Improving Dissolution and Cytotoxicity by Forming Multidrug Crystals
Abstract
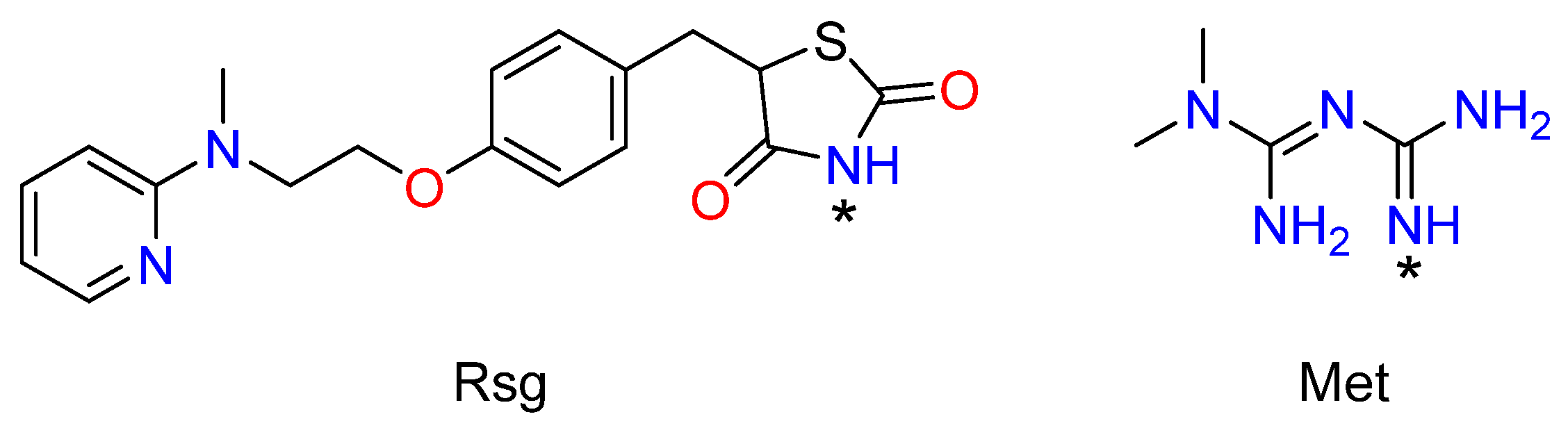
1. Introduction
2. Results
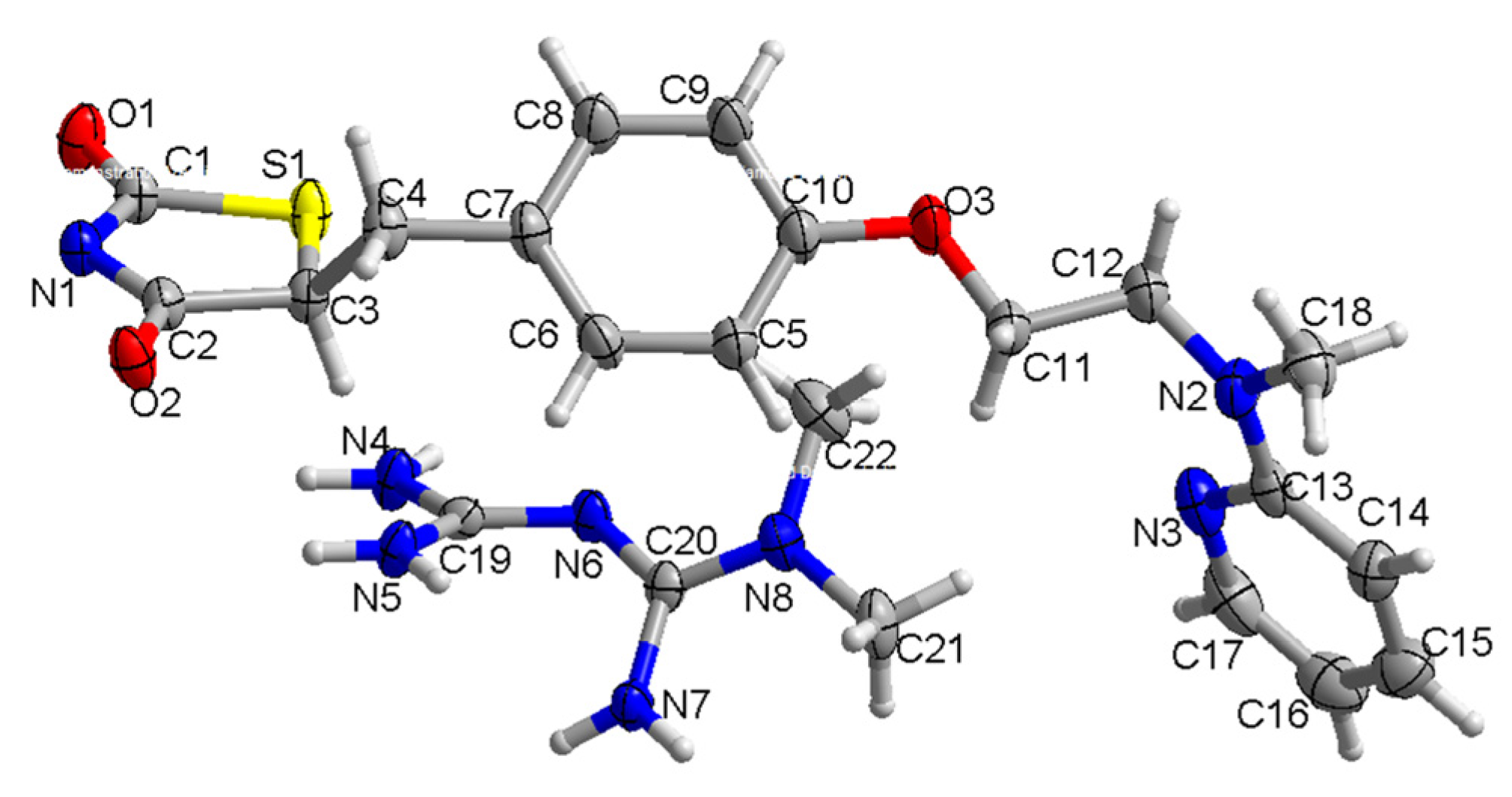
2.1. The Characterization of Crystal Structure
2.2. The Enhancement of the Dissolution Rate
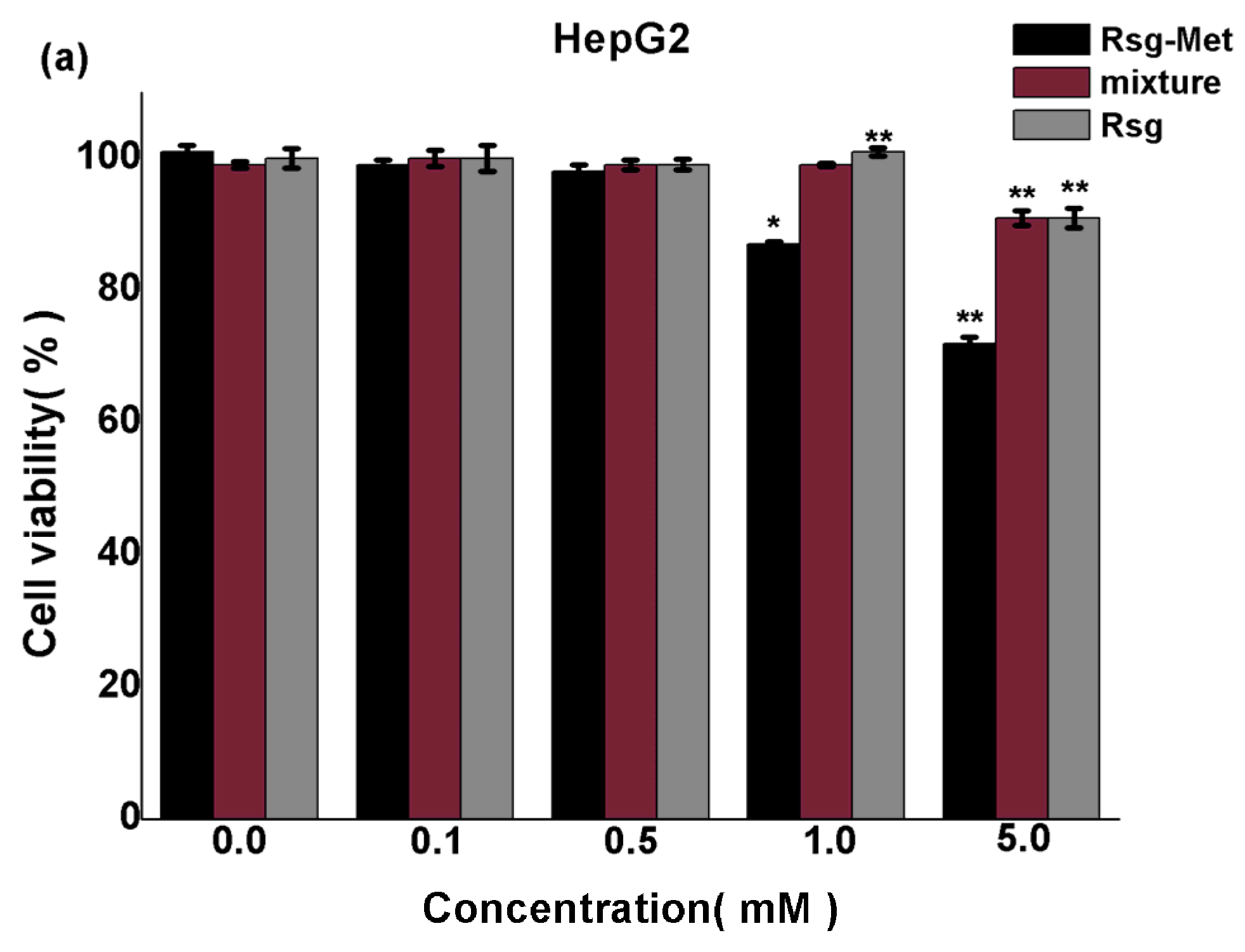
2.3. The Cytotoxicity on Liver Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Solvent Evaporation Method
4.3. Powder X-ray Diffraction (PXRD)
4.4. Single Crystal X-ray Diffraction(SCXRD)
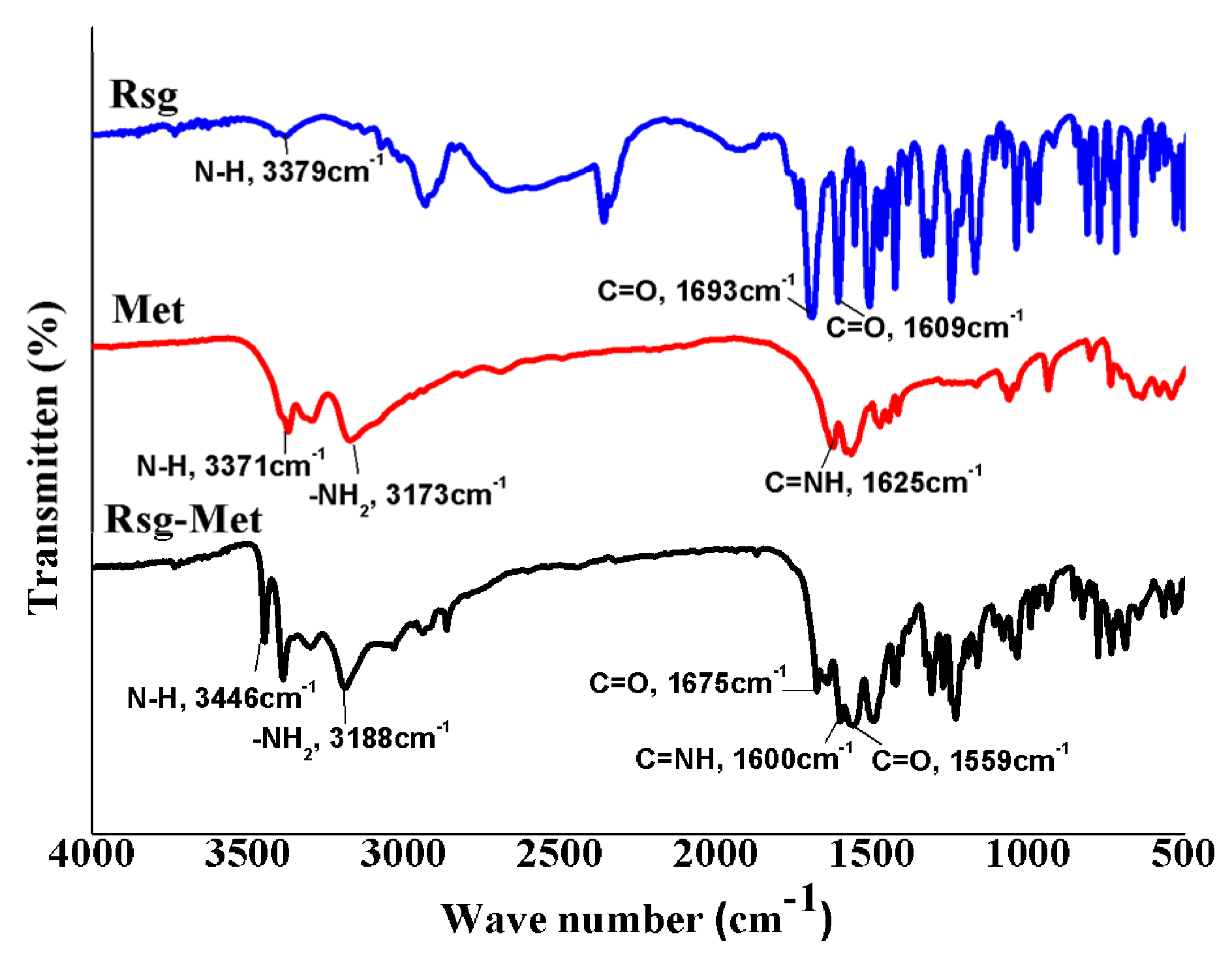
4.5. Fourier-Transform Infrared (FT-IR)
4.6. Dissolution Rate
4.7. MTT Assay
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- America Diabetes Association. Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42, S1–S193. [Google Scholar] [CrossRef]
- Lehmann, J.M.; Moore, L.B.; Smith-Oliver, T.A.; Wilkison, W.O.; Willson, T.M.; Kliewer, S.A. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J. Biol. Chem. 1995, 270, 12953–12956. [Google Scholar] [CrossRef] [PubMed]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M. Management of hyperglycemia in type 2 diabetes, 2015: A patient-centered approach: Update to a position statement of the American Diabetes Association and the European Association for the Study of diabetes. Diabetes Care 2015, 38, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Maruthur, N.M.; Tseng, E.; Hutfless, S.; Wilson, L.M.; Suarez, C.C.; Berger, Z. