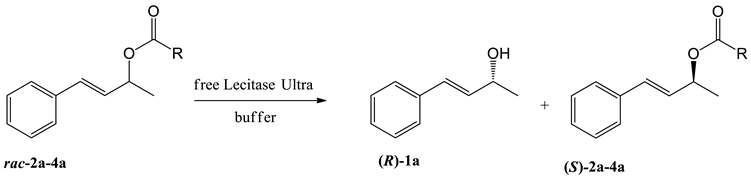
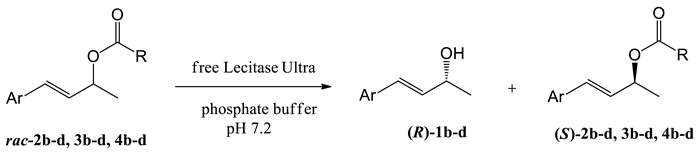
Abstract
The influence of buffer type, co-solvent type, and acyl chain length was investigated for the enantioselective hydrolysis of racemic 4-arylbut-3-en-2-yl esters using Lecitase™ Ultra (LU). Immobilized preparations of the Lecitase™ Ultra enzyme had significantly higher activity and enantioselectivity than the free enzyme, particularly for 4-phenylbut-3-en-2-yl butyrate as the substrate. Moreover, the kinetic resolution with the immobilized enzyme was achieved in a much shorter time (24–48 h). Lecitase™ Ultra, immobilized on cyanogen bromide-activated agarose, was particularly effective, producing, after 24 h of reaction time in phosphate buffer (pH 7.2) with acetone as co-solvent, both (R)-alcohols and unreacted (S)-esters with good to excellent enantiomeric excesses (ee 90–99%). These conditions and enzyme were also suitable for the kinetic separation of racemic (E)-4-phenylbut-3-en-2-yl butyrate analogs containing methyl substituents on the benzene ring (4b,4c), but they did not show any enantioselectivity toward (E)-4-(4’-methoxyphenyl)but-3-en-2-yl butyrate (4d).
1. Introduction
Lecitase™ Ultra (LU) is a commercially available chimeric enzyme that is the product of the fusion of lipase genes from Thermomyces lanuginosus and phospholipase A1 genes from Fusarium oxysporum [1]. This enzyme combines the stability of lipase and activity of phospholipase A1 and was initially designed for the degumming of plant oils during rafination [2,3,4,5,6]. In this process, phospholipids contained in the oil phase are hydrolyzed by Lecitase™ Ultra at the sn-1 position to lysophospholipids, which migrate to the aqueous phase. Since then, this fundamental reaction catalyzed by Lecitase™ Ultra has also found other food and none-food applications i.a. hydrolysis of soy phosphatidylcholine to produce lysophosphatidylcholine [7,8] and α-glycerophosphocholine [9,10], modification of flour in bread making [11,12,13], and rafination of oils used in the production of biodiesel [14,15]. Except for hydrolytic activity, Lecitase™ Ultra is also able to catalyze esterification, acidolysis, and alcoholysis, which has found application in the production of oils enriched with omega-3 fatty acids [16] as well as in the production of diglycerides [17,18,19,20] and structured phospholipids [21,22,23,24,25,26,27]. Due to its wide enzymatic activity, the application of Lecitase™ Ultra has been further extended to the production of flavor low-chain esters i.a. methyl benzoate and methyl butanoate [28] and regioselective hydrolysis of peracetylated mono- and disaccharides to produce key intermediates in the preparation of different glycoderivatives such as oligosaccharides, glycolipids, or glycopeptides [29].
One of the most popular uses of lipases in organic chemistry is the kinetic resolution of racemic mixtures [30,31,32]. Lecitase™ Ultra has been also employed for this goal and there are few reports concerning the application of Lecitase™ Ultra-catalyzed enantioselective hydrolysis of esters for the production of some important chiral drug intermediates. These applications covered the resolution of esters of 2-hydroxyacids with a phenyl ring including mandelates [33,34,35,36,37,38], resolution of glycidate esters [35,39,40], and N-acetyl-α-amino acid methyl esters [35]. Another example of an application in asymmetric synthesis was Lecitase™ Ultra-catalyzed regioselective hydrolysis of prochiral dimethyl 3-phenylglutarate [41].
Immobilization of enzymes on different supports is a useful tool to increase their stability, activity, and selectivity under operational conditions [42,43,44]. Due to a simpler separation of the biocatalyst from the reaction environment, such preparations may be reused in many catalytic cycles without losing their catalytic properties, which increases the economic efficiency of the industrial process [45]. However, in many cases, it is also related with a decrease in enzyme activity caused by various types of distortion, diffusion, and steric problems [46]. As Lecitase™ Ultra is commercially available only in its free form as the water solution, many attempts have been made to prepare an immobilized form of this enzyme for different purposes, for example, by entrapment in gelatin hydrogel [47] or in different calcium alginates [48], immobilization on the derivatives of cellulose [49,50], polystyrene resin [51], styrene-divinylbenzene beads [37,52], magnetic nanoparticles [53], or by encapsulation in AOT(bis(2-ethylhexyl) sulfosuccinate sodium salt)/isooctane reverse micelles [54]. In the processes of the kinetic resolution of racemates catalyzed by immobilized Lecitase™ Ultra, the best results were obtained using gelatin [35,39], various derivatives of agarose [33,36,38,41,55], hexyl- and butyl-toyopearl [34], or epoxy activated polymer Dilbead VWR coated with polyethylenimine (PEI) and crosslinked with glutaraldehyde [40].
In previous investigations, kinetic resolutions of racemic esters through their Lecitase™ Ultra-catalyzed enantioselective hydrolysis has been limited mainly to the esters of chiral carboxylic acids [34,35,39,41]. However, since a large variety of chiral esters possess the stereogenic center in the nucleophilic part of molecule, to extend the substrate scope and increase the usability of this enzyme in the asymmetric synthesis, we employed Lecitase™ Ultra for the biocatalytic resolution of racemic 4-arylbut-3-en-2-ol esters. In our working group, these alcohols are the subject of special interest as chiral precursors of the optically active lactones with antiproliferative activity [56,57]. Likewise, they have been used in the production of chiral drugs [58,59].
In our previous paper, we reported the preliminary results of the free Lecitase™ Ultra-mediated kinetic resolution of model racemic esters of (E)-4-phenylbut-3-en-2-ol through their enantioselective hydrolysis, achieving moderate to good enantiopurity of the products [60]. Here, we present the results of our further investigations in this field, aiming to increase the process efficacy through optimization of some reaction conditions (pH of the reaction medium, addition of organic co-solvent) as well as by enzyme immobilization. To determine the substrate specificity and extend the application of Lecitase™ Ultra, the hydrolysis of some analogs of (E)-4-phenylbut-3-en-3-ol esters differing in aromatic fragment of the molecule was also studied.
2. Results and Discussion
Eleven known (2a–d, 3a–d and 4a,b,d) and one new (4c) racemic allyl esters were obtained in high yields (96–98%) by esterification of corresponding known [61,62,63] (E)-4-arylbut-3-en-2-ols (1a–d) with appropriate acid chlorides, according to the standard procedure (Scheme 1).
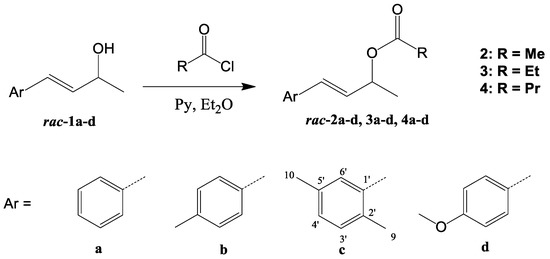
Scheme 1.
General scheme for the synthesis of racemic esters 2a–d, 3a–d and 4a–d.
Studies toward the kinetic resolution of the prepared compounds were started for the free Lecitase™ Ultra (LU)-catalyzed hydrolysis of model 4-phenylbut-3-en-2-ol esters (2a–4a) to investigate the influence of the length of an acyl chain, type of co-solvent, and type of buffer on the process stereoselectivity (Table 1).

Table 1.
Free Lecitase™ Ultra-catalyzed hydrolysis of racemic esters 2a–4a.
