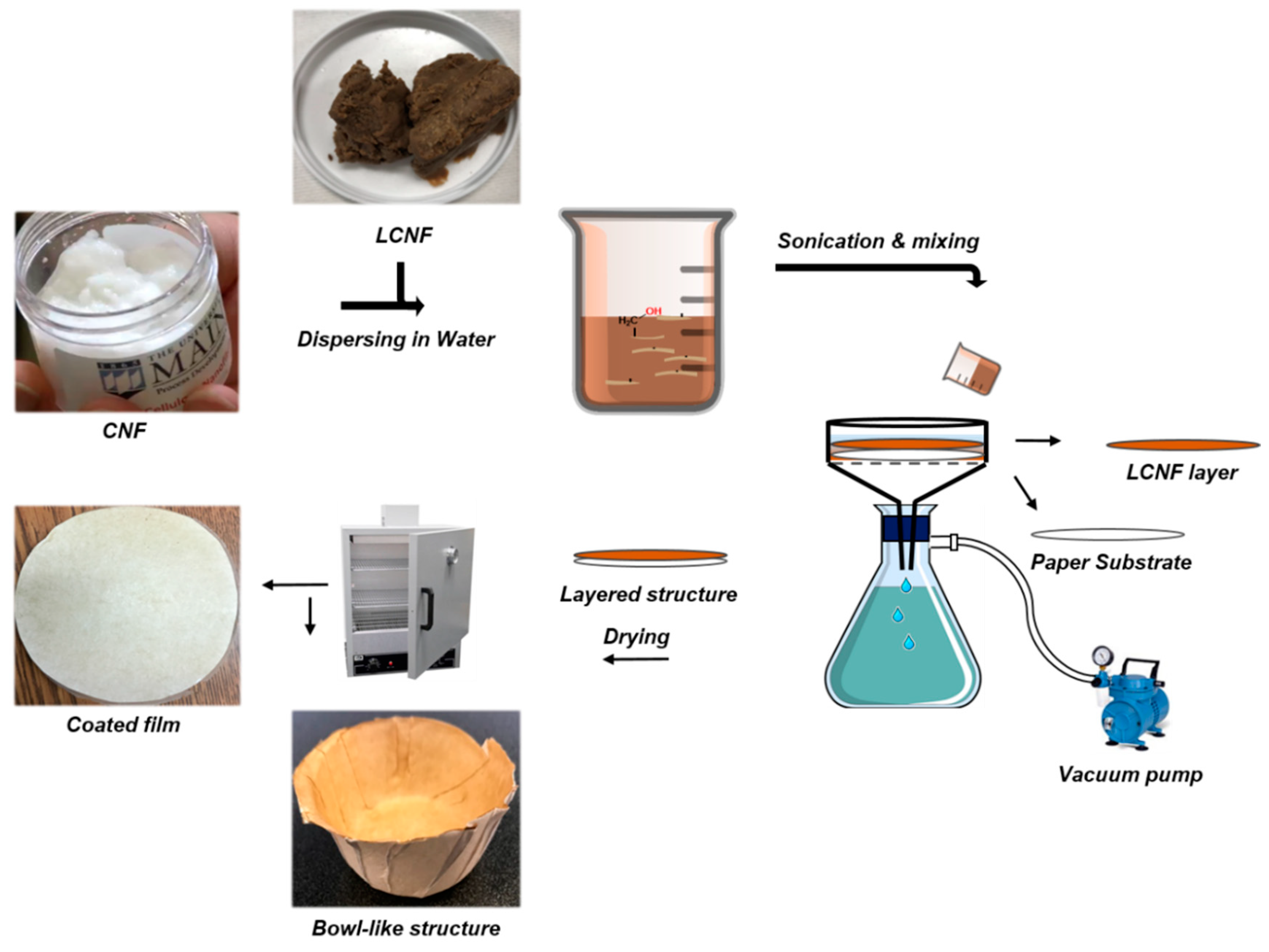
4.2. Preparation of Coated Films and Characterization
4.3. Surface Free Energy
4.4. Thermogravimetric Analysis (TGA)
4.5. Mechanical Attributes of the Films
4.6. Microscopic Analysis; SEM, TEM and Optical Microscopy
4.7. Particle Size Distribution Analysis
4.8. Oil Resistance
4.9. Water Vapor Transmission Rate
4.10. Oxygen Transmission Rate
Author Contributions
Funding
Conflicts of Interest
References
- Sheng, J.; Li, J.; Zhao, L. Fabrication of grease resistant paper with non-fluorinated chemicals for food packaging. Cellulose 2019, 26, 6291–6302. [Google Scholar] [CrossRef]
- Tayeb, A.H.; Amini, E.; Ghasemi, S.; Tajvidi, M. Cellulose Nanomaterials—Binding Properties and Applications: A Review. Molecules 2018, 23, 2684. [Google Scholar] [CrossRef] [PubMed]
- Kjellgren, H.; Gällstedt, M.; Engström, G.; Järnström, L. Barrier and surface properties of chitosan-coated greaseproof paper. Carbohydr. Polym. 2006, 65, 453–460. [Google Scholar] [CrossRef]
- Tarrés, Q.; Oliver-Ortega, H.; Ferreira, P.J.; Àngels Pèlach, M.; Mutjé, P.; Delgado-Aguilar, M. Towards a new generation of functional fiber-based packaging: cellulose nanofibers for improved barrier, mechanical and surface properties. Cellulose 2018, 25, 683–695. [Google Scholar] [CrossRef]
- Butt, C.M.; Muir, D.C.G.; Mabury, S.A. Biotransformation pathways of fluorotelomer-based polyfluoroalkyl substances: A review. Environ. Toxicol. Chem. 2014, 33, 243–267. [Google Scholar] [CrossRef] [PubMed]
- Vierke, L.; Staude, C.; Biegel-Engler, A.; Drost, W.; Schulte, C. Perfluorooctanoic acid (PFOA)-main concerns and regulatory developments in Europe from an environmental point of view. Environ. Sci. Eur. 2012, 24. [Google Scholar] [CrossRef]
- Wang, Z.; Cousins, I.T.; Scheringer, M.; Hungerbuehler, K. Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFAAs) and their precursors: Status quo, ongoing challenges and possible solutions. Environ. Int. 2015, 75, 172–179. [Google Scholar] [CrossRef]
- Holmquist, H.; Schellenberger, S.; van der Veen, I.; Peters, G.M.; Leonards, P.; Cousins, I. Properties, performance and associated hazards of state-of-the-art durable water repellent (DWR) chemistry for textile finishing. Environ. Int. 2016, 91, 251–264. [Google Scholar] [CrossRef]