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: A systematic review and meta-analysis. Ann. Intern. Med. 2016, 164, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Jalving, M.; Gietema, J.A.; Lefrandt, J.D.; Jong, S.; Reyners, A.K.L.; Gans, R.O.B. Metformin: Taking away the candy for cancer. Eur. J. Cancer 2010, 46, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, V.; Rosenstock, J.; Patwardhan, R.; Salzman, A. Effect of metformin and rosiglitazone combination therapy in patients with type 2 diabetes mellitus: A randomized controlled trial. J. Am. Med. Assoc. 2000, 283, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gao, C.; Fang, L.; Zhao, H.C.; Yao, S.K. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients: A meta-analysis. Scand. J. Gastroenterol. 2013, 48, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, M.; Meier, C.; Krähenbühl, S.; Jick, S.S.; Meier, C.R. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care 2010, 33, 1304–1308. [Google Scholar] [CrossRef]
- Lee, M.S.; Hsu, C.C.; Wahlqvist, M.L.; Tsai, H.N.; Chang, Y.H.; Huang, Y.C. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: A representative population respective cohort study of 800,000 individuals. BMC Cancer 2011, 11, 20–30. [Google Scholar] [CrossRef]
- Skinner, H.D.; Mccurdy, M.R.; Echeverria, A.E.; Lin, S.H.; Welsh, J.W.; Reilly, M.S. Metformin use and improved response to therapy in esophageal adenocarcinoma. Acta Oncol. 2013, 52, 1002–1009. [Google Scholar] [CrossRef]
- Govindarajan, R.; Ratnasinghe, L.; Simmons, D.L.; Siegel, E.R.; Midathada, M.V.; Kim, L. Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J. Clin. Oncol. 2007, 25, 1476–1481. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, N. PPARγ activator Rosiglitazone inhibits cell migration via upregulation of PTEN in human hepatocarcinoma cell line BEL-7404. Cancer Biol. Ther. 2006, 5, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Leavens, K.F.; Birnbaum, M.J. Insulin signaling to hepatic lipid metabolism in health and disease. Crit. Rev. Biochem. Mol. 2011, 46, 200–215. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Kemp, B.E. AMPK in Health and Disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar] [CrossRef] [PubMed]
- Denko, N.C. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat. Rev. Cancer 2008, 8, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Elstrom, R.L.; Bauer, D.E.; Buzzai, M. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004, 64, 3892–3899. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Horike, N.; Sakoda, H.; Kushiyama, A. AMP-activated protein kinase activation increases phosphorylation of glycogen synthase kinase 3beta and thereby reduces cAMP-responsive element transcriptional activity and phosphoenolpyruvate carboxykinase C gene expression in the liver. J. Biol. Chem. 2008, 283, 33902–33910. [Google Scholar] [CrossRef] [PubMed]
- Cool, B.; Zinker, B.; Chiou, W. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006, 3, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Arshag, D.M. Rosiglitazone. Drugs 1999, 57, 921–930. [Google Scholar]
- Cheng, C.L.; Yu, L.X.; Lee, H.L.; Yang, C.Y.; Lue, C.S.; Chou, C.H. Biowaiver extension potential to BCS Class III high solubility-low permeability drugs: Bridging evidence for metformin immediate-release tablet. Eur. J. Pharm. Sci. 2004, 22, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Laura, J.M.; Clifford, J.B.; Ewan, R.P. Metformin and the gastrointestinal tract. Diabetologia 2016, 59, 426–435. [Google Scholar]
