Two New Apotirucallane-Type Triterpenoids from the Pericarp of Toona sinensis and Their Ability to Reduce Oxidative Stress in Rat Glomerular Mesangial Cells Cultured under High-Glucose Conditions
Abstract
1. Introduction
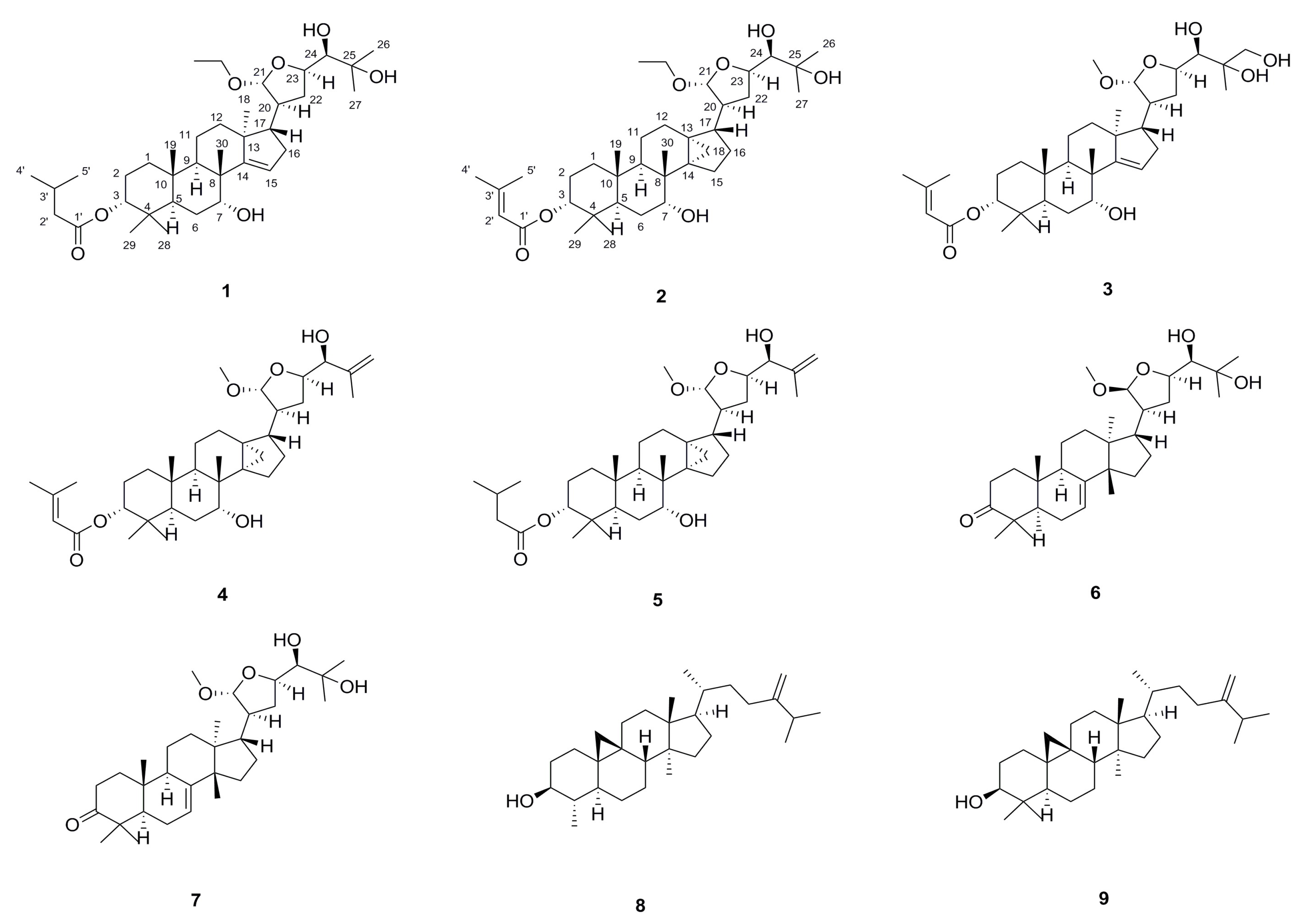
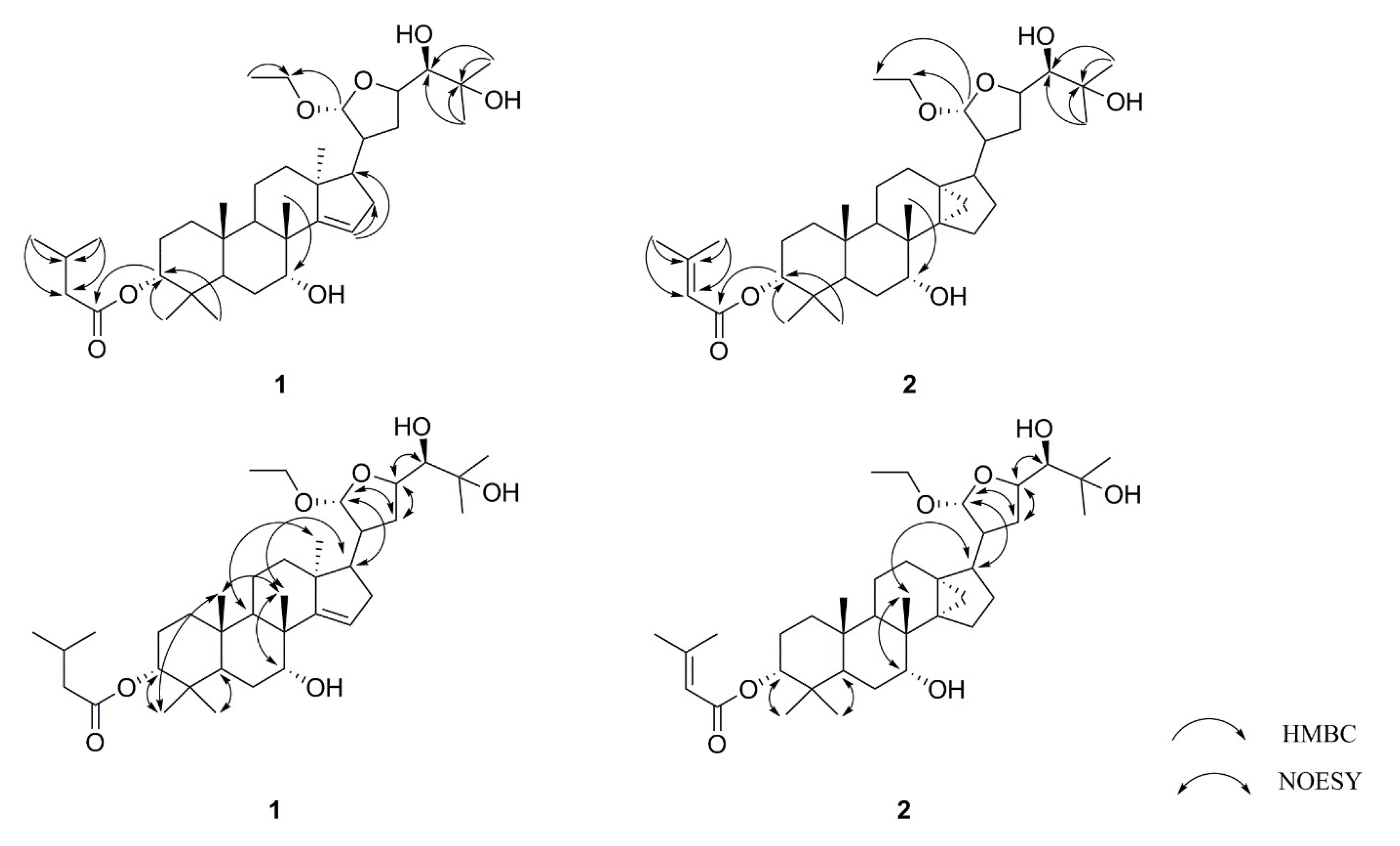
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. Toonasinensin A (1)
3.3.2. Toonasinensin B (2)
3.4. Cytotoxicity Assay
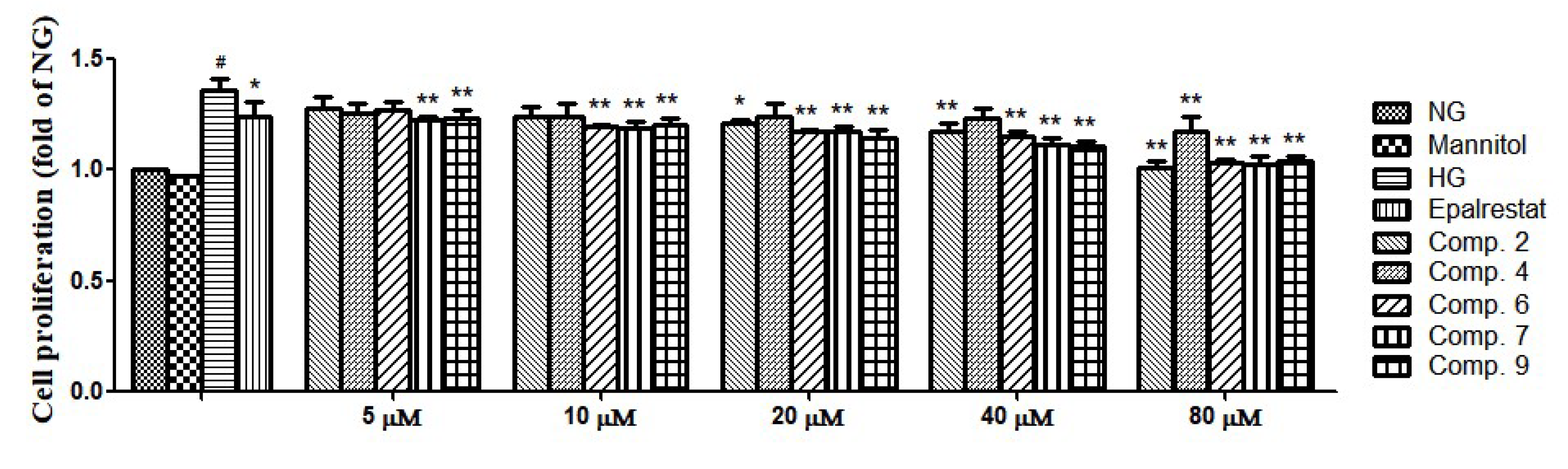
3.5. Cell Proliferation Assay
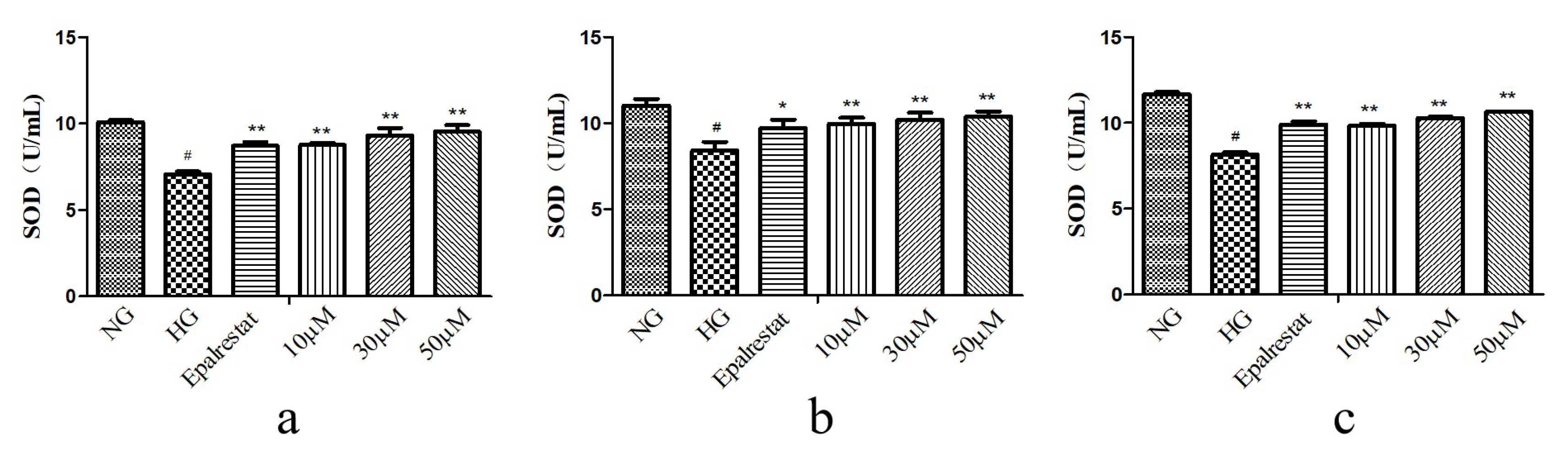
3.6. In Vitro Antioxidant Activity of SOD, MDA, and ROS
3.6.1. SOD
3.6.2. MDA
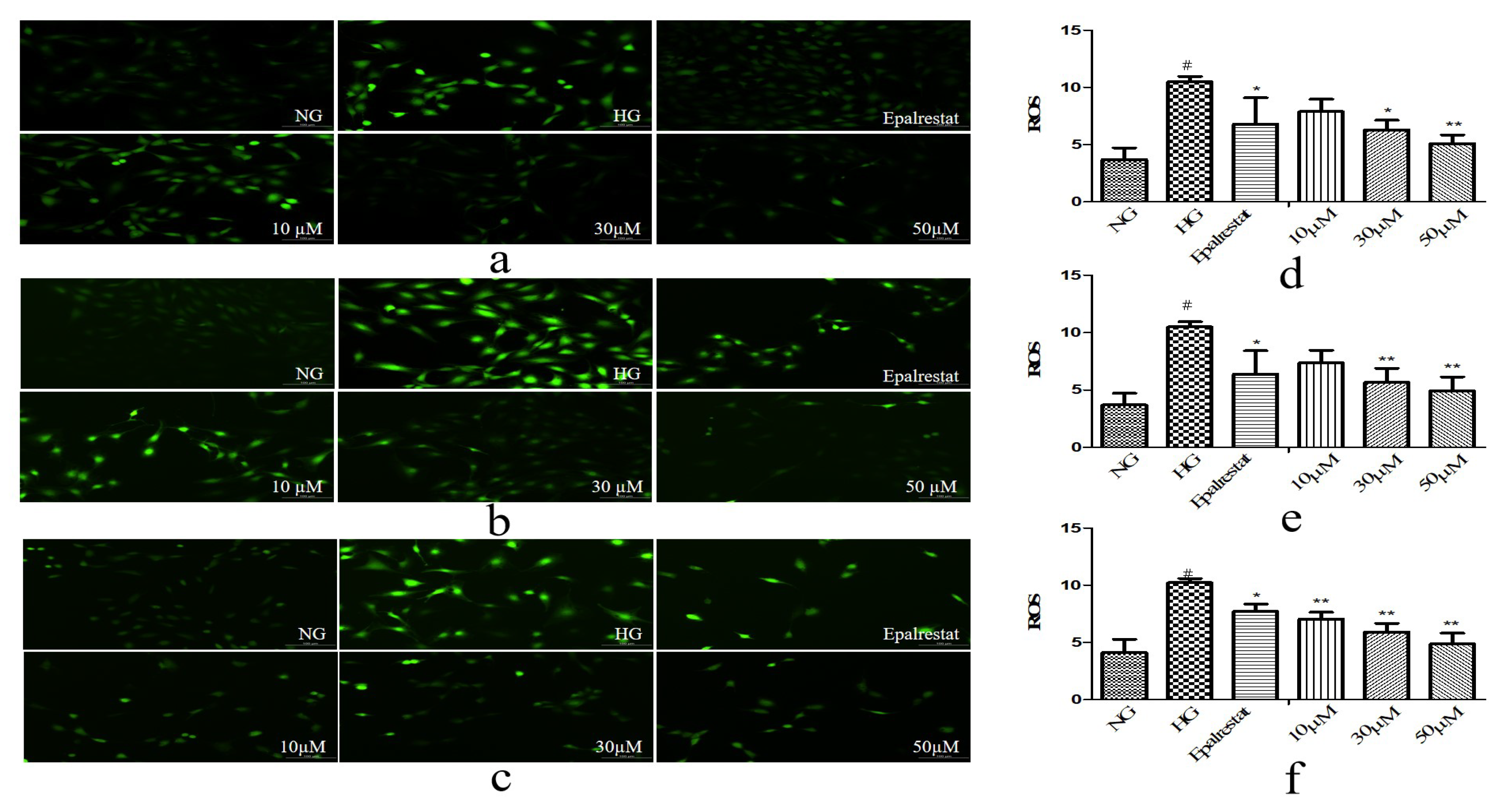
3.6.3. ROS
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dong, A.; Tan, X.Y.; Kong, Q.; Zhang, M.Z. Protective Effect and Mechanism of Schisandra Chinensis Ethanol Extract on Oxidative Stress in Diabetic Nephropathy Mice. Chin. Tradit. Herb. Drugs 2019, 50, 6038–6044. [Google Scholar]
- Li, S.Y.; Li, W.J.; Wu, R.Y.; Sargsyan, D.; Raskin, I.; Kong, A.N. Epigenome and transcriptome study of moringa isothiocyanate in mouse kidney mesangial cells induced by high glucose, a potential model for diabetic-induced nephropathy. AAPS J. 2019, 22, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Li, Y.; Wang, Y.; Zhao, K.X.; Chi, Y.Q.; Wang, B.X. Pyrroloquinoline