Comparative Studies on Polysaccharides, Triterpenoids, and Essential Oil from Fermented Mycelia and Cultivated Sclerotium of a Medicinal and Edible Mushroom, Poria Cocos
Abstract
1. Introduction
2. Results and Discussion
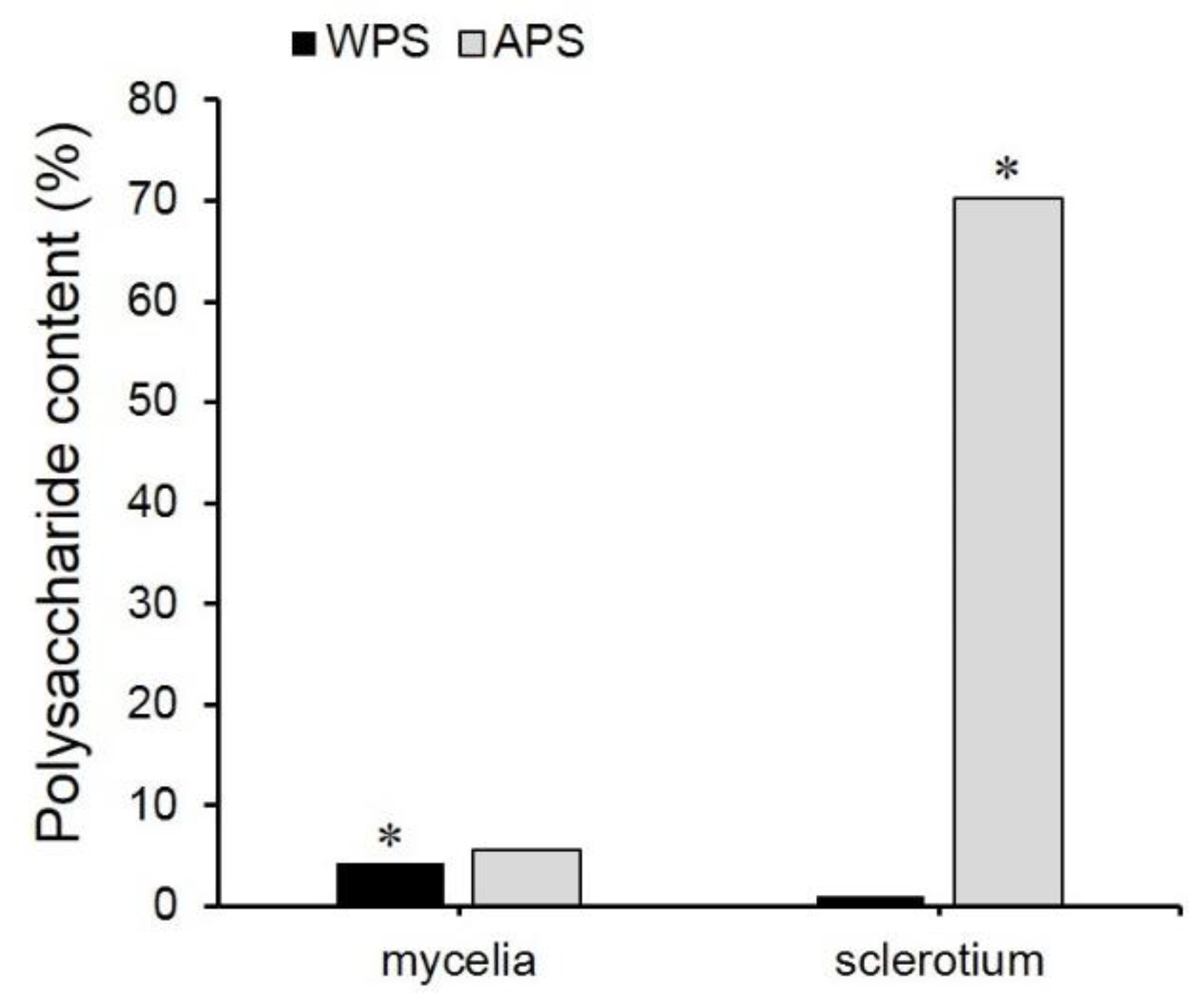
2.1. EPS, WPS, and APS Content Determination
2.2. Monosaccharide Composition Determination
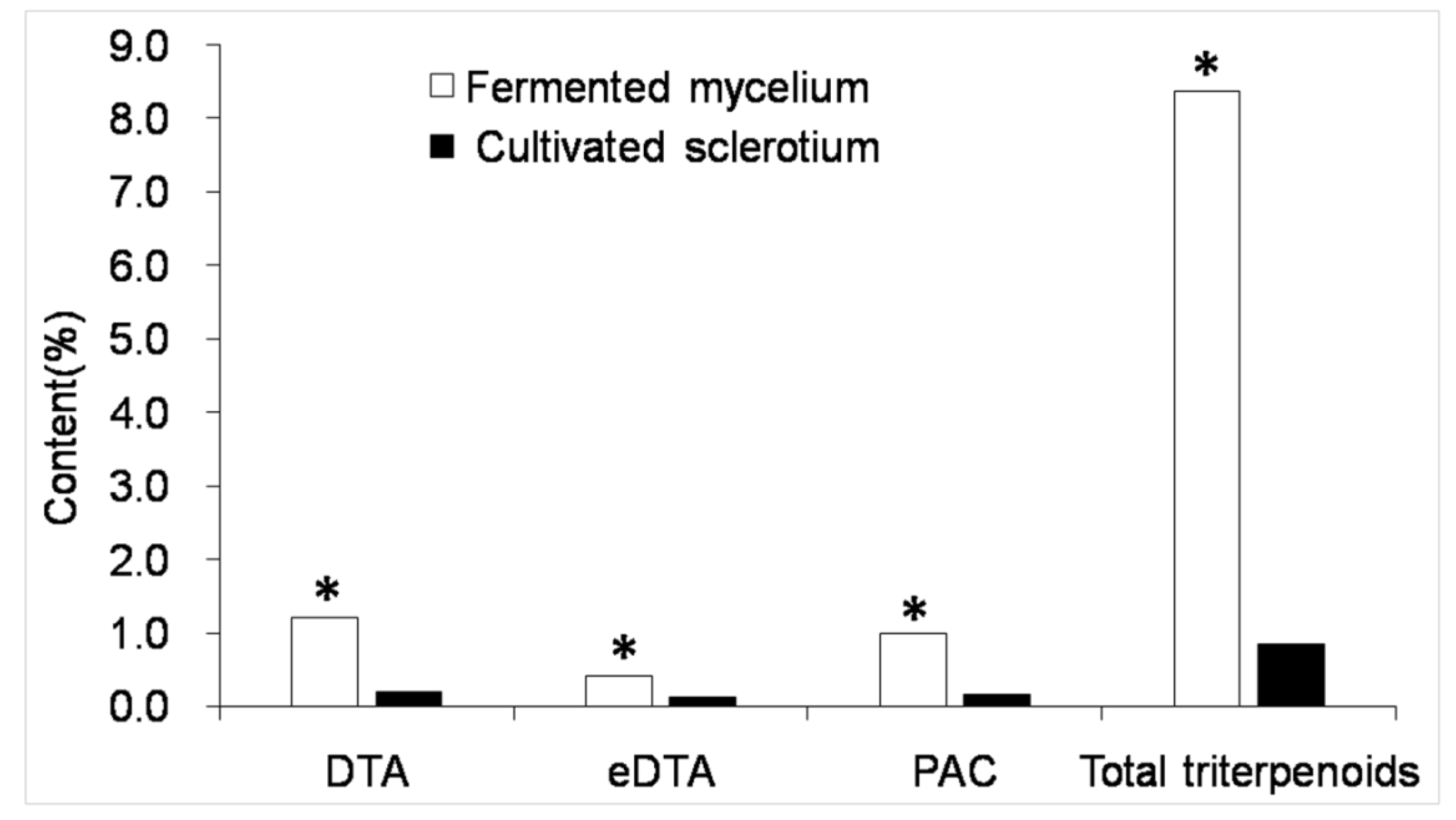
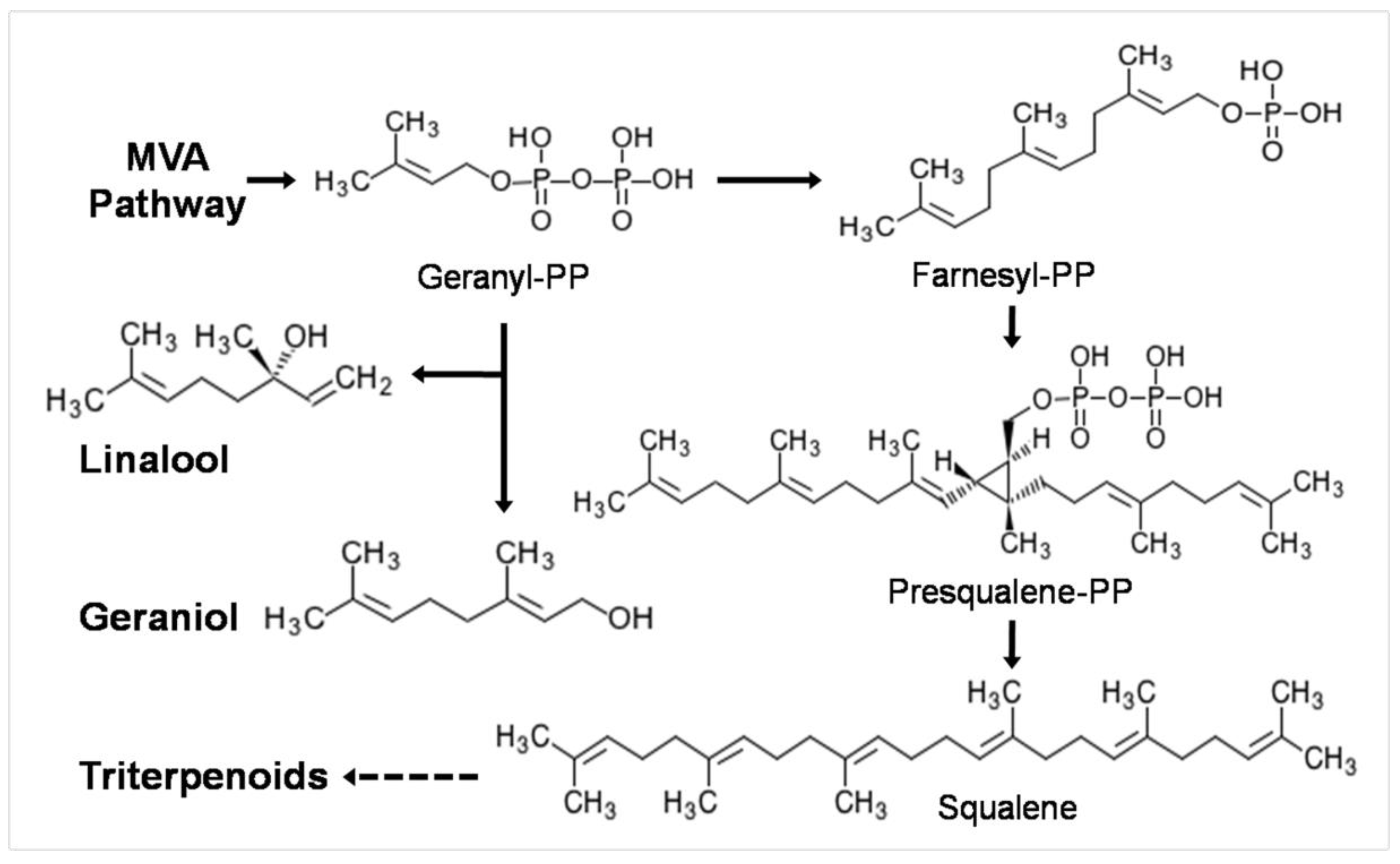
2.3. Triterpenoid Content Determination
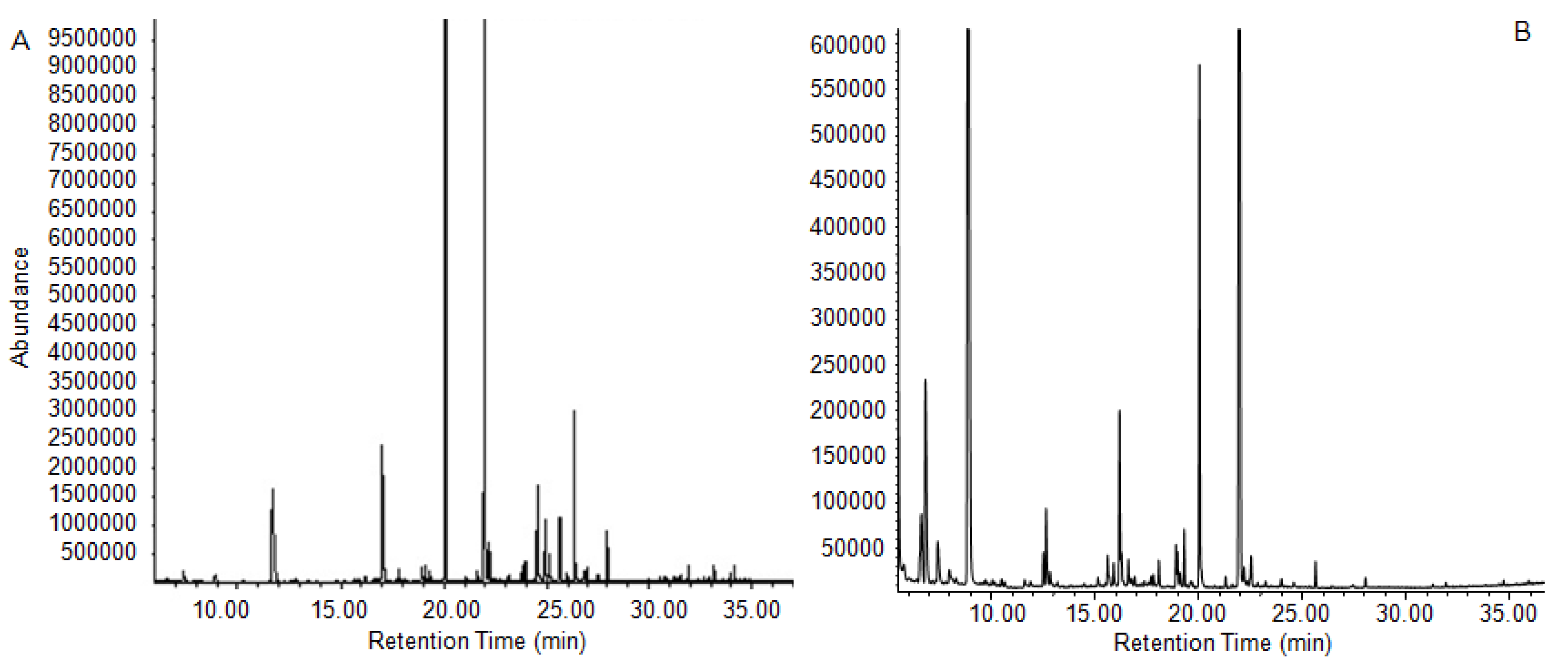
2.4. Chemical Composition of Essential Oils from the Fermentation Solution
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Fungus Isolation and Culture Conditions
3.3. Measurements of Biomass and Preparation of EPS, WPS, and APS
3.4. Determination of β-d-Glucose Equivalence of EPS, WPS and APS
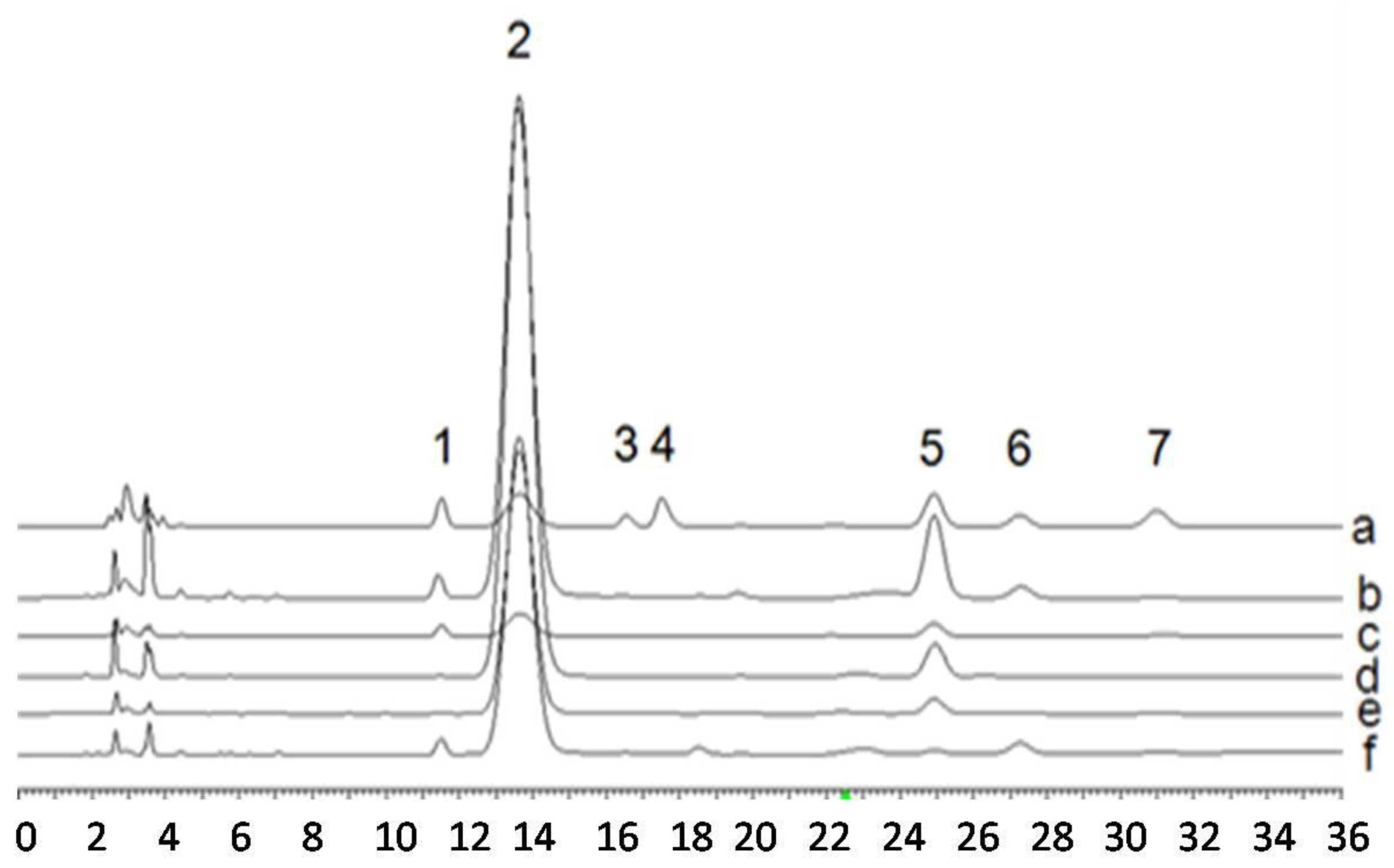
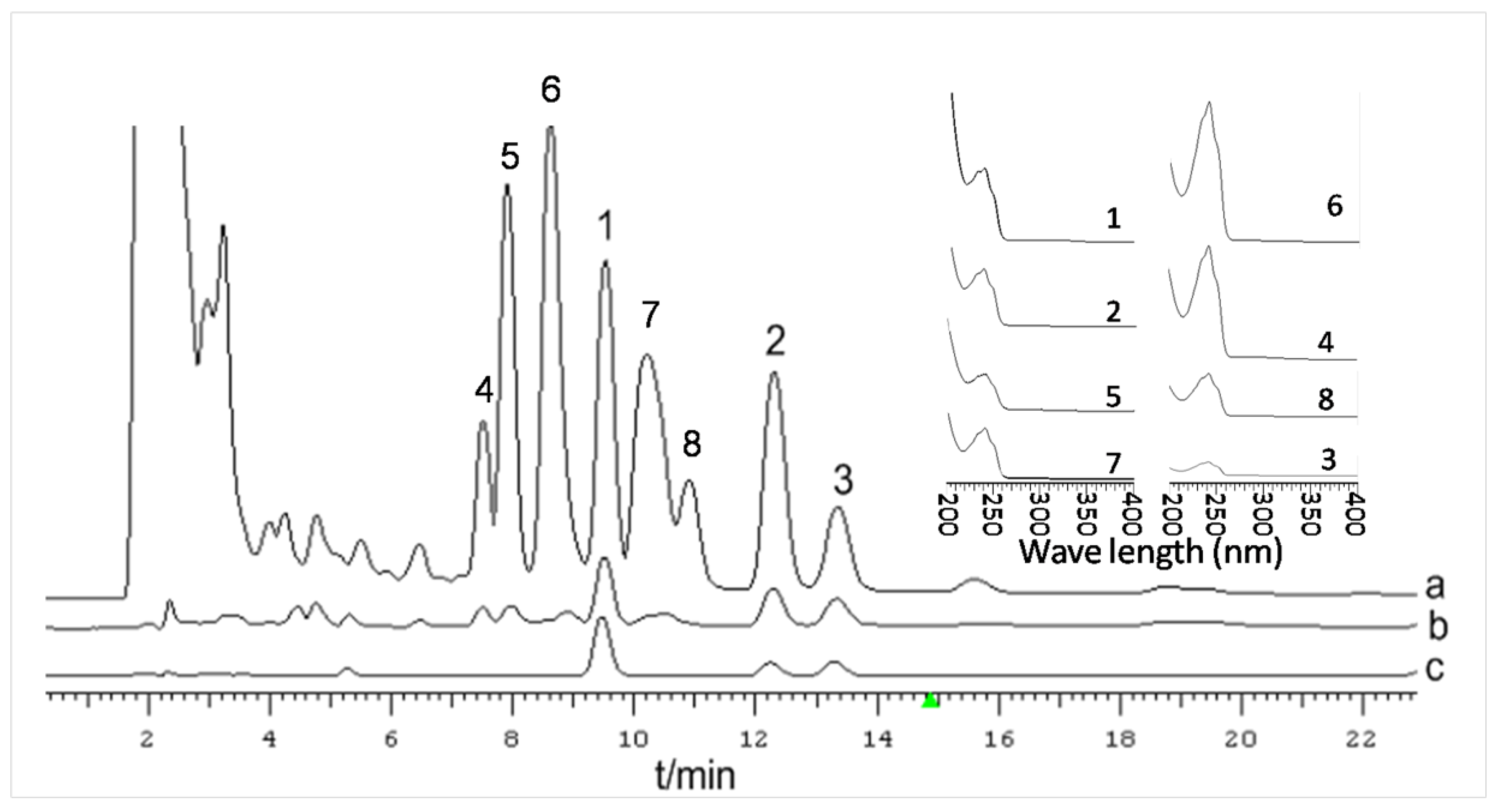
3.5. Monosaccharide Composition Analysis
3.6. Determination of DTC, eDTC and PAC
3.7. HS-GC/MS Analysis of Essential Oil from Fermented Media
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, J.-S.; Ji, S.-L.; Ren, M.-F.; He, Y.-L.; Jing, X.-R.; Xu, J.-W. Enhanced accumulation of individual ganoderic acids in a submerged culture of Ganoderma lucidum by the overexpression of squalene synthase gene. Biochem. Eng. J. 2014, 90, 178–183. [Google Scholar] [CrossRef]
