Synthesis of Natural (−)-Antrocin and Its Enantiomer via Stereoselective Aldol Reaction
Abstract
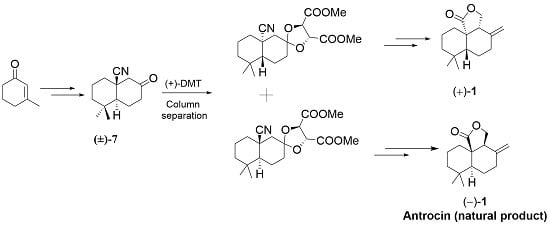
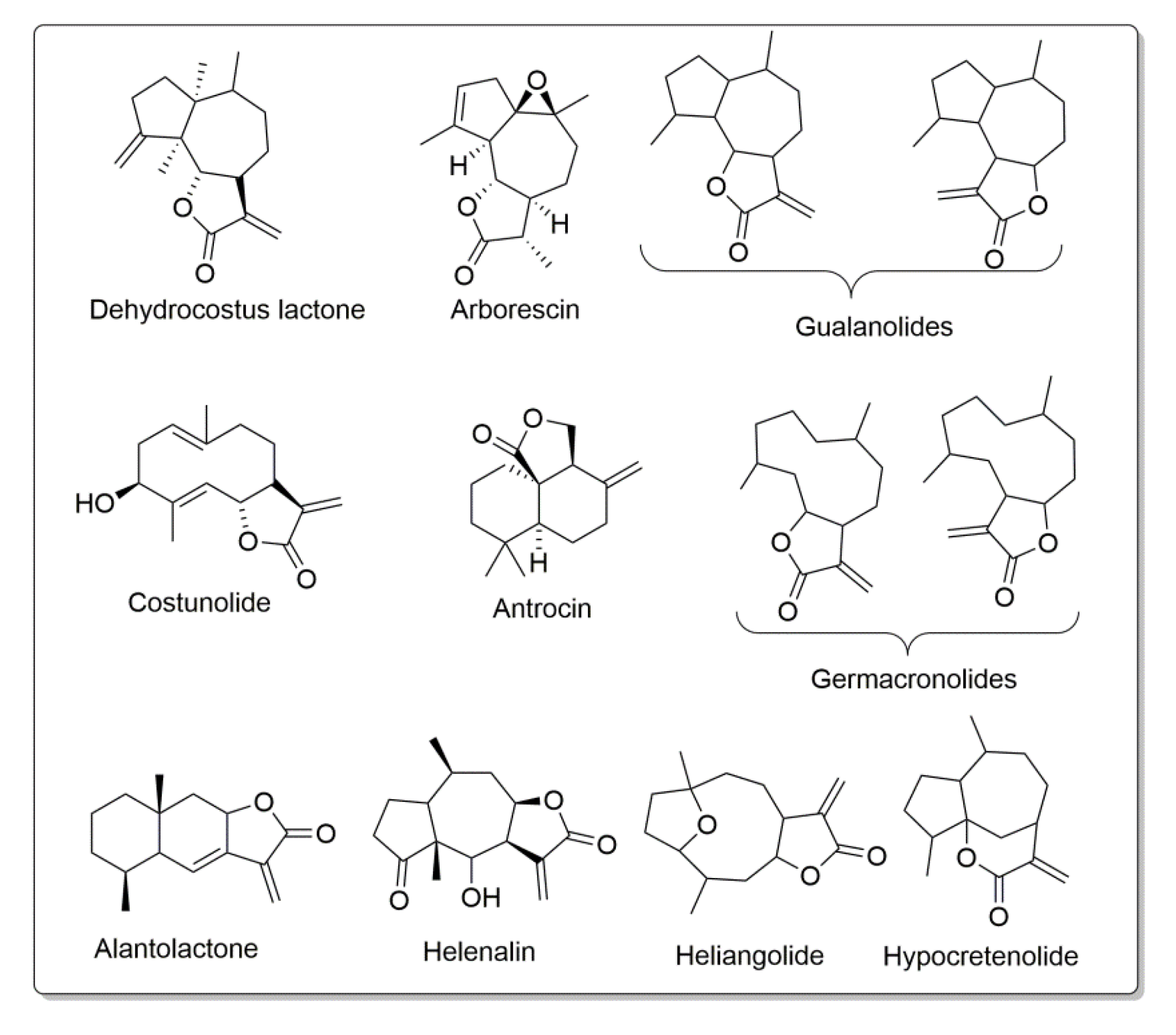
1. Introduction
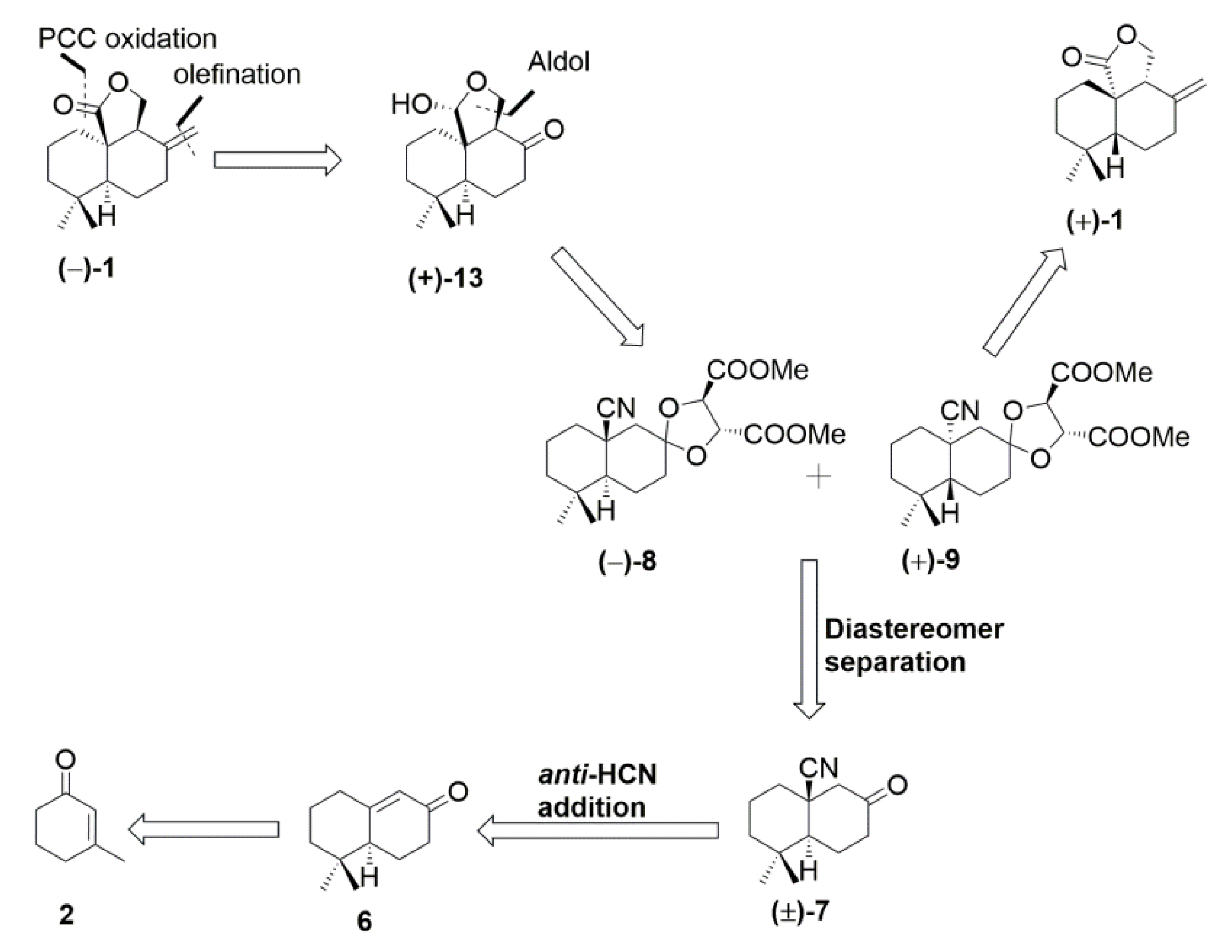
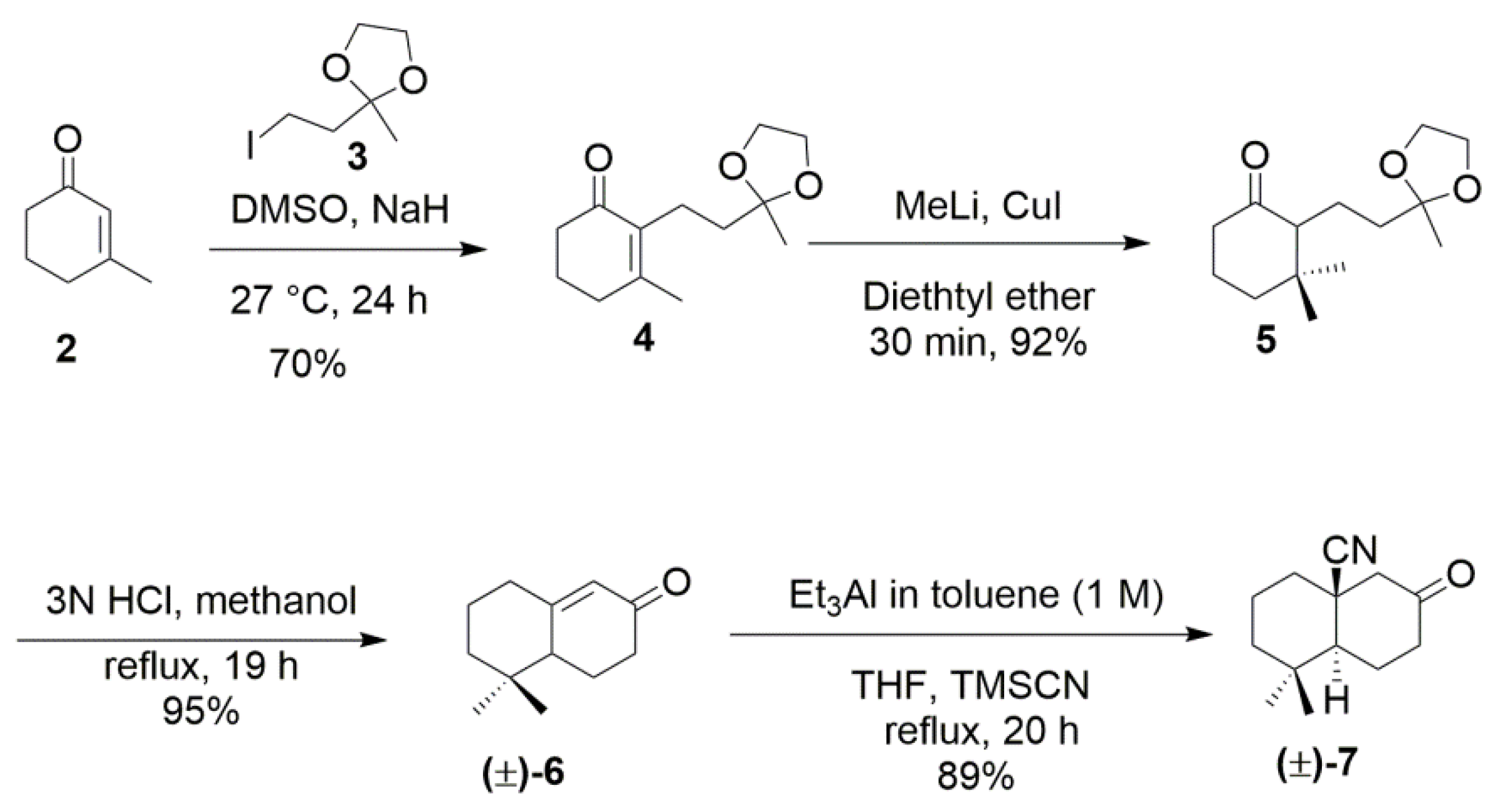
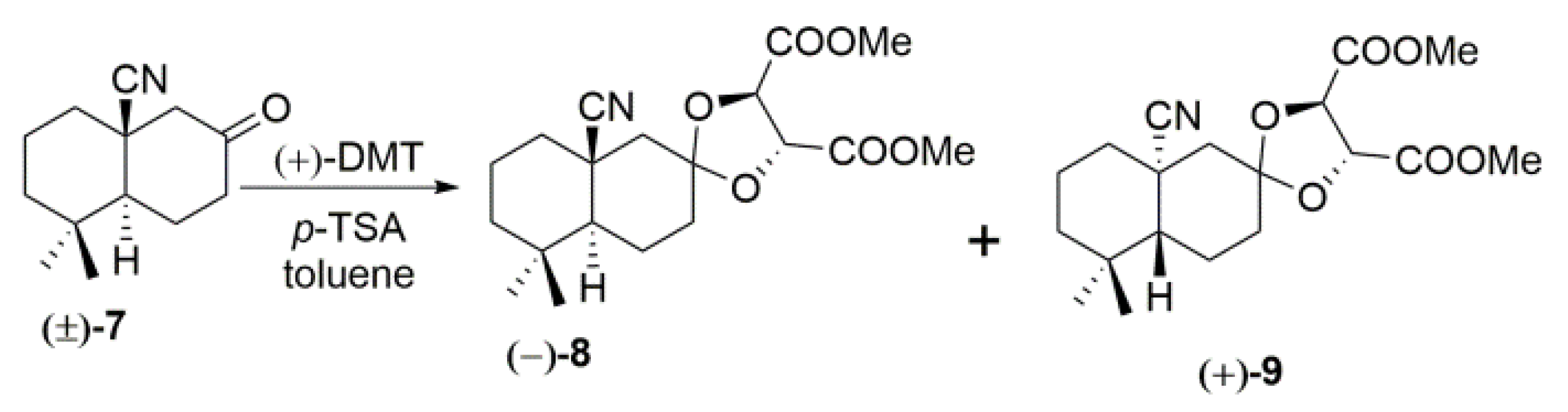
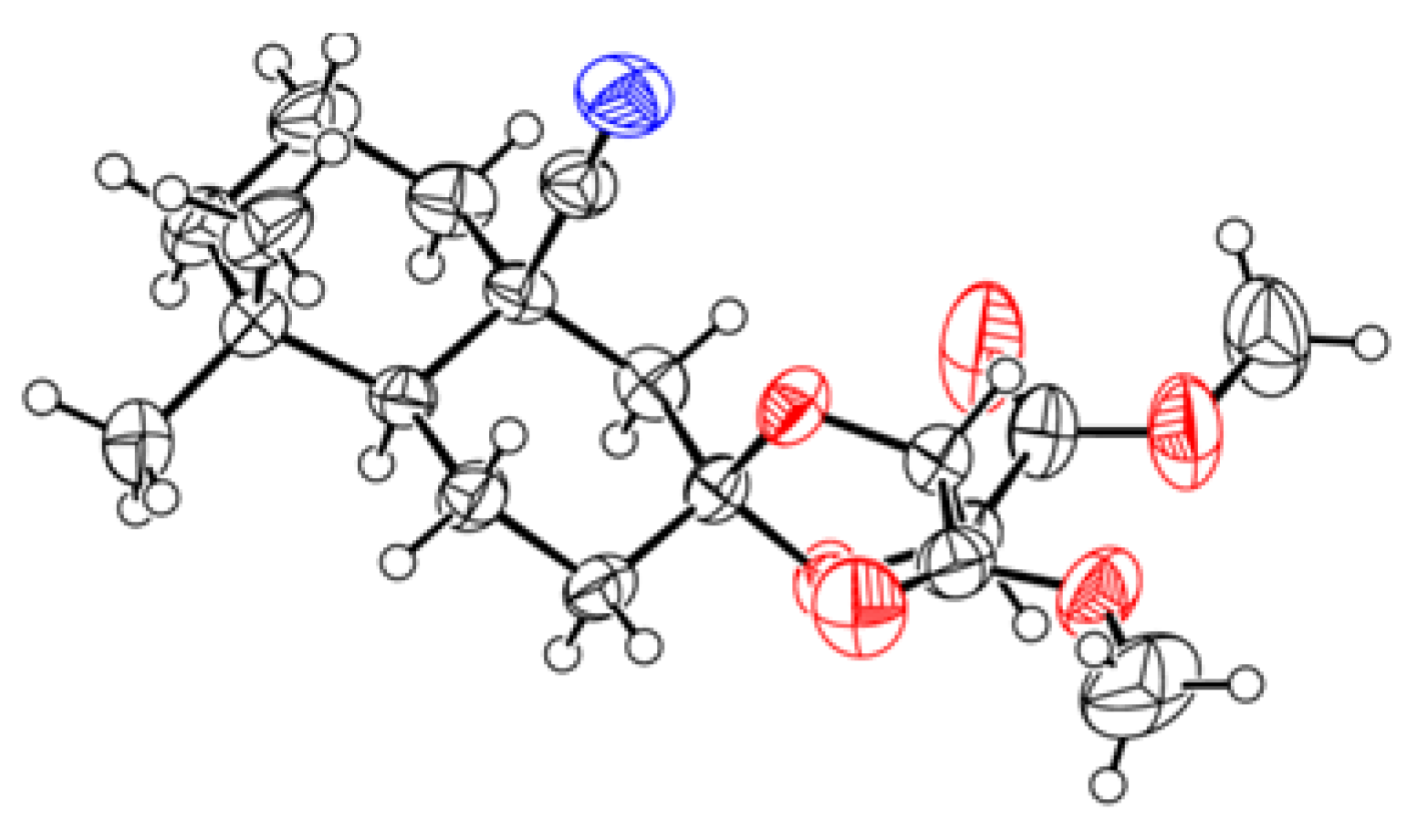
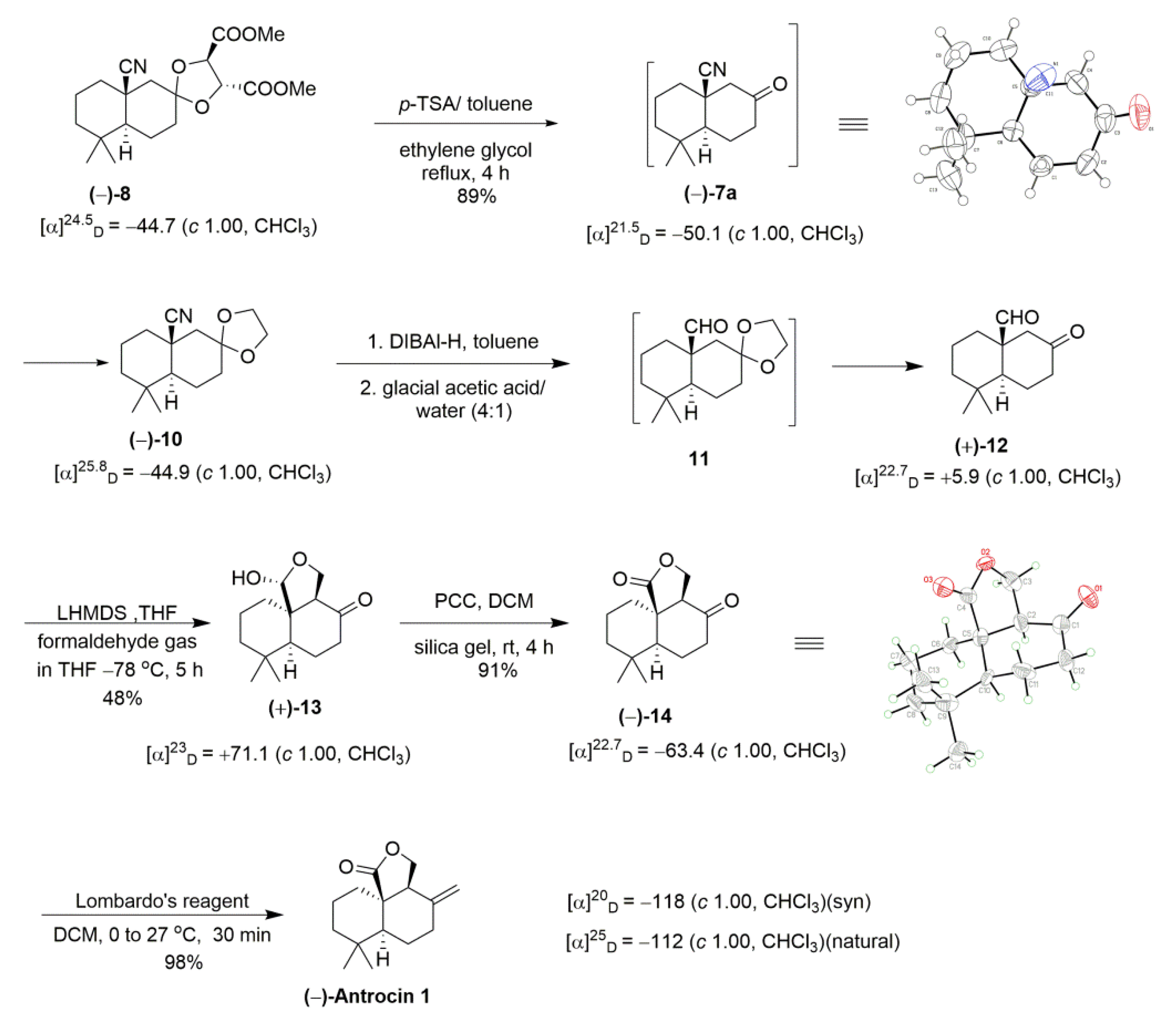
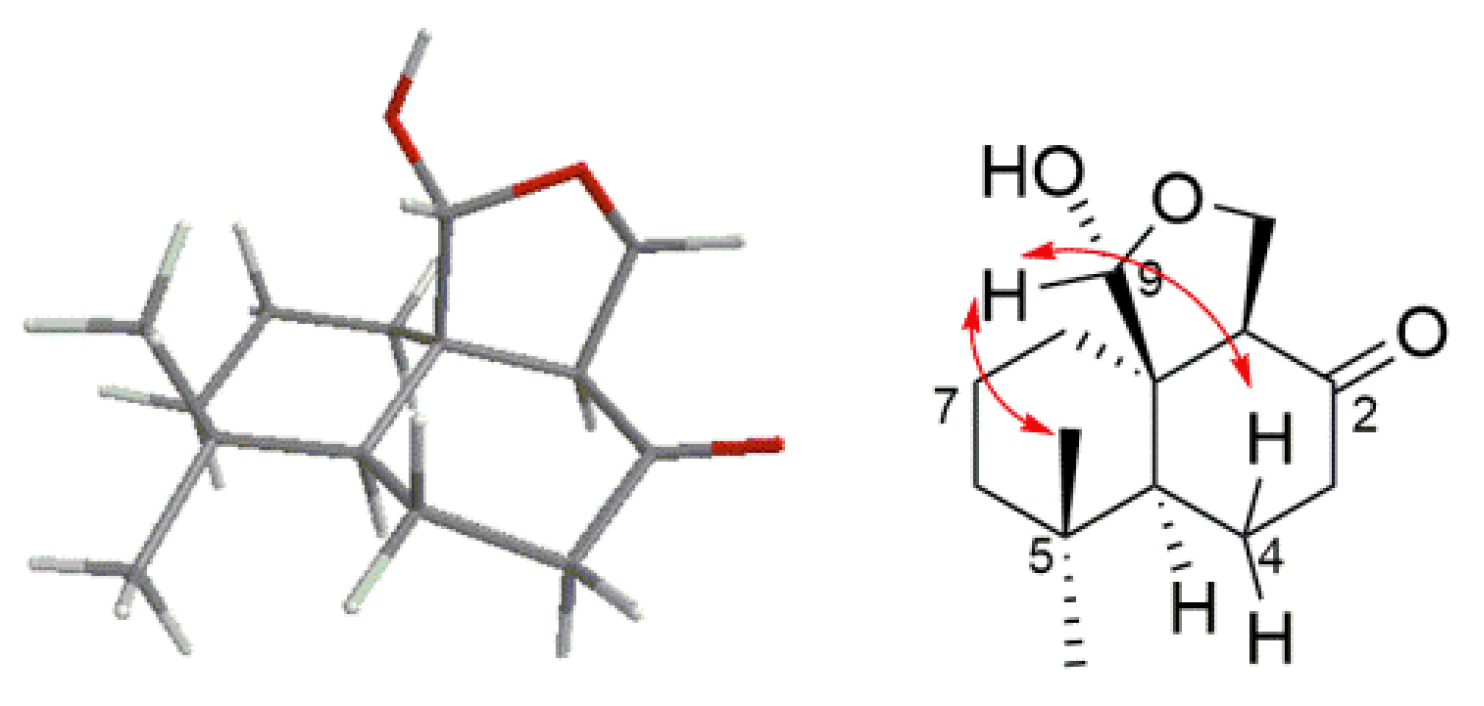
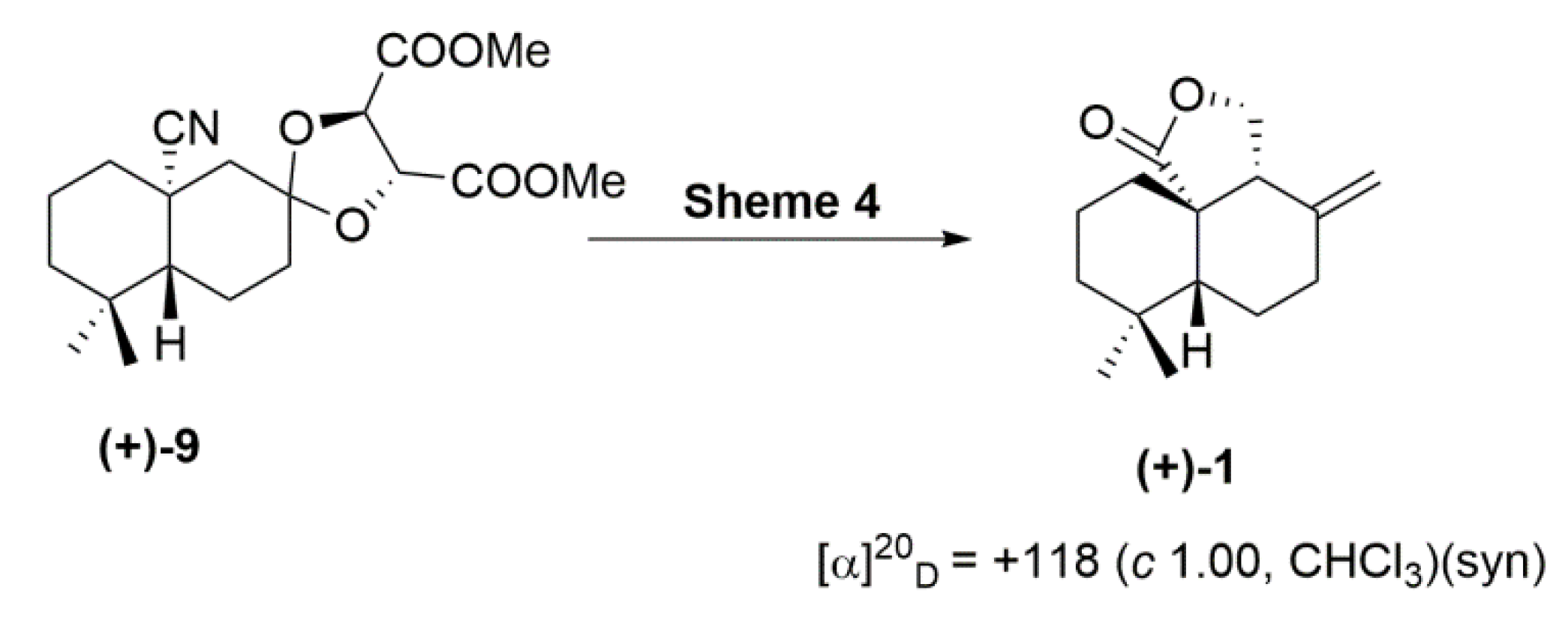
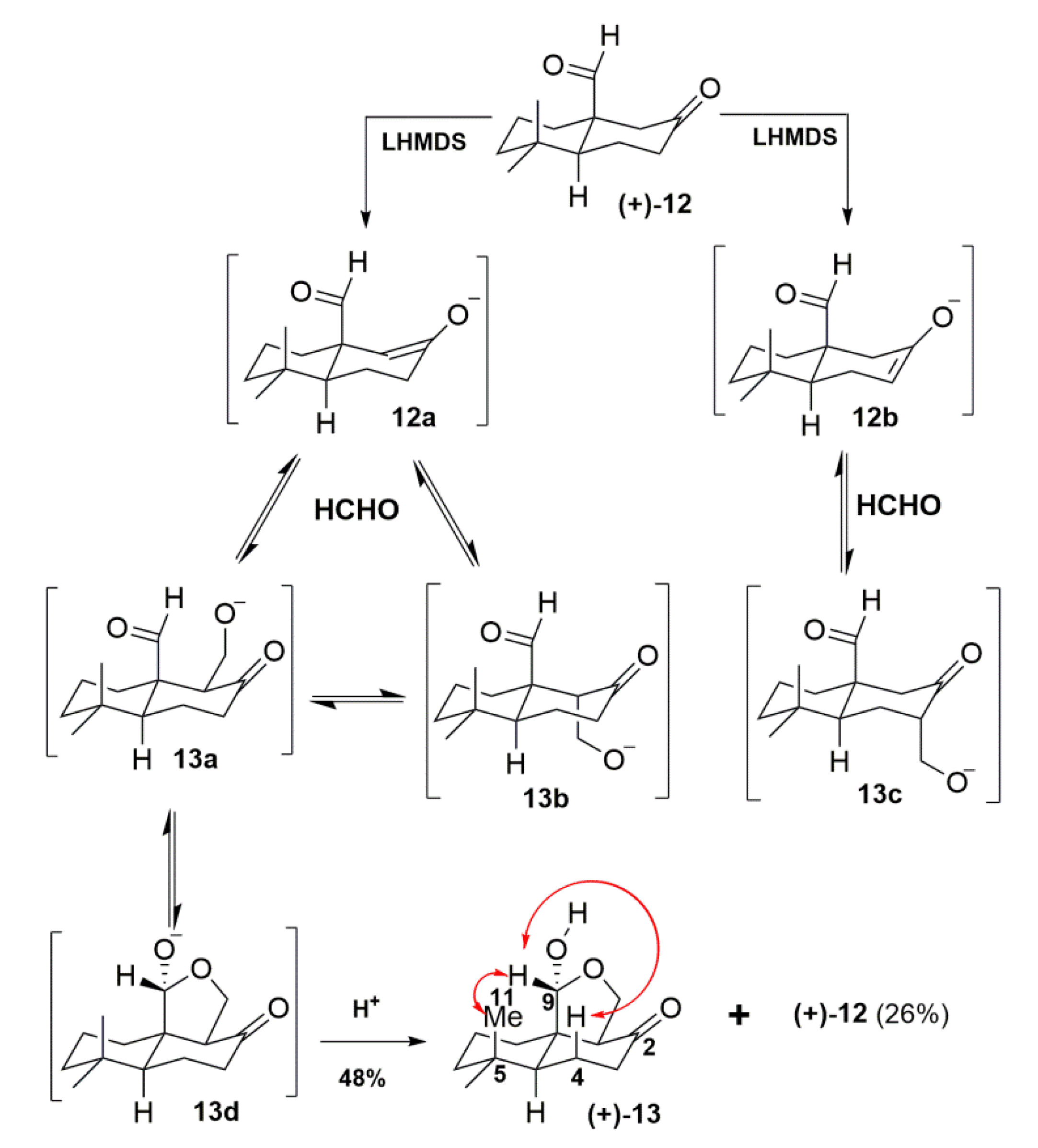
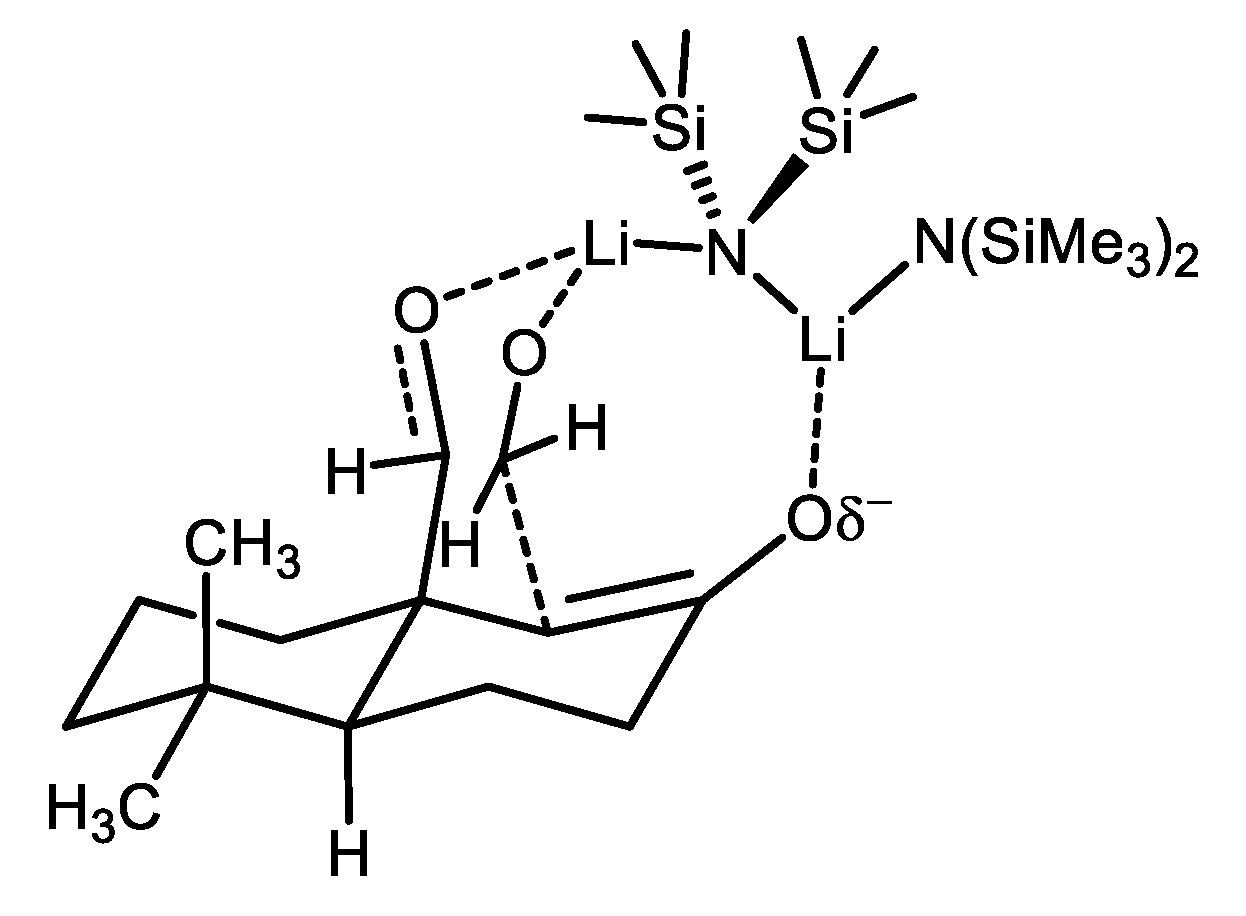
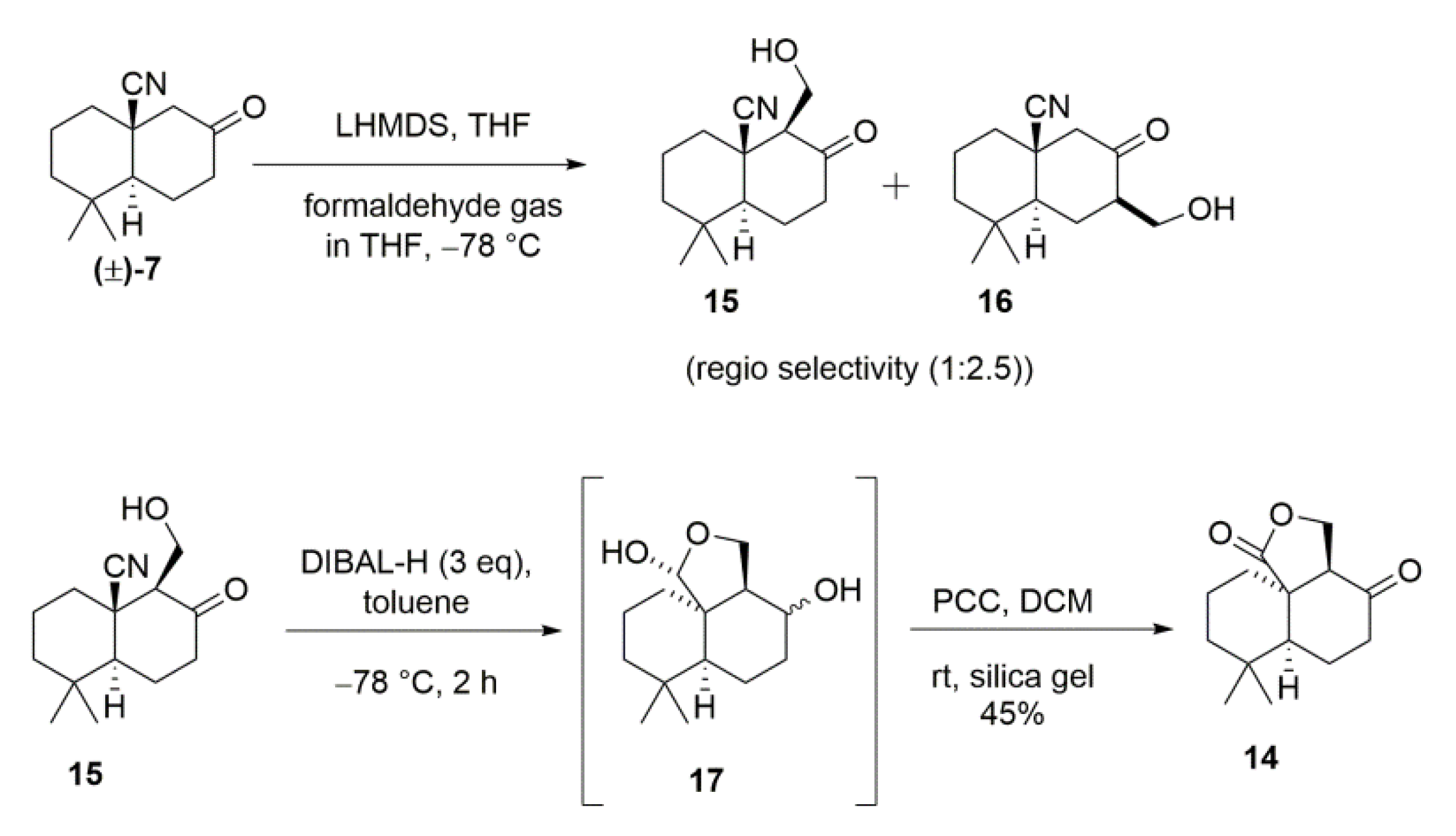
2. Results and Discussion
3. Conclusions
4. Experimental
General Conditions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neerman, M.F. Sesquiterpene lactones: A diverse class of compounds found in essential oils possessing antibacterial and antifungal properties. Int. J. Aromatherapy 2003, 13, 114–120. [Google Scholar] [CrossRef]
- Seaman, F.C.; Funk, V.A. Cladistic analysis of complex natural products: Developing transformation series from sesquiterpene lactone data. Taxon 1983, 32, 1–27. [Google Scholar] [CrossRef][Green Version]
- Jansen, B.J.M.; de Groot, A. The occurrence and biological activity of drimane sesquiterpenoids. Nat. Prod. Rep. 1991, 8, 309–318. [Google Scholar] [CrossRef]
