Investigation of the Absorption of Nanosized lamotrigine Containing Nasal Powder via the Nasal Cavity
Abstract
1. Introduction
2. Results and Discussion
2.1. Determination of Particle Size and Image Analysis (SEM)
2.2. Structural Investigations
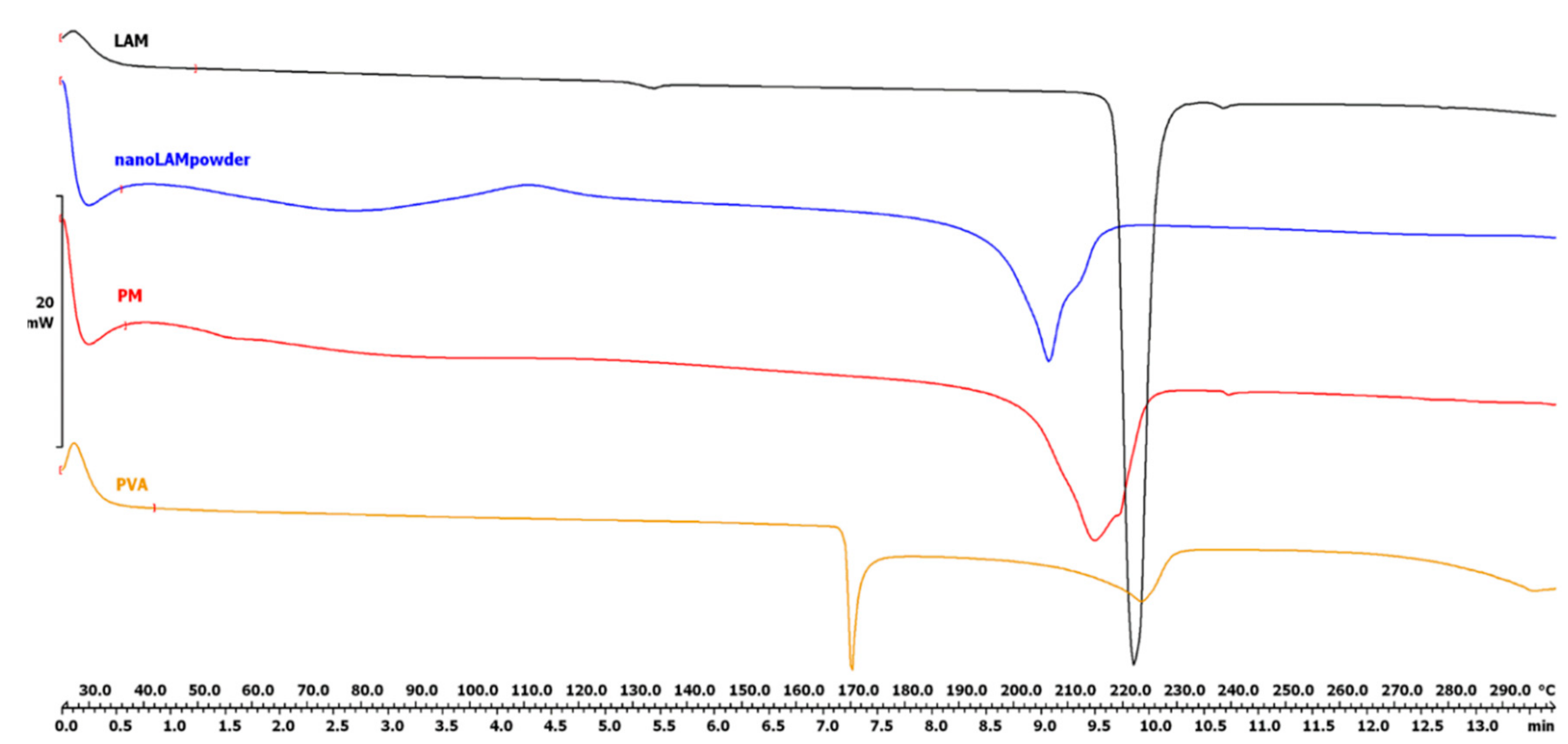
2.2.1. Differential Scanning Calorimetry (DSC)
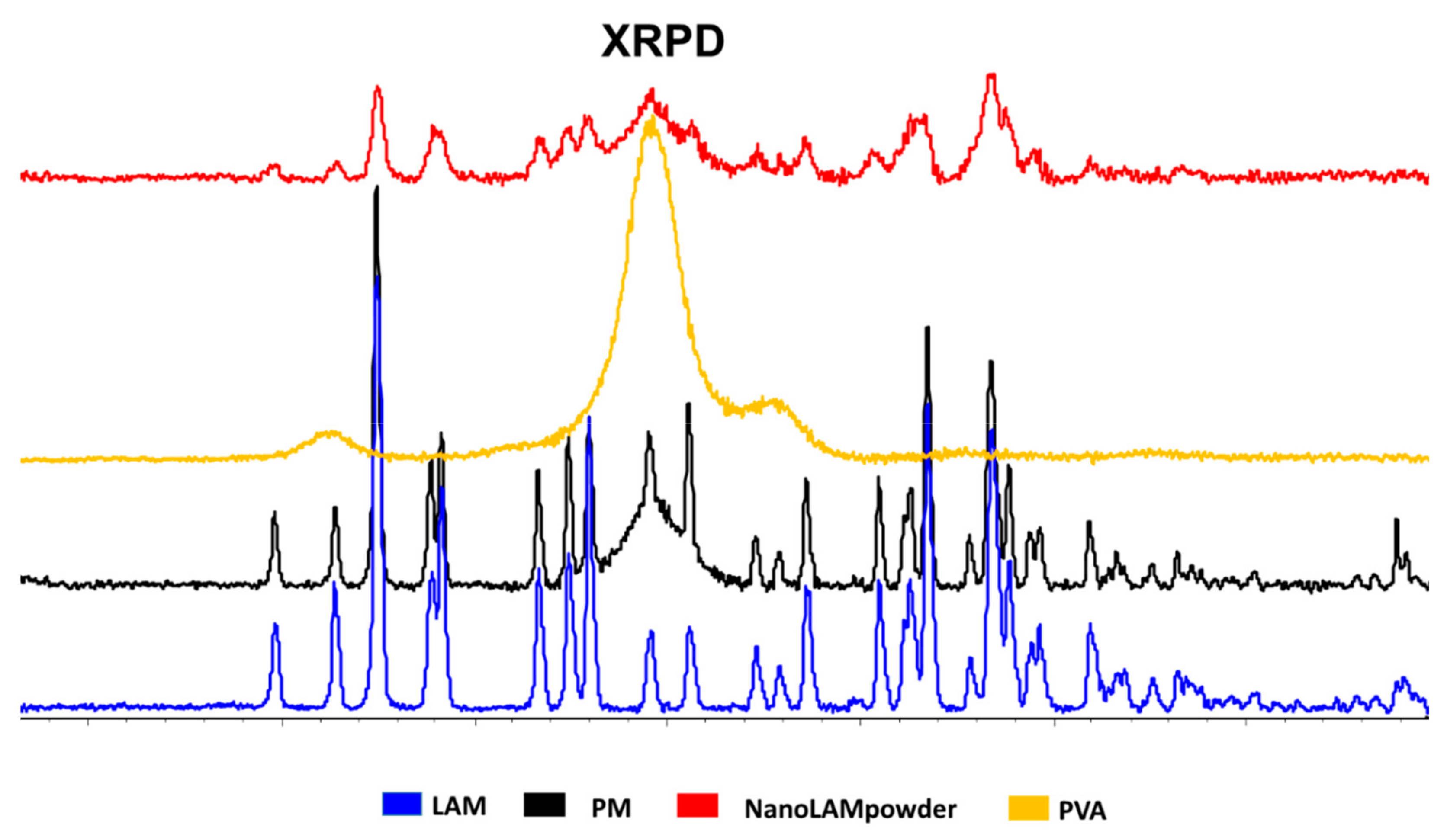
2.2.2. XRPD
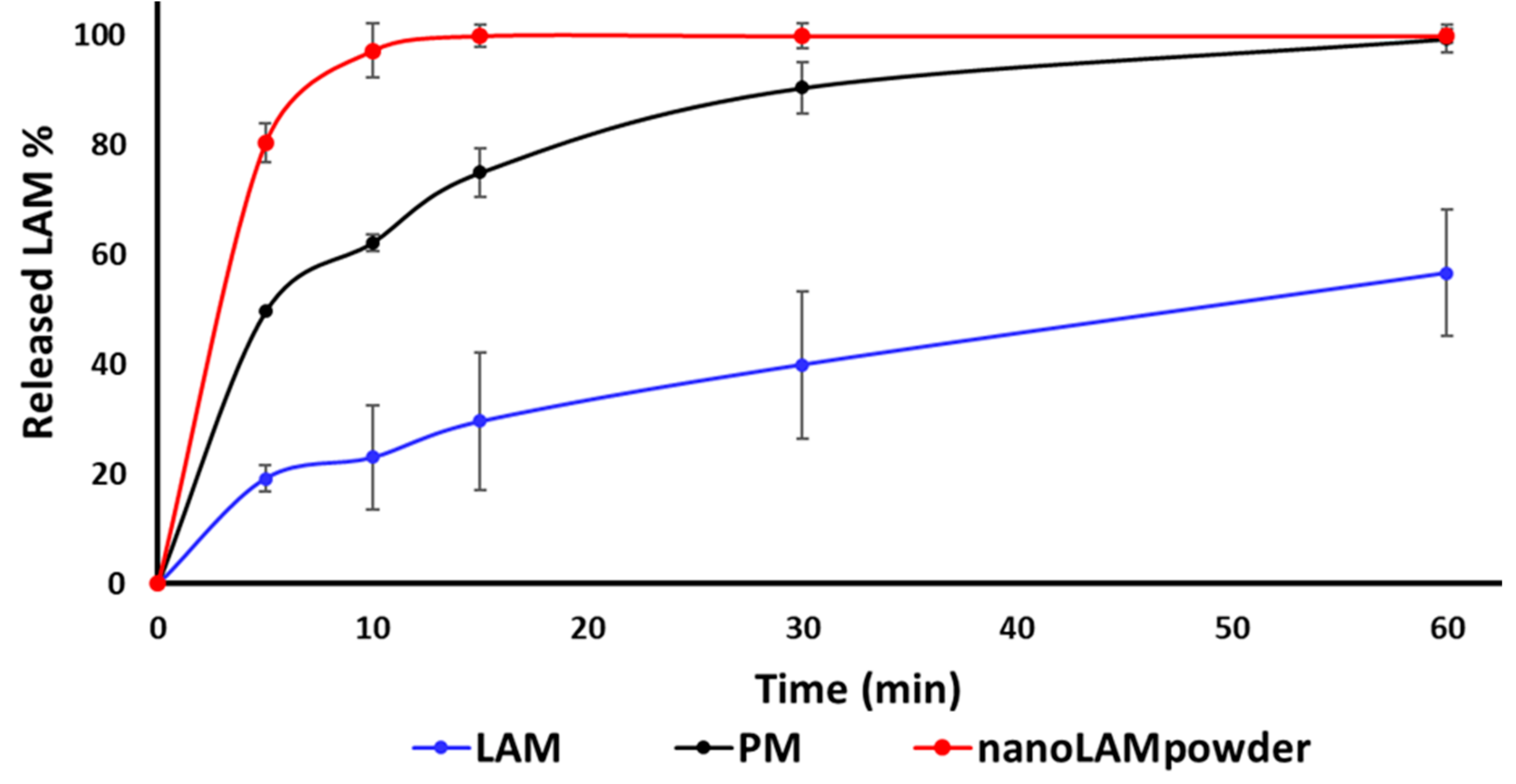
2.3. In Vitro Dissolution Study
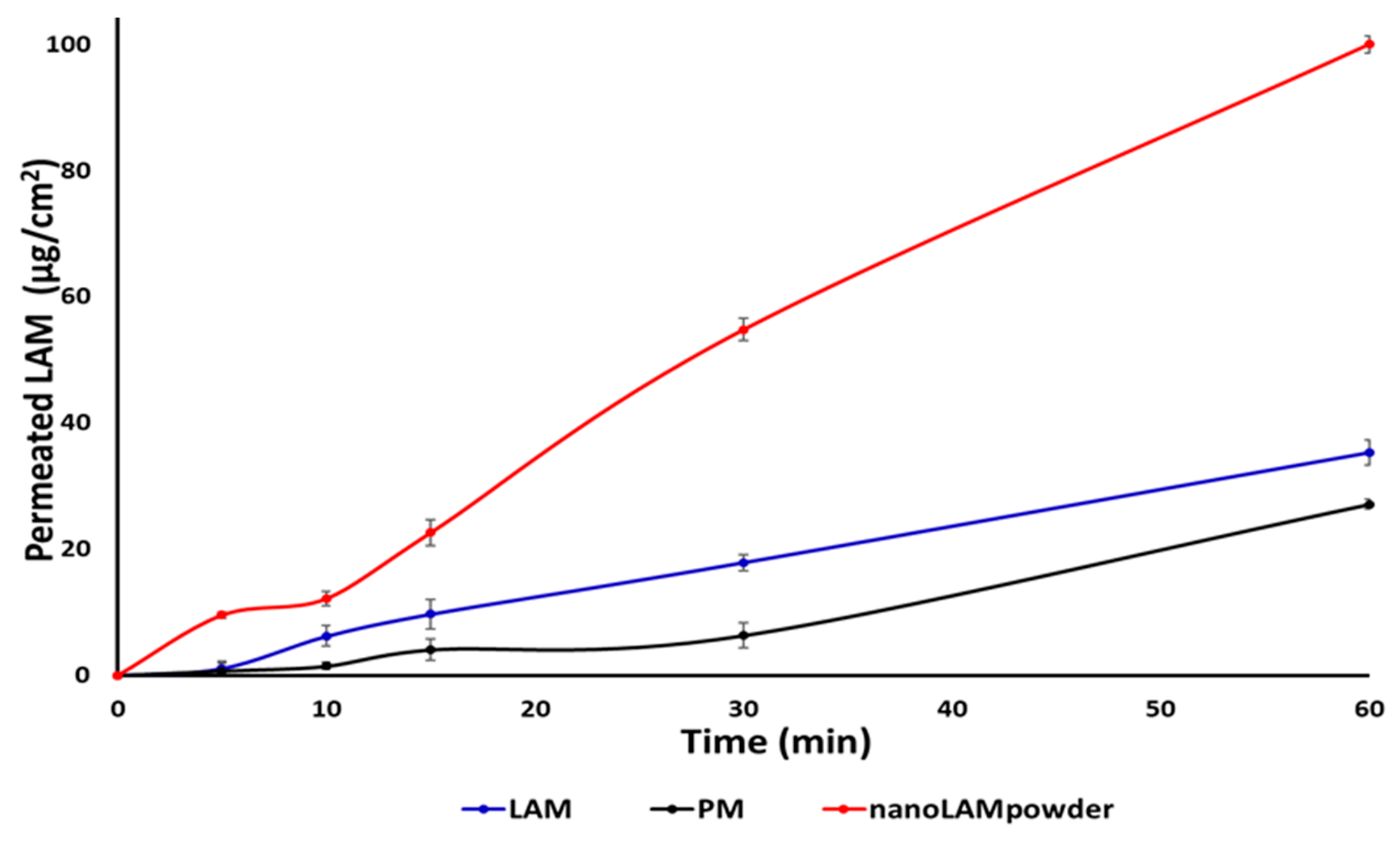
2.4. In Vitro Permeability Study
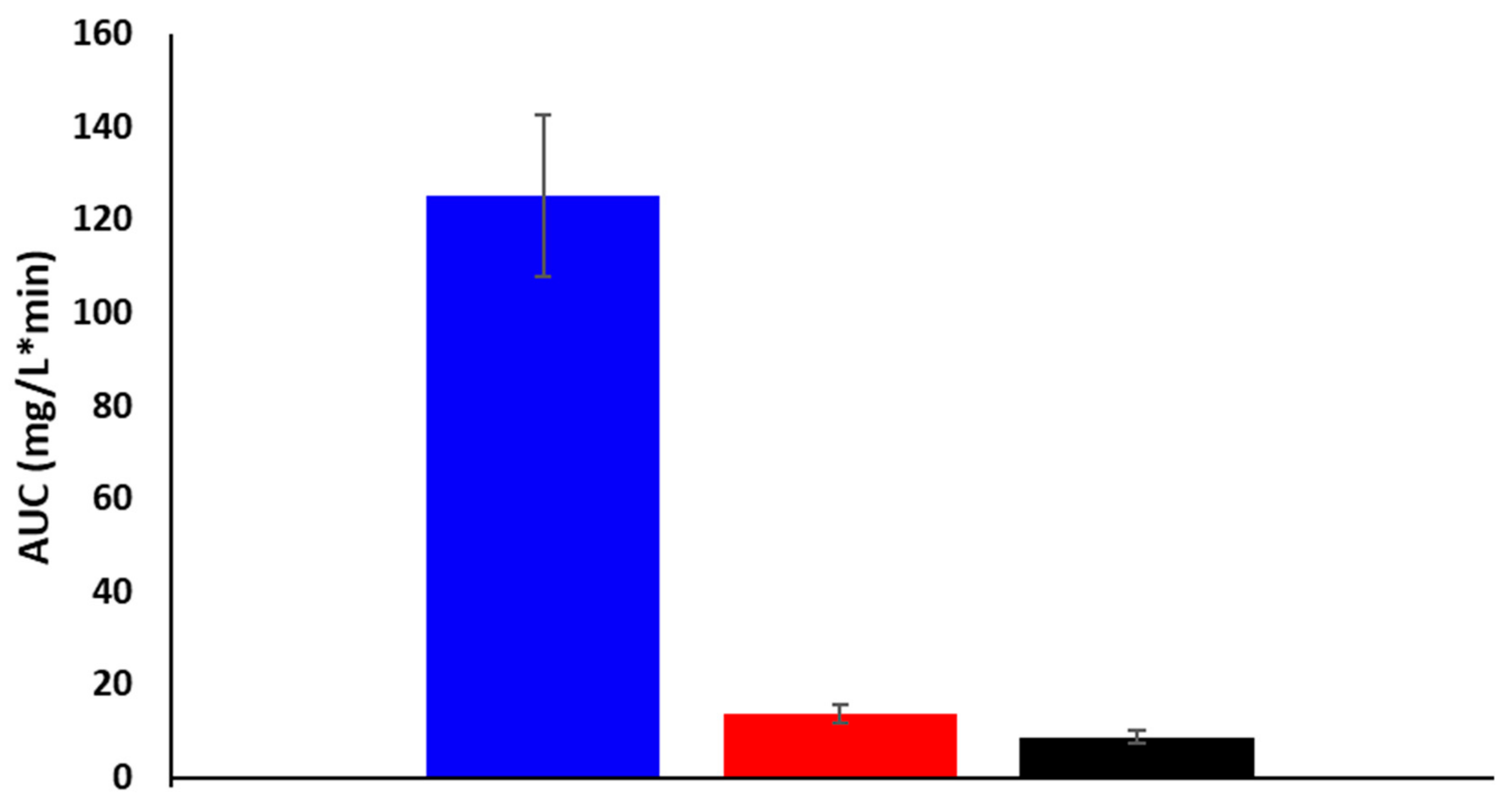
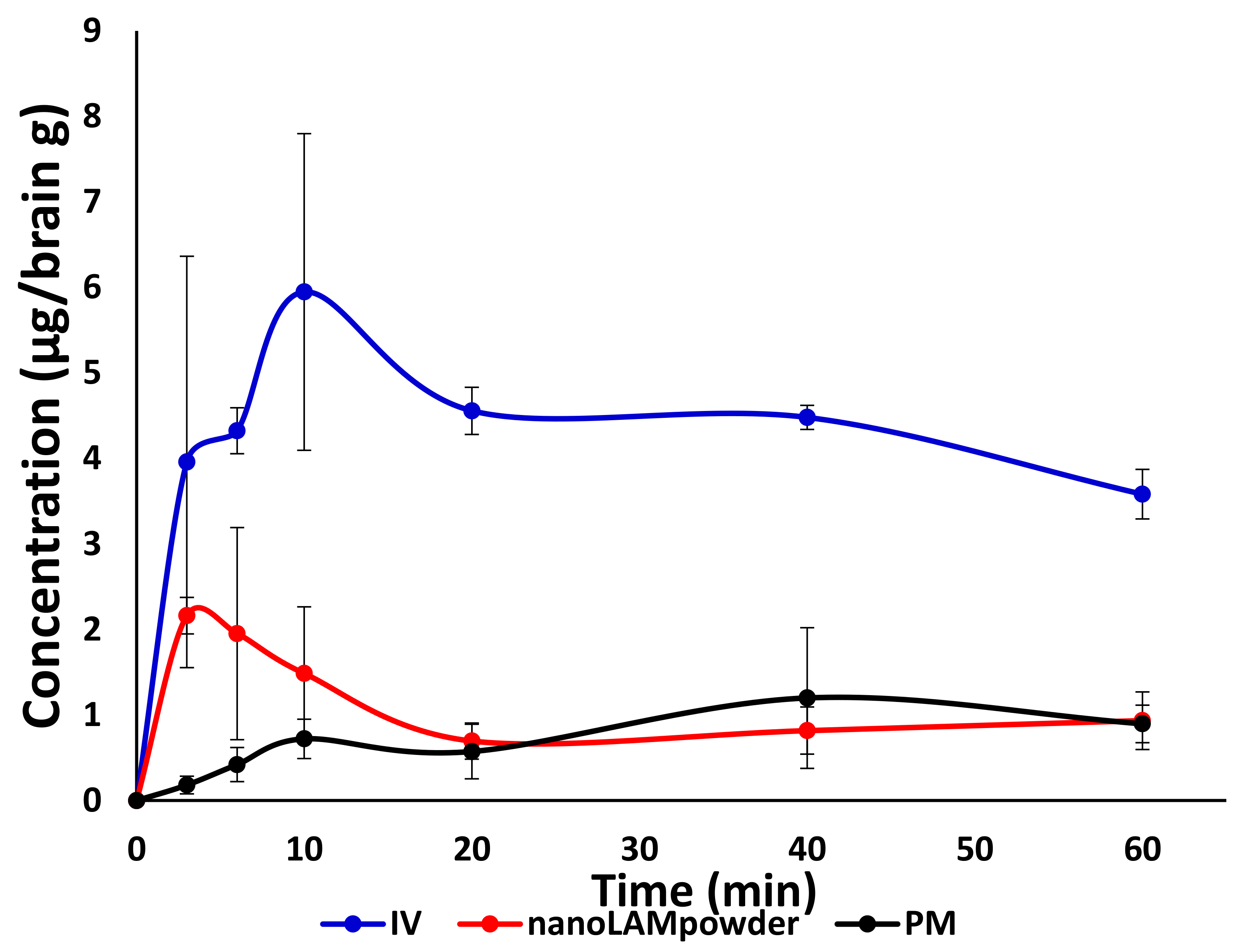
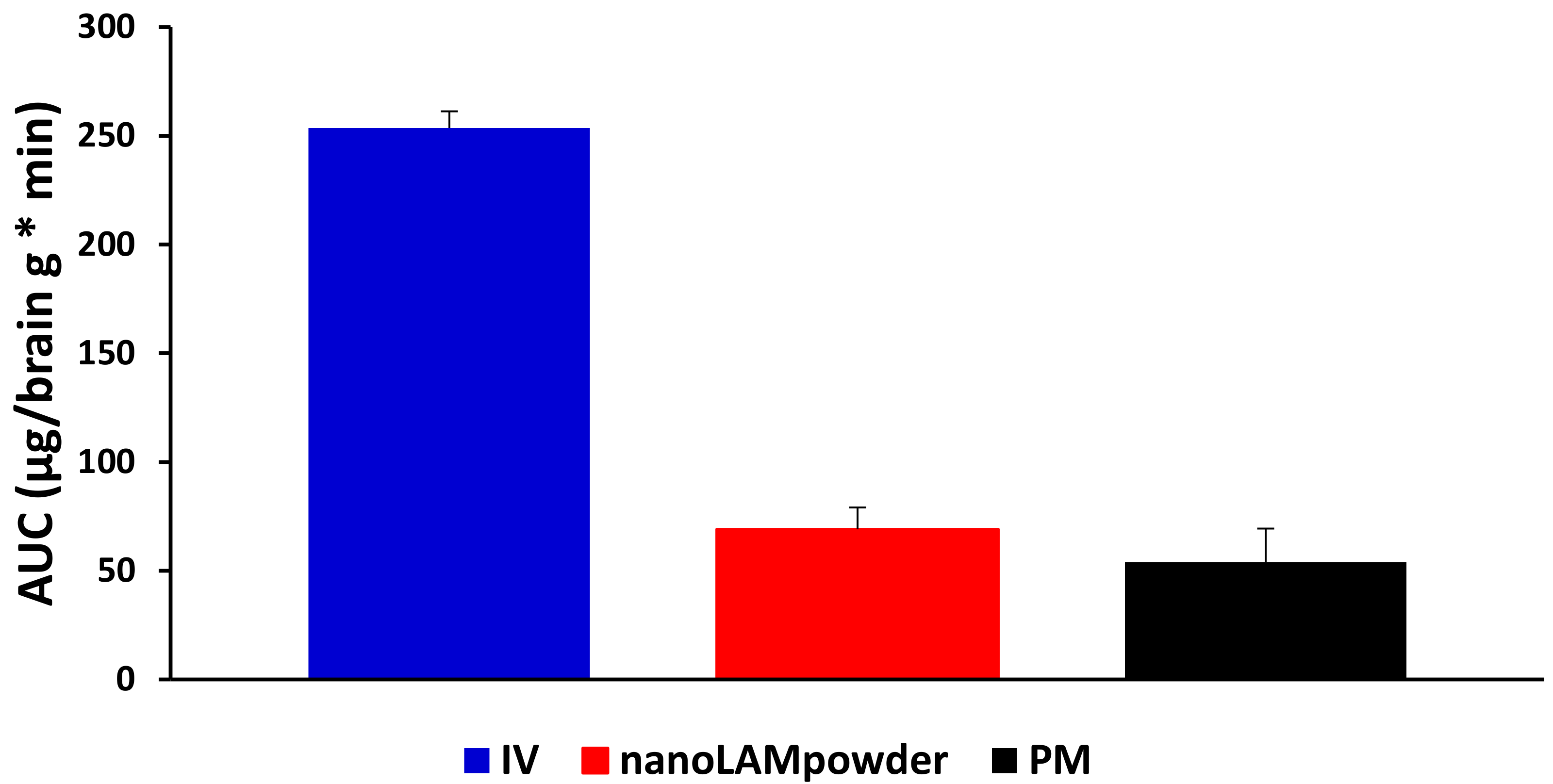
2.5. In Vivo Studies
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.3. Determination of Particle Size and Image Analysis (SEM)
3.4. Structural Investigations
3.4.1. Differential scanning calorimetry (DSC)
3.4.2. X-Ray Powder Diffraction (XRPD)
3.5. In Vitro Dissolution Study
3.6. In Vitro Permeability Study
3.7. In Vivo Studies
3.7.1. IN Administration, Blood Sample Collection, and Brain Removal
3.7.2. Plasma Sample Preparation
3.7.3. Brain Tissue Sample Preparation
3.7.4. LC-MS/MS
3.7.5. Sample Preparation of Rat Plasma and Brain
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nasare, L.; Niranjane, K.; Nagdevte, A.; Mohril, S. Nasal Drug Delivery System: An Emerging Approach for Brain Targeting. World J. Pharm. Pharmaceut. Sci. 2014, 3, 539–553. [Google Scholar]
- Prommer, E.; Thompson, L. Intranasal fentanyl for pain control: Current status with a focus on patient considerations. Patient Prefer. Adherence 2011, 5, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Penke, Z.; Sipos, E.; Kurunczi, A.; Ku, L.; Veszelka, S.; Penke, B.; Szabo, P. Intranasal Delivery of Human b-Amyloid Peptide in Rats: Effective Brain Targeting. Cell. Mol. Neurobiol. 2010, 405–413. [Google Scholar]
