Simultaneous Optimization of the Ultrasonic Extraction Method and Determination of the Antioxidant Activities of Hydroxysafflor Yellow A and Anhydrosafflor Yellow B from Safflower Using a Response Surface Methodology
Abstract
1. Introduction
2. Results
2.1. Single-Factor Experiments
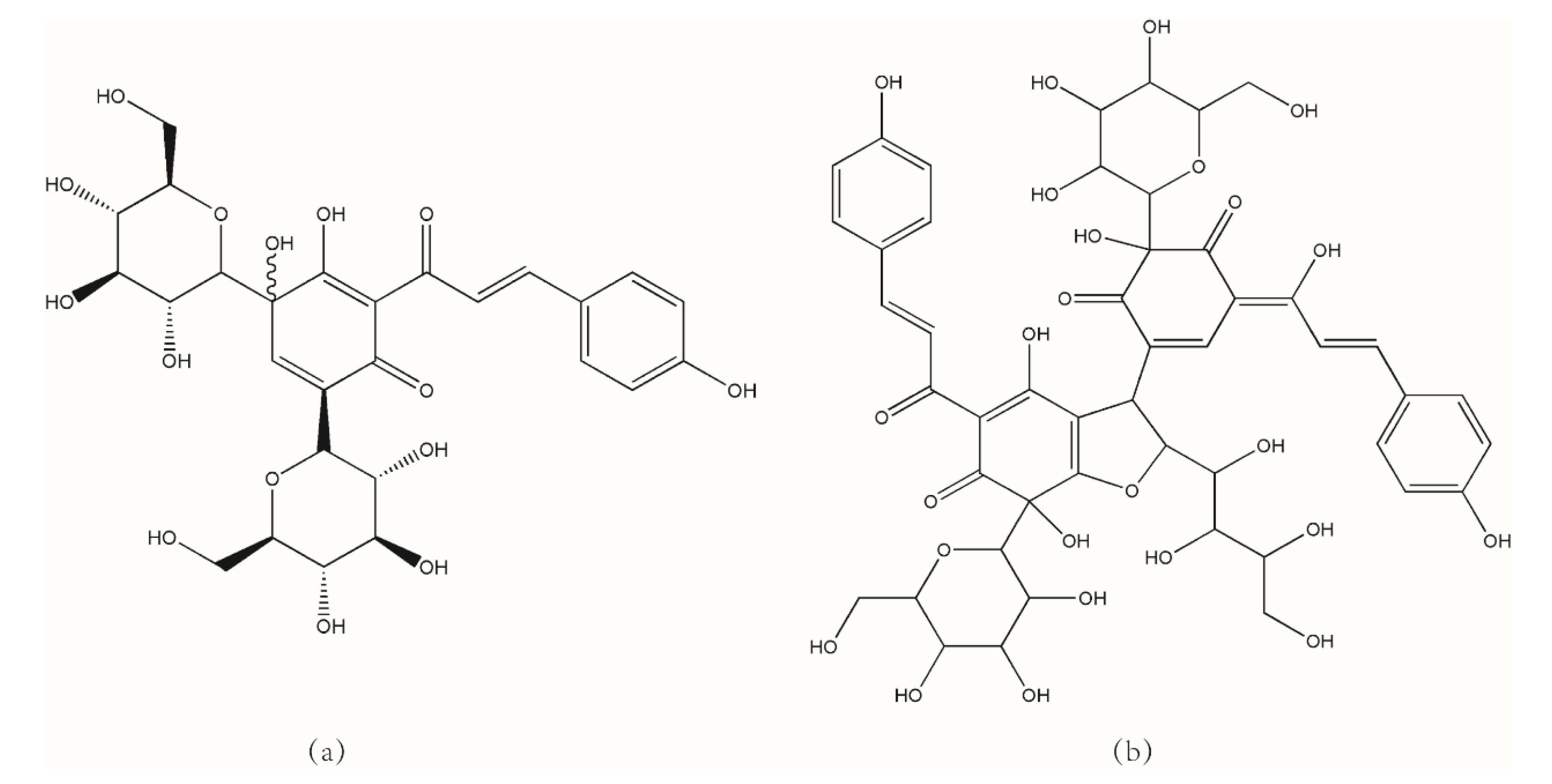
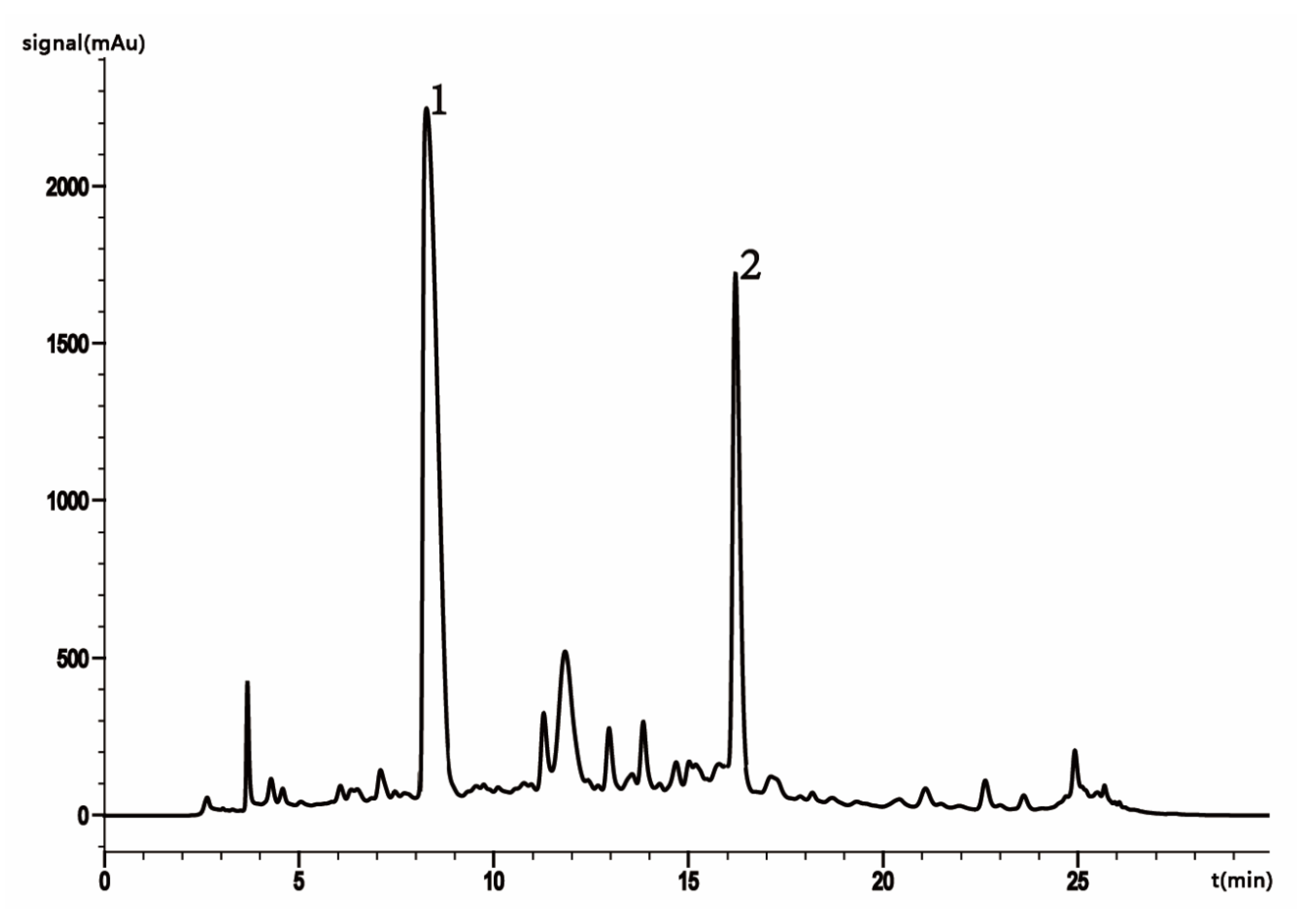
2.2. HPLC Analysis of HSYA and AHSYB
2.2.1. Linearity
2.2.2. Precision
2.2.3. Repeatability
2.3. Effect of Variables and Model Fitting
2.4. Optimal Processing Conditions and Verification of the Predictive Model
2.5. Comparison with Traditional Methods
2.6. In Vitro Antioxidant Activity
2.6.1. FRAP
2.6.2. DPPH Radical Scavenging Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Response Surface Methodology
4.2.1. Variables Selection
4.2.2. BBD for Extraction Optimization
4.3. The Ultrasonic Extraction Process
4.4. HPLC Analysis
4.4.1. Chromatographic Conditions
4.4.2. Preparation of Control Solutions
4.5. Calculation of the Comprehensive Evaluation Value
4.6. Determination of Antioxidant Activity
4.6.1. FRAP Assay
4.6.2. Measurement of DPPH Radical Scavenging Activity
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wan, Y.; Wang, M.; Fu, Q.; Wang, L.; Wang, D.; Zhang, K.; Xia, Z.; Gao, D. Novel dual functional monomers based molecularly imprinted polymers for selective extraction of myricetin from herbal medicines. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1097, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Choy, C.S.; Liu, Y.H.; Cheah, K.P.; Li, J.S.; Wang, J.T.; Yu, W.Y.; Lin, C.W.; Cheng, H.W.; Hu, C.M. Protective effect of dried safflower petal aqueous extract and its main constituent, carthamus yellow, against lipopolysaccharide-induced inflammation in RAW264.7 macrophages. J. Sci. Food Agric. 2011, 91, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, R.; Pu, X.; Sun, Y.; Zhao, X. Evaluation of the sub-chronic toxicity of a standardized flavonoid extract of safflower in rats. Regul. Toxicol. Pharmacol. 2017, 85, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Norris, L.E.; Collene, A.L.; Asp, M.L.; Hsu, J.C.; Liu, L.F.; Richardson, J.R.; Li, D.; Bell, D.; Osei, K.; Jackson, R.D.; et al. Comparison of dietary conjugated linoleic acid with safflower oil on body composition in obese postmenopausal women with type 2 diabetes mellitus. Am. J. Clin. Nutr. 2009, 90, 468–476. [Google Scholar] [CrossRef]
