Microfluidics in Haemostasis: A Review
Abstract
1. Introduction
2. Microfluidic Chip Design
2.1. Fluid Specific Considerations
2.1.1. Miscible and Immiscible Fluids
2.1.2. Shear Stress and Shear Rate
2.1.3. Viscosity
2.1.4. Additional Design Specifications
2.2. Transduction Methods
2.2.1. Electrical Impedance Dependent Detection Techniques
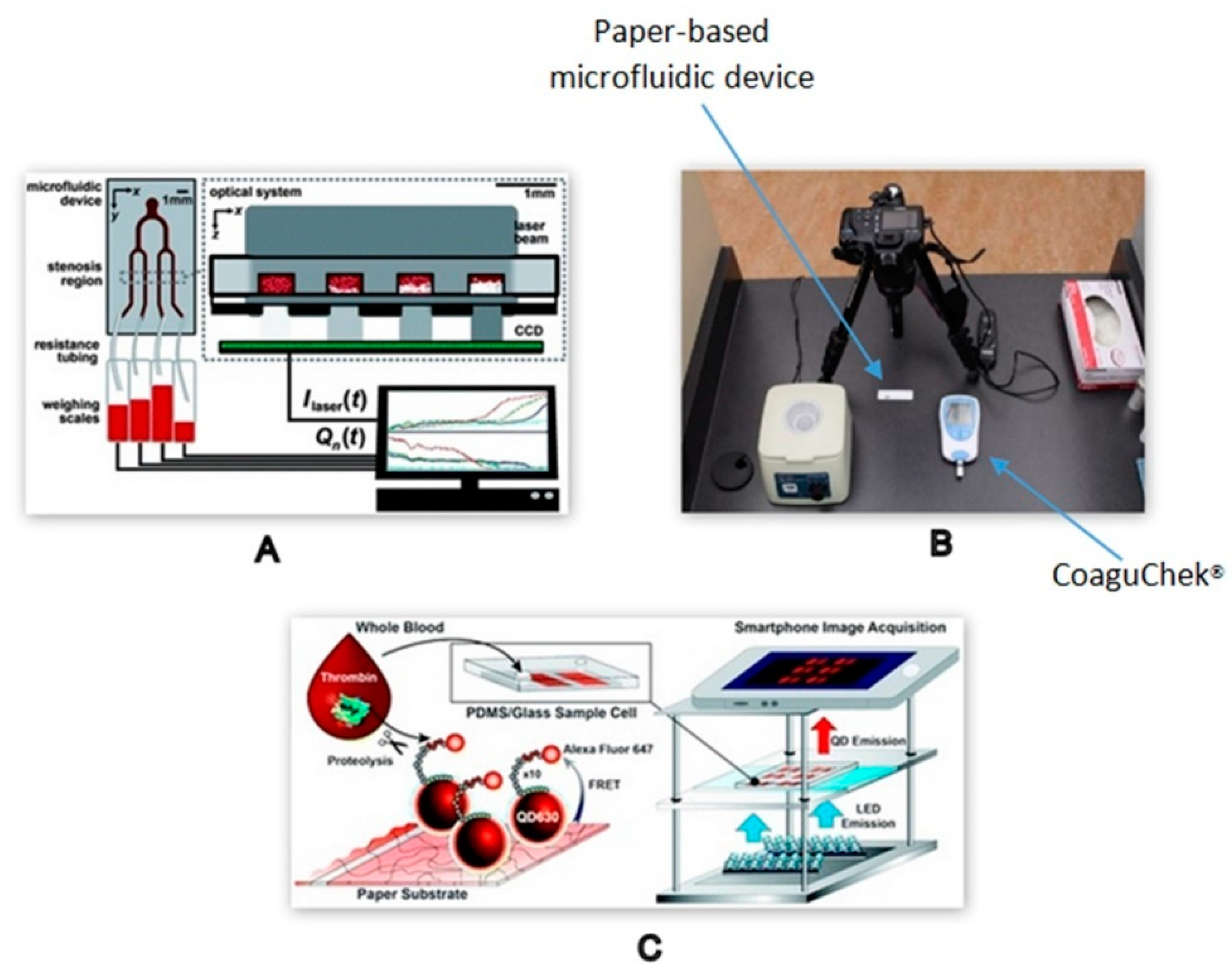
2.2.2. Optical Detection Method
Microscopic Methods
Non-Microscopic Optical Methods
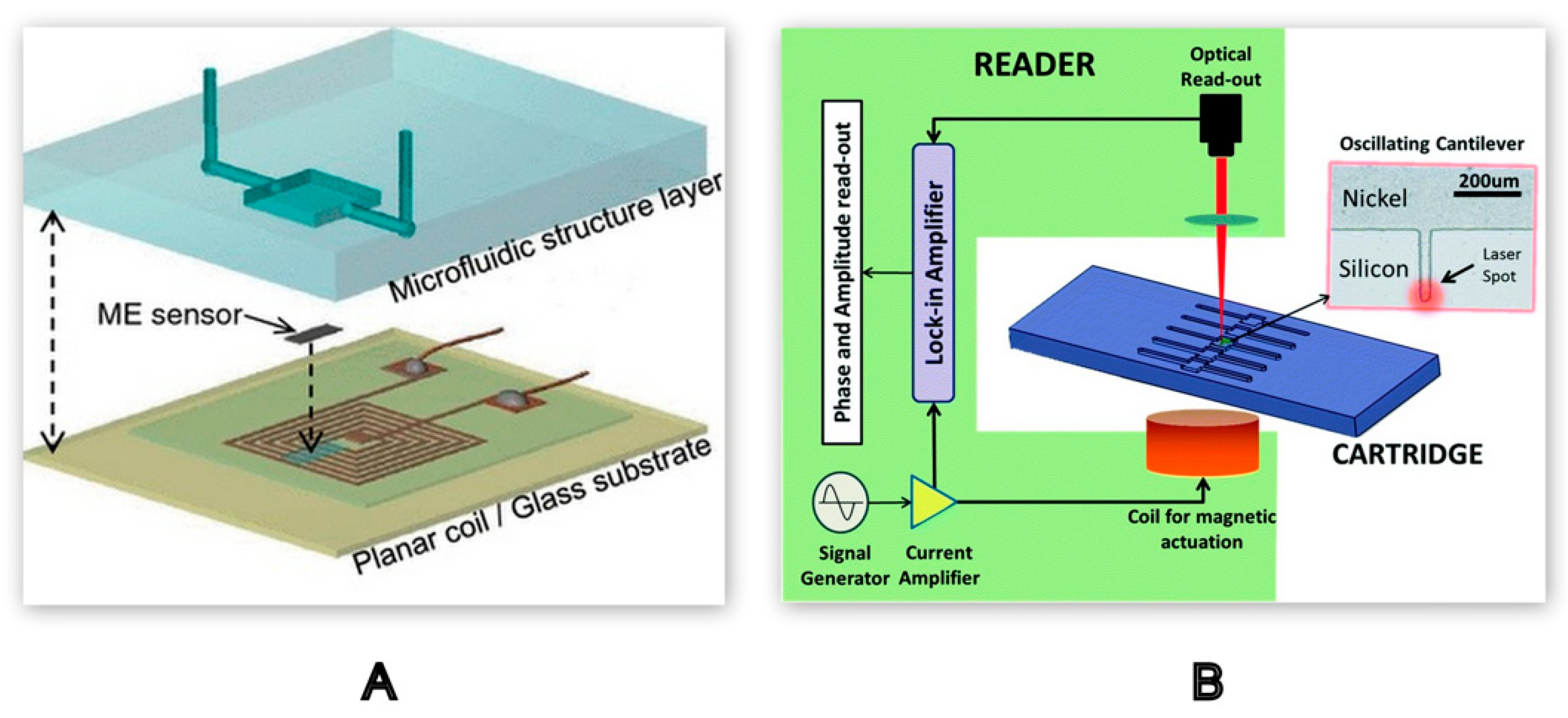
2.2.3. Mechanical Detection Methods
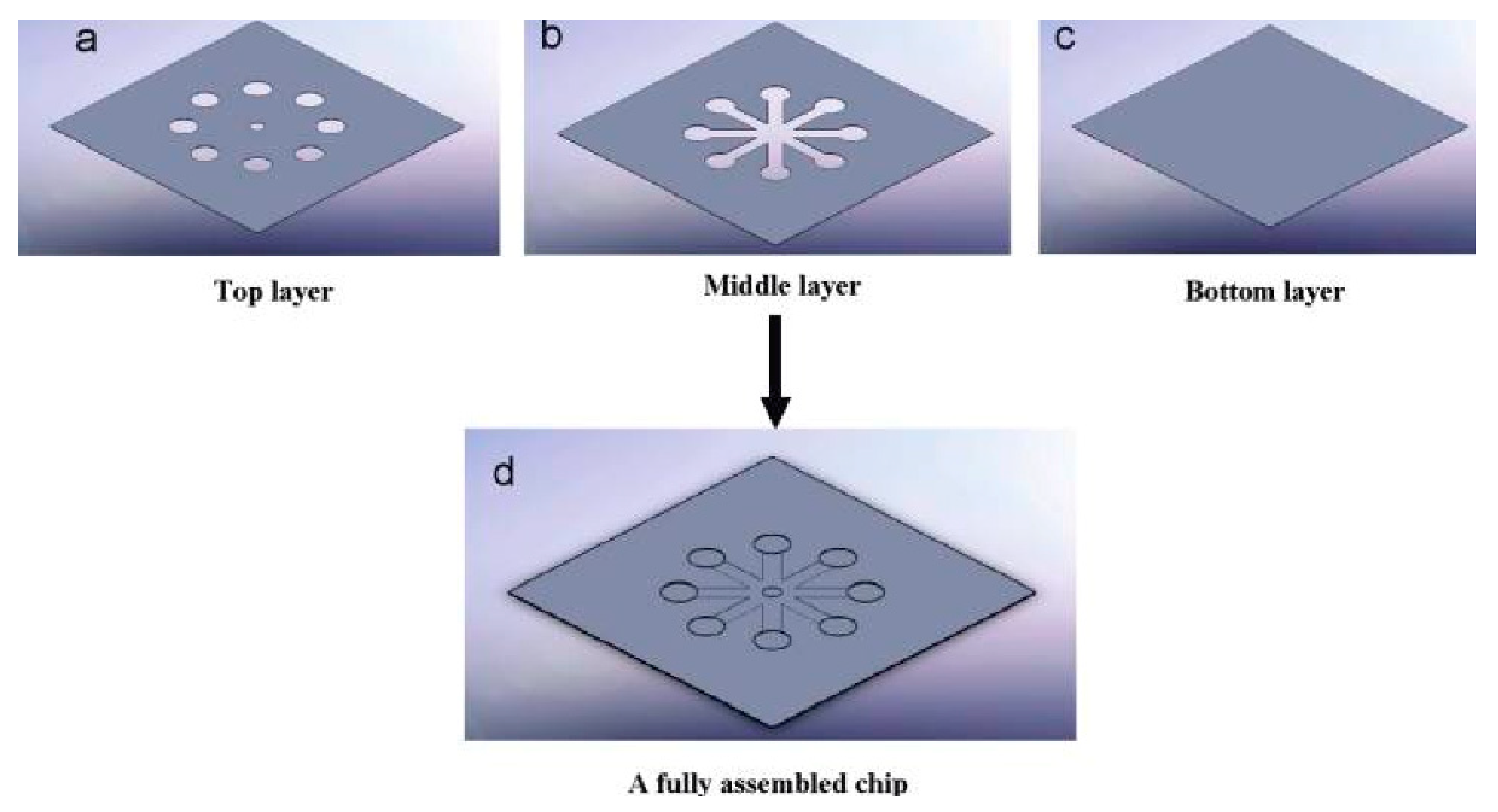
3. Microfluidic Device Fabrication
3.1. Materials for Device Fabrication
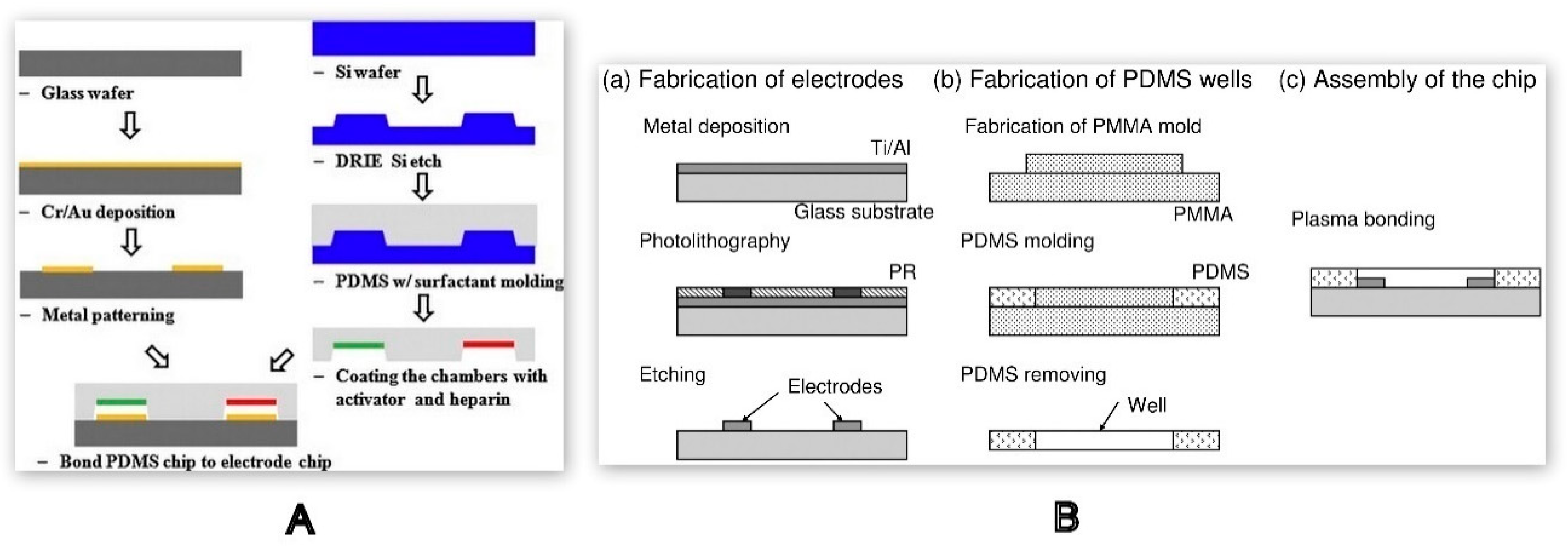
3.2. Fabrication Techniques
3.2.1. Direct Machining
Photolithography
Chemical Etching
Injection Moulding and Laser Processing
Laminate-based and Printing Techniques
3.2.2. Indirect Machining
3.3. Additional Consideration – Biomolecule Patterning
4. Commercially Available Devices
5. Conclusion and Future Perspective
Funding
Acknowledgments
Conflicts of Interest
References
- Runyon, M.K.; Johnson-Kerner, B.L.; Kastrup, C.J.; Van Ha, T.G.; Ismagilov, R.F. Propagation of Blood Clotting in the Complex Biochemical Network of Hemostasis Is Described by a Simple Mechanism. J. Am. Chem. Soc. 2007, 129, 7014–7015. [Google Scholar] [CrossRef] [PubMed]
- Wolberg, A.S.; Aleman, M.M.; Leidarman, K.; Machlus, K.R. Procoagulant Activity in Hemostasis and Thrombosis: Virchow’s Triad Revisited. Anesth. Analg. 2012, 114, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Neelamegham, S. Application of microfluidic devices in studies of thrombosis and hemostasis. Platelets 2017, 28, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Convery, N.; Gadegaard, N. 30 Years of Microfluidics. Micro Nano Eng. 2019, 2, 76–91. [Google Scholar] [CrossRef]
- Chiu, D.T.; de Mello, A.J.; Di Carlo, D.; Doyle, P.S.; Hansen, C.; Maceiczyk, R.M.; Wootton, R.C.R. Small but Perfectly Formed? Successes, Challenges, and Opportunities for Microfluidics in the Chemical and Biological Sciences. Chem 2017, 2, 201–223. [Google Scholar] [CrossRef]
- Mayr, L.M.; Bojanic, D. Novel trends in high-throughput screening. Curr. Opin. Pharmacol. 2009, 9, 580–588. [Google Scholar] [CrossRef]
- Sundberg, S.A.; Chow, A.; Nikiforov, T.; Wada, H.G. Microchip-based systems for target validation and HTS. Drug Discov. Today 2000, 5, S92–S103. [Google Scholar] [CrossRef]
- Raskob, G.E.; Angchaisuksiri, P.; Blanco, A.N.; Büller, H.; Gallus, A.; Hunt, B.J.; Hylek, E.M.; Kakkar, T.L.; Konstantinides, S.V.; McCumber, M.; et al. Thrombosis: A Major Contributor to Global Disease Burden. Semin. Thromb. Hemost. 2014, 40, 724–735. [Google Scholar] [CrossRef]
- Wendelboe, A.M.; Raskob, G.E. Global Burden of Thrombosis. Circ. Res. 2016, 118, 1340–1347. [Google Scholar] [CrossRef]
- Lowe, G.D.O. Common risk factors for both arterial and venous thrombosis. Br. J. Haematol. 2008, 140, 488–495. [Google Scholar] [CrossRef]
- Pandian, N.K.R.; Mannino, R.G.; Lam, W.A.; Jain, A. Thrombosis-on-a-chip: Prospective impact of microphysiological models of vascular thrombosis. Curr. Opin. Biomed. Eng. 2018, 5, 29–34. [Google Scholar] [CrossRef]
- Gutierrez, E.; Petrich, B.G.; Shanttil, S.J.; Ginsberg, M.H.; Groisman, A.; Kasirer-Friede, A. Microfluidic Devices for Studies of Shear-Dependent Platelet Adhesion. Lab Chip 2008, 8, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Gao, Y.; Zheng, M.; Xu, T.; Schoenhagen, P.; Jin, Z. Recent progress and market analysis of anticoagulant drugs. J. Thorac. Dis. 2018, 10, 2011–2025. [Google Scholar] [CrossRef] [PubMed]
- Walenga, J.M.; Hoppensteadt, D.A. Monitoring the New Antithrombotic Drugs. Semin. Thromb. Hemost. 2004, 30, 683–695. [Google Scholar] [CrossRef] [PubMed]
- De Caterina, R.; Husted, S.; Wallentin, L.; Agnelli, G.; Bachmann, F.; Baigent, C.; Jespersen, J.; Kristensen, S.D.; Montalescot, G.; Siegbahn, A.; et al. Anticoagulants in heart disease: Current status and perspectives. Eur. Heart J. 2007, 28, 880–913. [Google Scholar] [CrossRef]
- Bates, S.M. Coagulation Assays. Circulation 2005, 112, e53–e60. [Google Scholar] [CrossRef]
- Sobieraj-Teague, M.; Daniel, D.; Farrelly, B.; Coghlan, D.; Gallus, A. Accuracy and clinical usefulness of the CoaguChek S and XS Point of Care devices when starting warfarin in a hospital outreach setting. Thromb. Res. 2009, 123, 909–913. [Google Scholar] [CrossRef]
- Gardiner, C.; Williams, K.; Mackie, I.J.; Machin, S.J.; Cohen, H. Patient self-testing is a reliable and acceptable alternative to laboratory INR monitoring. Br. J. Haematol. 2005, 128, 242–247. [Google Scholar] [CrossRef]