- Sandri, G.; Bonferoni, M.C.; Rossi, S.; Ferrari, F.; Gibin, S.; Zambito, Y.; Di Colo, G.; Caramella, C. Nanoparticles based on N-trimethylchitosan: Evaluation of absorption properties using in vitro (Caco-2 cells) and ex vivo (excised rat jejunum) models. Eur. J. Pharm. Biopharm. 2007, 65, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Horvát, S.; Fehér, A.; Wolburg, H.; Sipos, P.; Veszelka, S.; Tóth, A.; Kis, L.; Kurunczi, A.; Balogh, G.; Kürti, L.; et al. Sodium hyaluronate as a mucoadhesive component in nasal formulation enhances delivery of molecules to brain tissue. Eur. J. Pharm. Biopharm. 2009, 72, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Warnken, Z.N.; Smyth, H.D.C.; Watts, A.B.; Weitman, S.; Kuhn, J.G.; Williams, R.O. Formulation and device design to increase nose to brain drug delivery. J. Drug Deliv. Sci. Technol. 2016, 35, 213–222. [Google Scholar] [CrossRef]
- Kürti, L.; Gáspár, R.; Márki, Á.; Kápolna, E.; Bocsik, A.; Veszelka, S.; Bartos, C.; Ambrus, R.; Vastag, M.; Deli, M.A.; et al. In vitro and in vivo characterization of meloxicam nanoparticles designed for nasal administration. Eur. J. Pharm. Sci. 2013, 50, 86–92. [Google Scholar] [CrossRef]
- Sun, K.; Yu, X.; Wang, A.; Wu, Z.; Bi, C.; Yan, X.; Chu, L.; Duan, D.; Xu, L. Lactoferrin-modified rotigotine nanoparticles for enhanced nose-to-brain delivery: LESA-MS/MS-based drug biodistribution, pharmacodynamics, and neuroprotective effects. Int. J. Nanomed. 2018, Volume 13, 273–281. [Google Scholar]
- Shinde, R.L.; Bharkad, G.P.; Devarajan, P.V. Intranasal microemulsion for targeted nose to brain delivery in neurocysticercosis: Role of docosahexaenoic acid. Eur. J. Pharm. Biopharm. 2015, 96, 363–379. [Google Scholar] [CrossRef]