- Peng, X.R.; Wang, X.; Dong, J.R.; Qin, X.J.; Li, Z.R.; Yang, H.; Zhou, L.; Qiu, M.H. Rare hybrid dimers with anti-acetylcholinesterase activities from a safflower (Carthamus tinctorius L.) seed oil cake. J. Agric. Food Chem. 2017, 65, 9453–9459. [Google Scholar] [CrossRef]
- Yao, D.; Wang, Z.; Miao, L.; Wang, L. Effects of extracts and isolated compounds from safflower on some index of promoting blood circulation and regulating menstruation. J. Ethnopharmacol. 2016, 191, 264–272. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, Q.; Yin, S.J.; Cai, L.; Yang, Y.X.; Liu, W.J.; Hu, Y.J.; Chen, H.; Yang, F.Q. Screening of blood-activating active components from Danshen-Honghua herbal pair by spectrum-effect relationship analysis. Phytomedicine 2019, 54, 149–158. [Google Scholar] [CrossRef]
- Liu, L.; Duan, J.A.; Tang, Y.; Guo, J.; Yang, N.; Ma, H.; Shi, X. Taoren-Honghua herb pair and its main components promoting blood circulation through influencing on hemorheology, plasma coagulation and platelet aggregation. J. Ethnopharmacol. 2012, 139, 381–387. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Song, H.; Xiong, Y.; Liu, D.; Bai, X. Hydroxysafflor yellow A suppresses angiogenesis of hepatocellular carcinoma through inhibition of p38 MAPK phosphorylation. Biomed. Pharmacother. 2019, 109, 806–814. [Google Scholar] [CrossRef]
- Zhu, H.J.; Wang, L.J.; Wang, X.Q.; Pan, H.; Li, N.S.; Yang, H.B.; Jin, M.; Zang, B.X.; Gong, F.Y. Hormone-sensitive lipase is involved in the action of hydroxysafflor yellow A (HYSA) inhibiting adipogenesis of 3T3-L1cells. Fitoterapia 2014, 93, 182–188. [Google Scholar] [CrossRef]
- Lu, Y.Q.; Luo, Y.; He, Z.F.; Chen, J.; Yan, B.L.; Wang, Y.; Yu, Q. Hydroxysafflor yellow A ameliorates homocysteine-induced Alzheimer-like pathologic dysfunction and memory/synaptic disorder. Rejuvenation Res. 2013, 16, 446–452. [Google Scholar] [CrossRef]
- Bai, J.; Zhao, J.; Cui, D.; Wang, F.; Song, Y.; Cheng, L.; Gao, K.; Wang, J.; Li, L.; Li, S.; et al. Protective effect of hydroxysafflor yellow A against acute kidney injury via the TLR4/NF-kappaB signaling pathway. Sci. Rep. 2018, 8, 9173. [Google Scholar] [CrossRef]
- Yang, F.; Li, J.; Zhu, J.; Wang, D.; Chen, S.; Bai, X. Hydroxysafflor yellow A inhibits angiogenesis of hepatocellular carcinoma via blocking ERK/MAPK and NF-kappaB signaling pathway in H22 tumor-bearing mice. Eur. J. Pharmacol. 2015, 754, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, D.P.; Qin, Z.; Wang, P.Y.; Hu, B.H.; Yu, J.G.; Zhao, Y.; Cai, B.; Chen, Y.L.; Lu, M.; et al. Protective cerebrovascular effects of hydroxysafflor yellow A (HSYA) on ischemic stroke. Eur. J. Pharmacol. 