- Levi, M.; Hunt, B.J. A critical appraisal of point-of-care coagulation testing in critically ill patients. J. Thromb. Haemost. 2015, 13, 1960–1967. [Google Scholar] [CrossRef]
- St-Louis, P. Status of point-of-care testing: Promise, realities, and possibilities. Clin. Biochem. 2000, 33, 427–440. [Google Scholar] [CrossRef]
- Prisco, D.; Paniccia, R. Point-of-care Testing of Hemostasis in Cardiac Surgery. Thromb. J. 2003, 1, 1. [Google Scholar] [CrossRef][Green Version]
- Ramaswamy, B.; Yeh, Y.T.T.; Zheng, S.Y. Microfluidic device and system for point-of-care blood coagulation measurement based on electrical impedance sensing. Sens. Actuators B Chem. 2013, 180, 21–27. [Google Scholar] [CrossRef]
- Lin, C.-H.; Liu, C.-Y.; Shih, C.-H.; Lu, C.-H. A sample-to-result system for blood coagulation tests on a microfluidic disk analyzer. Biomicrofluidics 2014, 8, 052105. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.F.; Rainey, P.; Castro-López, V.; O’Donnell, J.S.; Killard, A.J. A microfluidic anti-Factor Xa assay device for point of care monitoring of anticoagulation therapy. Analyst 2013, 138, 4769–4776. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Luppa, P.B.; Yeo, L.Y.; Ozcan, A.; Luong, J.H.T. Emerging Technologies for Next-Generation Point-of-Care Testing. Trends Biotechnol. 2015, 33, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P. Microfluidics—Downsizing large-scale biology. Nat. Biotechnol. 2001, 19, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Ferry, M.S.; Razinkov, I.A.; Hasty, J. Microfluidics for Synthetic Biology. In Methods in Enzymology; Voigt, C., Ed.; Elsevier: San Diego, CA, USA, 2011; Volume 497, pp. 295–372. [Google Scholar] [CrossRef]
- Ward, K.; Fan, Z.H. Mixing in microfluidic devices and enhancement methods. J. Micromech. Microeng. 2015, 25, 094001. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.J.; Ross, D.; Locascio, L.E. Rapid Microfluidic Mixing. Anal. Chem. 2002, 74, 45–51. [Google Scholar] [CrossRef]
- Shih, C.-H.; Lu, C.-H.; Wu, J.-H.; Lin, C.-H.; Wang, J.-M.; Lin, C.-Y. Prothrombin time tests on a microfluidic disc analyzer. Sens. Actuators B Chem. 2012, 161, 1184–1190. [Google Scholar] [CrossRef]
- Tice, J.D.; Song, H.; Lyon, A.D.; Ismagilov, R.F. Formation of Droplets and Mixing in Multiphase Microfluidics at Low Values of the Reynolds and the Capillary Numbers. Langmuir 2003, 19, 9127–9133. [Google Scholar] [CrossRef]
- Song, H.; Li, H.; Munson, M.S.; Van Ha, T.G.; Ismagilov, R.F. On-chip Titration of an Anticoagulant Argatroban and Determination of the Clotting Time within Whole Blood or Plasma Using a Plug-Based Microfluidic System. Anal. Chem. 2006, 78, 4839–4849. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Tao, D.; Ng, E.X.; Drum, C.L.; Liu, A.Q.; Chen, C.-H. Real-time measurement of thrombin generation using continuous droplet microfluidics. Biomicrofluidics 2014, 8, 052108. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.; Kita, A.; Leach, J.; Rounsevell, R.; Huang, J.N.; Moake, J.; Ware, R.E.; Fletcher, D.A.; Lam, W.A. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J. Clin. Investig. 2012, 122, 408–418. [Google Scholar] [CrossRef]
- He, Z.; Lin, J.-M. Recent Development of Cell Analysis on Microfludics. In Cell Analysis on Microfluidics; Lin, J.-M., Ed.; Springer Nature: Singapore, 2018; pp. 43–93. [Google Scholar] [CrossRef]
- Paszkowiak, J.J.; Dardik, A. Arterial wall shear stress: Observations from the Bench to the Bedside. Vasc. Endovasc. Surg. 2003, 37, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, T.G.; Stefanadis, C. Vascular wall shear stress: Basic principles and methods. Hell. J. Cardiol. 2005, 46, 9–15. [Google Scholar]
- Hathcock, J.J. Flow effects on Coagulation and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Graveline, A.; Waterhouse, A.; Vernet, A.; Flaumenhaft, R.; Ingber, D.E. A shear gradient-activated microfluidic device for automated monitoring of whole blood haemostasis and platelet function. Nat. Commun. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Neeves, K.B.; Maloney, S.F.; Fong, K.P.; Schmaier, A.A.; Kahn, M.L.; Brass, L.F.; Diamond, S.L. Microfluidic focal thrombosis model for measuring murine platelet deposition and stability: PAR4 signaling enhances shear-resistance of platelet aggregates. J. Thromb. Haemost. 2008, 6, 2193–2201. [Google Scholar] [CrossRef]
- Ranucci, M.; Laddomada, T.; Ranucci, M.; Baryshnikova, E. Blood viscosity during coagulation at different shear rates. Physiol. Rep. 2014, 2, e12065. [Google Scholar] [CrossRef]
- Lei, K.F.; Chen, K.-H.; Tsui, P.-H.; Tsang, N.-M. Real-Time Electrical Impedimetric Monitoring of Blood Coagulation Process under Temperature and Hematocrit Variations Conducted in a Microfluidic Chip. PLoS ONE 2013, 8, e76243. [Google Scholar] [CrossRef]
