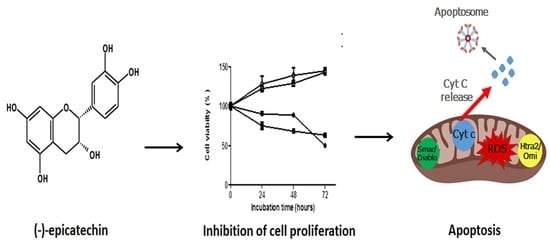
Apoptosis Induced by (−)-Epicatechin in Human Breast Cancer Cells is Mediated by Reactive Oxygen Species
Abstract
1. Introduction
2. Results
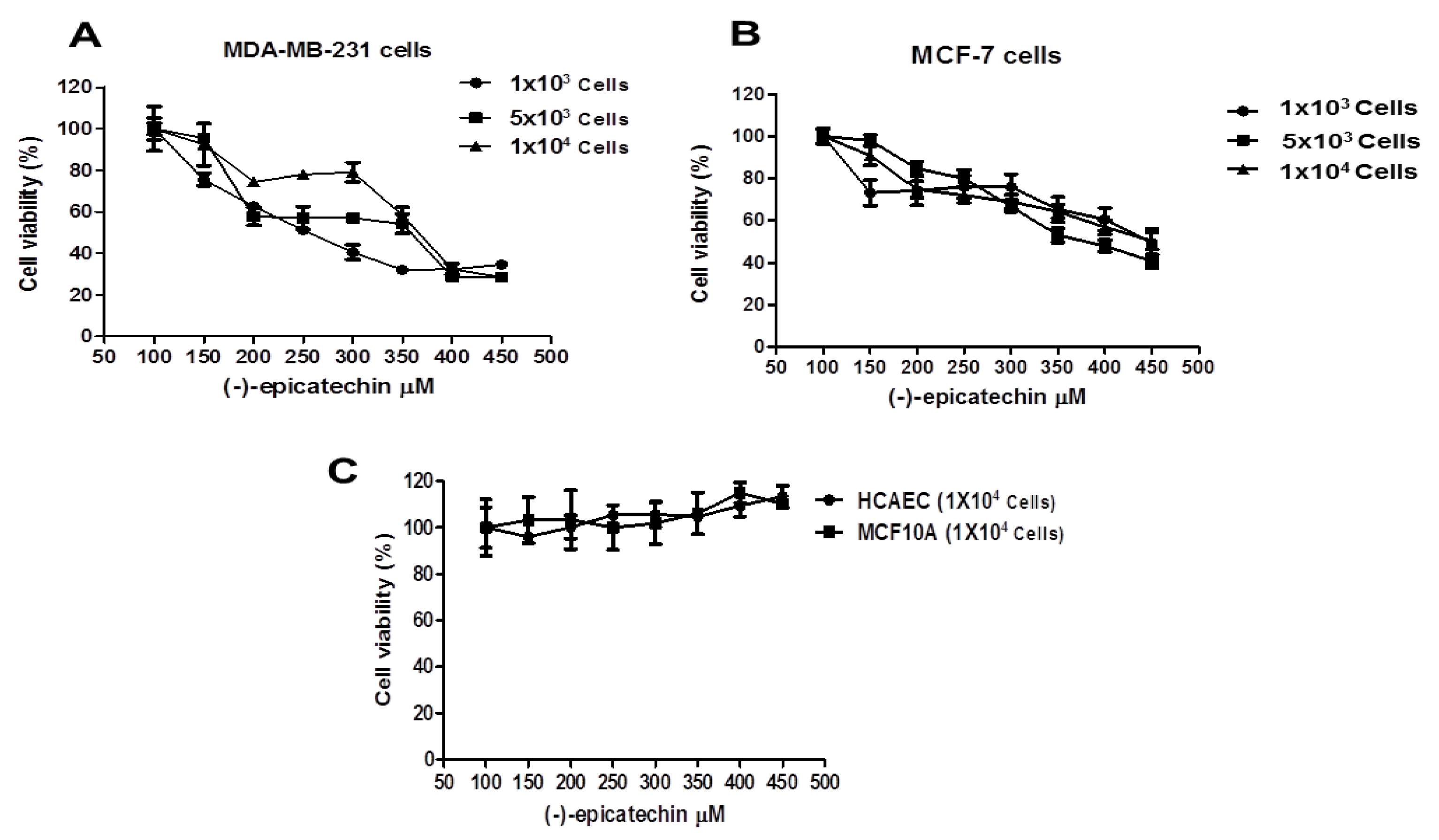
2.1. The Cell Viability of MDA-MB-231 and MCF-7 Cells is Inhibited by (–)-Epicatechin, Dependent on Its Concentration, and Independent of the Seeded Cell Number
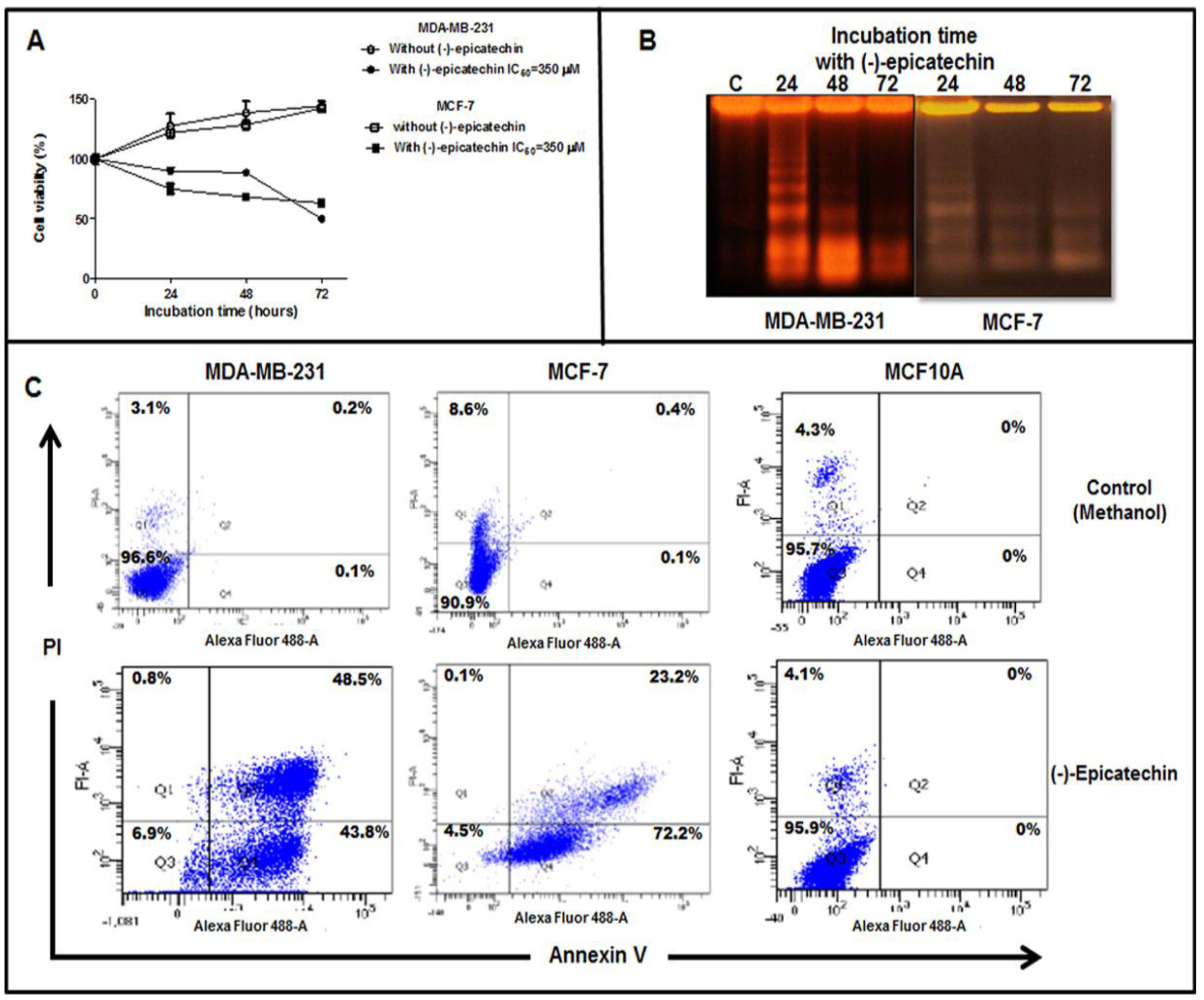
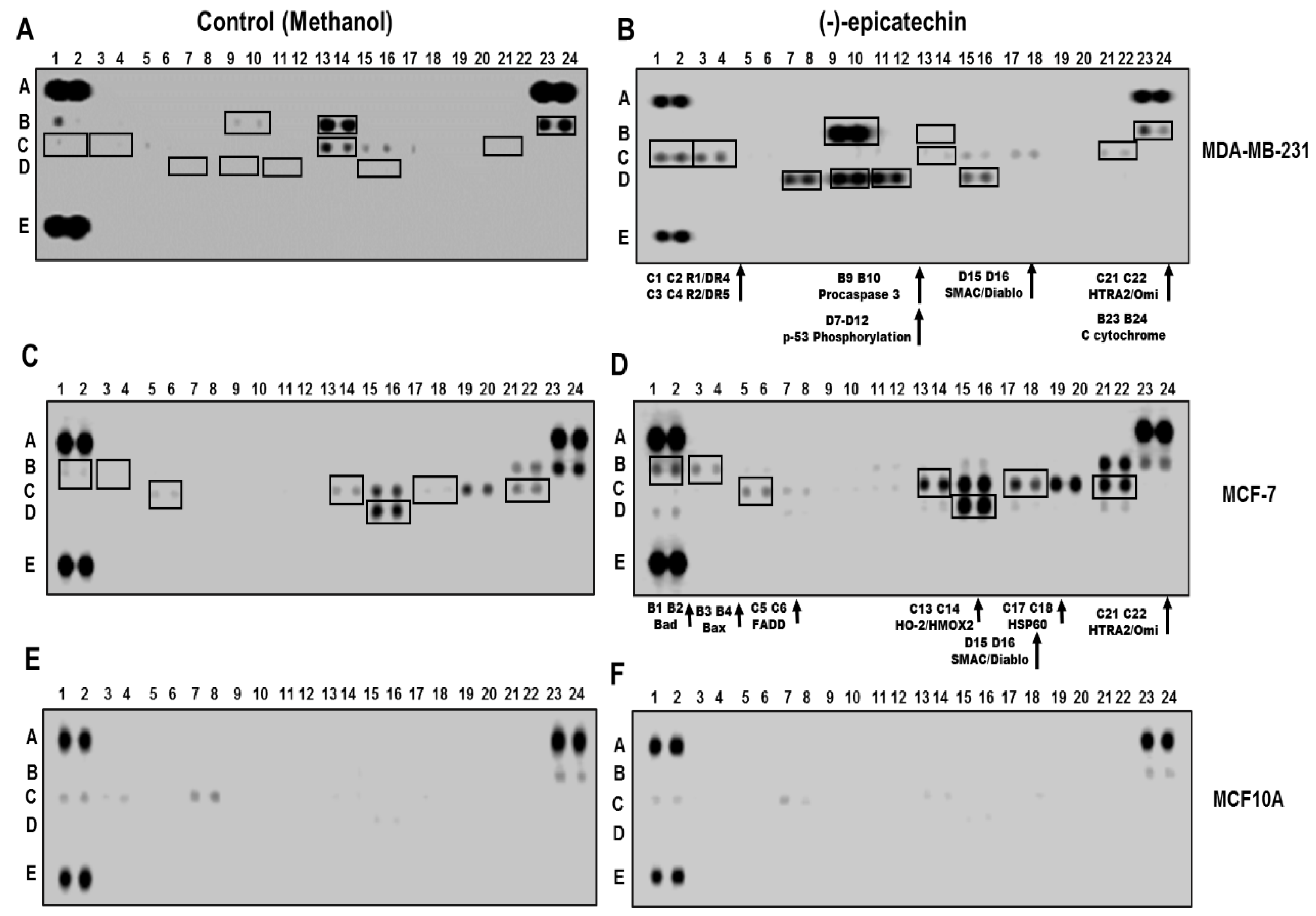
2.2. (−)-Epicatechin Induces Apoptosis in MDA-MB-231 and MCF-7 Breast Cancer Cells
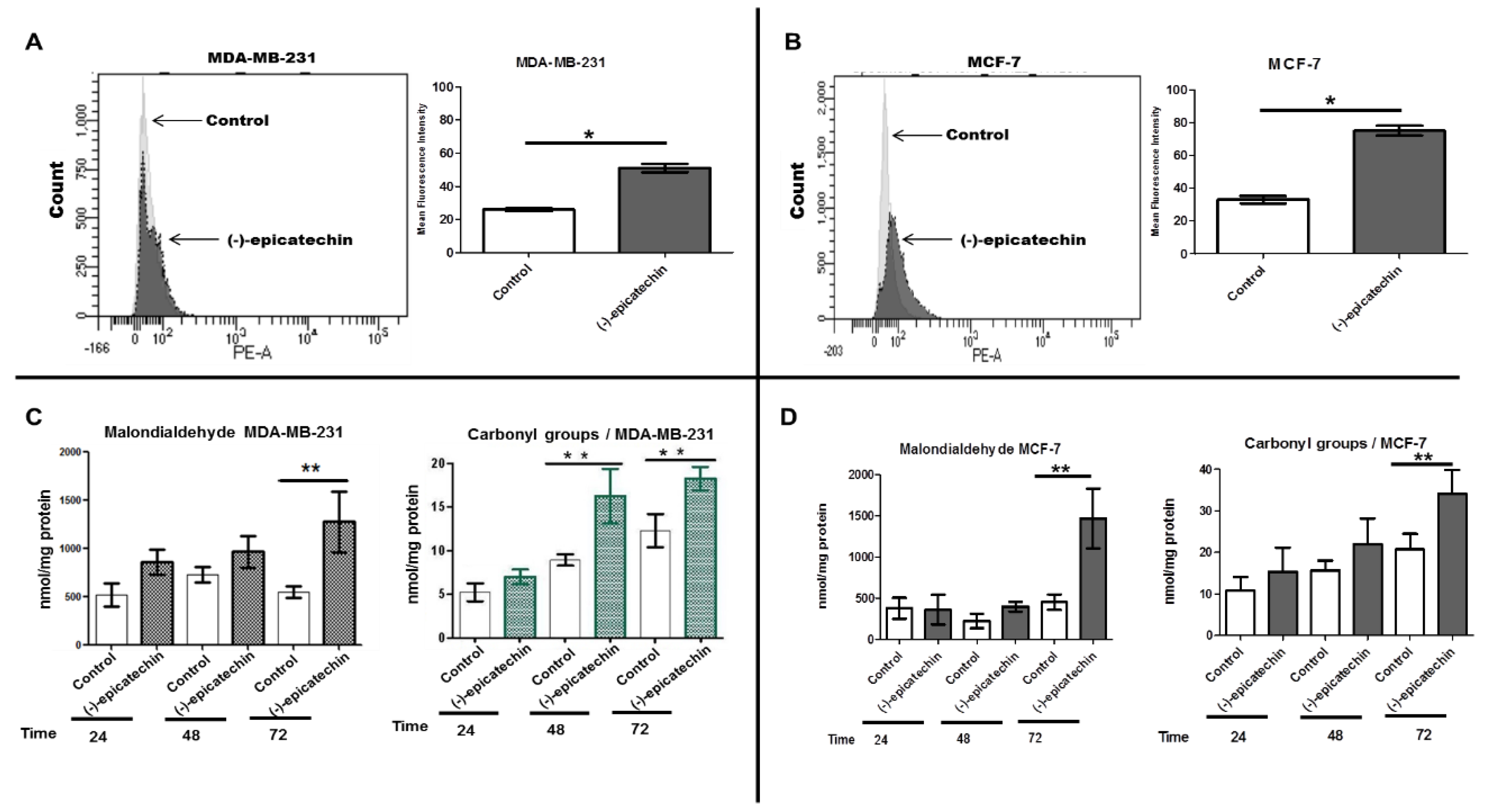
2.3. (−)-Epicatechin Induces ROS Production and Oxidative Damage in Breast Cancer Cells
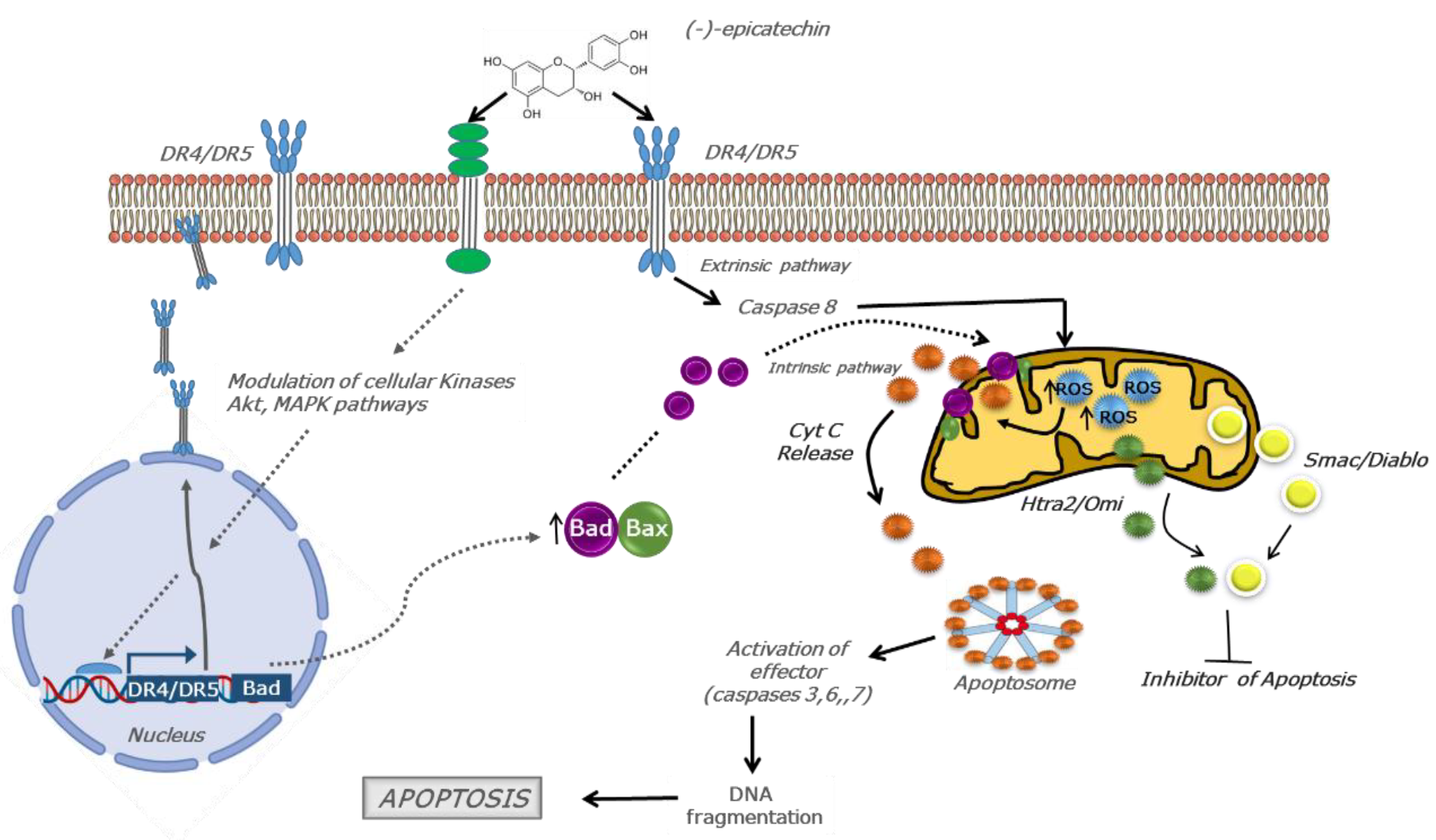
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and Treatment
4.3. Cell Proliferation Assays
4.4. Assessment of Apoptosis by DNA Fragmentation
4.5. Quantification of Cell Apoptosis by Annexin VFITC/PI Staining
4.6. Human Apoptosis Antibody Array
4.7. Measurement of Reactive Oxygen Species (ROS) and Biomarkers of Oxidative Damage
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2018, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Golubnitschaja, O.; Debald, M.; Yeghiazaryan, K.; Kuhn, W.; Pešta, M.; Costigliola, V.; Grech, G. Breast cancer epidemic in the early twenty-first century: Evaluation of risk factors, cumulative questionnaires and recommendations for preventive measures. Tumor Boil. 2016, 37, 12941–12957. [Google Scholar] [CrossRef]
