Analytical Technique Optimization on the Detection of β-cyclocitral in Microcystis Species
Abstract
1. Introduction
2. Results
2.1. Analysis of VOCs Using SPME and the Modified Extraction Methods for Confirmation of the Reproducibility of the Previous Results
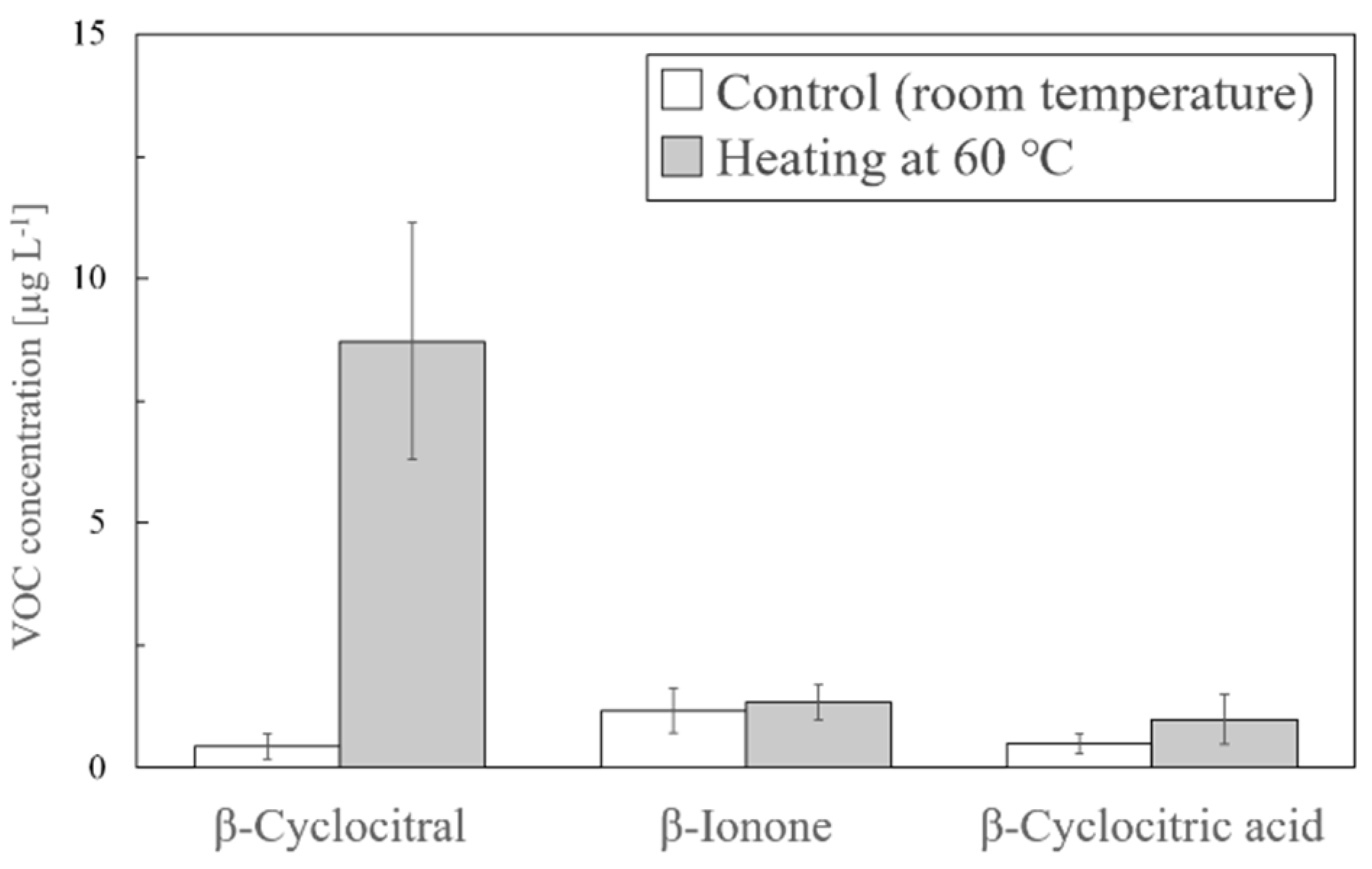
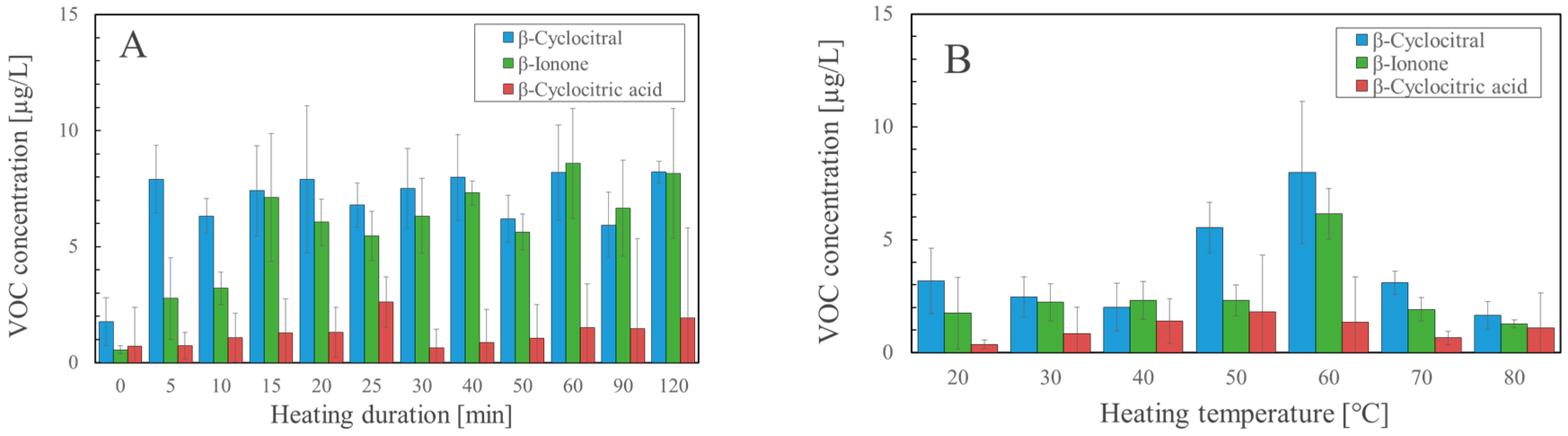
2.2. Factors Affecting the Formation of β-Cyclocitral during SPME
2.3. Optimal Heating Conditions for the Detection of β-Cyclocitral by the Solvent Extraction Method
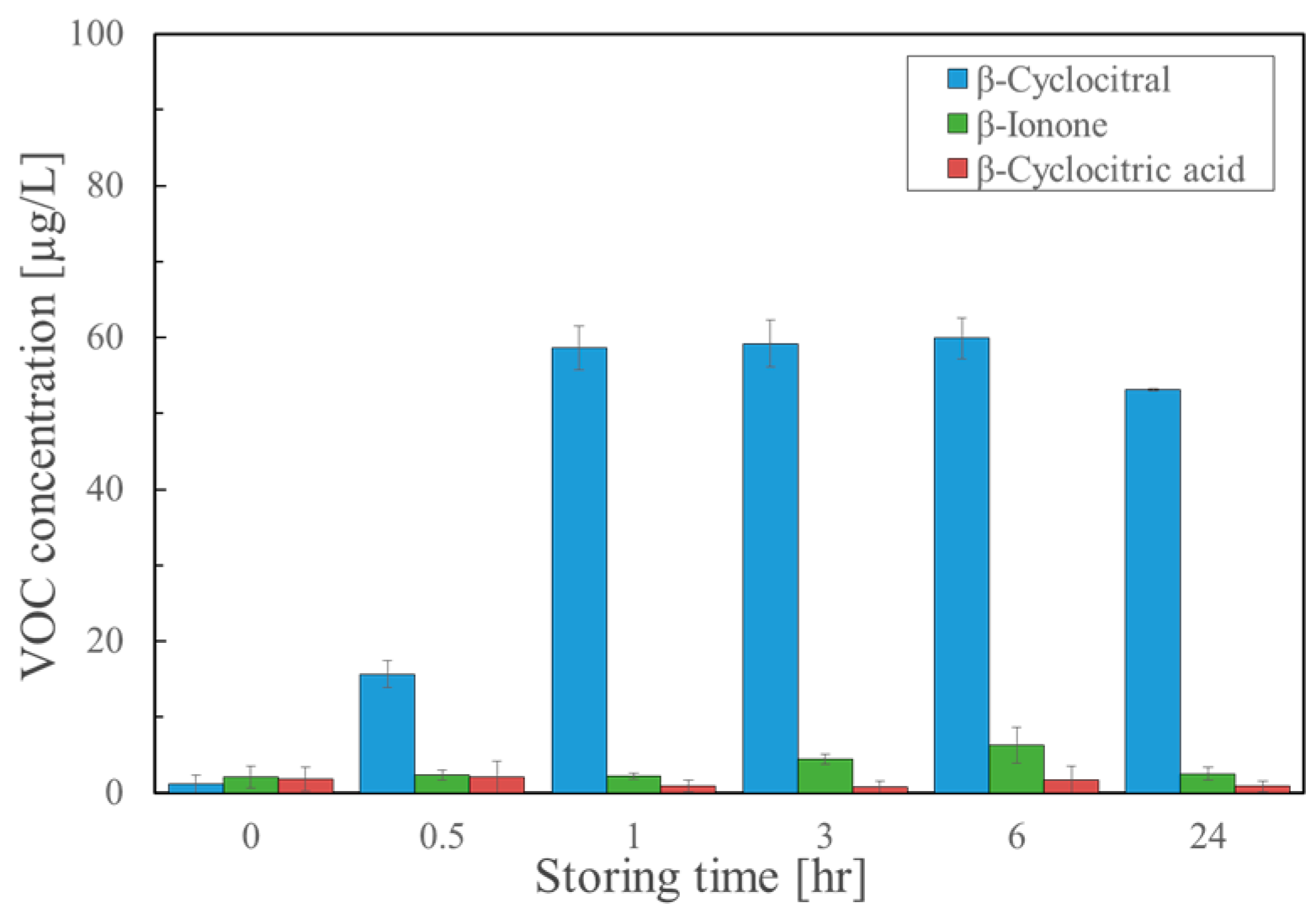
2.4. Effect of Acidification on the Formation of β-Cyclocitral
2.5. Comparison of the Established Methods with Typical SPME for Formation of β-Cyclocitral
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cyanobacteria Cultures
4.3. Analysis Procedure to Examine which Operations Affected the Formation of β-Cyclocitral during SPME
4.4. Analysis Procedure to Examine Optimal Heating Conditions for the Detection of β-cyclocitral by the Solvent Extraction Method
4.5. Analysis Procedure to Examine Effect of Storage Time after Acidification on the Formation of β-Cyclocitral
4.6. Comparison of the Established Methods with the Typical SPME for Formation of β-Cyclocitral
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. 2009, 1, 27–37. [Google Scholar] [CrossRef]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Jüttner, F.; Höflacher, B.; Wurster, K. Seasonal analysis of volatile organic biogenic substances (VOBS) in freshwater phytoplankton populations dominated by Dinobryon, Microcystis and Aphanizomenon. J. Phycol. 1986, 22, 169–175. [Google Scholar] [CrossRef]
- Jones, G.J.; Korth, W. In situ production of volatile odour compounds by river and reservoir phytoplankton populations in Australia. Water Sci. Technol. 1995, 31, 145–151. [Google Scholar] [CrossRef]