- Cakmak, O.; Ermek, E.; Kilinc, N.; Bulut, S.; Baris, I.; Kavakli, I.H.; Yaralioglu, G.G.; Urey, H. A cartridge based sensor array platform for multiple coagulation measurements from plasma. Lab Chip 2015, 15, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, D.; Pauletti, G.M.; Steckl, A.J. Blood coagulation screening using a paper-based microfluidic lateral flow device. Lab Chip 2014, 14, 4035–4041. [Google Scholar] [CrossRef] [PubMed]
- Iyer, J.K.; Otvos, R.A.; Kool, J.; Kini, R.M. Microfluidic Chip-Based Online Screening Coupled to Mass Spectrometry: Identification of Inhibitors of Thrombin and Factor Xa. J. Biomol. Screen. 2016, 21, 212–220. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Runyon, M.K.; Johnson-Kerner, B.L.; Ismagilov, R.F. Minimal Functional Model of Hemostasis in a Biomimetic Microfluidic System. Angew. Chem. Int. Ed. 2004, 43, 1531–1536. [Google Scholar] [CrossRef]
- Pires, N.M.M.; Dong, T.; Hanke, U.; Hoivik, N. Recent Developments in Optical Detection Technologies in Lab-on-a-Chip Devices for Biosensing Applications. Sensors 2014, 14, 15458–15479. [Google Scholar] [CrossRef]
- Viskari, P.J.; Landers, J.P. Unconventional detection methods for microfluidic devices. Electrophoresis 2006, 27, 1797–1810. [Google Scholar] [CrossRef]
- Zhu, Y.; Fang, Q. Analytical detection techniques for droplet microfluidics-A review. Anal. Chim. Acta 2013, 787, 24–35. [Google Scholar] [CrossRef]
- Schwarz, M.A.; Hauser, P.C. Recent developments in detection methods for microfabricated analytical devices. Lab Chip 2001, 1, 1–6. [Google Scholar] [CrossRef]
- Yi, C.; Zhang, Q.; Li, C.-W.; Yang, J.; Zhao, J.; Yang, M. Optical and electrochemical detection techniques for cell-based microfluidic systems. Anal. Bioanal. Chem. 2006, 384, 1259–1268. [Google Scholar] [CrossRef]
- Kuswandi, B.; Huskens, J.; Verboom, W. Optical sensing systems for microfluidic devices: A review. Anal. Chim. Acta 2007, 601, 141–155. [Google Scholar] [CrossRef]
- Wu, J.; Gu, M. Microfluidic sensing: State of the art fabrication and detection techniques. J. Biomed. Opt. 2011, 16, 080901. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, L.J.; Shore-Lesserson, L. Point-of-care coagulation testing and transfusion algorithms. Br. J. Anaesth. 2009, 103, i14–i22. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.-X. New applications of electrical impedance of human blood. J. Med. Eng. Technol. 1996, 20, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Libgot-Callé, R.; Ossant, F.; Gruel, Y.; Lermusiaux, P.; Patat, F. High Frequency Ultrasound Device to Investigate the Acoustic Properties of Whole Blood During Coagulation. Ultrasound Med. Biol. 2008, 34, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Ganter, M.T.; Hofer, C.K. Coagulation monitoring: Current techniques and clinical use of viscoelastic point-of-care coagulation devices. Anesth. Analg. 2008, 106, 1366–1375. [Google Scholar] [CrossRef]
- Evans, P.A.; Hawkins, K.; Lawrence, M.; Williams, R.L.; Barrow, M.S.; Thirumalai, N.; Williams, P.R. Rheometry and associated techniques for blood coagulation studies. Med. Eng. Phys. 2008, 30, 671–679. [Google Scholar] [CrossRef]
- Linnemann, B.; Schwonberg, H.; Mani, H.; Prochnow, S.; Lindhoff-Last, E. Standardization of light transmittance aggregometry for monitoring antiplatelet therapy: An adjustment for platelet count is not necessary. J. Thromb. Haemost. 2008, 6, 677–683. [Google Scholar] [CrossRef]
- Ur, A. Detection of Clot Retraction through Changes of the Electrical Impedance of Blood during Coagulation. Am. J. Clin. Pathol. 1971, 56, 713–718. [Google Scholar] [CrossRef]
- Ur, A. Analysis and Interpretation of the Impedance Blood Coagulation Curve. Am. J. Clin. Pathol. 1977, 67, 470–476. [Google Scholar] [CrossRef]
- Ur, A. Changes in the Electrical Impedance of Blood during Coagulation. Nature 1970, 226, 269–270. [Google Scholar] [CrossRef]
- Mohapatra, S.N.; Hill, D.W. The changes in blood resistivity with haematocrit and temperature. Eur. J. Intensive Care Med. 1975, 1, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Spence, N. Electrical impedance measurement as an endpoint detection method for routine coagulation tests. Br. J. Biomed. Sci. 2002, 59, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Meneghelo, Z.M.; Barroso, C.M.Q.; Liporace, I.L.; Cora, A.P. Comparison of the international normalized ratio levels obtained by portable coagulometer and laboratory in a clinic specializing in oral anticoagulation. Int. J. Lab. Hematol. 2015, 37, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Sambu, N.; Curzen, N. Monitoring the effectiveness of antiplatelet therapy: Opportunities and limitations. Br. J. Clin. Pharmacol. 2011, 72, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.W.; Chang, C.H.; Lin, C.H. High-throughput Fluorescence Detections in Microfluidic Systems. Genom. Med. Biomark. Health Sci. 2011, 3, 27–38. [Google Scholar] [CrossRef]