quinine protects HK-2 cells against high glucose-induced oxidative stress and apoptosis through Sirt3 and PI3K/Akt/FoxO3a signaling pathway. Biochem. Biophys. Res. Commun. 2019, 508, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Chen, S.C.; Lin, K.Y.; Wang, M.T.; Chen, Y.C.; Huang, H.C.; Cho, H.J.; Wang, L.; Kumar, K.J.; Hesu, Y.C. Antioxidant activities of aqueous leaf extracts of Toona sinensis on free radical-induced endothelial cell damage. J. Ethnopharmacology 2011, 137, 669–680. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, H.J.; Huang, B.M.; Chen, Y.C.; Chang, C.F. Polyphenol-rich extracts from Toona sinensis bark and fruit ameliorate free fatty acid-induced lipogenesis through AMPK and LC3 pathways. J. Clin. Med. 2019, 8, 1664. [Google Scholar] [CrossRef]
- Dong, X.J.; Zhu, Y.F.; Bao, G.H.; Hu, F.L.; Qin, G.W. New limonoids and a dihydrobenzofuran norlignan from the roots of Toona sinensis. Molecules 2013, 18, 2840–2850. [Google Scholar] [CrossRef]
- Mitsui, K.; Saito, H.; Yamamura, R.; Fukaya, H.; Hitotsuyanagi, Y.; Takeya, K. Apotirucallane and tirucallane triterpenoids from Cedrela sinensis. Chem. Pharm. Bull. 2007, 55, 1442–1447. [Google Scholar] [CrossRef]