In previous studies, the enantioselective hydrolysis of carboxylic acid esters catalyzed by LU was carried out in a phosphate buffer [33] or Tris-HCl buffer [35], therefore in our first experiments, we employed these two reaction media. (E)-4-Phenylbut-3-en-2-ol esters with different acyl chain lengths (2a–4a) were subjected to hydrolysis at room temperature, with acetone as a co-solvent. In all cases, a higher enantioselectivity of hydrolysis was noticed in the reactions carried out in the phosphate buffer (Entries 4–6). Higher enantiomeric excess of alcohol 1a and a shorter reaction time necessary to achieve the best resolution was observed with the elongation of the acyl chain. The best results were obtained for butyrate 4a; in this case, the highest enantioselectivity (E > 200) was observed after 96 h of reaction, resulting in the optically pure product (Entry 6). The positive effect of the acyl chain length of hydrolyzed esters 2a–4a on their kinetic resolution was a constant tendency, observed also in experiments carried out in Tris-HCl (Entries 1–3) as well as using other co-solvents (Entries 7–9 and 10–12). These investigations confirm the high specificity of Lecitase™ Ultra toward esters of butyric acid, which was earlier found by Mishra et al. [35]. In their experiments, Lecitase™ Ultra did not accept glycerol triacetate as the substrate but exhibited hydrolytic activity toward glycerol tributyrate.
The influence of the co-solvent on the kinetic resolution of esters 2a–4a was determined for the reactions carried out in the phosphate buffer. For each of the substrates, the results of the reactions with acetone as the co-solvent were compared with those carried out with the addition of dimethylformamide (DMF) and dimethyl sulfoxide (DMSO). In all cases, the highest enantioselectivity was observed when the co-solvent was acetone (Entries 4–6). In the hydrolysis of propionate 3a with the addition of acetone (Entry 5) and DMF (Entry 8), the enantiomeric excesses of both unreacted ester and the produced alcohol were comparable, while the addition of DMSO was the least effective (Entry 11). In the case of the hydrolysis of acetate 2a, the highest enantiomeric purity of both products was observed for the reaction with the addition of acetone (Entry 4), while the addition of DMSO resulted in slightly better results than the addition of DMF (Entries 7 and 10). Significant impact of the co-solvent on the enantioselectivity of the reaction was observed for the hydrolysis of butyrate 4a. In this case, a pure enantiomer of alcohol 1a was produced only in the presence of acetone (Entry 6); the addition of DMF (Entry 9) and DMSO (Entry 12) resulted in a decrease of ee for alcohol 1a to 87–88%. On the other hand, the highest ee of unreacted butyrate 4a (69%) was noticed using DMSO as the co-solvent (Entry 12).
In subsequent experiments, free Lecitase™ Ultra was applied to the hydrolysis of esters of (E)-4-phenylbut-3-en-2-ol analogues differing in the structure of the aromatic fragment (Table 2).

Table 2.
Hydrolysis of allyl esters with different phenyl ring substituents 2b–d, 3b–d, and 4b–d catalyzed by free Lecitase™ Ultra.
Based on the previous results of the kinetic resolution of esters 2a–4a, the procedure using phosphate buffer as the reaction medium and the addition of acetone as a co-solvent was applied. Regardless of the acyl chain length, esters of (E)-4-(4′-methoxyphenyl)but-3-en-2-ol (2d–4d) were hydrolyzed with no enantioselectivity (Entries 7–9), and for esters containing methyl groups (2b–4b, 2c–4c) lower enantioselectivities of the reactions were observed (E ≤ 18) compared to (E)-4-phenylbut-3-en-2-yl esters 2a–4a (Table 1, entries 4–6). The beneficial effect of the elongation of the acyl chain on the results of kinetic resolution was confirmed only in the group of esters with the p-methyl substituted phenyl ring (2b–4b). Better resolution was observed for compounds with a 2,5-dimethylsubstituted phenyl ring than for those with the p-methylphenyl substituent, which was particularly noticeable in the case of acetates and propionates.
The positive effect of immobilization on the activity and enantioselectivity of enzymatic reactions is known from the literature [42]. In the case of LU, this relationship was confirmed in the hydrolysis of α-hydroxy acids esters. The highest increase of enantioselectivity, from E = 4 for the free enzyme to E = 19.5 for the enzyme immobilized in gelatin was observed for the hydrolysis of ethyl 2-hydroxy-4-oxo-4-phenylbutyrate, resulting in optically pure unreacted ester at 60–65% conversion [35]. Taking this into consideration, in our further studies, immobilized preparations of Lecitase™ Ultra were used as biocatalysts of the kinetic resolution of esters of alcohols with a 4-arylbut-3-en-2-ol system. For this purpose, the commercial enzyme was immobilized on four carriers: calcium alginate; Supelite™ DAX-8 acrylic resin; cyanogen bromide-activated agarose; and modified bacterial cellulose. The activity of immobilized preparations (Table 3) was measured using p-nitrophenyl palmitate as a substrate [64]. The highest, comparable activity was determined for the preparations obtained after immobilization on cyanogen bromide-activated agarose (LU-CNBr) and acrylic resin (LU-DAX). Preparation LU-CNBr was previously used in the production of optically pure (S)-3-methyl phenylglutarate through the asymmetric hydrolysis of dimethyl 3-phenylglutarate (48 h, 80% yield) [41].

Table 3.
Activity of Lecitase™ Ultra (LU) preparations.
(E)-4-Phenylbut-3-en-2-yl acetate (2a), (E)-4-phenylbut-3-en-2-yl propionate (3a), and (E)-4-phenylbut-3-en-2-yl butyrate (4a) were subjected to kinetic resolution with immobilized preparations of LU and the results were compared with those obtained previously for the free enzyme (Figure 1, Figure 2 and Figure 3).
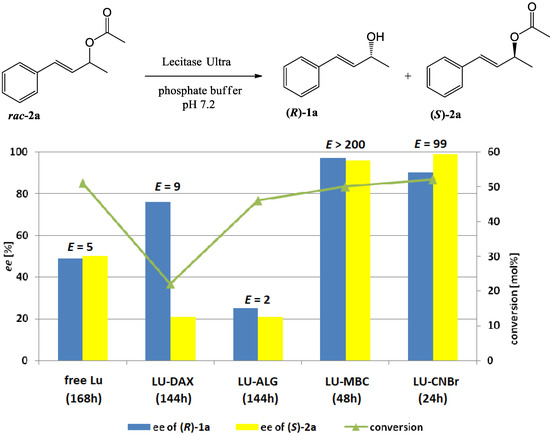
Figure 1.
The effect of enzyme immobilization on the conversion, enantioselectivity, and enantiopurity of the products of Lecitase™ Ultra-catalyzed hydrolysis of racemic acetate 2a (reaction scheme presented above)
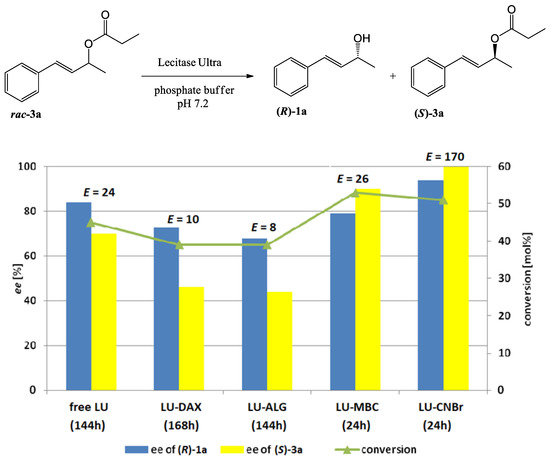
Figure 2.
The effect of enzyme immobilization on the conversion, enantioselectivity, and enantiopurity of the products of Lecitase™ Ultra-catalyzed hydrolysis of racemic propionate 3a (reaction scheme presented above).
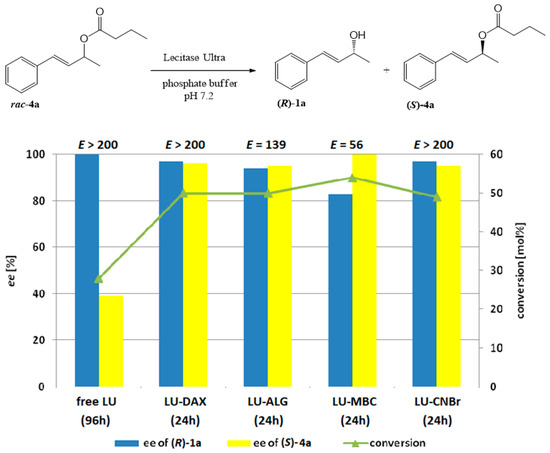
Figure 3.