- Paiè, P.; Martínez Vázquez, R.; Osellame, R.; Bragheri, F.; Bassi, A. Microfluidic Based Optical Microscopes on Chip. Cytom. Part A 2018, 93, 987–996. [Google Scholar] [CrossRef]
- Tovar-Lopez, F.J.; Rosengarten, G.; Westein, E.; Khoshmanesh, K.; Jackson, S.P.; Mitchell, A.; Nesbitt, W.S. A microfluidics device to monitor platelet aggregation dynamics in response to strain rate micro-gradients in flowing blood. Lab Chip 2010, 10, 291–302. [Google Scholar] [CrossRef]
- Colace, T.V.; Fogarty, P.F.; Panckeri, K.A.; Li, R.; Diamond, S.L. Microfluidic assay of hemophilic blood clotting: Distinct deficits in platelet and fibrin deposition at low factor levels. J. Thromb. Haemost. 2014, 12, 147–158. [Google Scholar] [CrossRef]
- Govindarajan, V.; Zhu, S.; Li, R.; Lu, Y.; Diamond, S.L.; Reifman, J.; Mitrophanov, A.Y. Impact of Tissue Factor Localization on Blood Clot Structure and Resistance under Venous Shear. Biophys. J. 2018, 114, 978–991. [Google Scholar] [CrossRef]
- Shen, F.; Kastrup, C.J.; Liu, Y.; Ismagilov, R.F. Threshold Response of Initiation of Blood Coagulation by Tissue Factor in Patterned Microfluidic Capillaries Is Controlled by Shear Rate. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 2035–2041. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Carrilho, E.; Thomas, S.W.; Sindi, H.; Whitesides, G.M. Simple Telemedicine for Developing Regions: Camera Phones and Paper-Based Microfluidic Devices for Real-Time, Off-Site Diagnosis. Anal. Chem. 2008, 80, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ku, D.N.; Forest, C.R. Microfluidic system for simultaneous optical measurement of platelet aggregation at multiple shear rates in whole blood. Lab Chip 2012, 12, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Hegener, M.A.; Li, H.; Han, D.; Steckl, A.J.; Pauletti, G.M. Point-of-care coagulation monitoring: First clinical experience using a paper-based lateral flow diagnostic device. Biomed. Microdevices 2017, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Petryayeva, E.; Algar, W.R. Single-step bioassays in serum and whole blood with a smartphone, quantum dots and paper-in-PDMS chips. Analyst 2015, 140, 4037–4045. [Google Scholar] [CrossRef] [PubMed]
- Alouidor, B.; Sweeney, R.E.; Tat, T.; Wong, R.K.; Yoon, J. Microfluidic Point-of-Care Ecarin-Based Clotting and Chromogenic Assays for Monitoring Direct Thrombin Inhibitors. J. Extracorpor. Technol. 2019, 51, 29–37. [Google Scholar]
- Puckett, L.G.; Barrett, G.; Kouzoudis, D.; Grimes, C.; Bachas, L.G. Monitoring blood coagulation with magnetoelastic sensors. Biosens. Bioelectron. 2003, 18, 675–681. [Google Scholar] [CrossRef]
- Grimes, C.A.; Roy, S.C.; Rani, S.; Cai, Q. Theory, Instrumentation and Applications of Magnetoelastic Resonance Sensors: A Review. Sensors 2011, 11, 2809–2844. [Google Scholar] [CrossRef]
- Chen, P.; Jiang, Q.; Horikawa, S.; Li, S. Magnetoelastic-Sensor Integrated Microfluidic Chip for the Measurement of Blood Plasma Viscosity. J. Electrochem. Soc. 2017, 164, B247–B252. [Google Scholar] [CrossRef]
- Nge, P.N.; Rogers, C.I.; Woolley, A.T. Advances in Microfluidic Materials, Functions, Integration, and Applications. Chem. Rev. 2013, 113, 2550–2583. [Google Scholar] [CrossRef]
- Ren, K.; Zhou, J.; Wu, H. Materials for Microfluidic Chip Fabrication. Acc. Chem. Res. 2013, 46, 2396–2406. [Google Scholar] [CrossRef]
- Quake, S.R.; Scherer, A. From Micro- to Nanofabrication with Soft Materials. Science 2000, 290, 1536–1540. [Google Scholar] [CrossRef] [PubMed]
- Mcdonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.; Schueller, O.J.; Whitesides, G.M. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Kuncová-Kallio, J.; Kallio, P.J. PDMS and its suitability for analytical microfluidic devices. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology, New York, NY, USA, 30 August–3 September 2006; Volume 1, pp. 2486–2489. [Google Scholar] [CrossRef]
- Mehling, M.; Tay, S. Microfluidic cell culture. Curr. Opin. Biotechnol. 2014, 25, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Borenstein, J.; Guiney, L.; Miller, R.; Sukavaneshvar, S.; Loose, C. Polybetaine modification of PDMS microfluidic devices to resist thrombus formation in whole blood. Lab Chip 2013, 13, 1963–1968. [Google Scholar] [CrossRef]
- Mukhopadhyay, R. When PDMS isn’t the best. Anal. Chem. 2007, 79, 3248–3253. [Google Scholar] [CrossRef]
- Berthier, E.; Young, E.W.K.; Beebe, D. Engineers are from PDMS-land, Biologists are from Polystyrenia. Lab Chip 2012, 12, 1224–1237. [Google Scholar] [CrossRef]