- MacLeod, M.; Breitholtz, M.; Cousins, I.; Wit, C.; Persson, L.; Rudén, C.; McLachlan, M. Identifying Chemicals That Are Planetary Boundary Threats. Environ. Sci. Technol. 2014, 48, 11057–11063. [Google Scholar] [CrossRef]
- Kihlman, M.; Aldaeus, F.; Chedid, F.; Germgård, U. Effect of various pulp properties on the solubility of cellulose in sodium hydroxide solutions. Holzforschung 2012, 66, 601–606. [Google Scholar] [CrossRef]
- Li, R.; Chang, C.; Zhou, J.; Zhang, L.; Gu, W.; Li, C.; Liu, S.; Kuga, S. Primarily Industrialized Trial of Novel Fibers Spun from Cellulose Dope in NaOH/Urea Aqueous Solution. Ind. Eng. Chem. Res. 2010, 49, 11380–11384. [Google Scholar] [CrossRef]
- Huber, T.; Pang, S.; Staiger, M.P. All-cellulose composite laminates. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1738–1745. [Google Scholar]
- Ma, J.; Wang, Z.; Zhou, X.; Xiao, H. Self-Reinforced Grease-Resistant Sheets Produced by Paper Treatment with Zinc Chloride Solution. BioResources 2015, 10, 8225–8237. [Google Scholar] [CrossRef][Green Version]
- Jost, V.; Kobsik, K.; Schmid, M.; Noller, K. Influence of plasticiser on the barrier, mechanical and grease resistance properties of alginate cast films. Carbohydr. Polym. 2014, 110, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, V.K.; Samyn, P. Bio-based coatings for paper applications. Coatings 2015, 5, 887–930. [Google Scholar] [CrossRef]
- Tayeb, A.H.; Tajvidi, M. Sustainable Barrier System via Self-Assembly of Colloidal Montmorillonite and Cross-linking Resins on Nanocellulose Interfaces. ACS Appl. Mater. Interfaces 2018, 11, 1604–1615. [Google Scholar] [CrossRef]
- Tayeb, P.; Tayeb, A.H. Nanocellulose applications in sustainable electrochemical and piezoelectric systems: A review. Carbohydr. Polym. 2019, 224, 115149. [Google Scholar] [CrossRef]
- Zheng, M.; Tajvidi, M.; Tayeb, A.H.; Stark, N.M. Effects of bentonite on physical, mechanical and barrier properties of cellulose nanofibril hybrid films for packaging applications. Cellulose 2019, 26, 5363–5379. [Google Scholar] [CrossRef]
- Wang, J.; Gardner, D.J.; Stark, N.M.; Bousfield, D.W.; Tajvidi, M.; Cai, Z. Moisture and Oxygen Barrier Properties of Cellulose Nanomaterial-Based Films. ACS Sustain. Chem. Eng. 2018, 6, 49–70. [Google Scholar] [CrossRef]
- Tayeb, A.H.; Latibari, A.J.; Tajdini, A.; Sepidehdam, S.M.J. The influence of pulp refining on de-inking potential and strength properties of ink jet printed paper. BioResources 2012, 7, 3837–3846. [Google Scholar]