2018, 818, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhao, H.Y.; Xu, M.; Zhou, L.; Guo, H.; Han, J.; Wang, B.R.; Guo, D.A. Qualitative evaluation and quantitative determination of 10 major active components in carthamus tinctorius L. by high-performance liquid chromatography coupled with diode array detector. J. Chromatogr. A 2009, 1216, 2063–2070. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Wang, L.Y.; Jin, W.T.; Tang, Y.P.; Jin, Y.; Shi, X.Q.; Shang, L.L.; Shang, E.X.; Duan, J.A. Comparative analysis of the effects of hydroxysafflor yellow a and anhydrosafflor yellow B in safflower series of herb pairs using prep-hplc and a selective knock-out approach. Molecules 2016, 21, 1480. [Google Scholar] [CrossRef]
- Yue, S.; Wu, L.; Qu, C.; Tang, Y.; Jin, Y.; Li, S.; Shen, J.; Shi, X.; Shan, C.; Cui, X.; et al. Development and validation of a UFLC–MS/MS method for the determination of anhydrosafflor yellow B in rat plasma and its application to pharmacokinetic study. J. Chromatogr. B 2015, 1003, 54–59. [Google Scholar] [CrossRef]
- Chang, C.W.; Yen, C.C.; Wu, M.T.; Hsu, M.C.; Wu, Y.T. Microwave-assisted extraction of cannabinoids in hemp nut using response surface methodology: Optimization and comparative study. Molecules 2017, 22, 1894. [Google Scholar] [CrossRef]
- Wong, K.H.; Li, G.Q.; Li, K.M.; Razmovski-Naumovski, V.; Chan, K. Optimisation of Pueraria isoflavonoids by response surface methodology using ultrasonic-assisted extraction. Food Chem. 2017, 231, 231–237. [Google Scholar] [CrossRef]
- Xie, G.; Li, R.; Han, Y.; Zhu, Y.; Wu, G.; Qin, M. Optimization of the extraction conditions for buddleja officinalis maxim. using response surface methodology and exploration of the optimum harvest time. Molecules 2017, 22, 1187. [Google Scholar] [CrossRef]
- Fang, X.; Gu, S.; Jin, Z.; Hao, M.; Yin, Z.; Wang, J. Optimization of ultrasonic-assisted simultaneous extraction of three active compounds from the fruits of forsythia suspensa and comparison with conventional extraction methods. Molecules 2018, 23, 2115. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.N.; Zhang, J.J.; Li, Y.; Meng, X.; Li, H.B. Microwave-Assisted Extraction of Phenolic Compounds from Melastoma sanguineum Fruit: Optimization and Identification. Molecules 2018, 23, 2498. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dang, J.; Wang, Q.; Yu, M.; Jiang, L.; Mei, L.; Shao, Y.; Tao, Y. Optimization of polysaccharides from Lycium ruthenicum fruit using RSM and its anti-oxidant activity. Int. J. Biol. Macromol. 2013, 61, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Bo, R.; Ma, X.; Feng, Y.; Zhu, Q.; Huang, Y.; Liu, Z.; Liu, C.; Gao, Z.; Hu, Y.; Wang, D. Optimization on conditions of Lycium barbarum polysaccharides liposome by RSM and its effects on the peritoneal macrophages function. Carbohydr. Polym. 2015, 117, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Oludemi, T.; Barros, L.; Prieto, M.A.; Heleno, S.A.; Barreiro, M.F.; Ferreira, I. Extraction of triterpenoids and phenolic compounds from Ganoderma lucidum: Optimization study using the response surface methodology. Food Funct. 2018, 9, 209–226. [Google Scholar] [CrossRef]
- Shu, G.; Shi, X.; Chen, H.; Ji, Z.; Meng, J. Optimization of goat milk with ACE inhibitory peptides fermented by lactobacillus bulgaricus LB6 using response surface methodology. Molecules 2017, 22, 2001. [Google Scholar] [CrossRef]
- Ferreres, F.; Grosso, C.; Gil-Izquierdo, A.; Valentao, P.; Mota, A.T.; Andrade, P.B. Optimization of the recovery of high-value compounds from pitaya fruit by-products using microwave-assisted extraction. Food Chem. 2017, 230, 463–474. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. Preparation, characterization, and optimization of altretamine-loaded solid lipid nanoparticles using Box-Behnken design and response surface methodology. Artif. Cells Nanomed. Biotechnol. 2014, 44, 571–580. [Google Scholar] [CrossRef]
- Ahmad, A.; Alkharfy, K.M.; Wani, T.A.; Raish, M. Application of Box-Behnken design for ultrasonic-assisted extraction of polysaccharides from paeonia emodi. Int. J. Biol. Macromol. 2015, 72, 990–997. [Google Scholar] [CrossRef]
- Qi, J.; Jin, X.; Huang, L.; Ping, Q. Simultaneous determination of hydroxysafflor yellow A and ferulic acid in rat plasma after oral administration of the co-extractum of Rhizoma chuanxiong and Flos Carthami by HPLC-diode array detector. Biomed. Chromatogr. 2007, 21, 816–822. [Google Scholar] [CrossRef]
- Nie, J.L.; Shi, C.; Yan, G.Y.; Pei, Y. Study on extraction process and content determination of safflor yellow. Adv. Mater. Res. 2013, 781–784, 663–667. [Google Scholar] [CrossRef]
- Ji, Y.; Guo, S.; Wang, B.; Yu, M. Extraction and determination of flavonoids in Carthamus tinctorius. Open Chem. 2018, 16, 1129–1133. [Google Scholar] [CrossRef]