- Gencturk, E.; Mutlu, S.; Ulgen, K.O. Advances in microfluidic devices made from thermoplastics used in cell biology and analyses. Biomicrofluidics 2017, 11, 051502. [Google Scholar] [CrossRef]
- Boone, T.D.; Fan, Z.H.; Hooper, H.H.; Ricco, A.J.; Tan, H.; Williams, S.J. Plastic Advances Microfluidic Devices. Anal. Chem. 2002, 74, 78A–86A. [Google Scholar] [CrossRef]
- Maji, D.; Suster, M.A.; Stavrou, E.; Gurkan, U.A.; Mohseni, P. Monitoring time course of human whole blood coagulation using a microfluidic dielectric sensor with a 3D capacitive structure. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 5904–5907. [Google Scholar] [CrossRef]
- Martínez, A.W. Microfluidic paper-based analytical deices: From POCKET to paper-based ELISA. Bioanalysis 2011, 3, 2589–2592. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-based microfluidic point-of-care diagnostic devices. Lab Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M.; Carrilho, E. Diagnostics for the Developing World: Microfluidic Paper-Based Analytical Devices. Anal. Chem. 2010, 82, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Haswell, S.J. Materials Matter in Microfluidic Devices. MRS Bull. 2006, 31, 95–99. [Google Scholar] [CrossRef]
- Velve-Casquillas, G.; Le Berre, M.; Piel, M.; Tran, P.T. Microfluidic tools for cell biological research. Nano Today 2010, 5, 28–47. [Google Scholar] [CrossRef] [PubMed]
- Attia, U.M.; Marson, S.; Alcock, J.R. Micro-injection moulding of polymer microfluidic devices. Microfluid. Nanofluid. 2009, 7, 1. [Google Scholar] [CrossRef]
- Becker, H. Polymer microfluidic devices. Talanta 2002, 56, 267–287. [Google Scholar] [CrossRef]
- Faustino, V.; Catarino, S.O.; Lima, R.; Minas, G. Biomedical microfluidic devices by using low-cost fabrication techniques: A review. J. Biomech. 2016, 49, 2280–2292. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Ju, X.-J.; Xie, R.; Liu, Z.; Chu, L.-Y. Fabrication of glass-based microfluidic devices with dry film photoresists as pattern transfer masks for wet etching. RSC Adv. 2015, 5, 5638–5646. [Google Scholar] [CrossRef]
- Zhao, X.-M.; Xia, Y.; Whitesides, G.M. Soft lithographic methods for nano-fabrication. J. Mater. Chem. 1997, 7, 1069–1074. [Google Scholar] [CrossRef]
- McDonald, J.C.; Whitesides, G.M. Poly(dimethylsiloxane) as a Material for Fabricating Microfluidic Devices. Acc. Chem. Res. 2002, 35, 491–499. [Google Scholar] [CrossRef]
- Matellan, C.; del Río Hernández, A.E. Cost-effective rapid prototyping and assembly of poly(methyl methacrylate) microfluidic devices. Sci. Rep. 2018, 8, 6971. [Google Scholar] [CrossRef]
- Xia, Y.; Si, J.; Li, Z. Fabrication techniques for microfluidic paper-based analytical devices and their applications for biological testing: A review. Biosens. Bioelectron. 2016, 77, 774–789. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M. Three-dimensional microfluidic devices fabricated in layered paper and tape. Proc. Natl. Acad. Sci. USA 2008, 105, 19606–19611. [Google Scholar] [CrossRef] [PubMed]
- Zaouk, R.; Park, B.Y.; Madou, M.J. Introduction to Microfabrication Techniques. In Microfluidic Techniques. Methods in Molecular BiologyTM; Minteer, S.D., Ed.; Humana Press: Totowa, NJ, USA, 2006; pp. 3–16. [Google Scholar] [CrossRef]
- Venkatakrishnan, K.; Ngoi, B.K.; Stanley, P.; Lim, L.E.; Tan, B.; Sivakumar, N. Laser writing techniques for photomask fabrication using a femtosecond laser. Appl. Phys. A Mater. Sci. Process. 2002, 74, 493–496. [Google Scholar] [CrossRef]
- Miyoshi, H.; Taniguchi, J. Fabrication of a high-resolution mask by using variable-shaped electron beam lithography with a non-chemically amplified resist and a post-exposure bake. Microelectron. Eng. 2015, 143, 48–54. [Google Scholar] [CrossRef]
- Tuomikoski, S.; Franssila, S. Free-standing SU-8 microfluidic chips by adhesive bonding and release etching. Sens. Actuators A Phys. 2005, 120, 408–415. [Google Scholar] [CrossRef]
- Alrifaiy, A.; Lindahl, O.A.; Ramser, K. Polymer-Based Microfluidic Devices for Pharmacy, Biology and Tissue Engineering. Polymers 2012, 4, 1349–1398. [Google Scholar] [CrossRef]
- Colace, T.V.; Tormoen, G.W.; McCarty, O.J.T.; Diamond, S.L. Microfluidics and Coagulation Biology. Annu. Rev. Biomed. Eng. 2013, 15, 283–303. [Google Scholar] [CrossRef]
- Iliescu, C.; Taylor, H.; Avram, M.; Miao, J.; Franssila, S. A practical guide for the fabrication of microfluidic devices using glass and silicon. Biomicrofluidics 2012, 6, 016505. [Google Scholar] [CrossRef]