The effect of enzyme immobilization on the conversion, enantioselectivity, and enantiopurity of the products of Lecitase™ Ultra-catalyzed hydrolysis of racemic butyrate 4a (reaction scheme presented above)
One can see that the use of LU-CNBr and LU-MBC significantly shortened the time needed to achieve the kinetic resolution of (E)-4-phenylbut-3-en-2-yl acetate (2a) up to 24 h and 48 h, respectively (Figure 1).
Likewise, the use of these preparations also had a positive effect on the parameters of kinetic resolution. The most spectacular improvement in the enantioselectivity of reaction (E > 200) was achieved using the LU-MBC preparation (Figure 1). In this case, very high enantiomeric excesses of alcohol 1a and unreacted acetate 2a were achieved of 97% and 96%, respectively. A highly enantioselective (E = 99) was also the reaction catalyzed by the LU-CNBr, in this case, after 24 h, the conversion exceeded 50%, which resulted in a lower ee of alcohol 1a (90%), but excellent ee of acetate 2a (99%). At a shorter reaction time (20 h, data not shown), when the conversion was close to 50%, the ee of alcohol 1a was higher, but a lower ee of acetate 2a was observed. The process of immobilization of the enzyme on Supelite™ DAX-8 acrylic resin and in calcium alginate did not bring the expected results. For the reaction catalyzed by LU-DAX, a slight increase of enantioselectivity was observed, affording alcohol 1a with 76% ee. However, the only 20% conversion degree achieved in this reaction resulted in significantly lower enantiomeric excess of unreacted ester 2a compared to the reaction catalyzed by free LU. Very low enantioselectivity of hydrolysis was observed in the case using Lecitase™ Ultra Alginate (LU-ALG).
Preparations LU-CNBr and LU-MBC were also the most efficient biocatalysts for the hydrolysis of propionate 3a (Figure 2).
The highest increase in the enantioselectivity of the reaction (E = 170) compared to the reaction catalyzed by the free enzyme was observed for LU-CNBr. Using this preparation allowed us to obtain enantiomerically pure propionate 3a and enantiomerically enriched alcohol 1a (ee 94%) after 24 h. The LU-MBC preparation hydrolyzed propionate 3a with an enantioselectivity comparable to that observed for the free LU-catalyzed reaction, but at a much higher rate. Accordingly, the enantiomers of the substrate were resolved in a significantly shorter time (24 h) and a 53% conversion of alcohol 1a and unreacted ester 3a was obtained with enantiomeric excesses of 79% and 90%, respectively. In the processes catalyzed by LU-DAX and LU-ALG, both the enantioselectivity of the reaction and the optical purities of unreacted propionate 3a and alcohol 1a were lower than those observed in free LU-catalyzed hydrolysis.
Particularly noteworthy are the results of the hydrolysis of (E)-4-phenylbut-3-en-2-yl butyrate (4a) (Figure 3).
For each preparation, the enzyme immobilization significantly increased its hydrolytic activity toward the substrate. Using preparations LU-CNBr, LU-DAX, and LU-ALG, kinetic resolution was achieved just after 24 h, affording alcohol 1a with ee in the range of 94–97% and unreacted ester 4a with 95–96% ee. A lower enantiomeric excess of alcohol 1a (ee 83%) was observed only when LU-MBC preparation was used; in this case, after 24 h, the conversion exceeded 50% and it was possible to obtain optically pure unreacted butyrate 4a.
Enantiomeric purities of the products of the kinetic resolution of racemic (E)-4-phenylbut-3-en-2-yl esters through Lecitase™ Ultra-catalyzed hydrolysis were comparable or higher than those obtained previously involving commercially available lipase preparations. Among the different lipases tested by Ghanem et al. [65], the most effective biocatalyst of the kinetic resolution of acetate 2a was lipase B from Candida antarctica (CALB). Optically pure alcohol 1a and unreacted acetate 2a with only 80% ee were obtained after 24 h of reaction in the phosphate buffer (pH 6.0) with the addition of toluene as the co-solvent. Similarly, in our earlier work, CALB was used to resolve racemic acetate 2a and propionate 3a in the reaction conditions analogous to those used in this study. A short time was needed to achieve kinetic resolution (6 h and 2 h, respectively), but significantly lower enantiomeric excess of unreacted ester was observed (80–85%) [60]. In other work, Thalén et al. [66] used CALB in the hydrolysis of enantiomerically enriched (S,E)-4-phenylbut-3-en-2-yl butyrate (ee 95%) to obtain optically pure butyrate 4a after 2 h of reaction carried out in a phosphate buffer (pH 7.2), but the reaction was carried out at high temperature (60 °C).
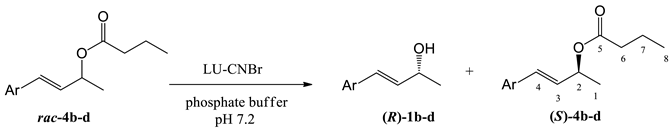
Taking into consideration that the most effective biocatalyst in the kinetic resolution of all 4-phenylbut-3-en-2-yl esters 2a–4a was LU-CNBr, it was decided to extend its scope of application to the kinetic resolution of butyrates 4b, 4c, and 4d containing the substituted benzene ring (Table 4), which have been hydrolyzed previously with free enzyme (Table 2).

Table 4.
Hydrolysis of butyrates 4b–d with different substituents on a benzene ring catalyzed by Lecitase™ Ultra immobilized on cyanogen bromide-activated agarose (LU-CNBr).
The preparation was an effective biocatalyst for the enantioselective hydrolysis of butyrate with the p-methylphenyl (4b) and 2,5-dimethylphenyl (4c) substituent. Significantly higher reaction rates and enantioselectivities were observed compared to the reactions catalyzed by free LU. Enantiomerically enriched alcohols (98% ee for 1b and 99% ee for 1c) and unreacted esters (89% ee for 4b and 99% ee for 4c) were obtained after 24 h or 48 h. On the other hand, the use of LU-CNBr did not affect the enantioselectivity of the hydrolysis of butyrate with the p-methoxyphenyl substituent (4d) and, similar to the reaction catalyzed by the free enzyme, both the alcohol 1d and unreacted ester 4d were racemic mixtures.
The aim of our last experiment was to determine the minimal enzyme dosage required for the effective kinetic resolution of the tested substrate in a 24 h reaction (Figure 4). The substrate of this reaction was (E)-4-phenylbut-3-en-2-yl acetate (2a) and hydrolysis was carried out using 100 mg of the starting ester. Regardless of an enzyme dosage, the enantioselectivity of reaction was consistently high (E > 200), but significant decrease of the conversion and the optical purity of unreacted ester 2a was observed when the enzyme dosage was lower than 0.03 U.
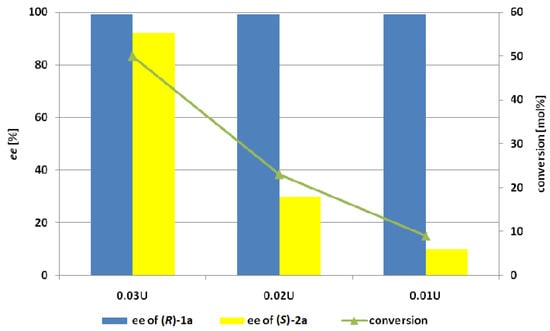
Figure 4.
The effect of the dose of Lecitase Ultra immobilized on cyanogen bromide-activated agarose (LU-CNBr) on the conversion and enantiopurity of the products after 24 h of hydrolysis of racemic acetate 2a in phosphate buffer (pH 7.2) and acetone as the co-solvent.
In order to prove the enantiopreference of Lecitase™ Ultra toward 4-arylbut-3-en-2-yl esters, alcohols and unreacted esters obtained after hydrolysis of butyrates 4a–4c by LU-CNBr were separated using preparative TLC and the configurations of known isolated isomers were unambiguously confirmed by comparing the signs of their specific rotation with the data in the literature (see Materials and Methods, paragraph 3.9.3). Similar to most lipases, Lecitase™ Ultra shows enantiopreference according to Kazlauskas’ rule, hydrolyzing (R)-enantiomers of tested substrates with a higher reaction rate, which results in the production of (R)-alcohols and (S)-enantiomers of unreacted esters.