- Lee, I.S.; Kim, H.J.; Youn, U.J.; Chen, Q.C.; Kim, J.P.; Ha, D.T.; Ngoc, T.M.; Min, B.S.; Lee, S.M.; Jung, H.J.; et al. Dihydrobenzofuran norlignans from the leaves of Cedrela sinensis A. Juss. Helv. Chim. Acta. 2010, 93, 272–276. [Google Scholar] [CrossRef]
- Chen, K.W.; Yang, R.Y.; Tsou, S.C.S.; Lo, C.S.C.; Ho, C.T.; Lee, T.C.; Wang, M.F. Analysis of antioxidant activity and antioxidant constituents of Chinese toon. J. Funct. Foods 2009, 1, 253–259. [Google Scholar] [CrossRef]
- Li, S.J.; Hu, Y.Q. Antioxidant activity of extracts from Toona sinensis leaves. Anhui Agricultural Sciences 2007, 35, 6807–6808. [Google Scholar]
- Xue, L.; Li, C.F. Preliminary pharmacodynamic experiment of total flavonoids from Toona sinensis buds. Shandong J. Tradit. Chin. Med. 2004, 23, 685–686. [Google Scholar]
- Chen, Y.K.; Ou, H.P.; Fang, C.L.; Yang, H.H.; Liu, S.B. Determination of antibacterial activity of pericarp of Toona sinensis and Ailanthus altissima in vitro. Sichuan Anim. Vet. Sci. 2011, 5, 26–27. [Google Scholar]
- Chen, Y.L.; Ruan, Z.P.; Lin, L.S.; Li, C.L. Research progress on chemical constituents and pharmacological effects of Toona sinensis. J. Changzhi Med. Coll. 2008, 22, 315–317. [Google Scholar]