- Zhao, S.S.; Leung, K.S.-Y. Quality evaluation of mycelial Antrodia camphorata using high-performance liquid chromatography (HPLC) coupled with diode array detector and mass spectrometry (DAD-MS). Chin. Med. 2010, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Liu, Y.-W.; Ker, Y.-B.; Wu, Y.-Y.; Lai, E.Y.; Chyau, C.-C.; Hseu, T.-H.; Peng, R.Y. Chemical Characterization and Anti-inflammatory Effect of Polysaccharides Fractionated from Submerge-Cultured Antrodia camphorata Mycelia. J. Agric. Food Chem. 2007, 55, 5007–5012. [Google Scholar] [CrossRef] [PubMed]
- Geethangili, M.; Tzeng, Y.-M. Review of Pharmacological Effects of Antrodia camphorata and Its Bioactive Compounds. Evid Based Complement Alternat. Med. 2011, 2011, 212641. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C. King of Ganoderma: Antrodia camphorata in Taiwan, 2nd ed.; Yuen Chi Jai Book Publishing: Taipei, Taiwan, 2008. [Google Scholar]
- Wu, D.-P.; Chiang, H.-C. Constituents of Antrodia Cinnamomea. J. Chin. Chem. Soc. 1995, 42, 797–800. [Google Scholar] [CrossRef]
- Lee, I.-H.; Huang, R.-L.; Chen, C.-T.; Chen, H.-C.; Hsu, W.-C.; Lu, M.-K. Antrodia camphorata polysaccharides exhibit anti-hepatitis B virus effects. Microbiol. Lett. 2002, 209, 63–67. [Google Scholar] [CrossRef]
- Hseu, Y.-C.; Chen, S.-C.; Chen, H.-C.; Liao, J.-W.; Yang, H.-L. Antrodia camphorata inhibits proliferation of human breast cancer cells in vitro and in vivo. Food Chem. Toxicol. 2008, 46, 2680–2688. [Google Scholar] [CrossRef]