- Shu, S.; Chen, B.; Zhou, M.; Zhao, X.; Xia, H.; Wang, M. De Novo Sequencing and Transcriptome Analysis of Wolfiporia cocos to Reveal Genes Related to Biosynthesis of Triterpenoids. PLoS ONE 2013, 8, e71350. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.-K.; Cheng, J.-J.; Lin, C.-Y.; Chang, C.-C. Purification, structural elucidation, and anti-inflammatory effect of a water-soluble 1, 6-branched 1, 3-alpha-D-galactan from cultured mycelia of Poria cocos. Food Chem. 2010, 118, 349–356. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L. Chain conformation of carboxymethylated derivatives of (1 -> 3)-beta-D-glucan from Poria cocos sclerotium. Carbohyd. Polym. 2006, 65, 504–509. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Cheung, P.C.K. Immunopotentiation and anti-tumor activity of carboxymethylated-sulfated beta-(1®3)-D-glucan from Poria cocos. Int. Immunopharmacol. 2010, 10, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Jin, Y.; Zhang, L.; Cheung, P.C.K.; Kennedy, J.F. Structure, molecular size and antitumor activities of polysaccharides from Poria cocos mycelia produced in fermenter. Carbohyd. Polym. 2007, 70, 324–333. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, X.-W. Two new lanostane triterpenoids from Poria cocos. J. Asian Nat. Prod. Res. 2008, 10, 323–328. [Google Scholar]