- Chen, L.; Zhang, J.X.; Wang, B.; Mu, S.Z.; Hao, X.J. Triterpenoids with anti-tobacco mosaic virus activities from Melia toosendan. Fitoterapia 2014, 97, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Jiang, F.L.; Huang, L.; Chen, Y.L.; Shao, L.Q.; Chai, C.B.; Wang, F.X.; Zuo, X.C. Effect of total flavonoids from Toona sinensis leaves on blood glucose in diabetic mice. Northwest Pharm. J. 2011, 4, 40–41. [Google Scholar]
- Mitsui, K.; Maejima, M.; Satio, H.; Fukaya, H.; Hitotsuyanagi, Y.; Takeya, K. Triterpenoids from Cedrela sinensis. Tetrahedron 2005, 61, 10569–10582. [Google Scholar] [CrossRef]
- Bai, Y.Y.; Jin, X.; Jia, X.H.; Tang, W.T.; Wang, X.J.; Zhao, Y.X. Two new apotirucallane-type isomeric triterpenoids from the root bark of Dictamnus dasycarpus with their anti-proliferative activity. Phytochem. Lett. 2014, 10, 118–122. [Google Scholar] [CrossRef]
- Lien, T.P.; Kamperdick, C.; Schmidt, J.; Adam, G.; Sung, T.V. Apotirucallane triterpenoids from Luvunga sarmentosa. Phytochemistry 2002, 60, 747–754. [Google Scholar] [CrossRef]
- Xu, J.Q.; Shen, Q.; Li, J.; Hu, L.H. Dammaranes from Gynostemma pentaphyllum and synthesis of their derivatives as inhibitors of protein tyrosine phosphatase 1B. Bioorg. Med. Chem. 2010, 18, 3934–3939. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, J.S.; Gu, Y.C.; Kong, L.Y. Cytotoxic and anti-inflammatory triterpenoids from Toona ciliata. J. Nat. Prod. 2012, 75, 538–546. [Google Scholar] [CrossRef]