- Liu, R.H. Dietary Bioactive Compounds and Their Health Implications. J. Food Sci. 2013, 78, A18–A25. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Boyd, L.; Walker, L.C. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br. J. Cancer 2011, 105, S77–S81. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Wang, H. Cancer Preventive Activities of Tea Catechins. Molecules 2016, 21, 1679. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, G.; Fan, B.; Zhou, Y.; Zhou, X.; Wei, L.; Zhang, J. Green tea (−)-epigallocatechin-3-gallate down-regulates VASP expression and inhibits breast cancer cell migration and invasion by attenuating Rac1 activity. Eur. J. Pharmacol. 2009, 606, 172–179. [Google Scholar] [CrossRef]
- Khokhar, S.; Magnusdottir, S.G. Total phenol, catechin, and caeine contents of teas commonly consumed in the United Kingdom. J. Agric. Food Chem. 2002, 50, 565–570. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar] [CrossRef]
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Kim, H.-S.; Quon, M.; Kim, J. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Boil. 2014, 2, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Webster, N.; Cao, J.; Shao, A. The safety of green tea and green tea extract consumption in adults – Results of a systematic review. Regul. Toxicol. Pharmacol. 2018, 95, 412–433. [Google Scholar] [CrossRef] [PubMed]
- Iacopini, P.; Baldi, M.; Storchi, P.; Sebastiani, L. Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. J. Food Compos. Anal. 2008, 21, 589–598. [Google Scholar] [CrossRef]
- Azizah, O.; Abbe, M.M.J.; Kong, K.W.; Amin, I.; Nawalyah, A.G.; Ilham, A.; Othman, A.; Jalil, A.M.M.; Weng, K.K.; Ismail, A.; et al. Epicatechin content and antioxidant capacity of cocoa beans from four different countries. Afr. J. Biotechnol. 2010, 9, 1052–1059. [Google Scholar] [CrossRef]
- Vasconcelos, P.C.D.P.; Seito, L.N.; Di Stasi, L.C.; Hiruma-Lima, C.A.; Pellizzon, C. Epicatechin Used in the Treatment of Intestinal Inflammatory Disease: An Analysis by Experimental Models. Evidence-Based Complement. Altern. Med. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (–)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef]
- Kim, D.; Mollah, M.L.; Kim, K. Induction of apoptosis of SW480 human colon cancer cells by (-)-epicatechin isolated from Bulnesia sarmienti. Anticancer. Res. 2012, 32, 5353–5362. [Google Scholar]
- Papież, M.; Baran, J.; Bukowska-Straková, K.; Wiczkowski, W. Antileukemic action of (−)-epicatechin in the spleen of rats with acute myeloid leukemia. Food Chem. Toxicol. 