- Cai, T.-G.; Cai, Y. Triterpenes from the Fungus Poria cocos and Their Inhibitory Activity on Nitric Oxide Production in Mouse Macrophages via Blockade of Activating Protein-1 Pathway. Chem. Biodivers. 2011, 8, 2135–2143. [Google Scholar] [CrossRef]
- Yang, C.-H.; Zhang, S.-F.; Liu, W.-Y.; Zhang, Z.-J.; Liu, J.-H. Two New Triterpenes from the Surface Layer of Poria cocos. Helv. Chim. Acta 2009, 92, 660–667. [Google Scholar] [CrossRef]
- Yang, L.; Qin, B.; Feng, S.; Liu, S.; Song, X. A new triterpenoid from traditional Chinese medicine Poria cocos. J. Chem. Res. 2010, 34, 553–554. [Google Scholar] [CrossRef]
- Lu, Z.-H.; Wu, S.-W.; Tang, J.; Zhang, Y.; Jing, L. The correlation between polysaccharides isolated from the sclerotium of Poria cocos and different habitat and processing methods. Food Sci. Technol. 2006, 31, 107–111. [Google Scholar]
- Ke, R.; Lin, S.; Chen, Y.; Ji, C.; Shu, Q. Analysis of chemical composition of polysaccharides from Poria cocos Wolf and its anti-tumor activity by NMR spectroscopy. Carbohyd. Polym. 2010, 80, 31–34. [Google Scholar]
- Sun, S.-S.; Wang, K.; Ma, K.; Bao, L.; Liu, H.-W. An insoluble polysaccharide from the sclerotium of Poria cocos improves hyperglycemia, hyperlipidemia and hepatic steatosis in ob/ob mice via modulation of gut microbiota. Chin. J. Nat. Med. 2019, 17, 3–14. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Yang, Z.; Zhu, Y.; Wu, Y.; Huang, J.; Mao, J. Oxidation of beta-glucan extracted from Poria cocos and its physiological activities. Carbohyd. Polym. 2011, 85, 798–802. [Google Scholar] [CrossRef]
- Feng, Y.-L.; Lei, P.; Tian, T.; Yin, L.; Chen, D.-Q.; Chen, H.; Mei, Q.; Zhao, Y.-Y.; Lin, R.-C. Diuretic activity of some fractions of the epidermis of Poria cocos. J. Ethnopharmacol. 2013, 150, 1114–1118. [Google Scholar] [CrossRef]
- Akihisa, T.; Uchiyama, E.; Kikuchi, T.; Tokuda, H.; Suzuki, T.; Kimura, Y. Anti-Tumor-Promoting Effects of 25-Methoxyporicoic Acid A and Other Triterpene Acids from Poria cocos. J. Nat. Prod. 2009, 72, 1786–1792. [Google Scholar] [CrossRef]
- Ríos, J. Chemical Constituents and Pharmacological Properties of Poria cocos. Planta Med. 2011, 77, 681–691. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Z.-Z.; Xiao, W.; Zhang, L.-Q.; Bi, K.-S.; Jia, Y. UPLC characteristic chromatographic profile of Poria. Chin. J. Chin. Mater. Med. 2012, 37, 966–968. [Google Scholar]
- Zhang, Y.; Kong, Y. Optimizing liquid fermentation conditions for polysaccharide production by Poria cocos. China Brew. 2009, 208, 96–99. [Google Scholar]
- Li, Y.; Wan, D.-G.; Pei, J.; Chen, X. Study on Poria on Liquid Fermentation Conditions. J. Chengdu Univ. Tradit Chin. Med. 2005, 28, 52–55. [Google Scholar]