- Yasir, M.; Sara, U.V.S. Solid lipid nanoparticles for nose to brain delivery of haloperidol: In vitro drug release and pharmacokinetics evaluation. Acta Pharm. Sin. B 2014, 4, 454–463. [Google Scholar] [CrossRef]
- Patel, M.R.; Patel, R.B.; Bhatt, K.K.; Patel, B.G.; Gaikwad, R.V. Paliperidone microemulsion for nose-to-brain targeted drug delivery system: Pharmacodynamic and pharmacokinetic evaluation. Drug Deliv. 2016, 23, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.; Singh, M.; Ranjan, O.P.; Nayak, Y.; Garg, S.; Shavi, G.V.; Nayak, U.Y. Development of risperidone liposomes for brain targeting through intranasal route. Life Sci. 2016, 163, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Gavini, E.; Hegge, A.B.; Rassu, G.; Sanna, V.; Testa, C.; Pirisino, G.; Karlsen, J.; Giunchedi, P. Nasal administration of Carbamazepine using chitosan microspheres: In vitro/in vivo studies. Int. J. Pharm. 2006, 307, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Mishra, N. Importance of Lipid Nanoparticles in the Treatment of Epilepsy: A Focus on Nasal Delivery. J. Pharm. Sci. Innov. 2014, 3, 199–207. [Google Scholar] [CrossRef]
- Chen, R.P. From Nose to Brain: The Promise of Peptide Therapy for Alzheimer’s Disease and Other Neurodegenerative Diseases. J. Alzheimer’s Dis. Parkinsonism 2017, 7, 2–4. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.R.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Intranasal Piperine-Loaded Chitosan Nanoparticles as Brain-Targeted Therapy in Alzheimer’s Disease: Optimization, Biological Efficacy, and Potential Toxicity. J. Pharm. Sci. 2015, 104, 3544–3556. [Google Scholar] [CrossRef]
- Muntimadugu, E.; Dhommati, R.; Jain, A.; Challa, V.G.S.; Shaheen, M.; Khan, W. Intranasal delivery of nanoparticle encapsulated tarenflurbil: A potential brain targeting strategy for Alzheimer’s disease. Eur. J. Pharm. Sci. 2016, 92, 224–234. [Google Scholar] [CrossRef]