- Kolodziejczyk-Czepas, J.; Bijak, M.; Saluk, J.; Ponczek, M.B.; Zbikowska, H.M.; Nowak, P.; Tsirigotis-Maniecka, M.; Pawlaczyk, I. Radical scavenging and antioxidant effects of Matricaria chamomilla polyphenolic-polysaccharide conjugates. Int. J. Biol. Macromol. 2015, 72, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Shi, C.; Cao, J.; Sun, Y.; Zhao, X.; Guo, Y.; Wang, C.; Lei, H.; Jiang, H.; Ablat, N.; et al. Neuroprotective effects of a standardized flavonoid extract of safflower against neurotoxin-induced cellular and animal models of parkinson’s disease. Sci. Rep. 2016, 6, 22135. [Google Scholar] [CrossRef]
- Tian, J.; Li, G.; Liu, Z.; Fu, F. Hydroxysafflor yellow A inhibits rat brain mitochondrial permeability transition pores by a free radical scavenging action. Pharmacology 2008, 82, 121–126. [Google Scholar] [CrossRef]
- Tamminga, G.; Paulitsch-Fuchs, A.; Jansen, G.; Euverink, G. Development and validation of an alternative parameter for quantification of signals emitted by fluorescently labelled bacteria in microscopic images. J. Microbiol. Methods 2019, 166, 105717. [Google Scholar] [CrossRef]
- Lengler, J.; Steger, A. Note on the coefficient of variations of neuronal spike trains. Biol. Cybern. 2017, 111, 229–235. [Google Scholar] [CrossRef]
- Foure, A.; Ogier, A.C.; Le Troter, A.; Vilmen, C.; Feiweier, T.; Guye, M.; Gondin, J.; Besson, P.; Bendahan, D. Diffusion properties and 3D architecture of human lower leg muscles assessed with ultra-high-field-strength diffusion-tensor MR imaging and tractography: Reproducibility and sensitivity to sex difference and intramuscular variability. Radiology 2018, 287, 592–607. [Google Scholar] [CrossRef]
- Projean, D.; Minh Tu, T.; Ducharme, J. Rapid and simple method to determine morphine and its metabolites in rat plasma by liquid chromatography–mass spectrometry. J. Chromatogr. B 2003, 787, 243–253. [Google Scholar] [CrossRef]
- Miyazaki, S.; Hamaoki, M.; Nagata, A. Development of enzyme-linked immunosorbent assay for human cardiac myosin light chain I. Methods Find. Exp. Clin. Pharmacol. 2005, 27, 323–326. [Google Scholar]
- Wang, G.; Lei, Z.; Zhong, Q.; Wu, W.; Zhang, H.; Min, T.; Wu, H.; Lai, F. Enrichment of caffeic acid in peanut sprouts and evaluation of its in vitro effectiveness against oxidative stress-induced erythrocyte hemolysis. Food Chem. 2017, 217, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Pan, S. Effects of various factors of ultrasonic treatment on the extraction yield of all-trans-lycopene from red grapefruit (Citrus paradise Macf.). Ultrason. Sonochem. 