- Li, Y.; Yang, S.; Li, C.; Yang, W.-Q.; Wan, D.-G. Compound medicinal medium of flask liquid fermentation of Poria cocos and its chemical constituents. Chin. Tradit. Herb. Drugs. 2012, 43, 1519–1522. [Google Scholar]
- Jin, J.; Zhou, R.-R.; Xie, J.; Ye, H.-X.; Liang, X.-J.; Zhong, C.; Shen, B.-B.; Qin, Y.; Zhang, S.-H.; Huang, L.-Q. Insights into triterpene acids in fermented mycelia of edible fungus Poria cocos by a comparative study. Molecules 2019, 24, 1331. [Google Scholar] [CrossRef] [PubMed]
- Bernard, M.; Latge, J.P. Aspergillus fumigatus cell wall: Composition and biosynthesis. Med. Mycol. 2001, 39, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Latge, J.-P. The cell wall: A carbohydrate armour for the fungal cell. Mol. Microbiol. 2007, 66, 279–290. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, L.-N.; Zhang, M.; Chen, L.; Cheung, P.C.K.; Oi, V.E.C.; Lin, Y.-L. Antitumor activities of heteropolysaccharides of Poria cocos mycelia from different strains and culture media. Carbohyd. Res. 2003, 338, 1517–1521. [Google Scholar] [CrossRef]
- Wang, Y.F.; Zhang, M.; Ruan, D.; Shashkov, A.S.; Kilcoyne, M.; Savage, A.V.; Zhang, L. Chemical components and molecular mass of six polysaccharides isolated from the sclerotium of Poria cocos. Carbohyd. Res. 2004, 339, 327–334. [Google Scholar] [CrossRef]