- Vaibhavkumar, A.; Jagtap, G.; Vidyasagar, S.C. Solubility enhancement of rosiglitazone by using melt sonocrystallization technique. J. Ultrasound 2014, 17, 27–32. [Google Scholar]
- Zhang, T.; Deng, H.; GUO, Y. Study on dissolution of rosiglitazone maleate capsules from different manufacturers. Pract. Pharm. Clin. Remedies 2008, 3, 189–190. [Google Scholar]
- Blonde, L.; Dailey, G.E.; Jabbour, S.A.; Reasner, C.A.; Mills, D.J. Gastrointestinal tolerability of extended-release metformin tablets compared to immediate-release metformin tablets: Results of a retrospective cohort study. Curr. Med. Res. Opin. 2004, 20, 565–572. [Google Scholar] [CrossRef]
- Scott, D.E.; Coyne, A.G.; Hudson, S.A.; Abell, C. Fragment-Based Approaches in Drug Discovery and Chemical Biology. Biochemistry 2012, 51, 4990–5003. [Google Scholar] [CrossRef]
- Brittain, H.G. Pharmaceutical cocrystals: The coming wave of new drug substances. J. Pharm. Sci. 2013, 102, 311–317. [Google Scholar] [CrossRef]
- Cinčić, D.; Friščić, T.; Jones, W. A cocrystallisation-based strategy to construct isostructural solids. New J. Chem. 2008, 32, 1776–1781. [Google Scholar] [CrossRef]
- Aitipamula, S.; Vangala, V.R.; Chow, P.S.; Tan, R.B.H. Cocrystal Hydrate of an Antifungal Drug, Griseofulvin, with Promising Physicochemical Properties. Cryst. Growth Des. 2012, 12, 5858–5863. [Google Scholar] [CrossRef]
- Sarma, B.; Chen, J.; His, H.Y.; Myerson, A.S. Solid forms of pharmaceuticals: Polymorphs, salts and cocrystals. Korean J. Chem. Eng. 2011, 28, 315–322. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Beatty, A.M.; Helfrich, B.A. “Total Synthesis” Supramolecular Style: Design and Hydrogen-Bond-Directed Assembly of Ternary Supermolecules. Angew. Chem. Int. Ed. 2001, 40, 3240–3242. [Google Scholar] [CrossRef]