2010, 48, 3391–3397. [Google Scholar] [CrossRef]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Hüttemann, M. Molecular Mechanisms and Therapeutic Effects of (−)-Epicatechin and Other Polyphenols in Cancer, Inflammation, Diabetes, and Neurodegeneration. Oxidative Med. Cell. Longev. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Luca, S.V.; Miron, A. Flavor Chemistry of Cocoa and Cocoa Products-An Overview. Compr. Rev. Food Sci. Food Saf. 2015, 15, 73–91. [Google Scholar] [CrossRef]
- Odle, T.G. Adverse effects of breast cancer treatment. Radiol. Technol. 2014, 85, 297–319. [Google Scholar]
- Escandón, R.A.; Del Campo, M.; López-Solis, R.; Obreque-Slier, E.; Toledo, H. Antibacterial effect of kaempferol and (−)- epicatechin on Helicobacter pyroli. Eur Food Res. Technol. 2016, 242, 1495–1502. [Google Scholar] [CrossRef]
- I Dower, J.; Geleijnse, J.M.; Gijsbers, L.; Zock, P.; Kromhout, D.; Hollman, P.C. Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: A randomized, double-blind, placebo-controlled, crossover trial. Am. J. Clin. Nutr. 2015, 101, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.J.; Choudhry, F.; Peacey, E.; Perkinton, M.S.; Richardson, J.C.; Howlett, D.R.; Lichtenthaler, S.F.; Francis, P.; Williams, R. Dietary (−)-epicatechin as a potent inhibitor of βγ-secretase amyloid precursor protein processing. Neurobiol. Aging 2014, 36, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Hwang-Bo, H.; Lee, W.S.; Nagappan, A.; Kim, H.J.; Panchanathan, R.; Park, C.; Chang, S.