- Hubbe, M.; Pruszynski, P. Greaseproof Paper Products: A Review Emphasizing Ecofriendly Approaches. bioresources 2020, 15, 1978–2004. [Google Scholar]
- Delgado-Aguilar, M.; González, I.; Tarrés, Q.; Pèlach, M.À.; Alcalà, M.; Mutjé, P. The key role of lignin in the production of low-cost lignocellulosic nanofibres for papermaking applications. Ind. Crops Prod. 2016, 86, 295–300. [Google Scholar] [CrossRef]
- Rojo, E.; Peresin, M.S.; Sampson, W.W.; Hoeger, I.C.; Vartiainen, J.; Laine, J.; Rojas, O.J. Comprehensive elucidation of the effect of residual lignin on the physical, barrier, mechanical and surface properties of nanocellulose films. Green Chem. 2015, 17, 1853–1866. [Google Scholar] [CrossRef]
- Bian, H.; Chen, L.; Dai, H.; Zhu, J.Y. Integrated production of lignin containing cellulose nanocrystals (LCNC) and nanofibrils (LCNF) using an easily recyclable di-carboxylic acid. Carbohydr. Polym. 2017, 167, 167–176. [Google Scholar] [CrossRef]
- Nair, S.S.; Yan, N. Effect of high residual lignin on the thermal stability of nanofibrils and its enhanced mechanical performance in aqueous environments. Cellulose 2015, 22, 3137–3150. [Google Scholar] [CrossRef]
- Farooq, M.; Zou, T.; Riviere, G.; Sipponen, M.H.; Österberg, M. Strong, Ductile, and Waterproof Cellulose Nanofibril Composite Films with Colloidal Lignin Particles. Biomacromolecules 2019, 20, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Buono, P.; Ruch, D.; Dubois, P.; Wu, L.; Wang, W.J. Biodegradable UV-Blocking Films through Core-Shell Lignin-Melanin Nanoparticles in Poly(butylene adipate- co-terephthalate). ACS Sustain. Chem. Eng. 2019, 7, 4147–4157. [Google Scholar] [CrossRef]
- Nair, S.; Kuo, P.Y.; Chen, H.; Yan, N. Investigating the effect of lignin on the mechanical, thermal, and barrier properties of cellulose nanofibril reinforced epoxy composite. Ind. Crops Prod. 2017, 100, 208–217. [Google Scholar] [CrossRef]
- Horseman, T.; Tajvidi, M.; Diop, C.I.K.; Gardner, D.J. Preparation and property assessment of neat lignocellulose nanofibrils (LCNF) and their composite films. Cellulose 2017, 24, 2455–2468. [Google Scholar] [CrossRef]
- Mazhari Mousavi, S.M.; Afra, E.; Tajvidi, M.; Bousfield, D.W.; Dehghani-Firouzabadi, M. Cellulose nanofiber/carboxymethyl cellulose blends as an efficient coating to improve the structure and barrier properties of paperboard. Cellulose 2017, 24, 3001–3014. [Google Scholar] [CrossRef]