- Agrawal, M.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Chougule, M.B.; Shoyele, S.A.; Alexander, A. Nose-to-brain drug delivery: An update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J. Control. Release 2018, 281, 139–177. [Google Scholar] [CrossRef]
- Vasa, D.M.; Buckner, I.S.; Cavanaugh, J.E.; Wildfong, P.L.D. Improved Flux of Levodopa via Direct Deposition of Solid Microparticles on Nasal Tissue. AAPS Pharmscitech 2017, 18, 904–912. [Google Scholar] [CrossRef]
- Rinaldi, F.; Seguella, L.; Gigli, S.; Hanieh, P.N.; Del Favero, E.; Cantù, L.; Pesce, M.; Sarnelli, G.; Marianecci, C.; Esposito, G.; et al. Pentasomes: An innovative nose-to-brain pentamidine delivery blunts MPTP parkinsonism in mice. J. Control. Release 2019, 294, 17–26. [Google Scholar] [CrossRef]
- Gavini, E.; Rassu, G.; Ferraro, L.; Beggiato, S.; Alhalaweh, A.; Velaga, S.; Marchetti, N.; Bandiera, P.; Giunchedi, P.; Dalpiaz, A. Influence of polymeric microcarriers on the in vivo intranasal uptake of an anti-migraine drug for brain targeting. Eur. J. Pharm. Biopharm. 2013, 83, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Scherließ, R.; Mönckedieck, M.; Young, K.; Trows, S.; Buske, S.; Hook, S. First in vivo evaluation of particulate nasal dry powder vaccine formulations containing ovalbumin in mice. Int. J. Pharm. 2015, 479, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Trows, S.; Scherließ, R. Carrier-based dry powder formulation for nasal delivery of vaccines utilizing BSA as model drug. Powder Technol. 2016, 292, 223–231. [Google Scholar] [CrossRef]
- Sabir, F.; Ismail, R.; Csoka, I. Nose-to-brain delivery of antiglioblastoma drugs embedded into lipid nanocarrier systems: Status quo and outlook. Drug Discov. Today 2019. [Google Scholar] [CrossRef] [PubMed]