- Lee, Y.-P.; Tsai, W.-C.; Ko, C.-J.; Rao, Y.K.; Yang, C.-R.; Chen, D.-R.; Yang, M.-H.; Yang, C.-C.; Tzeng, Y.-M. Anticancer Effects of Eleven Triterpenoids Derived from Antrodia camphorata. Anticancer Res. 2012, 32, 2727–2734. [Google Scholar]
- Ao, Z.-H.; Xu, Z.-H.; Lu, Z.-M.; Xu, H.-Y.; Zhang, X.-M.; Dou, W.-F. Niuchangchih (Antrodia camphorata) and its potential in treating liver diseases. J. Ethnopharmacol. 2009, 121, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Wu, A.T.H.; Tzeng, D.T.W.; Huang, C.-C.; Tzeng, Y.-M.; Chao, T.-Y. Antrocin, a bioactive component from Antrodia cinnamomea, suppresses breast carcinogenesis and stemness via downregulation of β-catenin/Notch1/Akt signaling. Phytomedicine 2019, 52, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Hung-Chen, C.; De-Peng, W.; Cherng, I.W.; Chuen-Her, U. A sesquiterpene lactone, phenyl and biphenyl compounds from Antrodia cinnamomea. Phytochemistry 1995, 39, 613–616. [Google Scholar] [CrossRef]
- Rao, Y.K.; Wu, A.T.H.; Geethangili, M.; Huang, M.-T.; Chao, W.-J.; Wu, C.-H.; Deng, W.-P.; Yeh, C.-T.; Tzeng, Y.-M. Identification of Antrocin from Antrodia camphorata as a Selective and Novel Class of Small Molecule Inhibitor of Akt/mTOR Signaling in Metastatic Breast Cancer MDA-MB-231 Cells. Chem. Res. Toxicol. 2011, 24, 238–245. [Google Scholar] [CrossRef]
- Chen, C.-C.; Shiao, Y.-J.; Lin, R.-D.; Shao, Y.-Y.; Lai, M.-N.; Lin, C.-C.; Ng, L.-T.; Kuo, Y.-H. Neuroprotective Diterpenes from the Fruiting Body of Antrodia camphorata. J. Nat. Prod. 2006, 69, 689–691. [Google Scholar] [CrossRef] [PubMed]