- Phung, O.J.; Sood, N.A.; Sill, B.E.; Coleman, C.I. Oral anti-diabetic drugs for the prevention of type 2 diabetes. Diabet. Med. 2011, 28, 948–964. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.; Kapelouzou, A.; Tsanikidis, H.; Vitta, I.; Liapis, C.D.; Sailer, N. Effects of rosiglitazone/metformin fixed-dose combination therapy and metformin monotherapy on serum vaspin, adiponectin and IL-6 levels in drug-naive patients with type 2 diabetes. Exp. Clin. Endocrinol. Diabetes 2010, 119, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Vanderpoel, D.R.; Hussein, M.A.; Watsonheidari, T.; Perry, A. Adherence to a fixed-dose combination of rosiglitazone maleate/metformin hydrochloride in subjects with type 2 diabetes mellitus: A retrospective database analysis. Clin. Ther. 2004, 26, 2066–2075. [Google Scholar] [CrossRef]
- Dujic, T.; Causevic, A.; Bego, T.; Malenica, M.; Velija, A.Z.; Pearson, E.R.; Semiz, S. Organic cation transporter 1 variants and gastrointestinal side effects of metformin in patients with Type 2 diabetes. Diabet. Med. 2016, 33, 511–514. [Google Scholar] [CrossRef]
- He, X.X.; Tu, S.M.; Lee, M.H.; Yeung, S.C. Thiazolidinediones and metformin associated with improved survival of diabetic prostate cancer patients. Ann. Oncol. 2011, 22, 2640–2645. [Google Scholar] [CrossRef]
- Kahn, B.B.; Alquier, T.; Carling, D.; Hardie, D.G. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005, 1, 15–25. [Google Scholar] [CrossRef]