-H.; Kim, N.D.; Leem, S.; Chang, Y.; et al. Morin enhances auranofin anticancer activity by up-regulation of DR4 and DR5 and modulation of Bcl-2 through reactive oxygen species generation in Hep3B human hepatocellular carcinoma cells. Phytotherapy Res. 2019, 33, 1384–1393. [Google Scholar] [CrossRef]
- Gillissen, B.; Wendt, J.; Richter, A.; Richter, A.; Müer, A.; Overkamp, T.; Gebhardt, N.; Preissner, R.; Belka, C.; Dörken, B.; et al. Endogenous Bak inhibitors Mcl-1 and Bcl-xL: Differential impact on TRAIL resistance in Bax-deficient carcinoma. J. Cell Boil. 2010, 188, 851–862. [Google Scholar] [CrossRef]
- Quast, S.-A.; Berger, A.; Eberle, J. ROS-dependent phosphorylation of Bax by wortmannin sensitizes melanoma cells for TRAIL-induced apoptosis. Cell Death Dis. 2013, 4, e839. [Google Scholar] [CrossRef]
- Cain, K.; Bratton, S.B.; Cohen, G.M. The Apaf-1 apoptosome: A large caspase-activating complex. Biochimie 2002, 84, 203–214. [Google Scholar] [CrossRef]
- Yu, B.; Sun, X.; Shen, H.-Y.; Gao, F.; Fan, Y.-M.; Sun, Z.-J. Expression of the apoptosis-related genes BCL-2 and BAD in human breast carcinoma and their associated relationship with chemosensitivity. J. Exp. Clin. Cancer Res. 2010, 29, 107. [Google Scholar] [CrossRef]