- Satchwill, T.; Watson, S.B.; Dixon, E. Odourous algal-derived alkenes: Differences in stability and treatment responses in drinking water. Water Sci. Technol. 2007, 55, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.B.; Monis, P.; Baker, P.; Giglio, S. Biochemistry and genetics of taste- and odor-producing cyanobacteria. Harmful Algae 2016, 54, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Ohta, A.; Iwata, C.; Horikawa, A.; Tsuji, K.; Ito, E.; Ikai, Y.; Harada, K.-I. Lysis of cyanobacteria with volatile organic compounds. Chemosphere 2008, 71, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Jüttner, F.; Watson, S.B.; von Elert, E.; Köster, O. β-cyclocitral, a grazer defence signal unique to the cyanobacterium. Microcystis. J. Chem. Ecol. 2010, 36, 1387–1397. [Google Scholar] [CrossRef]
- Zhang, K.; Lin, T.F.; Zhang, T.; Li, C.; Gao, N. Characterization of typical taste and odor compounds formed by Microcystis aeruginosa. Environ. Sci. 2013, 25, 1539–1548. [Google Scholar] [CrossRef]
- Arii, S.; Tsuji, K.; Tomita, K.; Hasegawa, M.; Bober, B.; Harada, K.-I. Cyanobacterial blue color formation during lysis process under natural conditions. Appl. Environ. Microbiol. 2015, 81, 2667–2675. [Google Scholar] [CrossRef]
- Harada, K.-I.; Ozaki, K.; Tsuzuki, S.; Kato, H.; Hasegawa, M.; Kuroda, E.K.; Arii, S.; Tsuji, K. Blue color formation of cyanobacteria with β-cyclocitral. J. Chem. Ecol. 2009, 35, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Hasegawa, M.; Arii, S.; Tsuji, K.; Bober, B.; Harada, K.-I. Characteristic oxidation behavior of β-cyclocitral from the cyanobacterium Microcystis. Environ. Sci. Poll. Res. 2016, 23, 1998–2006. [Google Scholar] [CrossRef] [PubMed]
- Ramel, H.; Mialoundama, A.; Havaux, M. Nonenzymic carotenoid oxidation and photooxidative stress signaling in plants. J. Exp. Bot. 2013, 64, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Auldridge, M.E.; McCarty, D.R.; Klee, H.J. Plant carotenoid cleavage oxygenases and their apocarotenoid products. Plant Biol. 2006, 9, 315–321. [Google Scholar] [CrossRef]