- Han, B.H.; Yang, H.O.; Kang, Y.-H.; Suh, D.-Y.; Go, H.J.; Song, W.-J.; Kim, Y.C.; Park, M.K. In Vitro Platelet-Activating Factor Receptor Binding Inhibitory Activity of Pinusolide Derivatives: A Structure−Activity Study. J. Med. Chem. 1998, 41, 2626–2630. [Google Scholar] [CrossRef]
- Chiang, Y.-M.; Liu, H.-K.; Lo, J.-M.; Chien, S.-C.; Chan, Y.-F.; Lee, T.-H.; Su, J.-K.; Kuo, Y.-H. Cytotoxic Constituents of the Leaves of Calocedrus Formosana. J. Chin. Chem. Soc. 2003, 50, 161–166. [Google Scholar] [CrossRef]
- Smith, L.; Watson, M.B.; O’Kane, S.L.; Drew, P.J.; Lind, M.J.; Cawkwell, L. The analysis of doxorubicin resistance in human breast cancer cells using antibody microarrays. Mol. Cancer. Ther. 2006, 5, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Fang, L.; Tan, C.; Shi, L.; Zhang, W.; Li, C.-C.; Luo, T.; Yang, Z. Total Syntheses of Drimane-Type Sesquiterpenoids Enabled by a Gold-Catalyzed Tandem Reaction. J. Am. Chem. Soc. 2011, 133, 14944–14947. [Google Scholar] [CrossRef]
- Li, F.-Z.; Li, S.; Zhang, P.-P.; Huang, Z.-H.; Zhang, W.-B.; Gong, J.; Yang, Z. A chiral pool approach for asymmetric syntheses of (−)-antrocin, (+)-asperolide C, and (−)-trans-ozic acid. Chem. Comm. 2016, 52, 12426–12429. [Google Scholar] [CrossRef]
- Huang, S.-H.; Liang, K.-H.; Lu, J.-S.; Chang, W.-S.; Su, M.-D.; Yang, T.-F. Total Synthesis of (+)-Antrocin and Its Diastereomer and Clarification of the Absolute Stereochemistry of (−)-Antrocin. J. Org. Chem. 2017, 82, 9576–9584. [Google Scholar] [CrossRef] [PubMed]