- Kumar, V.; Elfving, A.; Koivula, H.; Bousfield, D.; Toivakka, M. Roll-to-Roll Processed Cellulose Nanofiber Coatings. Ind. Eng. Chem. Res. 2016, 55, 3603–3613. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, X.; Yang, Q.; Song, X.; Qin, C.; Wang, S.; Li, K. Effects of residual lignin on composition, structure and properties of mechanically defibrillated cellulose fibrils and films. Cellulose 2019, 26, 1577–1593. [Google Scholar] [CrossRef]
- Hong, S.; Song, Y.; Yuan, Y.; Lian, H.; Liimatainen, H. Production and characterization of lignin containing nanocellulose from luffa through an acidic deep eutectic solvent treatment and systematic fractionation. Ind. Crops Prod. 2020, 143, 111913. [Google Scholar] [CrossRef]
- Huang, Y.; Nair, S.S.; Chen, H.; Fei, B.; Yan, N.; Feng, Q. Lignin-Rich Nanocellulose Fibrils Isolated from Parenchyma Cells and Fiber Cells of Western Red Cedar Bark. ACS Sustain. Chem. Eng. 2019, 7, 15607–15616. [Google Scholar] [CrossRef]
- Wolf, C.; Angellier-Coussy, H.; Gontard, N.; Doghieri, F.; Guillard, V. How the shape of fillers affects the barrier properties of polymer/non-porous particles nanocomposites: A review. J. Memb. Sci. 2018, 556, 393–418. [Google Scholar] [CrossRef]
- Tayeb, A.H.; Hubbe, M.A.; Tayeb, P.; Pal, L.; Rojas, O.J. Soy Proteins As a Sustainable Solution to Strengthen Recycled Paper and Reduce Deposition of Hydrophobic Contaminants in Papermaking: A Bench and Pilot-Plant Study. ACS Sustain. Chem. Eng. 2017, 5, 7211–7219. [Google Scholar] [CrossRef]
- Meier, M.M.; Kanis, L.A.; de Lima, J.C.; Pires, A.T.N.; Soldi, V. Poly(caprolactone triol) as plasticizer agent for cellulose acetate films: influence of the preparation procedure and plasticizer content on the physico-chemical properties. Polym. Adv. Technol. 2004, 15, 593–600. [Google Scholar] [CrossRef]
- Spence, K.L.; Venditti, R.A.; Rojas, O.J.; Habibi, Y.; Pawlak, J.J. The effect of chemical composition on microfibrillar cellulose films from wood pulps: water interactions and physical properties for packaging applications. Cellulose 2010, 17, 835–848. [Google Scholar] [CrossRef]
- Paunonen, S. Strength and barrier enhancements of cellophane and cellulose derivative films: A Review: BioResources. BioResources 2013, 8, 3098–3121. [Google Scholar] [CrossRef]