- Jin, H.; Lee, W.S.; Eun, S.Y.; Jung, J.H.; Park, H.-S.; Kim, G.; Choi, Y.H.; Ryu, C.H.; Jung, J.M.; Hong, S.C.; et al. Morin, a flavonoid from Moraceae, suppresses growth and invasion of the highly metastatic breast cancer cell line MDA-MB-231 partly through suppression of the Akt pathway. Int. J. Oncol. 2014, 45, 1629–1637. [Google Scholar] [CrossRef]
- Peng, S.-F.; Lee, C.-Y.; Hour, M.-J.; Tsai, S.-C.; Kuo, D.-H.; Chen, F.-A.; Shieh, P.-C.; Yang, J.-S. Curcumin-loaded nanoparticles enhance apoptotic cell death of U2OS human osteosarcoma cells through the Akt-Bad signaling pathway. Int. J. Oncol. 2013, 44, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Lee, W.S.; Go, S.-I.; Nagappan, A.; Han, M.H.; Hong, S.H.; Kim, G.S.; Kim, G.Y.; Kwon, T.K.; Ryu, C.-H.; et al. Morin, a Flavonoid from Moraceae, Induces Apoptosis by Induction of BAD Protein in Human Leukemic Cells. Int. J. Mol. Sci. 2014, 16, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Zhang, L.; Duan, Y.; Zhang, M.; Wang, G.; Zhang, J.; Zhao, Z. The differential susceptibilities of MCF-7 and MDA-MB-231 cells to the cytotoxic effects of curcumin are associated with the PI3K/Akt-SKP2-Cip/Kips pathway. Cancer Cell Int. 2014, 14, 126. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.J.; Das Gupta, S.; Wahler, J.; Suh, N. Role of dietary bioactive natural products in estrogen receptor-positive breast cancer. Semin. Cancer Boil. 2016, 170–191. [Google Scholar] [CrossRef] [PubMed]