- Havaux, M. Carotenoid oxidation products as stress signals in plants. Plant J. 2013, 79, 597–606. [Google Scholar] [CrossRef]
- Cui, H.; Wang, Y.; Qin, S. Genomewide analysis of carotenoid cleavage dioxygenases in unicellular and filamentous cyanobacteria. Comp. Funct. Genom. 2012, 2012, 164690. [Google Scholar] [CrossRef]
- Fallon, R.D.; Brock, T.D. Lytic organisms and photooxidative effects: Influence on blue-green algae cyanobacteria in Lake Mendota, Wisconsin. Appl. Emviron. Microbiol. 1979, 38, 499–505. [Google Scholar] [CrossRef]
- Crayton, M.A. Toxic Cyanobacteria Blooms: A Field/Laboratory Guide. Office of Environmental Health Assessments; Washington State Department of Health: Olympia, WA, USA, 1993. [Google Scholar]
- Paerl, H.W.; Fulton, R.S.; Moisander, P.H.; Dyble, J. Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Sci. World J. 2001, 1, 76–113. [Google Scholar] [CrossRef]
- Fujise, D.; Tsuji, K.; Fukushima, N.; Kawai, K.; Harada, K.-I. Analytical aspects of cyanobacterial volatile organic compounds for investigation of their production behavior. J. Chromatogr. A 2010, 1217, 6122–6125. [Google Scholar] [CrossRef]
- Arii, S.; Tsuji, K.; Tomita, K.; Hasegawa, M.; Yamashita, R.; Bober, B.; Harada, K.-I. Densification of cyanobacteria from a lake leading to production of β-cyclocitral and related VOCs and species change. Phycol. Res. 2018, 66, 161–166. [Google Scholar] [CrossRef]
- Reynolds, C.S. Phytoplankton assemblages and their periodicity in stratifying lake systems. Holarctic Ecol. 1980, 3, 141–159. [Google Scholar] [CrossRef]
- Watson, S.B. Cyanobacterial and eukaryotic algal odour compounds: Signals or by-products? A review of their biological activity. Phycologia 2003, 42, 332–350. [Google Scholar] [CrossRef]
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Merkle, S.; Kleeberg, K.K.; Fritsche, J. Recent developments and applications of sold phase microextraction (SPME) in food and environmental analysis—A review. Chromatography 2015, 2, 293–381. [Google Scholar] [CrossRef]