- Mistry, A.; Stolnik, S.; Illum, L. Nanoparticles for direct nose-to-brain delivery of drugs. Int. J. Pharm. 2009, 379, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Bartos, C.; Ambrus, R.; Kovács, A.; Gáspár, R.; Sztojkov-Ivanov, A.; Márki, Á.; Janáky, T.; T m si, F.; Kecskeméti, G.; Szabó-Révész, P. Investigation of absorption routes of meloxicam and its salt form from intranasal delivery systems. Molecules 2018, 23, 784. [Google Scholar] [CrossRef]
- Horvath, T.; Ambrus, R.; Volgyi, G.; Budai-Szucs, M.; Marki, A.; Sipos, P.; Bartos, C.; Seres, A.B.; Sztojkov-Ivanov, A.; Takacs-Novak, K.; et al. Effect of solubility enhancement on nasal absorption of meloxicam. Eur. J. Pharm. Sci. 2016, 95, 96–102. [Google Scholar] [CrossRef][Green Version]
- Kublik, H.; Vidgren, M.T. Nasal delivery systems and their effect on deposition and absorption. Adv. Drug Deliv. Rev. 1998, 29, 157–177. [Google Scholar] [CrossRef]
- Karashima, M.; Sano, N.; Yamamoto, S.; Arai, Y.; Yamamoto, K.; Amano, N.; Ikeda, Y. Enhanced pulmonary absorption of poorly soluble itraconazole by micronized cocrystal dry powder formulations. Eur. J. Pharm. Biopharm. 2017, 115, 65–72. [Google Scholar] [CrossRef]
- Parumasivam, T.; Chang, R.Y.K.; Abdelghany, S.; Ye, T.T.; Britton, W.J.; Chan, H.K. Dry powder inhalable formulations for anti-tubercular therapy. Adv. Drug Deliv. Rev. 2016, 102, 83–101. [Google Scholar] [CrossRef]
- Tiozzo Fasiolo, L.; Manniello, M.D.; Tratta, E.; Buttini, F.; Rossi, A.; Sonvico, F.; Bortolotti, F.; Russo, P.; Colombo, G. Opportunity and challenges of nasal powders: Drug formulation and delivery. Eur. J. Pharm. Sci. 2018, 113, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Furubayashi, T.; Tomisaki, M.; Kawakami, M.; Kimura, S.; Inoue, D.