- Zhang, L.N.; Chen, H.S.; Li, X. Studies on structure-activity relationship of acidic hetero-polysaccharide for Auricularia auricula-Judae. Chem. J. Chin. Univ. 1994, 15, 1231–1234. [Google Scholar]
- Hu, G.S.; Huang, C.G.; Jia, J.M. Accumulation of biomass and four triterpenoids in two-stage cultured Poria cocos mycelia and diuretic activity in rats. Chin. J. Nat. Med. 2017, 15, 265–270. [Google Scholar] [CrossRef]
- Lin, X.-Y. Dictionary of Flavor & Fragrance, 1st ed.; Chemical industry press: Beijing, China, 2007. [Google Scholar]
- Dar, T.A.; Uddin, M.; Khan, M.M.A.; Hakeem, K.; Jalee, H. Jasmonates counter plant stress: A Review. Environ. Exp. Bot. 2015, 115, 49–57. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Chen, C. Stimulated production of triterpenoids of Inonotus obliquus using methyl jasmonate and fatty acids. Ind. Crop. Prod. 2016, 85, 49–57. [Google Scholar] [CrossRef]
- Ren, A.; Qin, L.; Shi, L.; Dong, X.; Mu, D.S.; Li, Y.-X.; Zhao, M.-W. Methyl jasmonate induces ganoderic acid biosynthesis in the basidiomycetous fungus Ganoderma lucidum. Bioresource Technol. 2010, 101, 6785–6790. [Google Scholar] [CrossRef] [PubMed]
- KEGG PATHWAY: Terpenoid Backbone Biosynthesis-Reference. Available online: http://www.genome.jp/kegg-bin/show_pathway?map00900 (accessed on 10 July 2019).