- Spietelun, A.; Pilarczyk, M.; Kloskowski, A.; Namiesnik, J. Current trends in solid-phase microextraction (SPME) fibre coatings. Chem. Soc. Rev. 2010, 39, 4524–4537. [Google Scholar] [CrossRef]
- Barreira, L.; Duporte, G.; Ronkko, T.; Parshintsev, J.; Hartonen, K.; Hyrsky, L.; Heikkinen, E.; Jussila, M.; Kulmala, M.; Riekkola, M. Field measurements of biogenic volatile organic compounds in the atmosphere using solid-phase microextraction Arrow. Atmos. Meas. Tech. 2018, 11, 881–893. [Google Scholar] [CrossRef]
- Biajoli, A.F.P.; Augusto, F. Solid phase microextraction fibers coated with sol-gel aminopropylsilica/ polydimethylsiloxane: Development and its application to screening of beer headspace. Anal. Sci. 2008, 24, 1141–1146. [Google Scholar] [CrossRef]
- Harrison, P.J.; Bugg, T. Enzymology of the carotenoid cleavage dioxygenases: Reaction mechanisms, inhibition and biochemical roles. Arch. Biochem. Biophys. 2014, 544, 105–111. [Google Scholar] [CrossRef]
- Chia, M.A.; Jankowiak, J.G.; Kramer, B.J.; Goleski, J.A.; Huang, I.-S.; Zimba, P.V.; Bittencourt-Oliceira, M.C.; Gobler, C.J. Succession and toxicity of Microcystis and Anabaena (Dolichospermum) blooms are controlled by nutrient-dependent allelopathic interactions. Harmful Algae 2018, 74, 67–77. [Google Scholar] [CrossRef]
- Ichimura, T. Media for blue-green algae. In Methods in algalogical studies; Nishizawa, K., Chihara, M., Eds.; Kyoritsu: Tokyo, Japan, 1978; pp. 294–305. [Google Scholar]
Sample Availability: Samples of the compounds and all materials except β-cyclocitric acid are commercially available. β-Cyclocitric acid can be prepared from β-cyclocitral and is described in previous report [12]. |

| 2008 | 2008 | 2010 | 2010 | 2013 | 2014 | 2016 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total VOC | Filtrate VOC | Total VOC | Filtrate VOC | Total VOC | Filtrate VOC | Total VOC | Filtrate VOC | Total VOC | Filtrate VOC | Total VOC | Filtrate VOC | Total VOC | Filtrate VOC | |