3. Materials and Methods
3.1. Enzyme and Chemicals
Lecitase™ Ultra (LU, >10 000 U × g−1) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Racemic alcohols: (E)-4-phenylbut-3-en-2-ol (1a), (E)-4-(4′-methylphenyl)but-3-en-2-ol (1b), (E)-4-(2′,5′-dimethylphenyl)but-3-en-2-ol (1c), and (E)-4-(4′-methoxyphenyl)but-3-en-2-ol (1d) were synthesized according to the procedures described earlier [61,62,63]. Acetyl chloride (≥99%), propionyl chloride (98%), butyryl chloride (≥99%), sodium alginate, calcium chloride (≥96%), p-nitrophenyl palmitate (p-NPP), p-nitrophenol (p-NP), and polyethyleneimine (PEI) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Cyanogen bromide-activated agarose (Sepharose® 4B) was purchased from GE Healthcare Bio-Sciences AB (Uppsala, Sweden). Supelite™ DAX-8 was purchased from Supelco Analytical (Bellefonte, PA, USA). Sodium alginate was purchased from Sigma-Aldrich. Other chemicals were of analytical grade.
3.2. Analysis
Racemic esters 2a–d, 3a–d, and 4a–d were analyzed on an Agilent Technologies 6890N gas chromatograph (Santa Clara, CA, USA) equipped with hydrogen as the gas carrier, autosampler, split injection (50:1), and a flame ionization detector (FID) detector. Enantiomeric purity of the compounds was determined using a CP(Chrompack)-Chiral-Dex CB column (25 m × 0.25 mm × 0.25 m, Varian, Palo Alto, CA, USA). The temperature program for the products of hydrolysis of acetates 2a–d and propionates 3a–d are as follows: injector 280 °C, detector (FID) 280 °C, column 80–130 °C (0.5 °C × min−1), 130–200 °C (30 °C min−1), 200 °C (2 min); for the products of hydrolysis of butyrate 4a: injector 280 °C, detector (FID) 250 °C, column 80–120 °C (1 °C min−1), 120–140 °C (0.5 °C min−1); for the products of hydrolysis of butyrate 4b: injector 230 °C, detector (FID) 250 °C, column 40–160 °C (0.5 °C min−1), 160–190 °C (5 °C min−1); for the products of hydrolysis of butyrate 4c and 4d: injector 280 °C, detector (FID) 250 °C, column 40–140 °C (0.3 °C min−1), 140–180 °C (1 °C min−1). Analytical thin layer chromatography (TLC) was carried out on silica gel coated aluminum plates (DC-Alufolien Kieselgel 60 F254, Merck, Darmstadt, Germany). Compounds were visualized by spraying the plates with solution of 1% Ce(SO4)2 and 2% H3[P(Mo 3O10)4] in 10% H2SO4. Silica gel (Kieselgel 60, 230–400 mesh, Merck) was used as a stationary phase in the chromatographic purification of synthesized esters 2a–d, 3a–d, and 4a–d. Products of kinetic resolution were separated on preparative TLC silica gel glass plates (Uniplate™ UV254, layer thickness 1000 μm, 20 cm × 20 cm, Analtech, Newar, DE, USA). The optical rotations were measured on a Jasco P-2000-Na digital polarimeter (Easton, PA, USA) with an intelligent Remote Module (iRM) controller. The NMR spectra (1H NMR, 13C NMR, HMBC, and HMQC) were recorded on a Bruker Avance II 600 MHz spectrometer (Bruker, Rheinstetten, Germany). Samples were dissolved in CDCl3 and chemical shifts were referenced to residual solvent signals (δH = 7.26, δC = 77.0). The IR spectra were recorded on a Mattson IR 300 Thermo Nicolet spectrophotometer (Mattson, Waltham, MA, USA). High resolution mass spectrum (HRMS) of 4-(2′,5′-dimethylphenyl)but-3-en-2-yl butyrate (4c) was recorded on a Waters ESI-QTOF Premier XE spectrometer (Waters Corp., Millford, MA, USA) using the electron spray ionization (ESI) technique. The index of refraction was measured on an Abbe refractometer (Carl Zeiss, Jena, Germany).
3.3. Synthesis of Racemic Esters 2a–d, 3a–d, and 4a–d—General Procedure
Alcohol 1a–d (14 mmol) was dissolved in 100 mL of dry diethyl ether and 10 mL of pyridine. The mixture was stirred in an ice bath and 19 mmol of corresponding acylating agent (acetyl chloride, propionyl chloride, or butyryl chloride) was added dropwise. The reaction was continued at room temperature until the alcohol reacted completely (24 h, TLC). The reaction mixture was acidified with 1M HCl, the product was extracted with diethyl ether (3 × 40 mL) and separated by silica gel column chromatography (hexane:acetone, 10:1) to afford pure ester.
(E)-4-phenylbut-3-en-2-yl acetate (2a): Yield 96% (2.7 g), spectroscopic data consistent with those reported in the literature [67].
(E)-4-(4′-methylphenyl)but-3-en-2-yl acetate (2b): Yield 98% (2.6 g), spectroscopic data consistent with those reported in the literature [68].
(E)-4-(2′,5′-dimethylphenyl)but-3-en-2-yl acetate (2c): Yield 97% (2.8 g), spectroscopic data consistent with those reported in the literature [63].
(E)-4-(4’-methoxyphenyl)but-3-en-2-yl acetate (2d): Yield 98% (2.6 g), spectroscopic data consistent with those reported in the literature [68].
(E)-4-phenylbut-3-en-2-yl propionate (3a): Yield 96% (2.9 g), spectroscopic data consistent with those reported in the literature [69].
(E)-4-(4′-methylphenyl)but-3-en-2-yl propionate (3b): Yield 97% (2.9 g), spectroscopic data consistent with those reported in the literature [69].
(E)-4-(2’,5’-dimethylphenyl)but-3-en-2-yl propionate (3c): Yield 98% (3.0 g), spectroscopic data consistent with those reported in the literature [63].
(E)-4-(4’-methoxyphenyl)but-3-en-2-yl propionate (3d): Yield 97% (2.8 g), spectroscopic data consistent with those reported in the literature [70].
(E)-4-phenylbut-3-en-2-yl butyrate (4a): Yield 96% (2.7 g), spectroscopic data consistent with those reported in the literature [71].
(E)-4-(4′-methylphenyl)but-3-en-2-yl butyrate (4b): Yield 98% (2.9 g), spectroscopic data consistent with those reported in the literature [71].
(E)-4-(2,5-dimethylphenyl)but-3-en-2-yl butyrate (4c): Yield 97% (3.1 g), colorless liquid, = 1.5175; 1H NMR, δ: 0.96 (t, J = 7.8 Hz, 3H, CH3-8), 1.42 (d, J = 6.6 Hz, 3H, CH3-1), 1.68 (sextet, J = 7.8 Hz, 2H, CH2-7), 2.30, 2.31 (two s, 6H, CH3-9 and CH3-10), 2.29–2.32 (m, 2H, CH2-6), 5.55 (m, 1H, H-2), 6.06 (dd, J = 15.6 and 6.6 Hz, 1H, H-3), 6.79 (d, J = 15.6 Hz, 1H, H-4), 6.98 (m, 1H, H-4′), 7.03 (d, J = 7.8 Hz, 1H, H-3′), 7.25 (s, 1H, H-6′); 13C NMR, δ: 13.6 (C-8), 18.5 (C-7), 19.2 (C-9), 20.9 (C-10), 20.5 (C-1), 36.6 (C-6), 70.9 (C-2), 126.2 (C-6′), 128.5 (C-4′), 129.4 (C-4), 130.0 (C-3), 130.2 (C-3′), 132.6 (C-2′), 135.2, (C-1′), 135.4 (C-5′), 172.9 (C-5); IR (cm−1): 1735 (s), 1457 (m), 1182 (s), 1040 (s), 966 (s), 808 (m); HRMS: calcd for C16H22O2 [M + Na]+: 269.1517, found 269.1518.
(E)-4-(4′-methoxyphenyl)but-3-en-2-yl butyrate (4d): Yield 98% (3.0 g), spectroscopic data consistent with those reported in the literature [71].