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Nasal drug absorption from powder formulations: The effect of three types of hydroxypropyl cellulose (HPC). Eur. J. Pharm. Sci. 2017, 96, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.; Bortolotti, F.; Chiapponi, V.; Buttini, F.; Sonvico, F.; Invernizzi, R.; Quaglia, F.; Danesino, C.; Pagella, F.; Russo, P.; et al. Nasal powders of thalidomide for local treatment of nose bleeding in persons affected by hereditary hemorrhagic telangiectasia. Int. J. Pharm. 2016, 514, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Gieszinger, P.; Csóka, I.; Pallagi, E.; Katona, G.; Jójárt-Laczkovich, O.; Szabó-Révész, P.; Ambrus, R. Preliminary study of nanonized lamotrigine containing products for nasal powder formulation. Drug Des. Dev. Ther. 2017, 11, 2453–2466. [Google Scholar] [CrossRef] [PubMed]
- Gieszinger, P.; Tomuta, I.; Casian, T.; Bartos, C.; Szabó-Révész, P.; Ambrus, R. Definition and validation of the Design Space for co-milled nasal powder containing nanosized lamotrigine. Drug Dev. Ind. Pharm. 2018, 44, 1622–1630. [Google Scholar] [CrossRef]
- Mohana Raghava Srivalli, K.; Lakshmi, P.K.; Balasubramaniam, J. Design of a novel bilayered gastric mucoadhesive system for localized and unidirectional release of lamotrigine. Saudi Pharm. J. 2013, 21, 45–52. [Google Scholar] [CrossRef]
- Juenke, J.M.; Miller, K.A.; Ford, M.A.; McMillin, G.A.; Johnson-Davis, K.L. A comparison of two FDA approved lamotrigine immunoassays with ultra-high performance liquid chromatography tandem mass spectrometry. Clin. Chim. Acta 2011, 412, 1879–1882. [Google Scholar] [CrossRef]
- Saberi, R.-S.; Shahrokhian, S. Highly sensitive voltammetric determination of lamotrigine at highly oriented pyrolytic graphite electrode. Bioelectrochemistry 2012, 84, 38–43. [Google Scholar] [CrossRef]