| Method | SPME | SPME | SPME | SPME | S.E. | S.E. | SPME | S.E. | SPME | S.E. | ||||
| Cell number [cells/mL] | 6.2 × 104 | 3.1 × 104 | 4.2 × 107 | 7.6 × 107 | 2.0 × 105 | 8.5 × 105 | 2.0 × 107 | 8.4 × 106 | ||||||
| pH | 6.7 | 5.4 | 5.9 | 9.9 | 8.7 | 6.2 | 10.0 | 10.0 | ||||||
| β-Cyclocitral [µg/L] | 50 | 0.9 | 100 | 55 | 1400 | 3.2 | 52 | 1.2 | ND | ND | 136 | ND | 93 | ND |
| β-Cyclocitric acid [µg/L] | - | - | - | - | - | - | - | - | 52 | 120 | ND | 36 | - | 20 |
| β-Ionone [µg/L] | 9.6 | 7.6 | 98 | 15 | 300 | 1.6 | 26 | 0.5 | 120 | 150 | 15.7 | - | 22 | ND |
| Trial No. | Heat | Salt | Shake | β-Cyclocitral [µg/L] |
|---|---|---|---|---|
| 1) | + | + | + | 9.7 |
| 2) | + | + | - | 9.3 |
| 3) | + | - | + | 14.3 |
| 4) | + | - | - | 12.3 |
| 5) | - | + | + | 6.6 |
| 6) | - | + | - | 0 |
| 7) | - | - | + | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashita, R.; Bober, B.; Kanei, K.; Arii, S.; Tsuji, K.; Harada, K.-i. Analytical Technique Optimization on the Detection of β-cyclocitral in Microcystis Species. Molecules 2020, 25, 832. https://doi.org/10.3390/molecules25040832
Yamashita R, Bober B, Kanei K, Arii S, Tsuji K, Harada K-i. Analytical Technique Optimization on the Detection of β-cyclocitral in Microcystis Species. Molecules. 2020; 25(4):832. https://doi.org/10.3390/molecules25040832
Chicago/Turabian StyleYamashita, Ryuji, Beata Bober, Keisuke Kanei, Suzue Arii, Kiyomi Tsuji, and Ken-ichi Harada. 2020. "Analytical Technique Optimization on the Detection of β-cyclocitral in Microcystis Species" Molecules 25, no. 4: 832. https://doi.org/10.3390/molecules25040832
APA StyleYamashita, R., Bober, B., Kanei, K., Arii, S., Tsuji, K., & Harada, K.-i. (2020). Analytical Technique Optimization on the Detection of β-cyclocitral in Microcystis Species. Molecules, 25(4), 832. https://doi.org/10.3390/molecules25040832