- Ceriotti, L.; Weible, K.; de Rooij, N.F.; Verpoorte, E. Rectangular channels for lab-on-a-chip applications. Microelectron. Eng. 2003, 67–68, 865–871. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, N.-E.; Lee, J.; Park, J.S.; Park, H.D. Deep dry etching of borosilicate glass using SF6 and SF6/Ar inductively coupled plasmas. Microelectron. Eng. 2005, 82, 119–128. [Google Scholar] [CrossRef]
- Bu, M.; Melvin, T.; Ensell, G.J.; Wilkinson, J.S.; Evans, A.G.R. A new masking technology for deep glass etching and its microfluidic application. Sens. Actuators A Phys. 2004, 115, 476–482. [Google Scholar] [CrossRef]
- Simpson, P.C.; Woolley, A.T.; Mathies, R.A. Microfabrication Technology for the Production of Capillary Array Electrophoresis Chips. Biomed. Microdevices 1998, 1, 7–26. [Google Scholar] [CrossRef]
- Becker, H.; Gärtner, C. Polymer microfabrication methods for microfluidic analytical applications. Electrophoresis 2000, 21, 12–26. [Google Scholar] [CrossRef]
- Klank, H.; Kutter, J.P.; Geschke, O. CO2-laser micromachining and back-end processing for rapid production of PMMA-based microfluidic systems. Lab Chip 2002, 2, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Tennico, Y.H.; Hutanu, D.; Koesdjojo, M.T.; Bartel, C.M.; Remcho, V.T. On-Chip Aptamer-Based Sandwich Assay for Thrombin Detection Employing Magnetic Beads and Quantum Dots. Anal. Chem. 2010, 82, 5591–5597. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Shih, C.-H.; Lu, C.-H. A Fully Integrated Prothrombin Time Test on the Microfluidic Disk Analyzer. J. Nanosci. Nanotechnol. 2013, 13, 2206–2212. [Google Scholar] [CrossRef]
- Gale, B.; Jafek, A.; Lambert, C.; Goenner, B.; Moghimifam, H.; Nze, U.; Kamarapu, S. A Review of Current Methods in Microfluidic Device Fabrication and Future Commercialization Prospects. Inventions 2018, 3, 60. [Google Scholar] [CrossRef]
- Cassano, C.L.; Fan, Z.H. Laminated paper-based analytical devices (LPAD): Fabrication, characterization, and assays. Microfluid. Nanofluid. 2013, 15, 173–181. [Google Scholar] [CrossRef]
- Li, X.; Ballerini, D.R.; Shen, W. A perspective on paper-based microfluidics: Current status and future trends. Biomicrofluidics 2012, 6, 011301. [Google Scholar] [CrossRef]
- Lisowski, P.; Zarzycki, P.K. Microfluidic Paper-Based Analytical Devices (μPADs) and Micro Total Analysis Systems (μTAS): Development, Applications and Future Trends. Chromatographia 2013, 76, 1201–1214. [Google Scholar] [CrossRef]
- Carrilho, E.; Martinez, A.W.; Whitesides, G.M. Understanding Wax Printing: A Simple Micropatterning Process for Paper-Based Microfluidics. Anal. Chem. 2009, 81, 7091–7095. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tian, J.; Garnier, G.; Shen, W. Fabrication of paper-based microfluidic sensors by printing. Colloids Surfaces B Biointerfaces 2010, 76, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Forouzan, O.; Brown, T.P.; Shevkoplyas, S.S. Integrated separation of blood plasma from whole blood for microfluidic paper-based analytical devices. Lab Chip 2012, 12, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.M.K.; Gitlin, I.; Stroock, A.D.; Whitesides, G.M. Components for integrated poly(dimethylsiloxane) microfluidic systems. Electrophoresis 2002, 23, 3461–3473. [Google Scholar] [CrossRef]
- Rogers, J.A.; Nuzzo, R.G. Recent progress in soft lithography. Mater. Today 2005, 8, 50–56. [Google Scholar] [CrossRef]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid Prototyping of Microfluidic Systems in Poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef]
- Meyer dos Santos, S.; Zorn, A.; Guttenberg, Z.; Picard-Willems, B.; Kläffling, C.; Nelson, K.; Klinkhardt, U.; Harder, S. A novel μ-fluidic whole blood coagulation assay based on Rayleigh surface-acoustic waves as a point-of-care method to detect anticoagulants. Biomicrofluidics 2013, 7, 056502. [Google Scholar] [CrossRef]
- Blawas, A.S.; Reichert, W.M. Protein patterning. Biomaterials 1998, 19, 595–609. [Google Scholar] [CrossRef]
- Barbulovic-Nad, I.; Lucente, M.; Sun, Y.; Zhang, M.; Wheeler, A.R.; Bussmann, M. Bio-Microarray Fabrication Techniques—A Review. Crit. Rev. Biotechnol. 2006, 26, 237–259. [Google Scholar] [CrossRef]
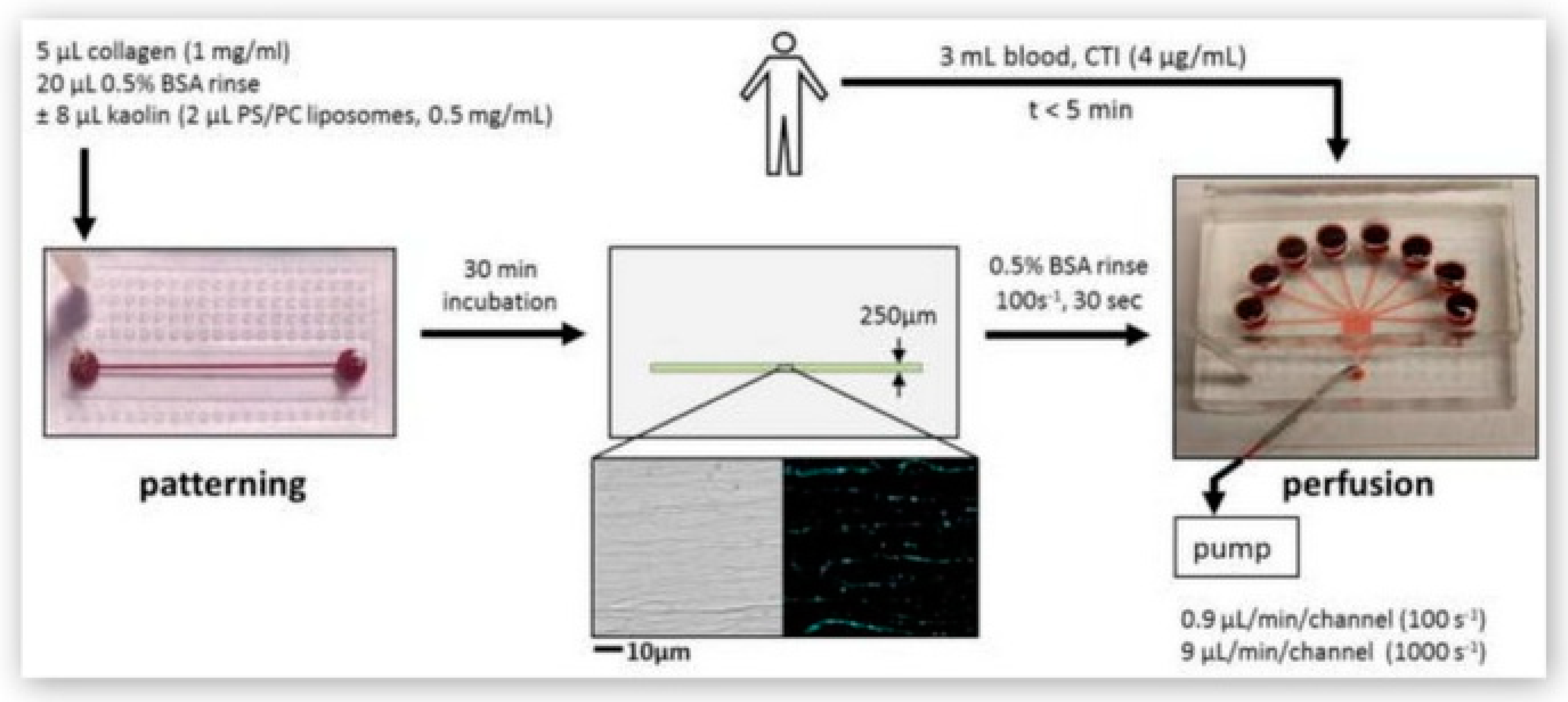
- Zhu, S.; Diamond, S.L. Contact activation of blood coagulation on a defined kaolin/collagen surface in a microfluidic assay. Thromb. Res. 2014, 134, 1335–1343. [Google Scholar] [CrossRef]
- Point of Care/POC Diagnostics Market Worth $46.7 Billion by 2024. Available online: https://www.marketsandmarkets.com/PressReleases/point-of-care-diagnostic.asp (accessed on 27 December 2019).
- Chin, C.D.; Linder, V.; Sia, S.K. Commercialization of microfluidic point-of-care diagnostic devices. Lab Chip 2012, 12, 2118–2134. [Google Scholar] [CrossRef] [PubMed]