3.4. Determination of Enzyme Activity
The enzymatic activity of the free and immobilized preparations of Lecitase™ Ultra was determined according to the procedure described by Pencreac’h and Baratti [72] with some modifications, based on the amount of p-nitrophenol released during the hydrolysis of p-nitrophenyl palmitate. The reaction mixture consisted of 75 µL of 1 mM p-NPP, 5.75 mL of 10 mM Tris/HCl buffer (pH 8.0), and 50 µL of free lipase solution or 30 mg of immobilized lipase. The mixture was kept at 37 °C for 130 min and the reaction was terminated by the addition of cold ethanol. The absorbance of released p-nitrophenol was measured on a Cintra 101 spectrophotometer, GBC Scientific Equipment at 410 nm. One international unit of p-NPP activity (1 U) was defined as the amount of enzyme necessary to produce 1 µmol of p-NP per minute under the conditions described above.
3.5. Immobilization of Lecitase™ Ultra by Entrapment in Calcium Alginate
Procedure of immobilization described by Blandino et al. was used with some modifications [73]. Lecitase™ Ultra (2.2 mL) was added to 10 mL of 6% of sodium alginate solution and stirred for 1 h on a magnetic stirrer. The mixture was dropped using a syringe into 500 mL of 0.3 M CaCl2. Formed beads of immobilized preparation were left for 1 h in the solution. Finally, the preparation was filtered, washed with distilled water to remove the residual CaCl2, and freeze-dried for 24 h using an LYO GT2-Basic lyophilizer at 10 mbar. As a result, 0.55 g of Lecitase™ Ultra immobilized in calcium alginate (LU-CA) was obtained and stored at 4 °C until use.
3.6. Immobilization of Lecitase™ Ultra by Adsorption on Polyacrylic Resin (Supelite™ DAX-8)
Immobilization of the enzyme was carried out using the method described by Egger et al. [74] with some modifications. Polyacrylic resin Supelite™ DAX-8 (20 g) was washed with Tris-HCl buffer and distilled water (4 × 20 mL). The carrier was left at room temperature for 24 h, and 1 g of pretreated support was placed in a twisted 20 mL vial containing 10 mL of Tris-HCl buffer and 4 mL of Lecitase™ Ultra. The mixture was shaken on a laboratory end-over-end shaker for 4 days. Immobilized enzyme was filtered, washed with Tris-HCl buffer (2 × 10 mL) and freeze-dried as described in paragraph 3.5 (24 h). Finally, 0.8 g of Lecitase™ Ultra immobilized on Supelite ™ DAX-8 (LU-DAX) was obtained and stored at 4 °C until use.
3.7. Immobilization of Lecitase™ Ultra by Covalent Bonds on Cyanogen Bromide-Activated Agarose (CNBr)
Immobilization of the enzyme was carried out according to the modified protocol reported by Dos Santos [36]. Cyanogen bromide-activated agarose (3 g) was successively washed on a Schott G3 funnel with 600 mL of cold 1 mM HCl in several portions for 30 min and then with 50 mL of coupling buffer (0.1M NaHCO3, 0.5 M NaCl, pH 8.3). Commercial Lecitase™ Ultra (2 mL) in a 250 mL Erlenmayer flask was diluted in 12 mL of coupling buffer and 3 g of prepared wet carrier was added. The mixture was shaken on a rotary shaker for 1 h at room temperature, filtered on a Schott G3 funnel, and rinsed with 50 mL of coupling buffer. The immobilized enzyme was transferred back to the Erlenmayer flask and incubated with 50 mL of 1 M ethanolamine (pH 8.0) at room temperature for 2 h, filtered on a Schott G3 funnel, then washed with acetate buffer (pH 4.0, 4 × 10 mL) and finally with 50 mL of coupling buffer. After freeze-drying (24 h), 2.8 g of Lecitase™ Ultra immobilized on cyanogen bromide-activated agarose (LU-CNBr) was obtained and stored at 4 °C until use.
3.8. Immobilization of Lecitase™ Ultra by Covalent Bonds on Modified Bacterial Cellulose (MBC)
Bacterial cellulose spheres produced by shaking cultures of Komagataeibacter xylinus and modified with polyethyleneimine and ferromagnetic particles were used as a carrier for enzyme immobilization. The detailed procedure of the carrier preparation as well as Lecitase™ Ultra immobilization has been described earlier [50]. Briefly, prior to immobilization, the carrier was activated with 1% glutaraldehyde solution in 100 mM phosphate buffer (pH 7.0) by shaking at a roller shaker. After activation, the modified bacterial cellulose (MBC) was collected using a magnetic separator and rinsed with phosphate buffer to remove an excess of glutaraldehyde. Next, 10 mL of enzyme solution was added to 5 mL of activated carrier and the mixture was incubated at 4 °C at a roller shaker. After 24 h, the immobilized enzyme was collected using a magnetic separator and the supernatant was removed. The preparation was washed with phosphate buffer and incubated at 4 °C for 1 h with NaBH4 solution in the phosphate buffer. Finally, 4 g of Lecitase™ Ultra immobilized on modified bacterial cellulose (LU-MBC) was collected using a magnetic separator and flushed successively with phosphate buffer containing 100 mM NaCl, 0.25% Triton-X100, and phosphate buffer. The preparation was freeze-dried for 24 h and stored at 4 °C until use.
3.9. Hydrolysis of Esters 2a–d, 3a–d and 4a–d Catalyzed by free or Immobilized Lecitase™ Ultra
3.9.1. General Procedure
A total of 0.06 U of free enzyme Lecitase™ Ultra (2 mL) or 0.06 U of immobilized preparation of this enzyme (1.33 g LU-MBC or 1.15 g LU-DAX-8, or 1.56 g LU-CA or 1.05 g LU-CNBr) and 0.2 g of ester dissolved in 0.5 mL of organic solvent (acetone, DMF or DMSO) were placed in 10 mL screw cap glass vials containing 3.5 mL of the phosphate buffer (pH 7.2) or Tris/HCl buffer (pH 8.2). Vials were shaken at 750 rpm on a magnetic stirrer at 20 °C. Samples (0.6 mL) of the reaction mixture were taken at different time intervals and extracted with diethyl ether (2 × 2 mL). In the case of the reaction with the immobilized enzyme, the extracts were filtrated through diatomaceous earth (Celite 560) to completely remove an immobilized biocatalyst. The extracts were dried and solvent was removed by evaporation in vacuo. Before chiral gas chromatography (CGC) samples taken from the reaction mixture were treated with propionyl or acetyl chloride to derivatize inseparable enantiomers of alcohols 1a–d into the corresponding esters as described earlier [60].
3.9.2. The Effect of Dosage of Lecitase Ultra immobilized Cyanogen Bromide-Activated Agarose (LU-CNBr)
Different dosages of LU-CNBr and (E)-4-phenylbut-3-en-2-yl acetate 2a (0.2g) in 0.5 mL of acetone were placed in 10 mL screw cap glass vials containing 3.5 mL of the phosphate buffer (pH 7.2). Vials were shaken at 750 rpm on magnetic stirrer for 24 h. Samples (0.6 mL) from the reaction mixture were taken after 24 h, filtrated through Celite 560, and prepared for chiral GC as described in paragraph 3.9.1.
3.9.3. Isolation of Products Obtained by Hydrolysis of Esters 4a–4c—General Procedure
Hydrolysis of butyrate 4a–4c (0.2 g) in the phosphate buffer was carried out as described in paragraph 3.9.2 using LU-CNBr (0.06 U). After 24 h of reaction, the products were extracted with ether diethyl (2 × 5 mL) and the combined organic layers were filtered through Celite 560, washing the adsorbent with 8 mL of diethyl ether. The extract was dried over anhydrous magnesium sulfate, the solvent was evaporated in vacuo, and the mixture of products was separated by preparative TLC (hexane: acetone, 10:1).
After hydrolysis of ester 4a (0.2 g, 0.9 mmol) the following products were obtained:
(S,E)-4-phenylbut-3-en-2-yl butyrate ((S)-4a): yield 45% (0.09 g), ee 96%, = −51.3 (c 0.36; CHCl3); lit. [71]: = −111.3 (c 1.0; CHCl3, ee 97%).