- Tai, D.-F.; Angamuthu, V.; Tzeng, Y.-M. Process for preparing optically active antrocin. U.S. Patent US0096324 A1, 18 April 2013. [Google Scholar]
- Hosmer, C.A.; Comber, R.N.; Brouillette, W.J. Syntheses of alpha.-, beta.-, and gamma.-substituted carnitines via beta.-keto esters. J. Org. Chem. 1985, 50, 3627–3631. [Google Scholar] [CrossRef]
- Feringa, B.L. Trimethylsilyl-Halide-Mediated Conjugated Addition to Allylic Acetals. Syn. Comm. 1985, 15, 87–89. [Google Scholar] [CrossRef][Green Version]
- Lee, H.M.; Nieto-Oberhuber, C.; Shair, M.D. Enantioselective Synthesis of (+)-Cortistatin A, a Potent and Selective Inhibitor of Endothelial Cell Proliferation. J. Am. Chem. Soc. 2008, 130, 16864–16866. [Google Scholar] [CrossRef] [PubMed]
- House, H.O.; Wilkins, J.M. Reactions involving electron transfer. 12. Effects of solvent and substituents upon the ability of lithium diorganocuprates to add to enones. J. Org. Chem. 1978, 43, 2443–2454. [Google Scholar] [CrossRef]
- Spessard, S.J.; Stoltz, B.M. Progress toward the Synthesis of Garsubellin A and Related Phloroglucins: The Direct Diastereoselective Synthesis of the Bicyclo[3.3.1]nonane Core. Org. Lett. 2002, 4, 1943–1946. [Google Scholar] [CrossRef][Green Version]
- Taber, D.F.; Sikkander, M.I.; Storck, P.H. Enantioselective Synthesis of (+)-Majusculone. J. Org. Chem. 2007, 72, 4098–4101. [Google Scholar] [CrossRef][Green Version]