- Lukic, S.; Jovic, N.; Milosevic, Z.; Bjelakovic, B.; Peric, Z.; Spasic, M. Outcome prediction of initial lamotrigine monotherapy in adult patients with newly diagnosed localization related epilepsies. Epilepsy Res. 2014, 108, 295–304. [Google Scholar] [CrossRef]
- Rani, A.P.; Veesam, H. Full factorial design in formulation of lamotrigine suspension using locust bean gum. Int. J. Chem. Sci. 2013, 11, 751–760. [Google Scholar]
- Shinde, V.R.; Shelake, M.R.; Shetty, S.S.; Chavan-Patil, A.B.; Pore, Y.V.; Late, S.G. Enhanced solubility and dissolution rate of lamotrigine by inclusion complexation and solid dispersion technique. J. Pharm. Pharmacol. 2008, 60, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Soltanpour, S.; Jouyban, A. Solubility of lamotrigine in binary and ternary mixtures of N-methyl pyrrolidone and water with polyethylene glycols 200, 400, and 600 at 298.2K. J. Mol. Liq. 2013, 180, 1–6. [Google Scholar] [CrossRef]
- Lalani, J.; Patil, S.; Kolate, A.; Lalani, R.; Misra, A. Protein-Functionalized PLGA Nanoparticles of Lamotrigine for Neuropathic Pain Management. AAPS Pharmscitech 2015, 16, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Serralheiro, A.; Alves, G.; Fortuna, A.; Falcão, A. Direct nose-to-brain delivery of lamotrigine following intranasal administration to mice. Int. J. Pharm. 2015, 490, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Garnett, W.R. Lamotrigine: Pharmacokinetics. J. Child. Neurol. 1997, 12, S10–S15. [Google Scholar] [CrossRef]
- EMC. Available online: https://www.medicines.org.uk/emc/product/6093/smpc (accessed on 26 February 2020).
- Rault, J.; Gref, R.; Ping, Z.H.; Nguyen, Q.T.; Néel, J. Glass transition temperature regulation effect in a poly(vinyl alcohol)-water system. Polymer 1995, 36, 1655–1661. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| Particle Size of Product (µm) | Particle Size of LAM in the Products (nm) | |