(R,E)-4-phenylbut-3-en-2-ol ((R)-1a): yield 47% (0.063 g), ee 97%, = +19.6 (c 1.35; CH2Cl2); lit. [65]: = +19.9 (c 1.6; CH2Cl2, ee 96%)
After hydrolysis of ester 4b (0.2 g, 0.8 mmol) the following products were isolated: (S,E)-4-(4′-methylphenyl)but-3-en-2-yl butyrate ((S)-4b): yield 45% (0.089 g), ee 89%, = −108.1 (c 0.39; CHCl3), lit. [71]: = −115.9 (c 1.0; CHCl3, ee 98%)
(R,E)- 4-(4′-methylphenyl)but-3-en-2-ol ((R)-1b): yield 47% (0.066 g), ee 98%, = +22.7 (c 1.22; CH2Cl2), lit [69]: = +22.5 (c 1.5; CH2Cl2, ee 96%)
Hydrolysis of ester 4c (0.2 g, 0.8 mmol) afforded the following products: (S,E)-4-(2′,5′-dimethylphenyl)but-3-en-2-yl butyrate ((S)-4c): yield 48% (0.096 g), ee 99%, = −46.2 (c 0.38; CH2Cl2).
(R,E)-4-(2′,5′-dimethylphenyl)but-3-en-2-ol ((R)-1c): yield 43% (0.062 g), ee 99%, = +16.5 (c 0.37; CH2Cl2); lit. [63]: = +16.2 (c 1.7; CH2Cl2, ee 98%).
4. Conclusions
Results of this survey allowed for the extension of the scope of application for enzyme Lecitase™ Ultra in the asymmetric synthesis. This biocatalyst proved to be an attractive alternative to the commonly used commercial lipase preparations in the kinetic resolution of 4-arylbut-3-en-2-yl esters through their enantioselective hydrolysis. In the reactions catalyzed by the free enzyme, very good kinetic resolution was obtained in reactions carried out in the phosphate buffer, with the addition of acetone as a co-solvent. The enantioselectivity of hydrolysis increased along with the acyl chain length. The significant increase in the optical purity of unreacted substrates and products (up to 90–99%) as well as the reduction of a reaction time necessary for optimal kinetic resolution was achieved using immobilized preparations (up to 24–48 h). Particularly effective was the enzyme immobilized on cyanogen bromide-activated agarose (LU-CNBr). This preparation also catalyzed effective kinetic resolution of substrates containing methyl substituents on phenyl ring (4b,4c), however, it did not show any enantioselectivity toward 4-(4’-methoxyphenyl)but-3-en-2-yl butyrate (4d).
Further research will be aimed at the extension of the scope of the applicability of Lecitase™ Ultra not only in the hydrolysis of structurally diverse esters, but also in the kinetic resolution of alcohols through their transesterification.
Author Contributions
Conceptualization, A.L. and W.G.; Investigation, A.L., R.D., M.S.; Validation, W.G.; Writing—original draft, A.L.; Writing—Review & Editing, A.L., W.G., and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financed by the statutory activities of the Department of Chemistry, Wrocław University of Environmental and Life Sciences. Article Processing Charge (APC) was financed under the Leading Research Groups support project from the subsidy increased for the period 2020–2025 in the amount of 2% of the subsidy referred to Art. 387 (3) of the Law of 20 July 2018 on Higher Education and Science, obtained in 2019.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Ortiz, C.; Berenguer-Murcia, Á.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Lecitase Ultra: A phospholipase with great potential in biocatalysis. Mol. Catal. 2019, 473, 110405. [Google Scholar] [CrossRef]
- Yang, B.; Wang, Y.H.; Yang, J.G. Optimization of enzymatic degumming process for rapeseed oil. JAOCS J. Am. Oil Chem. Soc. 2006, 83, 653–658. [Google Scholar] [CrossRef]
- Manjula, S.; Jose, A.; Divakar, S.; Subramanian, R. Degumming rice bran oil using phospholipase-A1. Eur. J. Lipid Sci. Technol. 2011, 113, 658–664. [Google Scholar] [CrossRef]
- Lamas, D.L.; Crapiste, G.H.; Constenla, D.T. Changes in quality and composition of sunflower oil during enzymatic degumming process. LWT Food Sci. Technol. 2014, 58, 71–76. [Google Scholar] [CrossRef]
- Sampaio, K.A.; Zyaykina, N.; Wozniak, B.; Tsukamoto, J.; De Greyt, W.; Stevens, C.V. Enzymatic degumming: Degumming efficiency versus yield increase. Eur. J. Lipid Sci. Technol. 2015, 117, 81–86. [Google Scholar] [CrossRef]
- Li, Z.; Liu, H.; Zhao, G.; Wang, P.; Wang, L.; Wu, H.; Fang, X.; Sun, X.; Wu, X.; Zheng, Z. Enhancing the performance of a phospholipase A1 for oil degumming by bio-imprinting and immobilization. J. Mol. Catal. B Enzym. 2016, 123, 122–131. [Google Scholar] [CrossRef]
- Baeza-Jiménez, R.; López-Martínez, L.X.; Otero, C.; Kim, I.H.; García, H.S. Enzyme-Catalysed hydrolysis of phosphatidylcholine for the production of lysophosphatidylcholine. J. Chem. Technol. Biotechnol. 2013, 88, 1859–1863. [Google Scholar] [CrossRef]
- Lim, C.W.; Kim, B.H.; Kim, I.H.; Lee, M.W. Modeling and optimization of phospholipase A1-catalyzed hydrolysis of phosphatidylcholine using response surface methodology for lysophosphatidylcholine production. Biotechnol. Prog. 2015, 31, 35–41. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Wang, X. Enzymatic preparation of L-α-glycerylphosphorylcholine in an aqueous medium. Eur. J. Lipid Sci. Technol. 2012, 114, 1254–1260. [Google Scholar] [CrossRef]
- Bang, H.J.; Kim, I.H.; Kim, B.H. Phospholipase A1-catalyzed hydrolysis of soy phosphatidylcholine to prepare L-α-glycerylphosphorylcholine in organic-aqueous media. Food Chem. 2016, 190, 201–206. [Google Scholar] [CrossRef]
- Gerits, L.R.; Pareyt, B.; Masure, H.G.; Delcour, J.A. Native and enzymatically modified wheat (Triticum aestivum L.) endogenous lipids in bread making: A focus on gas cell stabilization mechanisms. Food Chem. 2015, 172, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Melis, S.; Pauly, A.; Delcour, J.A. Impact of lipases with different substrate specificity in wheat flour separation on the properties of the resultant gluten. J. Cereal Sci. 2017, 77, 291–296. [Google Scholar] [CrossRef]
- Melis, S.; Pauly, A.; Gerits, L.R.; Pareyt, B.; Delcour, J.A. Lipases as processing aids in the separation of wheat flour into gluten and starch: Impact on the lipid population, gluten agglomeration, and yield. J. Agric. Food Chem. 2017, 65, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Gofferjé, G.; Motulewicz, J.; Stäbler, A.; Herfellner, T.; Schweiggert-Weisz, U.; Flöter, E. Enzymatic degumming of crude jatropha oil: Evaluation of impact factors on the removal of phospholipids. JAOCS J. Am. Oil Chem. Soc. 2014, 91, 2135–2141. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.; Du, W.; Dai, L.; Liu, D. Combined phospholipase and lipase catalysis for biodiesel production from phospholipids-containing oil. Biotechnol. Bioprocess Eng. 2015, 20, 965–970. [Google Scholar] [CrossRef]
- Moharana, T.R.; Byreddy, A.R.; Puri, M.; Barrow, C.; Rao, N.M. Selective enrichment of omega-3 fatty acids in oils by phospholipase A1. PLoS ONE 2016, 11, e0151370. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, M.; Song, K.; Wang, L.; Tang, S.; Riley, W.W. Partial hydrolysis of soybean oil by phospholipase A1 (Lecitase Ultra). Food Chem. 2010, 121, 1066–1072. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Hu, C.; Cao, Q.; Yang, X.; Zhao, M. Preparation of diacylglycerol-enriched oil from free fatty acids using Lecitase Ultra-catalyzed esterification. JAOCS J. Am. Oil Chem. Soc. 2011, 88, 1557–1565. [Google Scholar] [CrossRef]