2013, 20, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Shirsath, S.R.; Sable, S.S.; Gaikwad, S.G.; Sonawane, S.H.; Saini, D.R.; Gogate, P.R. Intensification of extraction of curcumin from curcuma amada using ultrasound assisted approach: Effect of different operating parameters. Ultrason. Sonochem. 2017, 38, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Li, L.; Zhang, H.L.B.; Liu, C. Preparative separation of hydroxyl safflower yellow A and anhydrosafflor yellow B in plant extract of carthamus tinctorius L. by reverse-phase medium-pressure liquid chromatography. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 1947–1958. [Google Scholar] [CrossRef]
- Wang, K.; Xie, X.; Zhang, Y.; Huang, Y.; Zhou, S.; Zhang, W.; Lin, Y.; Fan, H. Combination of microwave-assisted extraction and ultrasonic-assisted dispersive liquid-liquid microextraction for separation and enrichment of pyrethroids residues in Litchi fruit prior to HPLC determination. Food Chem. 2018, 240, 1233–1242. [Google Scholar] [CrossRef]
- Cheng, Z.; Yang, Y.; Liu, Y.; Liu, Z.; Zhou, H.; Hu, H. Two-steps extraction of essential oil, polysaccharides and biphenyl cyclooctene lignans from Schisandra chinensis Baill fruits. J. Pharm. Biomed. Anal. 2014, 96, 162–169. [Google Scholar] [CrossRef]
- Xie, J.H.; Dong, C.J.; Nie, S.P.; Li, F.; Wang, Z.J.; Shen, M.Y.; Xie, M.Y. Extraction, chemical composition and antioxidant activity of flavonoids from Cyclocarya paliurus (Batal.) Iljinskaja leaves. Food Chem. 2015, 186, 97–105. [Google Scholar] [CrossRef]
- Zamri, N.; Abdullah, L. A New linguistic variable in interval type-2 fuzzy entropy weight of a decision making method. Proc. Comput. Sci. 2013, 24, 42–53. [Google Scholar] [CrossRef][Green Version]
- Zou, Z.H.; Yun, Y.; Sun, J.N. Entropy method for determination of weight of evaluating indicators in fuzzy synthetic evaluation for water quality assessment. J. Environ. Sci. 2006, 18, 1020–1023. [Google Scholar] [CrossRef]
- Paz, M.; Gullon, P.; Barroso, M.F.; Carvalho, A.P.; Domingues, V.F.; Gomes, A.M.; Becker, H.; Longhinotti, E.; Delerue-Matos, C. Brazilian fruit pulps as functional foods and additives: Evaluation of bioactive compounds. Food Chem. 2015, 172, 462–468. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, C.; Hu, J.; He, M.; Bao, J.; Wang, K.; Li, P.; Chen, M.; Wan, J.; Su, H.; et al. Ultrasound-assisted extraction, antioxidant and anticancer activities of the polysaccharides from rhynchosia minima root. Molecules 2015, 20, 20901–20911. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| Source | Sum of Squares | Degree of Freedom | Mean Square | f-Value |