- Scherbakov, A.; Andreeva, O.E. Apigenin Inhibits Growth of Breast Cancer Cells: The Role of ERα and HER2/neu. Acta Naturae 2015, 7, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Kabała-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Iriti, M.; Wojtyczka, R.D.; Buszman, E.; Stojko, J. Flavonoids, bioactive components of propolis, exhibit cytotoxic activity and induce cell cycle arrest and apoptosis in human breast cancer cells MDA-MB-231 and MCF-7 – a comparative study. Cell. Mol. Boil. 2018, 64, 1–10. [Google Scholar] [CrossRef]
- Mirzapur, P.; Khazaei, M.R.; Moradi, M.T.; Khazaei, M. Apoptosis induction in human breast cancer cell lines by synergic effect of raloxifene and resveratrol through increasing proapoptotic genes. Life Sci. 2018, 205, 45–53. [Google Scholar] [CrossRef]
- Blandino, G.; Valenti, F.; Sacconi, A.; Di Agostino, S. Wild type- and mutant p53 proteins in mitochondrial dysfunction: Emerging insights in cancer disease. Semin. Cell Dev. Boil. 2020, 98, 105–117. [Google Scholar] [CrossRef]
- Jan, R.; Chaudhry, G.-E.-S. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Boil. Chem. 1951, 193, 265–275. [Google Scholar]