- Zhang, J.; Zhang, Q.; Wang, J.; Shi, X.; Zhang, Z. Analysis of the monosaccharide composition of fucoidan by precolumn derivation HPLC. Chin. J. Oceanol. Limnol. 2009, 27, 578–582. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Man | Rha | Glcu | Glc | Gal | Ara | |
|---|---|---|---|---|---|---|
| EPS (Me) | 27.01 | 2.07 | 16.94 | 12.87 | 38.48 | 2.63 |
| APS (My) | 24.85 | 0.32 | - | 59.81 | 3.51 | 11.51 |
| WPS (My) | 9.11 | 1.25 | - | 67.74 | 19.81 | 2.09 |
| APS (S) | 2.23 | - | - | 97.11 | - | 0.66 |
| WPS (S) | 4.48 | - | - | 89.19 | 5.17 | 1.16 |
| Rt (min) | Compound Identified | Formula | Peak Area Percentage (%) | |
|---|---|---|---|---|
| Culture Media | Fresh External Layer | |||
| Alcohols | 47.49 | 63.18 | ||
| 7.378 | 1-Butanol | C4H10O | 1.29 | - |
| 8.835 | 1-Butanol, 3-methyl | C5H12O | - | 11.93 |
| 8.943 | 1-Butanol, 2-methyl | C5H12O | 1.06 | 29.90 |
| 12.646 | 1-Pentanol, 4-methyl | C6H14O | - | 0.59 |
| 12.659 | 1-Hexanol | C6H14O | - | 0.76 |
| 15.706 | Glycerin | C3H8O3 | - | 0.57 |
| 15.909 | 1-Heptanol | C7H16O | - | 0.23 |
| 16.202 | 1-Octen-3-ol | C8H16O | 0.25 | 4.74 |
| 16.915 | Phloroglucitol | C6H12O3 | 0.27 | - |
| 16.921 | 3,4-Dimethyl-3-hexanol | C8H18O | - | 0.31 |
| 17.825 | 1-Hexanol, 2-ethyl | C8H18O | 0.61 | - |
| 18.098 | Eucalyptol | C10H18O | - | 0.24 |
| 18.919 | 4-Heptanol, 4-methyl | C8H18O | 0.80 | - |
| 19.001 | 2-Octen-1-ol, (Z) | C8H16O | 0.16 | 0.27 |
| 19.11 | 1-Octanol | C8H18O | 0.83 | - |
| 19.161 | Bicyclo[3.1.0]hexan-2-ol, 2-methyl -5-(1-methylethyl)-, (1. alpha., 2. beta., 5. alpha.) | C10H18O | - | 0.69 |
| 19.313 | α-terpineol | C10H18O | - | 1.51 |
| 20.064 | Linalool | C10H18O | 35.72 | 9.65 |
| 20.127 | Phenylethyl Alcohol | C8H10O | - | 0.88 |
| 22.189 | 1-Nonanol | C9H20O | 0.63 | - |
| 22.5 | Terpinen-4-ol | C10H18O | 0.16 | 0.91 |
| 24.53 | Geraniol | C10H18O | 5.16 | - |
| 25.961 | 2-Pentadecanol | C15H32O | 0.29 | - |
| 27.609 | 3-Tetradecyn-1-ol | C14H26O | 0.23 | - |
| Aldehyde | 1.78 | 2.33 | ||
| 7.423 | Heptaldehyde | C7H14O | - | 2.33 |
| 11.844 | 2-Hexenal, (E) | C6H10O | 0.47 | - |
| 17.704 | Benzeneacetaldehyde | C8H8O | 0.21 | - |
| 21.623 | Benzaldehyde, 3-ethyl | C9H10O | 0.57 | - |
| 22.093 | Isophthalaldehyde | C8H6O2 | 0.30 | - |
| 30.568 | 5-Methyl-2-phenyl-2-hexenal | C13H16O | 0.22 | - |
| Esters | 34.27 | 21.47 | ||
| 12.512 | 1-Methoxy-2-propyl acetate | C6H12O3 | - | 0.86 |
| 12.843 | 1-Butanol, 3-methyl, acetate | C7H14O2 | 0.14 | - |
| 16.622 | Pentanoic acid, 2-hydroxy-3-methyl-, methyl ester | C7H14O3 | 0.28 | - |
| 19.313 | Ethyl 2-(5-methyl-5-vinyltetrahydrofuran-2-yl) propan- 2-yl carbonate | C13H22O4 | 0.68 | - |
| 21.966 | Benzeneacetic acid, methyl ester | C9H10O2 | 31.29 | 19.98 |
| 23.951 | Benzeneacetic acid, ethyl ester | C10H12O2 | 0.92 | - |
| 25.599 | Bornyl acetate | C12H20O2 | - | 0.23 |
| 28.01 | Isobornyl propionate | C13H22O2 | - | 0.40 |
| 32.667 | 3,7,11-Trimethyl-3-hydroxy-6,10-dodecadien-1-yl acetate | C17H30O3 | 0.10 | - |
| 34.162 | Methyl jasmonate | C13H20O3 | 0.70 | - |
| 40.657 | Dibutyl phthalate | C16H22O4 | 0.15 | - |
| Alkenes | 11.73 | 0.00 | ||
| 16.756 | beta-Myrcene | C10H16 | 0.16 | - |
| 26.394 | Cyclooctene, 3-methyl | C9H16 | 8.14 | - |
| 26.865 | 5-Undecene, 6-methyl | C12H24 | 0.43 | - |