- Del Nobile, M.; Fava, P.; Piergiovanni, L. Water transport properties of cellophane flexible films intended for food packaging applications. J. Food Eng. 2002, 53, 295–300. [Google Scholar] [CrossRef]
- Aulin, C.; Salazar-Alvarez, G.; Lindström, T. High strength, flexible and transparent nanofibrillated cellulose–nanoclay biohybrid films with tunable oxygen and water vapor permeability. Nanoscale 2012, 4, 6622–6628. [Google Scholar] [CrossRef]
- Aulin, C.; Gällstedt, M.; Lindström, T. Oxygen and oil barrier properties of microfibrillated cellulose films and coatings. Cellulose 2010, 17, 559–574. [Google Scholar] [CrossRef]
- Kumar, V.; Bollström, R.; Yang, A.; Chen, Q.; Chen, G.; Salminen, P.; Bousfield, D.; Toivakka, M. Comparison of nano- and microfibrillated cellulose films. Cellulose 2014, 21, 3443–3456. [Google Scholar] [CrossRef]
- Nair, S.; Zhu, J.; Deng, Y.; Ragauskas, A. High performance green barriers based on nanocellulose. Sustain. Chem. Process. 2014, 2, 23. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, S. The impact of low-surface-energy functional groups on oil fouling resistance in membrane distillation. J. Memb. Sci. 2017, 527, 68–77. [Google Scholar] [CrossRef]
- Udoetok, I.A.; Dimmick, R.M.; Wilson, L.D.; Headley, J.V. Adsorption properties of cross-linked cellulose-epichlorohydrin polymers in aqueous solution. Carbohydr. Polym. 2016, 136, 329–340. [Google Scholar] [CrossRef]
- Pratt, D.Y.; Wilson, L.D.; Kozinski, J.A.; Mohart, A.M. Preparation and sorption studies of β-cyclodextrin/epichlorohydrin copolymers. J. Appl. Polym. Sci. 2010, 116, 2982–2989. [Google Scholar] [CrossRef]
- Yu, J.; Paterson, N.; Blamey, J.; Millan, M. Cellulose, xylan and lignin interactions during pyrolysis of lignocellulosic biomass. Fuel 2017, 191, 140–149. [Google Scholar] [CrossRef]
- Smook, G.A. Handbook for Pulp & Paper Technologists, 3rd ed.; Angus Wilde Publications: Vancouver, BC, Canada, 2002; ISBN 0969462859. [Google Scholar]
- TAPPI T 538. Roughness of Paper and Paperboard (Sheffield Method); TAPPI Press: Atlanta, GA, USA, 2016. [Google Scholar]
- Kaelble, D.H. Dispersion-Polar Surface Tension Properties of Organic Solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]
- TAPPI T 559 cm-12. Grease Resistance Test for Paper and Paperboard; TAPPI Press: Atlanta, GA, USA, 2012. [Google Scholar]