- Zheng, L.; Yang, W.; Wu, F.; Wang, C.; Yu, L.; Tang, L.; Qiu, B.; Li, Y.; Guo, L.; Wu, M.; et al. Prognostic Significance of AMPK Activation and Therapeutic Effects of Metformin in Hepatocellular Carcinoma. Clin. Cancer Res. 2013, 19, 5372–5380. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X.; Shan, X.; Chen, K.; Tang, H. Rosiglitazone metformin adduct inhibits hepatocellular carcinoma proliferation via activation of AMPK/p21 pathway. Cancer Cell Int. 2019, 19, 13. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |




| D-H...A | d(D-H)/Å | d(H...A)/Å | d(D...A)/Å | D-H-A/° |
|---|---|---|---|---|
| N(4)-H(4C)...N(6) 1 | 0.847 | 2.263 | 3.110 | 178 |
| N(4)-H(4D)...N(1) 2 | 0.88 | 2.00 | 2.720 | 138 |
| N(5)-H(5A)...O(1) 3 | 0.84 | 2.14 | 2.988 | 178 |
| N(5)-H(5B)...O(1) 2 | 0.91 | 2.19 | 3.083 | 167 |
| N(7)-H(7A)...O(2) 4 | 0.86 | 2.26 | 3.086 | 161 |
| N(7)-H(7B)...N(4) 3 | 0.93 | 1.89 | 2.758 | 154 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, X.; Jiang, L.; Zhou, J.; Guan, X.; Wang, J.; Xiang, P.; Pan, J.; Hu, X. Improving Dissolution and Cytotoxicity by Forming Multidrug Crystals. Molecules 2020, 25, 1343. https://doi.org/10.3390/molecules25061343
Bian X, Jiang L, Zhou J, Guan X, Wang J, Xiang P, Pan J, Hu X. Improving Dissolution and Cytotoxicity by Forming Multidrug Crystals. Molecules. 2020; 25(6):1343. https://doi.org/10.3390/molecules25061343
Chicago/Turabian StyleBian, Xufei, Lan Jiang, Jing Zhou, Xiaoshu Guan, Jingyu Wang, Peng Xiang, Junyi Pan, and Xiangnan Hu. 2020. "Improving Dissolution and Cytotoxicity by Forming Multidrug Crystals" Molecules 25, no. 6: 1343. https://doi.org/10.3390/molecules25061343
APA StyleBian, X., Jiang, L., Zhou, J., Guan, X., Wang, J., Xiang, P., Pan, J., & Hu, X. (2020). Improving Dissolution and Cytotoxicity by Forming Multidrug Crystals. Molecules, 25(6), 1343. https://doi.org/10.3390/molecules25061343