| 27.501 | Cycloundecene (Z) | C11H20 | 0.22 | - |
| 27.997 | Nonane, 3-methylene | C10H20 | 2.20 | - |
| 33.17 | 1-Heptene, 4-methyl | C8H16 | 0.57 | - |
| Others | 4.73 | 11.05 | ||
| 12.684 | 1-Aziridineethanol | C4H9NO | 0.13 | - |
| 15.623 | 6-methyl- 5-hepten-2-one | C8H14O | - | 0.40 |
| 15.693 | N-Methoxy-N-methylacetamide | C4H9NO2 | 0.35 | - |
| 18.919 | Vinyl ethyl sulfoxide | C4H8OS | - | 0.32 |
| 18.919 | Hydrazine, 1,1-dipropyl | C6H16N2 | - | 1.04 |
| 19.307 | Ylangene | C15H24 | - | 0.28 |
| 22.138 | 3-Pyridinecarboxylic acid, 4-hydroxy | C6H5NO3 | - | 0.18 |
| 22.144 | 1,3-Dimethyl-1-cyclohexene | C8H14 | - | 1.05 |
| 23.169 | Bicyclo[3.1.1]hept-3-en-2-one, 4,6,6-trimethyl-, (1S) | C10H14O | - | 7.32 |
| 24.641 | 1,1’-Bicyclopentyl | C10H18 | 0.33 | - |
| 25.102 | Ethanone, 1-(3,4-dimethylphenyl) | C10H12O | 1.35 | - |
| 25.611 | 2-Undecanone | C11H22O | 2.07 | - |
| 31.255 | Phenol, 2,4-bis(1,1-dimethylethyl) | C14H22O | 0.17 | - |
| 31.935 | Naphthalene, 1,2,3,5,6,8a-hexahydro-4,7-dimethyl-1- (1-methylethyl), (1S-cis) | C15H24 | 0.32 | - |
| 34.671 | p-Hydroxyamphetamine | C9H13NO | - | 0.46 |
| Monosaccharide | RT (min) | Standard Formula | Linear Range (μg) | R2 |
|---|---|---|---|---|
| Mannose | 11.53 | Y = 2E-07X − 0.0053 | 0.01–0.125 | 0.9993 |
| Rhamnose | 16.56 | Y = 3E-07X − 0.0276 | 0.01–0.125 | 0.9992 |
| Glucuronide | 17.52 | Y = 3E-07X − 0.0861 | 0.0256–0.320 | 0.999 |
| Glucose | 24.93 | Y = 3E-07X − 0.0569 | 0.0532–0.665 | 0.9991 |
| Galactose | 27.27 | Y = 2E-07X − 0.0117 | 0.010–0.13 | 0.999 |
| Arabinose | 30.99 | Y = 2E-07X − 0.0054 | 0.01–0.125 | 0.9993 |
| Compound | RT (min) | Standard Formula | Linear Range (μg) | R2 |
|---|---|---|---|---|
| DTA | 9.527 | Y = 1E-06X − 0.0398 | 0.340–3.40 | 0.9952 |
| eDTA | 13.327 | Y = 9E-07X − 0.0022 | 0.091–0.91 | 0.9952 |
| PAC | 12.307 | Y = 1E-06X − 0.0044 | 0.080–0.80 | 0.9942 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Huang, C.; Zhao, Y.; Wang, L.; Yang, Y.; Wang, A.; Zhang, Y.; Hu, G.; Jia, J. Comparative Studies on Polysaccharides, Triterpenoids, and Essential Oil from Fermented Mycelia and Cultivated Sclerotium of a Medicinal and Edible Mushroom, Poria Cocos. Molecules 2020, 25, 1269. https://doi.org/10.3390/molecules25061269
Wang D, Huang C, Zhao Y, Wang L, Yang Y, Wang A, Zhang Y, Hu G, Jia J. Comparative Studies on Polysaccharides, Triterpenoids, and Essential Oil from Fermented Mycelia and Cultivated Sclerotium of a Medicinal and Edible Mushroom, Poria Cocos. Molecules. 2020; 25(6):1269. https://doi.org/10.3390/molecules25061269
Chicago/Turabian StyleWang, Dongdong, Chonggui Huang, Ye Zhao, Lin Wang, Yongcheng Yang, Anhua Wang, Yang Zhang, Gaosheng Hu, and Jingming Jia. 2020. "Comparative Studies on Polysaccharides, Triterpenoids, and Essential Oil from Fermented Mycelia and Cultivated Sclerotium of a Medicinal and Edible Mushroom, Poria Cocos" Molecules 25, no. 6: 1269. https://doi.org/10.3390/molecules25061269
APA StyleWang, D., Huang, C., Zhao, Y., Wang, L., Yang, Y., Wang, A., Zhang, Y., Hu, G., & Jia, J. (2020). Comparative Studies on Polysaccharides, Triterpenoids, and Essential Oil from Fermented Mycelia and Cultivated Sclerotium of a Medicinal and Edible Mushroom, Poria Cocos. Molecules, 25(6), 1269. https://doi.org/10.3390/molecules25061269