|---|---|---|---|---|
| Model a | 0.063 | 14 | 4.508 × 10−3 | 6.400 ** |
| A | 2.946 × 10−3 | 1 | 2.946 × 10−3 | 4.180 |
| B | 1.297 × 10−3 | 1 | 1.297 × 10−3 | 1.840 |
| C | 7.123 × 10−3 | 1 | 7.123 × 10−3 | 10.110 ** |
| D | 2.528 × 10−6 | 1 | 2.528 × 10−6 | 3.587 × 10−3 |
| AB | 3.023 × 10−4 | 1 | 3.023 × 10−4 | 0.430 |
| AC | 5.795 × 10−5 | 1 | 5.795 × 10−5 | 0.082 |
| AD | 4.814 × 10−5 | 1 | 4.814 × 10−5 | 0.068 |
| BC | 2.250 × 10−4 | 1 | 2.250 × 10−4 | 0.320 |
| BD | 3.440 × 10−3 | 1 | 3.440 × 10−3 | 4.880 * |
| AD | 5.795 × 10−5 | 1 | 5.795 × 10−5 | 0.082 |
| A2 | 6.046 × 10−3 | 1 | 6.046 × 10−3 | 8.580 * |
| B2 | 2.521 × 10−3 | 1 | 2.521 × 10−3 | 3.580 |
| C2 | 0.041 | 1 | 0.041 | 58.120 ** |
| D2 | 9.648 × 10−3 | 1 | 9.648 × 10−3 | 13.690 ** |
| Residual | 0.011 | 15 | 7.047 × 10−4 | |
| Lack of fit | 0.011 | 10 | 1.054 × 10−3 | 157.100 |
| R2 | 0.857 | |||
| R2adj. | 0.723 | |||
| Std. Dev. | 0.027 | |||
| Mean | 1.00 | |||
| CV (%) | 2.65 |
| Verification Experiment | Extraction Yield (%) | Comprehensive Evaluation Value (Y) | Average Value | |
|---|---|---|---|---|
| HSYA (Y1) | AHSYB (Y2) | |||
| 1 | 1.80 | 0.42 | 1.08 | 1.08 |
| 2 | 1.81 | 0.40 | 1.07 | |
| 3 | 1.82 | 0.42 | 1.09 | |
| 4 | 1.82 | 0.42 | 1.09 | |
| Concentration of Safflower Extract (mg/mL) | |||||
|---|---|---|---|---|---|
| Antioxidant Activity | 2.19 | 4.38 | 8.75 | 17.50 | 35.00 |
| FRAP (mM/(1 mg/mL)) | 0.35 | 0.65 | 0.99 | 1.79 | 3.01 |
| DPPH Radical Scavenging Activity (%) | 11.63% | 15.71% | 20.52% | 28.49% | 41.90% |
| Factors | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Temperature (°C, A) | 60 | 70 | 80 |
| Extraction Time (min, B) | 20 | 30 | 40 |
| Solvent-to-Material Ratio (mL/g, C) | 14 | 16 | 18 |
| Extraction Power (W, D) | 120 | 160 | 200 |
| Run | Temperature (°C, A) | Extraction Time (min, B) | Solvent-to-Material Ratio (mL/g, C) | Extraction Power (W, D) | Extraction Yield (%) | Comprehensive Evaluation Value (Y) | |
|---|---|---|---|---|---|---|---|
| HSYA (Y1) | AHSYB (Y2) | ||||||
| 1 | 1 | 0 | 0 | −1 | 1.55 | 0.37 | 0.93 |
| 2 | 0 | 0 | 0 | 0 | 1.78 | 0.42 | 1.07 |
| 3 | 0 | −1 | 1 | 0 | 1.65 | 0.34 | 0.97 |
| 4 | 0 | 0 | 1 | 1 | 1.59 | 0.32 | 0.93 |
| 5 | 0 | 0 | 0 | 0 | 1.77 | 0.42 | 1.07 |
| 6 | 0 | 0 | 1 | −1 | 1.58 | 0.35 | 0.94 |
| 7 | 1 | 1 | 0 | 0 | 1.72 | 0.38 | 1.02 |
| 8 | −1 | 0 | 0 | −1 | 1.72 | 0.39 | 1.03 |