- Foulis, P.R.; Wallach, P.M.; Adelman, H.M.; Sanford, B.H.; McCain, J.; Reed, D.; Schlede, C.M.; Kokseng, C.U.; Taylor, C.D. Performance of the Coumatrak System in a Large Anticoagulation Clinic. Am. J. Clin. Pathol. 1995, 103, 98–102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jennings, I.; Luddington, R.J.; Baglin, T. Evaluation of the Ciba Corning Biotrack 512 coagulation monitor for the control of oral anticoagulation. J. Clin. Pathol. 1991, 44, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Plesch, W.; Wolf, T.; Breitenbeck, N.; Dikkeschei, L.D.; Cervero, A.; Perez, P.L.; van den Besselaar, A.M.H.P. Results of the performance verification of the CoaguChek XS system. Thromb. Res. 2008, 123, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, M.; Sullivan, J.; Norbeck, A. Comparison of International Normalized Ratios provided by two point-of-care devices and laboratory-based venipuncture in a pharmacist-managed anticoagulation clinic. Am. J. Health-Syst. Pharm. 2010, 67, 1616–1622. [Google Scholar] [CrossRef]
- Alere Recalls INRatio® and INRatio2® PT/INR Monitoring System Due to Incorrect Test Results. Available online: https://www.fdanews.com/ext/resources/files/2016/08/08-25-16-Alertrecall.pdf?1515861497 (accessed on 27 December 2019).
- Andrew, M.; Ansell, J.L.; Becker, D.M.; Becker, R.C.; Triplett, D.A. Point-of-Care Prothrombin Time Measurement for Professional and Patient Self-Testing Use: A Multicenter Clinical Experience. Am. J. Clin. Pathol. 2001, 115, 288–296. [Google Scholar] [CrossRef]
- Van Den Besselaar, A.M.H.P.; Van Der Meer, F.J.M.; Abdoel, C.F.; Witteveen, E. Analytical accuracy and precision of two novel Point-of-Care systems for INR determination. Thromb. Res. 2015, 135, 526–531. [Google Scholar] [CrossRef]
- Harris, L.F.; Castro-López, V.; Killard, A.J. Coagulation monitoring devices: Past, present, and future at the point of care. TrAC Trends Anal. Chem. 2013, 50, 85–95. [Google Scholar] [CrossRef]
- McCahon, D.; Roalfe, A.; Fitzmaurice, D.A. An evaluation of a coagulation system (Xprecia Stride) for utilisation in anticoagulation management. J. Clin. Pathol. 2018, 71, 20–26. [Google Scholar] [CrossRef]
- Nicolau-Raducu, R.; Occhipinti, E.; Marshall, T.; Koveleskie, J.; Ganier, D.; Evans, B.; Daly, W.; Fish, B.; Cohen, A.J.; Reichman, T.W.; et al. Thromboprophylaxis with Heparin during Orthotopic Liver Transplantation: Comparison of Hepcon HMS Plus and Anti-Xa Assays for Low-Range Heparin. J. Cardiothorac. Vasc. Anesth. 2017, 31, 575–581. [Google Scholar] [CrossRef]
- Martin, C.L. i-STAT—Combining Chemistry and Haematology in PoCT. Clin. Biochem. Rev. 2010, 31, 81–84. [Google Scholar] [PubMed]
- Enriquez, L.J.; Shore-Lesserson, L. Coagulation and hematologic point-of-care testing. In Monitoring in Anesthesia and Perioperative Care; Reich, D.L., Kahn, R.A., Leibowitz, A.B., Mittnacht, A.J.C., Stone, M.E., Eisenkraft, J.B., Eds.; Cambridge University Press: New York, NY, USA, 2011; pp. 308–318. [Google Scholar] [CrossRef]
- Paniccia, R.; Priora, R.; Alessandrello Liotta, A.; Abbate, R. Platelet function tests: A comparative review. Vasc. Health Risk Manag. 2015, 11, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Mutlak, H.; Reyher, C.; Meybohm, P.; Papadopoulos, N.; Hanke, A.; Zacharowski, K.; Weber, C. Multiple Electrode Aggregometry for the Assessment of Acquired Platelet Dysfunctions during Extracorporeal Circulation. Thorac. Cardiovasc. Surg. 2014, 63, 021–027. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, E.J. Clinical utility of closure times using the platelet function analyzer-100/200. Am. J. Hematol. 2017, 92, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Savion, N.; Varon, D. Impact—The Cone and Plate(let) Analyzer: Testing Platelet Function and Anti-Platelet Drug Response. Pathophysiol. Haemost. Thromb. 2006, 35, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.K.; Kotha, J. The Utility of Platelet and Coagulation Testing of Antithrombotics: Fusing Science with Patient Care. Drug Dev. Res. 2013, 74, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Fabbro, M.; Winkler, A.M.; Levy, J.H. Technology: Is There Sufficient Evidence to Change Practice in Point-of-Care Management of Coagulopathy? J. Cardiothorac. Vasc. Anesth. 2017, 31, 1849–1856. [Google Scholar] [CrossRef]
- Theusinger, O.M.; Levy, J.H. Point of Care Devices for Assessing Bleeding and Coagulation in the Trauma Patient. Anesthesiol. Clin. 2013, 31, 55–65. [Google Scholar] [CrossRef]
- Murray, E.T.; Fitzmaurice, D.A.; McCahon, D. Point of care testing for INR monitoring: Where are we now? Br. J. Haematol. 2004, 127, 373–378. [Google Scholar] [CrossRef]
- Neeves, K.B.; Onasoga, A.A.; Wufsus, A.R. The use of microfluidics in hemostasis: Clinical diagnostics and biomimetic models of vascular injury. Curr. Opin. Hematol. 2013, 20, 417–423. [Google Scholar] [CrossRef]