- Nagata, W.; Yoshioka, M.; Hirai, S. Hydrocyanation. IV. New hydrocyanation methods using hydrogen cyanide and an alkylaluminum, and an alkylaluminum cyanide. J. Am. Chem. Soc. 1972, 94, 4635–4643. [Google Scholar] [CrossRef]
- Ihara, M.; Katsumata, A.; Egashira, M.; Suzuki, S.; Tokunaga, Y.; Fukumoto, K. Stereoselective Construction of the Diterpene Part of Indole Alkaloids, Radarins, by Way of Intramolecular Diels-Alder Reaction. J. Org. Chem. 1995, 60, 5560–5566. [Google Scholar] [CrossRef]
- Suryawanshi, S.N.; Fuchs, P.L. Bruceantin support studies. 10. Use of an axial alpha.-face control element in intramolecular conjugate additions: Synthesis of an ABCD tetracyclic bruceantin precursor. J. Org. Chem. 1986, 51, 902–921. [Google Scholar] [CrossRef]
- Utimoto, K.; Wakabayashi, Y.; Horiie, T.; Inoue, M.; Shishiyama, Y.; Obayashi, M.; Nozaki, H. Cyanotrimethylsilane as a versatile reagent for introducing cyanide functionality. Tetrahedron 1983, 39, 967–973. [Google Scholar] [CrossRef]
- Dailey, O.D.; Fuchs, P.L. Synthesis of a model for the BCE ring system of bruceantin. A caveat on the cyclohexene fwdarw. trans diaxial diol conversion. J. Org. Chem. 1980, 45, 216–236. [Google Scholar] [CrossRef]
- Furuichi, N.; Hata, T.; Soetjipto, H.; Kato, M.; Katsumura, S. Common synthetic strategy for optically active cyclic terpenoids having a 1,1,5-trimethyl-trans-decalin nucleus: Syntheses of (+)-acuminolide, (−)-spongianolide A, and (+)-scalarenedial. Tetrahedron 2001, 57, 8425–8442. [Google Scholar] [CrossRef]