- Zhou, F.; Ma, X.H.; Li, Z.J.; Li, W.; Zheng, W.M.; Wang, Z.B.; Zeng, X.M.; Sun, K.H.; Zhang, Y.H. Four new tirucallane triterpenoids from the fruits of Melia azedarach and their cytotoxic activities. Chem. Biodivers. 2016. [Google Scholar] [CrossRef]
- Wu, W.B.; Zhang, H.; Dong, S.H.; Sheng, L.; Wu, Y.; Li, J.; Yue, J.M. New triterpenoids with protein tyrosine phosphatase 1B inhibition from Cedrela odorata. J. Asian Nat. Prod. Res. 2014, 16, 709–716. [Google Scholar] [CrossRef]
- Yan, X.T.; Lee, S.H.; Li, W.; Jang, H.D.; Kim, Y.H. Terpenes and sterols from the fruits of Prunus mume and their inhibitory effects on osteoclast differentiation by suppressing tartrate-resistant acid phosphatase activity. Arch. Pharm. Res. 2015, 38, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Choi, S.U.; Lee, J.H.; Lee, D.U.; Lee, K.R. A new phenylpropane glycoside from the rhizome of Sparganium stoloniferum. Arch. Pharm. Res. 2010, 33, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Kimura, Y.; Tamura, T. Cycloartane triterpenes from the fruit peel of Musa sapientum. Phytochemistry 1998, 47, 1107–1110. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Fan, C.C.; Chen, B.P.; Shi, J. Resistin-like molecule beta (RELM-β) regulates proliferation of human diabetic nephropathy mesangial cells via mitogen-activated protein kinases (MAPK) signaling pathway. Med. Sci. Monit. 