- Branchford, B.R.; Ng, C.J.; Neeves, K.B.; Di Paola, J. Microfluidic technology as an emerging clinical tool to evaluate thrombosis and hemostasis. Thromb. Res. 2015, 136, 13–19. [Google Scholar] [CrossRef] [PubMed]
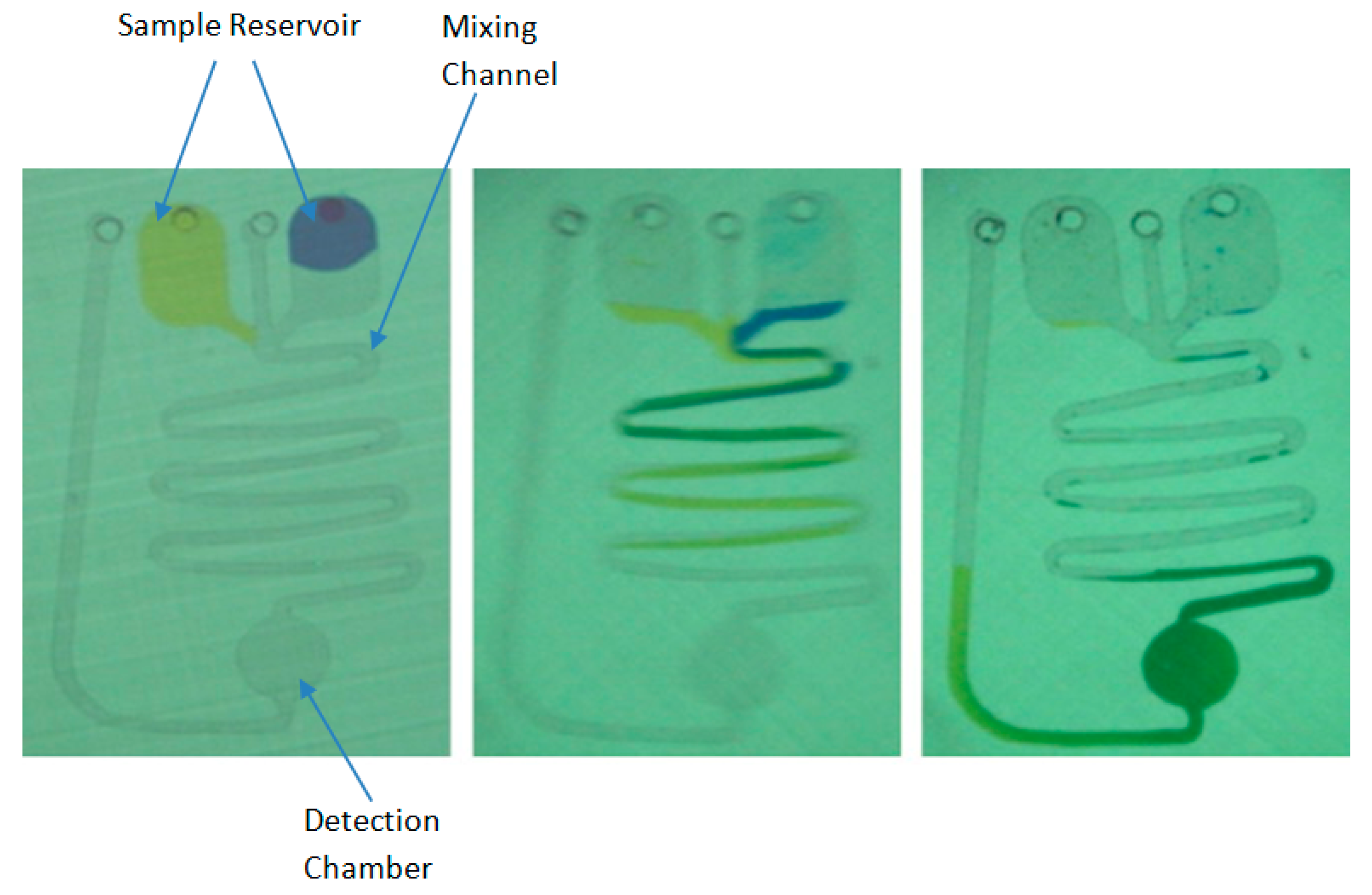
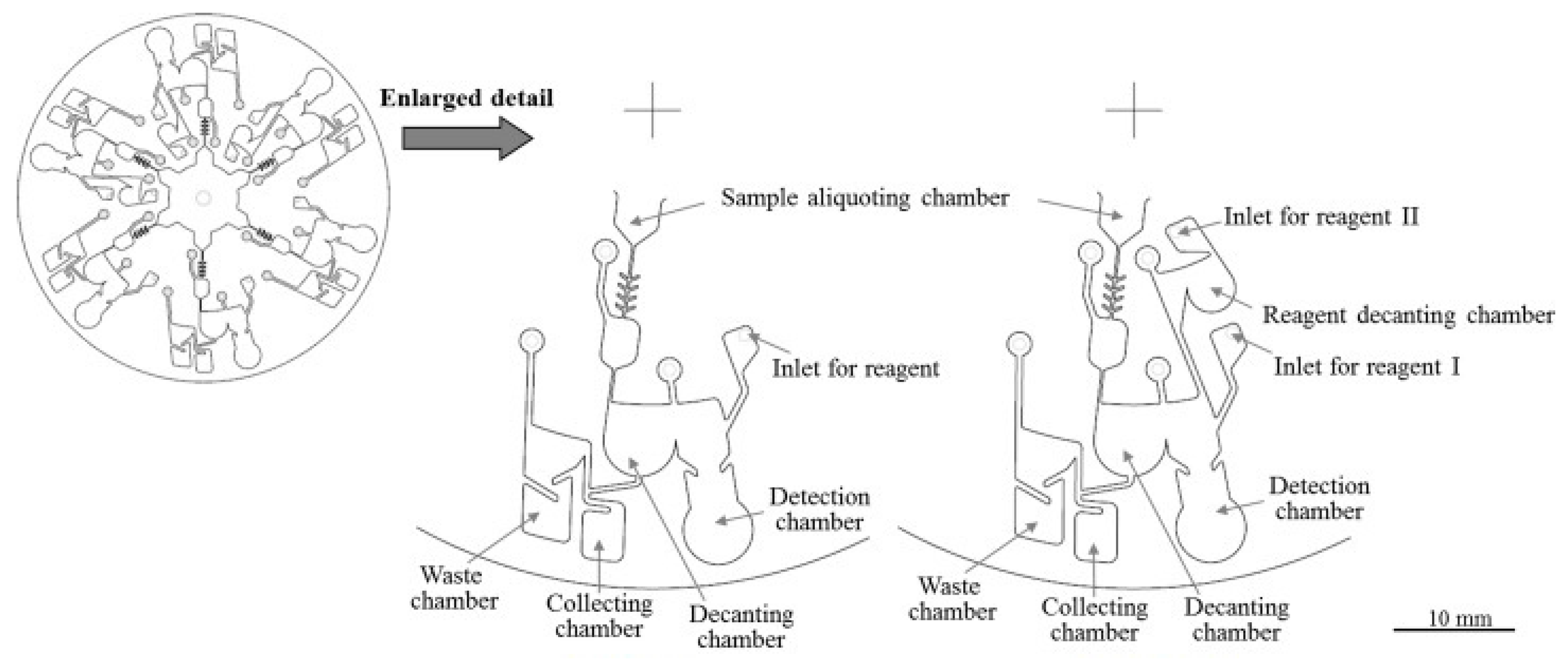
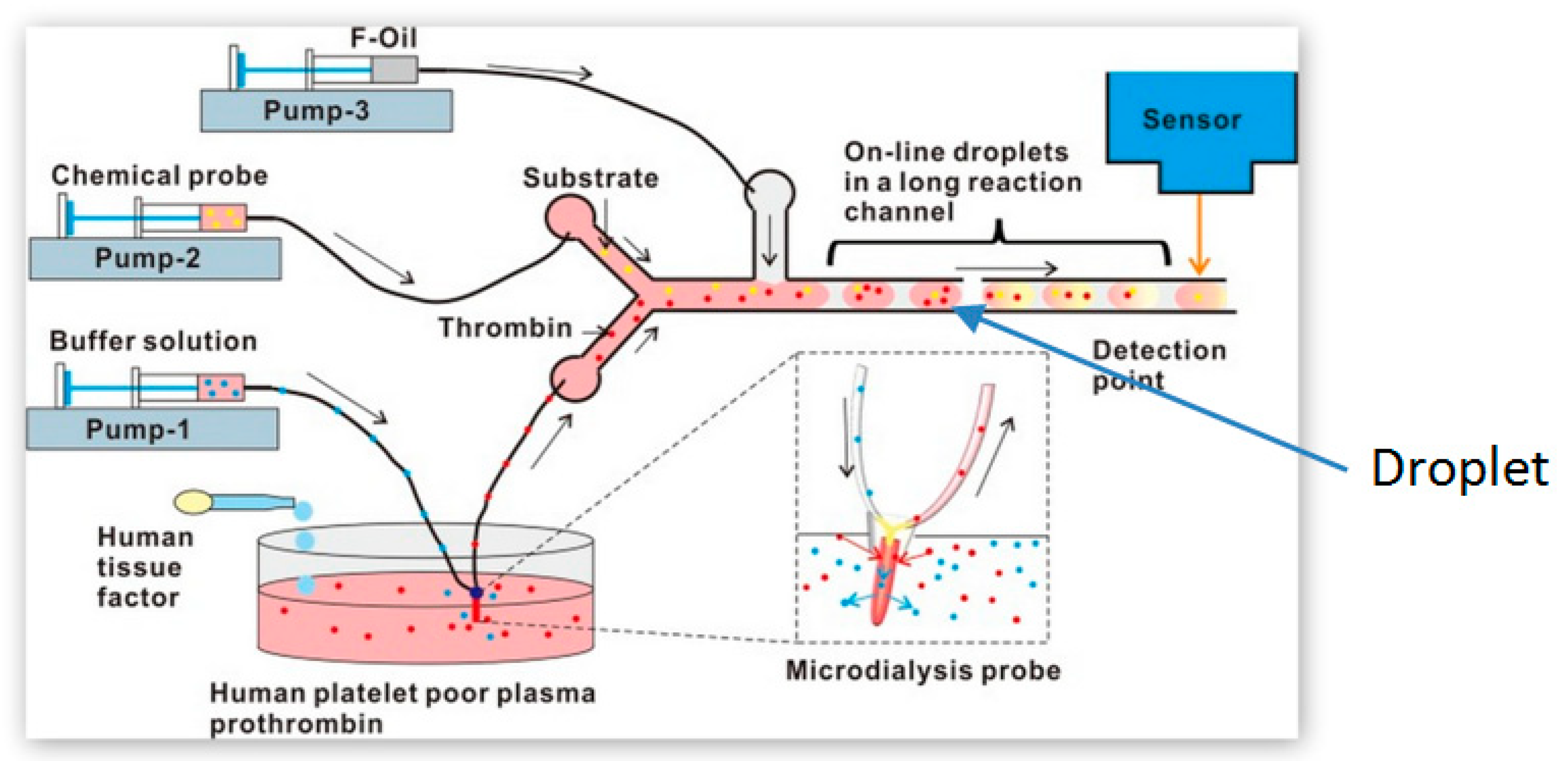
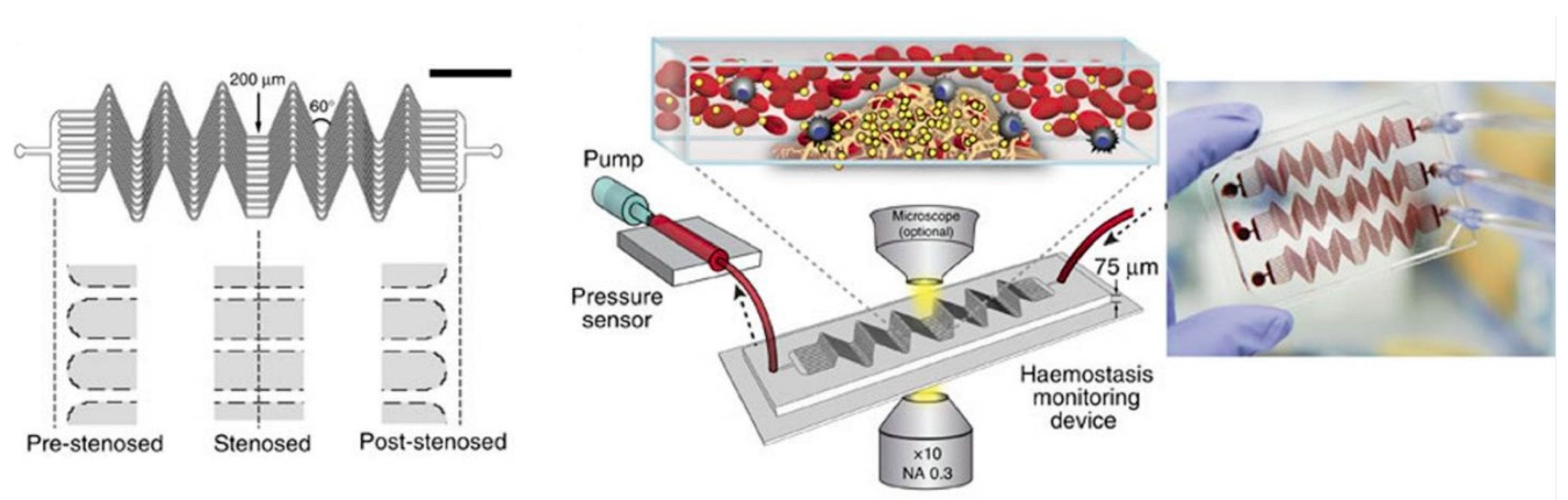
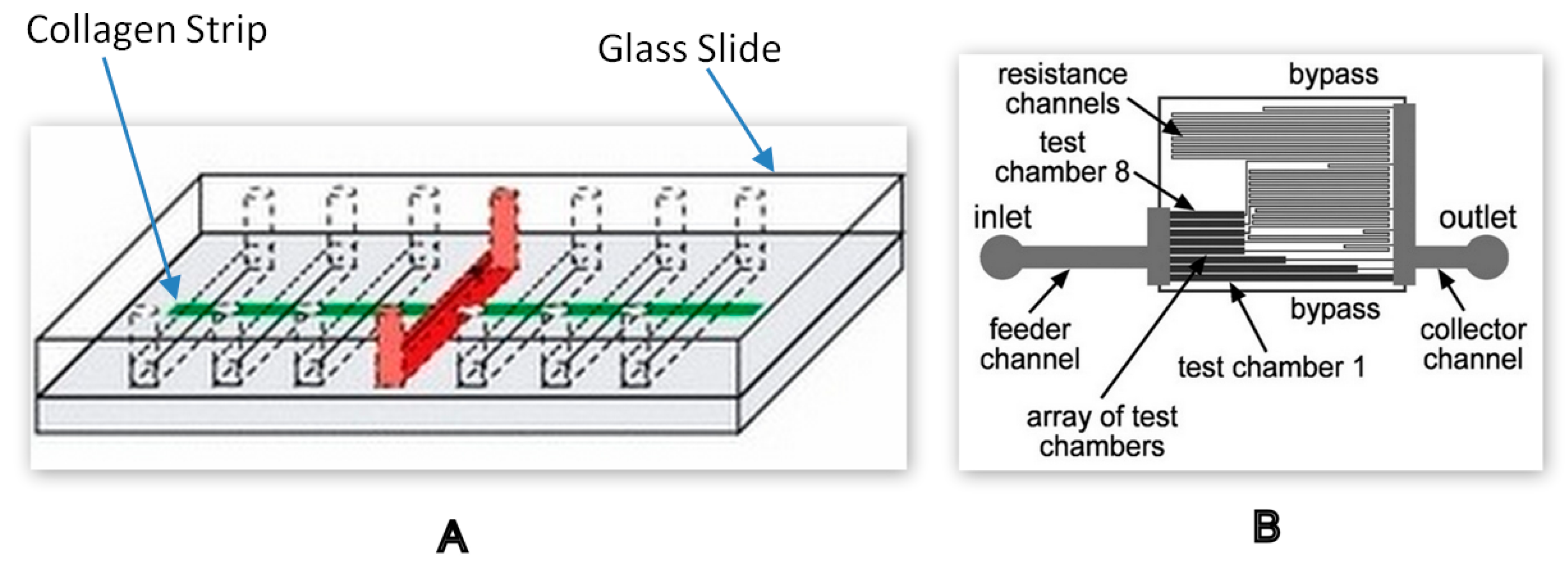
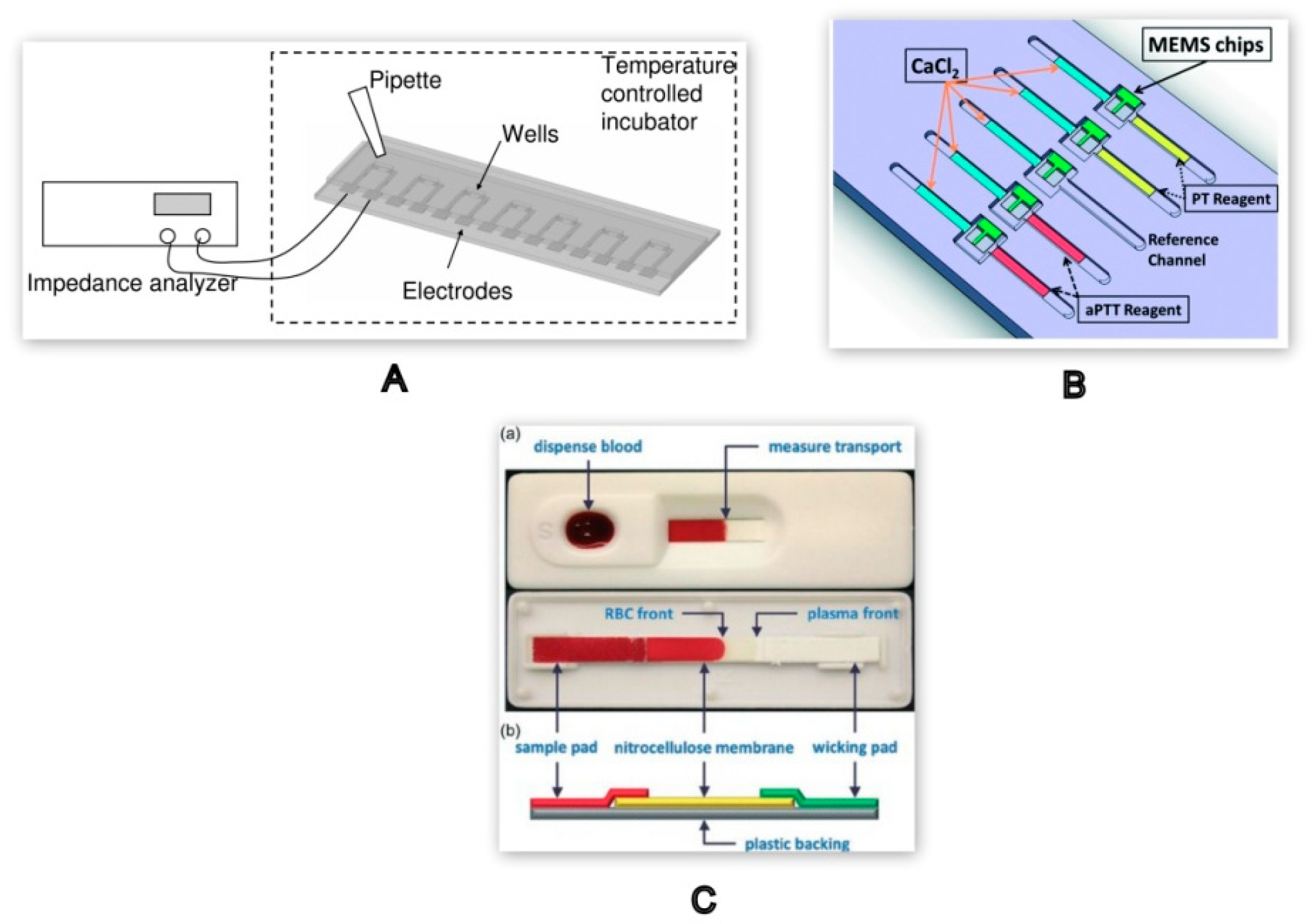
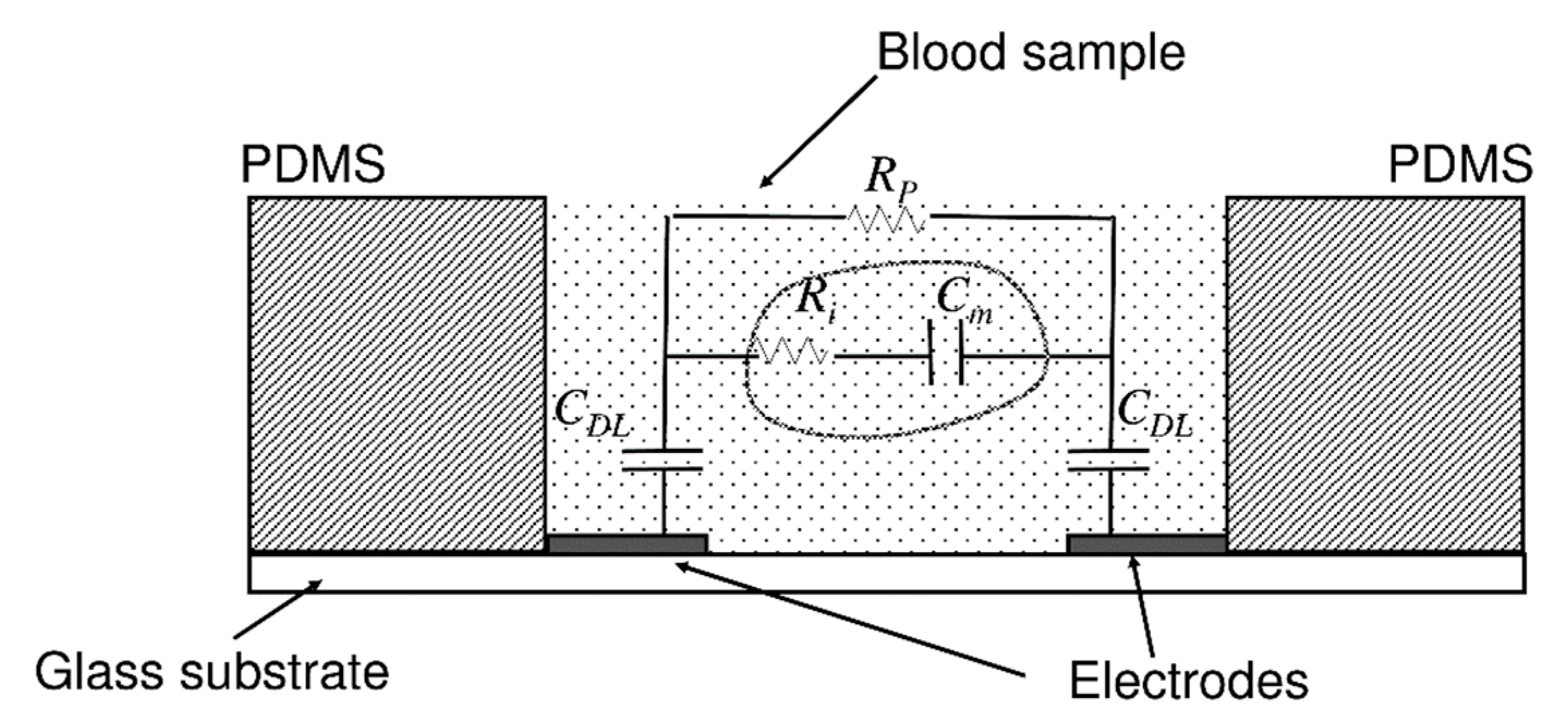
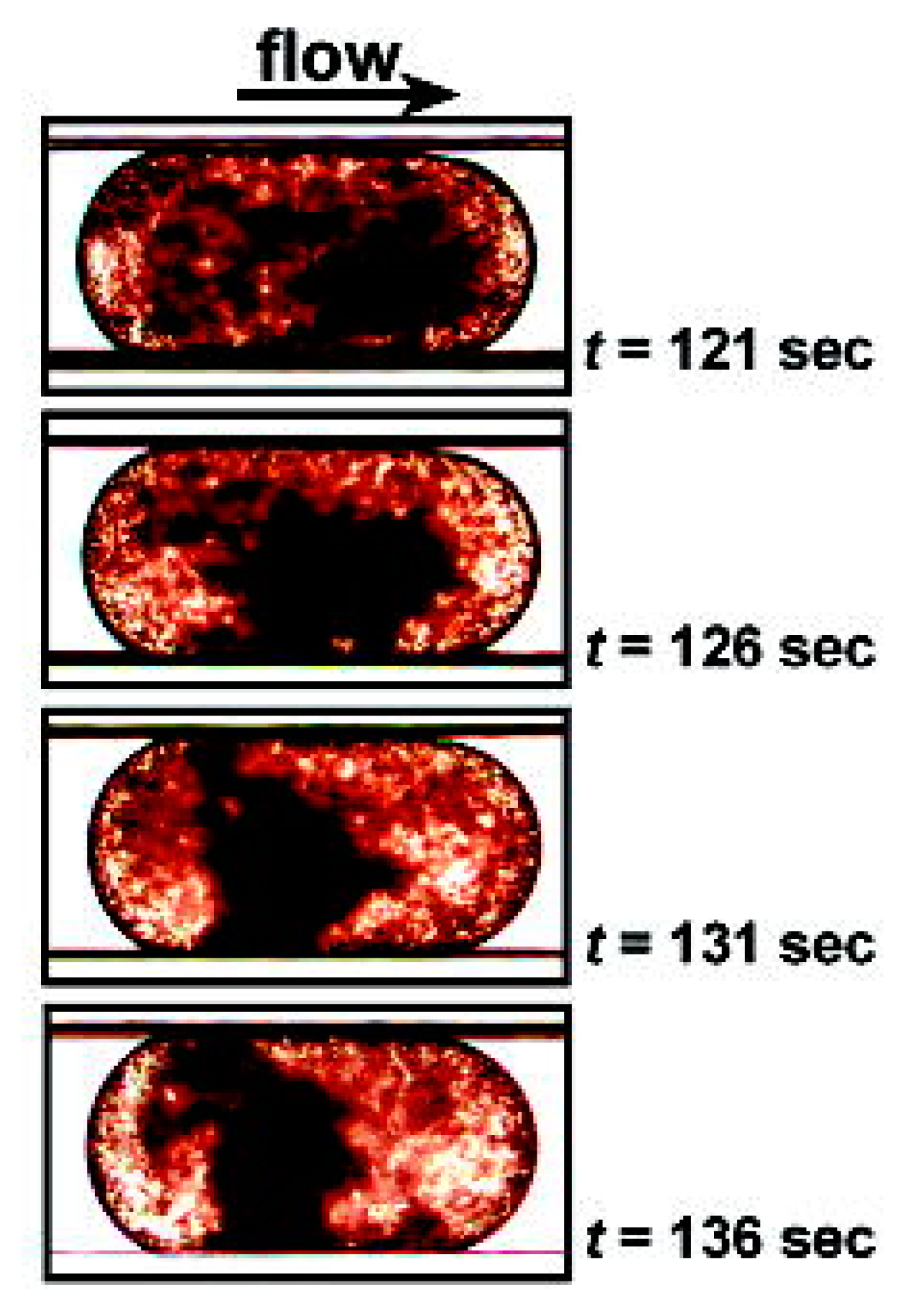

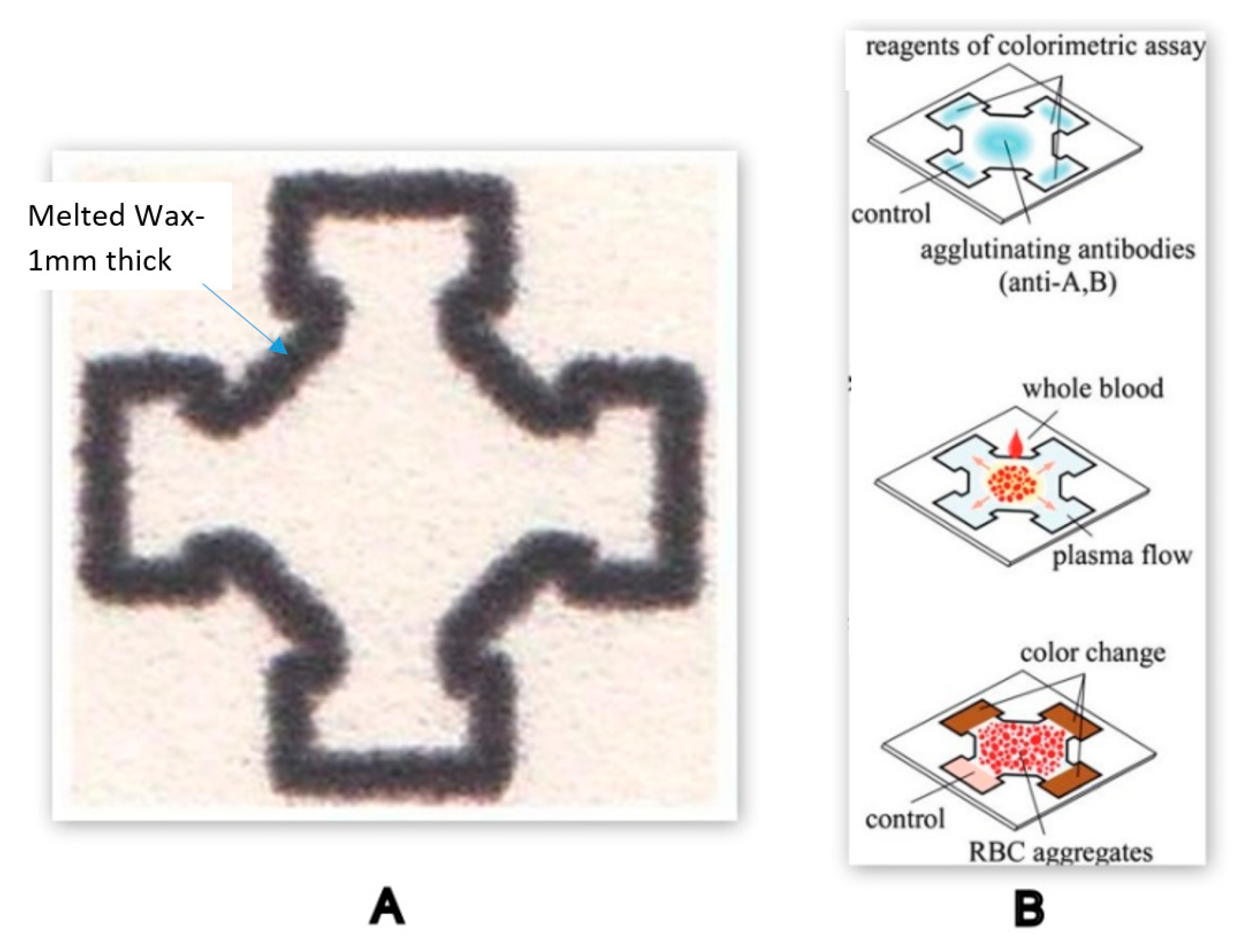

| PT/INR Monitoring Devices | ||
|---|---|---|
| Device | Features | Detection Method |
| Coumatrak (Biotrack, USA) | First Generation Device | Optical |
| Ciba Corning Biotrack 512 (Ciba Corning Diagnostics, USA) | First Generation Device | Optical |
| CoaguChek Series (Roche Diagnostics, Switzerland) | Second Generation small-sized handheld Devices Integrated with test strip quality check feature | Electro-chemical |
| iSTAT® PT/INR (Abbott Laboratories, USA) | Test cartridge integrated with sensors | Electro-chemical |
| INRatio/INRatio®2 (Alere Inc., USA) | Recalled in 2016 for erroneous results | Electro-mechanical (Electrical Impedance) |
| ProTime® Microcoagulation (International Technidyne Corp, USA) | Test cuvettes with micro-channels Integrated quality control system | Optical |
| Coag-Sense™ PT/INR Monitoring System (CoaguSense, Inc., USA) | Direct clot detection technology | Optical-mechanical |
| MicroINR® (iLine Microsystems S.L, Spain) | Test chip with one inlet and two micro-channels (Control and PT time test channel) | Optical |
| ProTime InRhythm™ (International Technidyne Corp, USA) | Pressure-driven clot formation and detection microchannel cuvette | Mechanical |
| CoagLite® and CoagMax® (Microvisk Technologies, UK) | MEMS-base | Electro-mechanical |
| Bio-AMD’s COAG (Bio-Alternative Medical Devices Ltd., UK) | Optical-magnetic mode of detection | Optical |
| Xprecia Stride™ Coagulation System (Siemens Healthineers, Germany) | Test strip consists of an electrochemical cell | Electro-chemical |
| ACT/aPTT Monitoring Devices | ||
| Device | Features | Detection Method |
| Hemochron Series (International Technidyne Corp, USA) | Test method – Test tube containing magnet and Test cuvettes preloaded with celite/kaolin/silica | Mechanical (First generation devices) Optical (Second generation devices) |
| HMS plus and ACT plus (Medtronic, USA) | Heparin Dosage Response assay | Optical |
| iSTAT (Abbott Laboratories, USA) | Multi-test analyser including PT/INR test with ACT and aPTT | Electro-chemical |
| Cascade POC and Actalyke® series (Helena Laboratories POC, USA) | Cascade POC - Paramagnetic particles-based assay Actalyke XL – Two-point clot detection technique | Optical-Mechanical |
| GEM® PCL Plus (Instrumentation Laboratory, USA) | Multi-test device for PT/INR, ACT and aPTT monitoring | Optical |
| Platelet Function Analysers | ||
| Device | Features | Detection Method |
| Multiplate® (Roche Diagnostics, Switzerland) | Impedance aggregometry and platelet reactivity assays | Electromechanical (Electrical Impedance) |
| PlateletWorks® (Helena Laboratories POC, USA) | Platelet aggregation and platelet reactivity monitoring based on platelet counts | Electromechanical (Electrical Impedance) |
| PFA-100/200 (Siemens Corporation, USA) | Flow based assay system | Mechanical |
| IMPACT-R Cone and Plate (Let) Analyser (DiaMed, Switzerland) | Shear stress dependent platelet adhesion monitoring | Optical |
| VerifyNow (Accumetrics, USA) | Electromagnetic induced mixing of sample and reagents | Optical |
| TEG® (Haemonetics Corp., USA) ROTEM® (Pentapharm GmbH, Germany) Sonoclot Analyser (Sienco Inc., USA) | Detailed Analysis of blood clot formation and lysis based on viscoelastic properties of blood | Mechanical |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jigar Panchal, H.; Kent, N.J.; Knox, A.J.S.; Harris, L.F. Microfluidics in Haemostasis: A Review. Molecules 2020, 25, 833. https://doi.org/10.3390/molecules25040833
Jigar Panchal H, Kent NJ, Knox AJS, Harris LF. Microfluidics in Haemostasis: A Review. Molecules. 2020; 25(4):833. https://doi.org/10.3390/molecules25040833
Chicago/Turabian StyleJigar Panchal, Heta, Nigel J Kent, Andrew J S Knox, and Leanne F Harris. 2020. "Microfluidics in Haemostasis: A Review" Molecules 25, no. 4: 833. https://doi.org/10.3390/molecules25040833
APA StyleJigar Panchal, H., Kent, N. J., Knox, A. J. S., & Harris, L. F. (2020). Microfluidics in Haemostasis: A Review. Molecules, 25(4), 833. https://doi.org/10.3390/molecules25040833