| 9 | 1 | 0 | 0 | 1 | 1.63 | 0.35 | 0.96 |
| 10 | 1 | −1 | 0 | 0 | 1.71 | 0.36 | 1.01 |
| 11 | 0 | 0 | 0 | 0 | 1.78 | 0.41 | 1.06 |
| 12 | −1 | 1 | 0 | 0 | 1.78 | 0.41 | 1.06 |
| 13 | 1 | 0 | 1 | 0 | 1.59 | 0.34 | 0.94 |
| 14 | 0 | −1 | 0 | −1 | 1.64 | 0.35 | 0.97 |
| 15 | −1 | 0 | −1 | 0 | 1.68 | 0.36 | 0.99 |
| 16 | 0 | 0 | 0 | 0 | 1.77 | 0.42 | 1.07 |
| 17 | 0 | 1 | 0 | 1 | 1.68 | 0.37 | 1.00 |
| 18 | −1 | 0 | 0 | 1 | 1.76 | 0.38 | 1.04 |
| 19 | 0 | −1 | 0 | 1 | 1.71 | 0.37 | 1.01 |
| 20 | 0 | −1 | −1 | 0 | 1.67 | 0.33 | 0.97 |
| 21 | 1 | 0 | −1 | 0 | 1.69 | 0.37 | 1.00 |
| 22 | 0 | 1 | −1 | 0 | 1.66 | 0.34 | 0.97 |
| 23 | −1 | 0 | 1 | 0 | 1.57 | 0.31 | 0.91 |
| 24 | 0 | 0 | −1 | −1 | 1.67 | 0.36 | 0.99 |
| 25 | 0 | 0 | 0 | 0 | 1.78 | 0.42 | 1.07 |
| 26 | 0 | 1 | 0 | −1 | 1.79 | 0.41 | 1.07 |
| 27 | 0 | 1 | 1 | 0 | 1.61 | 0.32 | 0.94 |
| 28 | 0 | 0 | −1 | 1 | 1.69 | 0.35 | 0.99 |
| 29 | 0 | 0 | 0 | 0 | 1.78 | 0.41 | 1.06 |
| 30 | −1 | −1 | 0 | 0 | 1.73 | 0.36 | 1.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yu, L.; Jin, W.; Li, C.; Wang, Y.; Wan, H.; Yang, J. Simultaneous Optimization of the Ultrasonic Extraction Method and Determination of the Antioxidant Activities of Hydroxysafflor Yellow A and Anhydrosafflor Yellow B from Safflower Using a Response Surface Methodology. Molecules 2020, 25, 1226. https://doi.org/10.3390/molecules25051226
Zhang Y, Yu L, Jin W, Li C, Wang Y, Wan H, Yang J. Simultaneous Optimization of the Ultrasonic Extraction Method and Determination of the Antioxidant Activities of Hydroxysafflor Yellow A and Anhydrosafflor Yellow B from Safflower Using a Response Surface Methodology. Molecules. 2020; 25(5):1226. https://doi.org/10.3390/molecules25051226
Chicago/Turabian StyleZhang, Yangyang, Li Yu, Weifeng Jin, Chang Li, Yu Wang, Haitong Wan, and Jiehong Yang. 2020. "Simultaneous Optimization of the Ultrasonic Extraction Method and Determination of the Antioxidant Activities of Hydroxysafflor Yellow A and Anhydrosafflor Yellow B from Safflower Using a Response Surface Methodology" Molecules 25, no. 5: 1226. https://doi.org/10.3390/molecules25051226
APA StyleZhang, Y., Yu, L., Jin, W., Li, C., Wang, Y., Wan, H., & Yang, J. (2020). Simultaneous Optimization of the Ultrasonic Extraction Method and Determination of the Antioxidant Activities of Hydroxysafflor Yellow A and Anhydrosafflor Yellow B from Safflower Using a Response Surface Methodology. Molecules, 25(5), 1226. https://doi.org/10.3390/molecules25051226