2017, 23, 3897–3903. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, F.T.; Shearer, G.C.; Atkinson, D.N. Lipoprotein-stimulated mesangial cell proliferation and gene expression are regulated by lipoprotein lipase. Kidney Int. 2001, 59, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Q.; Wang, Y.G.; Gao, G.Q. High glucose induces rat mesangial cells proliferation and MCP-1 expression via ROS-mediated activation of NF-κB pathway, which is inhibited by eleutheroside E. J. Recept. Signal Transduct. Res. 2016, 36, 152–157. [Google Scholar] [CrossRef]
- Chen, F.; Ma, Y.L.; Sun, Z.Q.; Zhu, X.G. Tangeretin inhibits high glucose-induced extracellular matrix accumulation in human glomerular mesangial cells. Biomed. Pharmacother. 2018, 102, 1077–1083. [Google Scholar] [CrossRef]
- Azevedo, B.C.; Roxo, M.; Borges, M.C.; Peixoto, H.; Crevelin, E.J.; Bertoni, B.W.; Contini, S.H.T.; Lopes, A.A.; França, S.C.; Pereira, A.M.S.; et al. Antioxidant activity of an aqueous leaf extract from and its major alkaloids mitraphylline and isomitraphylline in Caenorhabditis elegans. Molecules 2019, 24, 3299. [Google Scholar] [CrossRef]