- Gérard-Monnier, D.; Erdelmeier, I.; Régnard, K.; Moze-Henry, N.; Yadan, J.-C.; Chaudière, J. Reactions of 1-Methyl-2-phenylindole with Malondialdehyde and 4-Hydroxyalkenals. Analytical Applications to a Colorimetric Assay of Lipid Peroxidation. Chem. Res. Toxicol. 1998, 11, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.D.G.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereyra-Vergara, F.; Olivares-Corichi, I.M.; Perez-Ruiz, A.G.; Luna-Arias, J.P.; García-Sánchez, J.R. Apoptosis Induced by (−)-Epicatechin in Human Breast Cancer Cells is Mediated by Reactive Oxygen Species. Molecules 2020, 25, 1020. https://doi.org/10.3390/molecules25051020
Pereyra-Vergara F, Olivares-Corichi IM, Perez-Ruiz AG, Luna-Arias JP, García-Sánchez JR. Apoptosis Induced by (−)-Epicatechin in Human Breast Cancer Cells is Mediated by Reactive Oxygen Species. Molecules. 2020; 25(5):1020. https://doi.org/10.3390/molecules25051020
Chicago/Turabian StylePereyra-Vergara, Fernando, Ivonne María Olivares-Corichi, Adriana Guadalupe Perez-Ruiz, Juan Pedro Luna-Arias, and José Rubén García-Sánchez. 2020. "Apoptosis Induced by (−)-Epicatechin in Human Breast Cancer Cells is Mediated by Reactive Oxygen Species" Molecules 25, no. 5: 1020. https://doi.org/10.3390/molecules25051020
APA StylePereyra-Vergara, F., Olivares-Corichi, I. M., Perez-Ruiz, A. G., Luna-Arias, J. P., & García-Sánchez, J. R. (2020). Apoptosis Induced by (−)-Epicatechin in Human Breast Cancer Cells is Mediated by Reactive Oxygen Species. Molecules, 25(5), 1020. https://doi.org/10.3390/molecules25051020