- Bedane, A.H.; Eić, M.; Farmahini-Farahani, M.; Xiao, H. Water vapor transport properties of regenerated cellulose and nanofibrillated cellulose films. J. Memb. Sci. 2015, 493, 46–57. [Google Scholar] [CrossRef]
- ASTM D3985-17 Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor; ASTM International: West Conshohocken, PA, USA, 2017.
Sample Availability: Samples of the compounds are not available. |
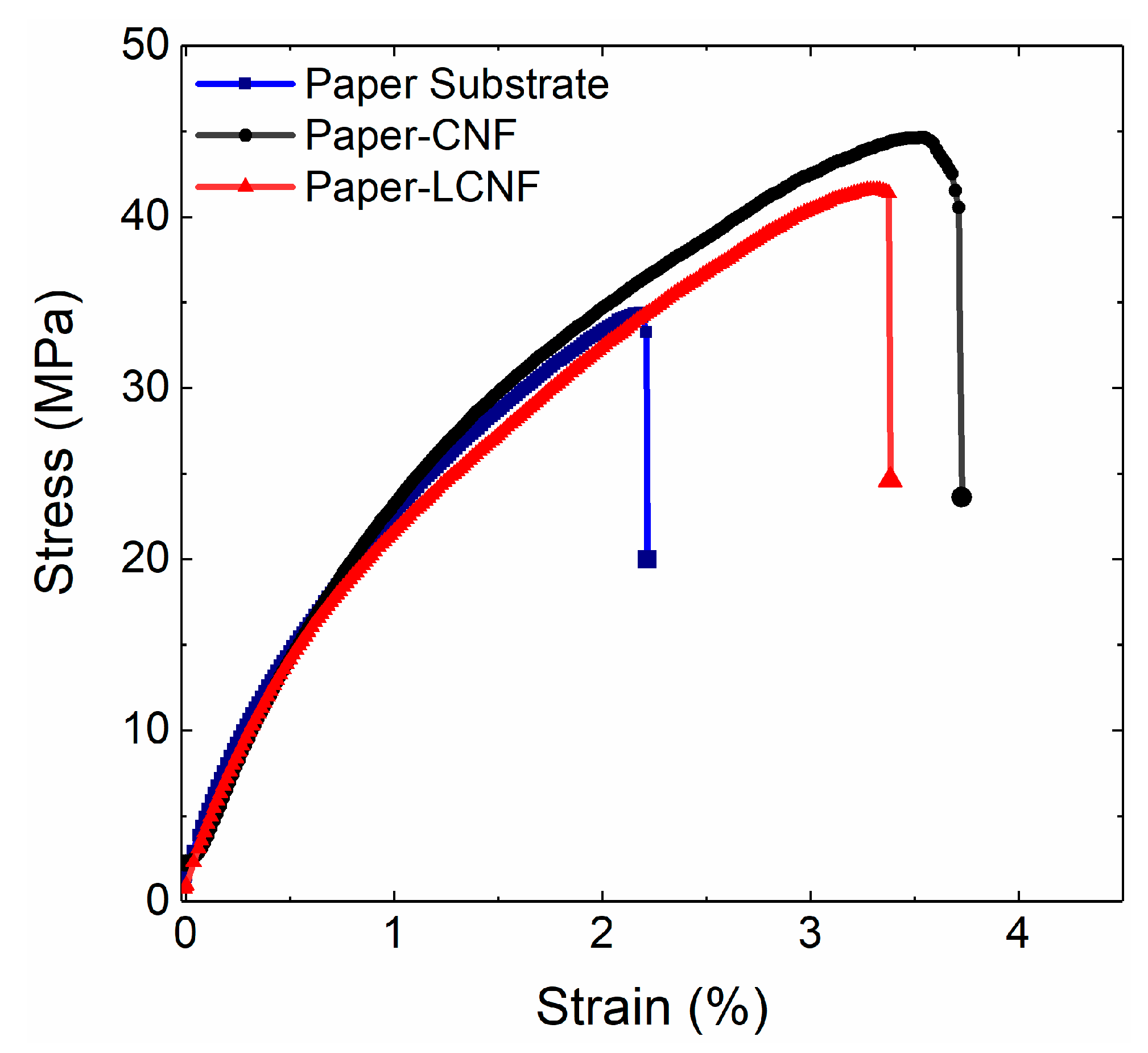

| Coating Materials | Tensile Strength (MPa) | Tensile Strength/Basis Weight (MPa·m2·g−1) | Tensile Strain (%) |
|---|---|---|---|
| Paper (78 g/m2) | 36 ± 3.2 | 0.46 | 2.3 ± 0.35 |
| Coated by CNF (94 g/m2) | 45 ± 3.8 | 0.48 | 3.5 ± 0.29 |
| Coated by LCNF (94 g/m2) | 41 ± 1.8 | 0.44 | 3.05 ± 0.31 |
| Sample Code | CNF | LCNF | ||||
|---|---|---|---|---|---|---|
| Relative Humidity% | 0 | 50 | 90 | 0 | 50 | 90 |
| OTR (cm3/m2/day) | 17.4 ± 1.1 | 19.9 ± 0.1 | 171 ± 2 | 26.6 ± 0.5 | 33.7 ± 0.7 | 302.5 ± 2.5 |
| Characteristic | Uncoated Paper | CNF | LCNF |
|---|---|---|---|
| Kit No | 0 | 12 | 12 |
| WVP (g.mm/m2.kPa.day) | 36.2 ± 0.5 | 5 ± 0.05 | 5.3 ± 0.1 |
| SFE (mN/m) | - | 46.3 ± 4.5 | 33.9 ± 4.8 |
| Disperse (mN/m) | - | 28.8 ± 2 | 28.7± 2.7 |
| Polar (mN/m) | - | 17.4 ± 2.5 | 5.1 ± 2 |
| WCA° | - | 59.4 ± 3.6 | 82.2 ± 4.7 |
| Porosity (Gurley s) | 14.5 ± 2.4 | - | - |
| Smoothness (SU) | 179 ± 11 | 140.8 ± 9 | 150 ± 6 |
| Thickness (µm) | 103 ± 2 | 120 ± 2 | 119 ± 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
H. Tayeb, A.; Tajvidi, M.; Bousfield, D. Paper-Based Oil Barrier Packaging using Lignin-Containing Cellulose Nanofibrils. Molecules 2020, 25, 1344. https://doi.org/10.3390/molecules25061344
H. Tayeb A, Tajvidi M, Bousfield D. Paper-Based Oil Barrier Packaging using Lignin-Containing Cellulose Nanofibrils. Molecules. 2020; 25(6):1344. https://doi.org/10.3390/molecules25061344
Chicago/Turabian StyleH. Tayeb, Ali, Mehdi Tajvidi, and Douglas Bousfield. 2020. "Paper-Based Oil Barrier Packaging using Lignin-Containing Cellulose Nanofibrils" Molecules 25, no. 6: 1344. https://doi.org/10.3390/molecules25061344
APA StyleH. Tayeb, A., Tajvidi, M., & Bousfield, D. (2020). Paper-Based Oil Barrier Packaging using Lignin-Containing Cellulose Nanofibrils. Molecules, 25(6), 1344. https://doi.org/10.3390/molecules25061344