- Cha, J.S.; Kim, E.J.; Kwon, O.O.; Kim, J.M. Chemoselective Reduction of Carbonyl Compounds with Diisopinocampheylchloroborane. Synlett 1995, 1995, 331–332. [Google Scholar] [CrossRef]
- Hori, K.; Hikage, N.; Inagaki, A.; Mori, S.; Nomura, K.; Yoshii, E. Total synthesis of tetronomycin. J. Org. Chem. 1992, 57, 2888–2902. [Google Scholar] [CrossRef]
- Zoretic, P.A.; Zhang, Y.; Fang, H.; Ribeiro, A.A.; Dubay, G. Advanced Tetracycles in a Stereoselective Approach to d,l-Spongiatriol and Related Metabolites: The Use of Radicals in the Synthesis of Angular Electrophores. J. Org. Chem. 1998, 63, 1162–1167. [Google Scholar] [CrossRef]
- Schlosser, M.; Jenny, T.; Guggisberg, Y. Monomeric Formaldehyde in Ethereal Solution. Synlett 1990, 1990, 704. [Google Scholar] [CrossRef]
- Kato, M.; Matsumura, Y.; Heima, K.; Fukamiya, N.; Kabuto, C.; Yoshikoshi, A. Total synthesis of dl-siccanin and dl-siccanochromene E. Tetrahedron 1987, 43, 711–722. [Google Scholar] [CrossRef]
- NOE spectroscopic date given in Supplementary Materials.
- Díaz, S.; Cuesta, J.; González, A.; Bonjoch, J. Synthesis of (−)-Nakamurol A and Assignment of Absolute Configuration of Diterpenoid (+)-Nakamurol, A. J. Org. Chem. 2003, 68, 7400–7406. [Google Scholar] [CrossRef]