|---|---|---|
| LAM | 6.57 ± 5.59 | - |
| PM | 16.39 ± 7.17 | Aggregated |
| nanoLAMpowder | 29.91 ± 15.85 | 97 ± 60 |
| J (µg/cm2/h) | Kp (cm/h) | |
|---|---|---|
| LAM | 35.29 | 0.011 |
| PM | 27.11 | 0.008 |
| nanoLAMpowder | 100 | 0.030 |
| abs. BA for brain (%) | AUCbrain/AUCblood | DTE | |
|---|---|---|---|
| IV injection | 100 | 2.02 | 1 |
| nano LAM powder | 39.84 | 5.06 | 2.49 |
| PM | 21.30 | 6.29 | 3.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrus, R.; Gieszinger, P.; Gáspár, R.; Sztojkov-Ivanov, A.; Ducza, E.; Márki, Á.; Janáky, T.; Tömösi, F.; Kecskeméti, G.; Szabó-Révész, P.; et al. Investigation of the Absorption of Nanosized lamotrigine Containing Nasal Powder via the Nasal Cavity. Molecules 2020, 25, 1065. https://doi.org/10.3390/molecules25051065
Ambrus R, Gieszinger P, Gáspár R, Sztojkov-Ivanov A, Ducza E, Márki Á, Janáky T, Tömösi F, Kecskeméti G, Szabó-Révész P, et al. Investigation of the Absorption of Nanosized lamotrigine Containing Nasal Powder via the Nasal Cavity. Molecules. 2020; 25(5):1065. https://doi.org/10.3390/molecules25051065
Chicago/Turabian StyleAmbrus, Rita, Péter Gieszinger, Róbert Gáspár, Anita Sztojkov-Ivanov, Eszter Ducza, Árpád Márki, Tamás Janáky, Ferenc Tömösi, Gábor Kecskeméti, Piroska Szabó-Révész, and et al. 2020. "Investigation of the Absorption of Nanosized lamotrigine Containing Nasal Powder via the Nasal Cavity" Molecules 25, no. 5: 1065. https://doi.org/10.3390/molecules25051065
APA StyleAmbrus, R., Gieszinger, P., Gáspár, R., Sztojkov-Ivanov, A., Ducza, E., Márki, Á., Janáky, T., Tömösi, F., Kecskeméti, G., Szabó-Révész, P., & Bartos, C. (2020). Investigation of the Absorption of Nanosized lamotrigine Containing Nasal Powder via the Nasal Cavity. Molecules, 25(5), 1065. https://doi.org/10.3390/molecules25051065