- Liu, N.; Wang, Y.; Zhao, Q.; Cui, C.; Fu, M.; Zhao, M. Immobilisation of Lecitase® Ultra for production of diacylglycerols by glycerolysis of soybean oil. Food Chem. 2012, 134, 301–307. [Google Scholar] [CrossRef]
- Liu, N.; Wang, Y.; Zhao, Q.; Zhao, M. Production of palm oil-based diacylglycerol using LecitaseTM Ultra-catalyzed glycerolysis and molecular distillation. Food Sci. Biotechnol. 2014, 23, 365–371. [Google Scholar] [CrossRef]
- Kim, I.H.; Garcia, H.S.; Hill, C.G. Phospholipase A1-catalyzed synthesis of phospholipids enriched in n-3 polyunsaturated fatty acid residues. Enzym. Microb. Technol. 2007, 40, 1130–1135. [Google Scholar] [CrossRef]
- Kim, I.H.; Garcia, H.S.; Hill, C.G. Synthesis of structured phosphatidylcholine containing n-3 PUFA residues via acidolysis mediated by immobilized phospholipase A1. JAOCS J. Am. Oil Chem. Soc. 2010, 87, 1293–1299. [Google Scholar] [CrossRef]
- Baeza-Jiménez, R.; Noriega-Rodríguez, J.A.; García, H.S.; Otero, C. Structured phosphatidylcholine with elevated content of conjugated linoleic acid: Optimization by response surface methodology. Eur. J. Lipid Sci. Technol. 2012, 114, 1261–1267. [Google Scholar] [CrossRef]
- Gan, L.J.; Wang, X.Y.; Yang, D.; Zhang, H.; Shin, J.A.; Hong, S.T.; Park, S.H.; Lee, K.T. Emulsifying properties of lecithin containing different fatty acids obtained by immobilized Lecitase Ultra-catalyzed reaction. JAOCS J. Am. Oil Chem. Soc. 2014, 91, 579–590. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.F.; Yang, B.; Li, D.M.; Wang, Y.H.; Wang, W.F. Production of structured phosphatidylcholine with high content of DHA/EPA by immobilized phospholipase A1-catalyzed transesterification. Int. J. Mol. Sci. 2014, 15, 15244–15258. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; No, D.S.; Kim, B.H.; Garcia, H.S.; Kim, Y.; Kim, I.H. Immobilized phospholipase A1-catalyzed modification of phosphatidylcholine with n-3 polyunsaturated fatty acid. Food Chem. 2014, 157, 132–140. [Google Scholar] [CrossRef]
- Xi, X.; Feng, X.; Shi, N.; Ma, X.; Lin, H.; Han, Y. Immobilized phospholipase A1-catalyzed acidolysis of phosphatidylcholine from Antarctic krill (Euphausia superba) for docosahexaenoic acid enrichment under supercritical conditions. J. Mol. Catal. B Enzym. 2016, 126, 46–55. [Google Scholar] [CrossRef]
- Gumel, A.M.; Annuar, M.S.M. Thermomyces lanuginosus lipase-catalyzed synthesis of natural flavor esters in a continuous flow microreactor. 3 Biotech 2016, 6, 24. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Filice, M.; Terreni, M.; Guisan, J.M.; Fernandez-Lafuente, R.; Palomo, J.M. Lecitase® Ultra as regioselective biocatalyst in the hydrolysis of fully protected carbohydrates. Strong modulation by using different immobilization protocols. J. Mol. Catal. B Enzym. 2008, 51, 110–117. [Google Scholar] [CrossRef]
- Ghanem, A.; Aboul-Enein, H.Y. Application of lipases in kinetic resolution of racemates. Chirality 2005, 17, 1–15. [Google Scholar] [CrossRef]
- Carvalho, A.C.L.D.M.; Fonseca, T.D.S.; De Mattos, M.C.; De Oliveira, M.D.C.F.; De Lemos, T.M.L.G.; Molinari, F.; Romano, D.; Serra, I. Recent advances in lipase-mediated preparation of pharmaceuticals and their intermediates. Int. J. Mol. Sci. 2015, 16, 29682–29716. [Google Scholar] [CrossRef] [PubMed]
- Angajala, G.; Pavan, P.; Subashini, R. Lipases: An overview of its current challenges and prospectives in the revolution of biocatalysis. Biocatal. Agric. Biotechnol. 2016, 7, 257–270. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Palomo, J.M.; Guisan, J.M.; Fernandez-Lafuente, R. Effect of the immobilization protocol in the activity, stability, and enantioselectivity of Lecitase® Ultra. J. Mol. Catal. B Enzym. 2007, 47, 99–104. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Cabrera, Z.; Godoy, C.; Fernandez-Lafuente, R.; Palomo, J.M.; Guisan, J.M. Interfacially activated lipases against hydrophobic supports: Effect of the support nature on the biocatalytic properties. Process Biochem. 2008, 43, 1061–1067. [Google Scholar] [CrossRef]
- Mishra, M.K.; Kumaraguru, T.; Sheelu, G.; Fadnavis, N.W. Lipase activity of Lecitase® Ultra: Characterization and applications in enantioselective reactions. Tetrahedron Asymmetry 2009, 20, 2854–2860. [Google Scholar] [CrossRef]
- Dos Santos, J.C.S.; Garcia-Galan, C.; Rodrigues, R.C.; de Sant’ Ana, H.B.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Improving the catalytic properties of immobilized Lecitase via physical coating with ionic polymers. Enzym. Microb. Technol. 2014, 60, 1–8. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Barbosa, O.; Hernandez, K.; Dos Santos, J.C.S.; Rodrigues, R.C.; Fernandez-Lafuente, R. Evaluation of styrene-divinylbenzene beads as a support to immobilize lipases. Molecules 2014, 19, 7629–7645. [Google Scholar] [CrossRef]
- Rueda, N.; Dos Santos, J.C.S.; Torres, R.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R. Improved performance of lipases immobilized on heterofunctional octyl-glyoxyl agarose beads. RSC Adv. 2015, 5, 11212–11222. [Google Scholar] [CrossRef]
- Mishra, M.K.; Harini, M.; Kumaraguru, T.; Lakshmi Prasanna, T.; Fadnavis, N.W. A porous vessel bioreactor for gel entrapped biocatalysts: Kinetic resolution of trans-methyl (4-methoxyphenyl)glycidate by Lecitase® Ultra in gelatin organogel (Gelozyme). J. Mol. Catal. B Enzym. 2011, 71, 56–62. [Google Scholar] [CrossRef]
- Kumaraguru, T.; Harini, T.; Basetty, S. Immobilization of Lecitase® Ultra on recyclable polymer support: Application in resolution of trans-methyl(4-methoxyphenyl)glycidate in organic solvents. Tetrahedron Asymmetry 2017, 28, 1612–1617. [Google Scholar] [CrossRef]
- Cabrera, Z.; Fernandez-Lorente, G.; Palomo, J.M.; Guisan, J.M.; Fernandez-Lafuente, R. Asymmetric hydrolysis of dimethyl 3-phenylglutarate catalyzed by Lecitase Ultra®. Effect of the immobilization protocol on its catalytic properties. Enzym. Microb. Technol. 2008, 43, 531–536. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Adlercreutz, P. Immobilisation and application of lipases in organic media. Chem. Soc. Rev. 2013, 42, 6406–6436. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Zhao, Y.; Noreen, S.; Shah, S.Z.H.; Bharagava, R.N.; Iqbal, H.M.N. Modifying bio-catalytic properties of enzymes for efficient biocatalysis: A review from immobilization strategies viewpoint. Biocatal. Biotransform. 2019, 37, 159–182. [Google Scholar] [CrossRef]
- Di Cosimo, R.; Mc Auliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef]
- Sheelu, G.; Kavitha, G.; Fadnavis, N.W. Efficient immobilization of Lecitase in gelatin hydrogel and degumming of rice bran oil using a spinning basket reactor. JAOCS J. Am. Oil Chem. Soc. 2008, 85, 739–748. [Google Scholar] [CrossRef]