- Toshima, H.; Oikawa, H.; Toyomasu, T.; Sassa, T. Total Synthesis of (+)-Albicanol and (+)-Albicanyl Acetate. Biosci. Biotechnol. Biochem. 2001, 65, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Skepper, C.K.; Quach, T.; Molinski, T.F. Total Synthesis of Enigmazole A from Cinachyrella enigmatica. Bidirectional Bond Constructions with an Ambident 2,4-Disubstituted Oxazole Synthon. J. Am. Chem. Soc. 2010, 132, 10286–10292. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Electronic Supplementary Information (ESI) available: Spectral data (1H, 13C NMR, and HRMS) of all compounds; CIF for (−)-7, (−)-8, and (−)-14 compounds crystallographic data material are available from the authors. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angamuthu, V.; Tai, D.-F. Synthesis of Natural (−)-Antrocin and Its Enantiomer via Stereoselective Aldol Reaction. Molecules 2020, 25, 831. https://doi.org/10.3390/molecules25040831
Angamuthu V, Tai D-F. Synthesis of Natural (−)-Antrocin and Its Enantiomer via Stereoselective Aldol Reaction. Molecules. 2020; 25(4):831. https://doi.org/10.3390/molecules25040831
Chicago/Turabian StyleAngamuthu, Venkatachalam, and Dar-Fu Tai. 2020. "Synthesis of Natural (−)-Antrocin and Its Enantiomer via Stereoselective Aldol Reaction" Molecules 25, no. 4: 831. https://doi.org/10.3390/molecules25040831
APA StyleAngamuthu, V., & Tai, D.-F. (2020). Synthesis of Natural (−)-Antrocin and Its Enantiomer via Stereoselective Aldol Reaction. Molecules, 25(4), 831. https://doi.org/10.3390/molecules25040831