- Yu, D.; Jiang, L.; Li, Z.; Shi, J.; Xue, J.; Kakuda, Y. Immobilization of phospholipase A1 and its application in soybean oil degumming. JAOCS J. Am. Oil Chem. Soc. 2012, 89, 649–656. [Google Scholar] [CrossRef]
- da Silva, F.B.; de Morais Júnior, W.G.; da Silva, C.V.; Vieira, A.T.; Batista, A.C.F.; de Faria, A.M.; Assunção, R.M.N. Preparation and characterization of cellulose triacetate as support for Lecitase Ultra immobilization. Molecules 2017, 22, 1930. [Google Scholar] [CrossRef]
- Drozd, R.; Szymańska, M.; Rakoczy, R.; Junka, A.; Szymczyk, P.; Fijałkowski, K. Functionalized magnetic bacterial cellulose beads as carrier for Lecitase® Ultra immobilization. Appl. Biochem. Biotechnol. 2019, 187, 176–193. [Google Scholar] [CrossRef]
- Liu, N.; Fu, M.; Wang, Y.; Zhao, Q.; Sun, W.; Zhao, M. Immobilization of LecitaseTM® Ultra onto a novel polystyrene DA-201 resin: Characterization and biochemical properties. Appl. Biochem. Biotechnol. 2012, 168, 1108–1120. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.S.; Garcia-Galan, C.; Danelli, D.; Paludo, N.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Use of Lecitase Ultra immobilized on styrene-divinylbenzene beads as catalyst of esterification reactions: Effects of ultrasounds. Catal. Today 2015, 255, 27–32. [Google Scholar] [CrossRef]
- Yu, D.; Ma, Y.; Xue, S.J.; Jiang, L.; Shi, J. Characterization of immobilized phospholipase A1 on magnetic nanoparticles for oil degumming application. LWT Food Sci. Technol. 2013, 50, 519–525. [Google Scholar] [CrossRef]
- Gonçalves, K.M.; Júnior, I.I.; Papadimitriou, V.; Zoumpanioti, M.; Leal, I.C.R.; De Souza, R.O.M.A.; Cordeiro, Y.; Xenakis, A. Nanoencapsulated Lecitase Ultra and Thermomyces lanuginosus lipase, a comparative structural study. Langmuir 2016, 32, 6746–6756. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galan, C.; Dos Santos, J.C.S.; Barbosa, O.; Torres, R.; Pereira, E.B.; Corberan, V.C.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Tuning of Lecitase features via solid-phase chemical modification: Effect of the immobilization protocol. Process Biochem. 2014, 49, 604–616. [Google Scholar] [CrossRef]
- Gładkowski, W.; Skrobiszewski, A.; Mazur, M.; Gliszczyńska, A.; Czarnecka, M.; Pawlak, A.; Obmińska-Mrukowicz, B.; Maciejewska, G.; Białońska, A. Chiral δ-iodo-γ-lactones derived from cuminaldehyde, 2,5-dimethylbenzaldehyde and piperonal: Chemoenzymatic synthesis and antiproliferative activity. Tetrahedron Asymmetry 2016, 27, 227–237. [Google Scholar] [CrossRef]
- Gładkowski, W.; Włoch, A.; Pawlak, A.; Sysak, A.; Białońska, A.; Mazur, M.; Mituła, P.; Maciejewska, G.; Obmińska-Mrukowicz, B.; Kleszczyńska, H. Preparation of enantiomeric β-(2′,5′-dimethylphenyl)bromolactones, their antiproliferative activity and effect on biological membranes. Molecules 2018, 23, 3035. [Google Scholar] [CrossRef]
- Brenna, E.; Caraccia, N.; Fuganti, C.; Fuganti, D.; Grasselli, P. Enantioselective synthesis of β-substituted butyric acid derivatives via orthoester Claisen rearrangement of enzymatically resolved allylic alcohols: Application to the synthesis of (R)-(-)-baclofen. Tetrahedron Asymmetry 1997, 8, 3801–3805. [Google Scholar] [CrossRef]
- Brenna, E.; Fuganti, C.; Grasselli, P.; Serra, S. Enzyme-mediated synthesis of (S)-and (R)-Verapamil. Eur. J. Org. Chem. 2001, 1349–1357. [Google Scholar] [CrossRef]
- Leśniarek, A.; Chojnacka, A.; Gładkowski, W. Application of Lecitase® Ultra-catalyzed hydrolysis to the kinetic resolution of (E)-4-phenylbut-3-en-2-yl esters. Catalysts 2018, 8, 423. [Google Scholar] [CrossRef]
- Mazur, M.; Gładkowski, W.; Wawrzeńczyk, C. Synthesis of halolactones with methoxyphenyl ring. Przem. Chem. 2011, 5, 918–922. [Google Scholar]
- Gładkowski, W.; Skrobiszewski, A.; Mazur, M.; Siepka, M.; Pawlak, A.; Obmińska-Mrukowicz, B.; Białońska, A.; Poradowski, D.; Drynda, A.; Urbaniak, M. Synthesis and anticancer activity of novel halolactones with β-aryl substituents from simple aromatic aldehydes. Tetrahedron 2013, 69, 10414–10423. [Google Scholar] [CrossRef]
- Gładkowski, W.; Gliszczyńska, A.; Siepka, M.; Czarnecka, M.; Maciejewska, G. Kinetic resolution of (E)-4-(2′,5′-dimethylphenyl)-but-3-en-2-ol and (E)-4-(benzo[d][1′,3′]dioxol-5′-yl)-but-3-en-2-ol through lipase-catalyzed transesterification. Tetrahedron Asymmetry 2015, 26, 702–709. [Google Scholar] [CrossRef]
- Mahdi, B.A.; Bhattacharya, A.; Gupta, A. Enhanced lipase production from Aeromonas sp. S1 using Sal deoiled seed cake as novel natural substrate for potential application in dairy wastewater treatment. J. Chem. Technol. Biotechnol. 2012, 87, 418–426. [Google Scholar] [CrossRef]
- Ghanem, A.; Schurig, V. Lipase-catalyzed access to enantiomerically pure (R)-and (S)-trans-4-phenyl-3-butene-2-ol. Tetrahedron Asymmetry 2003, 14, 57–62. [Google Scholar] [CrossRef]
- Thalén, L.K.; Sumic, A.; Bogár, K.; Norinder, J.; Persson, A.K.; Bäckvall, J.E. Enantioselective synthesis of α-methyl carboxylic acids from readily available starting materials via chemoenzymatic dynamic kinetic resolution. J. Org. Chem. 2010, 75, 6842–6847. [Google Scholar]
- Horn, A.; Kazmaier, U. Stereoselective modification of N-(α-Hydroxyacyl)-glycinesters via palladium-catalyzed allylic alkylation. Org. Lett. 2019, 21, 4595–4599. [Google Scholar] [CrossRef]
- Akai, S.; Hanada, R.; Fujiwara, N.; Kita, Y.; Egi, M. One-Pot synthesis of optically active allyl esters via lipase-vanadium combo catalysis. Org. Lett. 2010, 12, 4900–4903. [Google Scholar] [CrossRef]
- Gładkowski, W.; Skrobiszewski, A.; Mazur, M.; Siepka, M.; Białońska, A. Convenient chemoenzymatic route to optically active β-aryl-δ-iodo-γ-lactones and β-aryl-γ-iodo-δ-lactones with the defined configurations of stereogenic centers. Eur. J. Org. Chem. 2015, 2015, 605–615. [Google Scholar] [CrossRef]
- Skrobiszewski, A.; Gładkowski, W.; Maciejewska, G.; Wawrzeńczyk, C. Chemoenzymatic synthesis of trans-β-aryl-δ-hydroxy-γ-lactones and enzymatic kinetic resolution of their racemic mixtures. Molecules 2016, 21, 1552. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, H.K.; Park, J. (S)-selective dynamic kinetic resolution of allylic alcohols by enzyme-metal bicatalysis. Bull. Korean Chem. Soc. 2007, 28, 2096–2098. [Google Scholar]
- Pencreac’h, G.; Baratti, J.C. Hydrolysis of p-nitrophenyl palmitate in n-heptane by the Pseudomonas cepacia lipase: A simple test for the determination of lipase activity in organic media. Enzyme 1996, 18, 417–422. [Google Scholar]
- Blandino, A.; Macías, M.; Cantero, D. Formation of calcium alginate gel capsules: Influence of sodium alginate and CaCl2 concentration on gelation kinetics. J. Biosci. Bioeng. 1999, 88, 686–689. [Google Scholar] [CrossRef]
- Egger, D.; Wehtje, E.; Adlercreutz, P. Characterization and optimization of phospholipase A2 catalyzed synthesis of phosphatidylcholine. Biochim. Biophys. Acta Protein Struct. Mol. Enzym. 1997, 1343, 76–84. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 2a–2d, 3a–3d, 4a–4d are not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).