- Li, C.N.; Lan, M.; Lv, J.W.; Zhang, Y.; Gao, X.C.; Gao, X.; Dong, L.H.; Luo, G.M.; Zhang, H.; Sun, J.M. Screening of the hepatotoxic components in and their effects on rat liver BRL-3A cells. Molecules 2019, 24, 3920. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Liang, J.; Luan, G.X.; Zhang, S.D.; Zhuoma, Y.X.; Xie, J.X.; Zhou, W. Quantitative analyses of nine phenolic compounds and their antioxidant activities from thirty-seven varieties of raspberry grown in the qinghai-tibetan plateau region. Molecules 2019, 24, 3932. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Dong, X.M.; Zhang, M.; Cheng, Y.H.; Pei, C. Knockdown of ALK7 inhibits high glucose-imacol. Physiol. 2020, 47, 313–321. [Google Scholar]
- Xu, K.; Guo, L.Q.; Bu, H.X.; Wang, H. Daphnetin inhibits high glucose-induced extracellular matrix accumulation, oxidative stress and inflammation in human glomerular mesangial cells. J. Pharmacol. Sci. 2019, 139, 91–97. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–9 are available from the authors. |

| Pos. | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 34.6 | 1.40 m, 1.27 m | 33.6 | 1.38 m, 1.21 m |
| 2 | 23.8 | 1.95 m, 1.63 m | 22.5 | 1.97 m, 1.54 m |
| 3 | 79.7 | 4.65 m | 77.4 | 4.62 m |
| 4 | 37.3 | - | 36.3 | - |
| 5 | 43.0 | 2.07 m | 41.2 | 2.01 m |
| 6 | 23.8 | 2.03 m, 1.54 m | 24.5 | 1.68 m, 1.57 m |
| 7 | 73.7 | 3.94 br s | 74.0 | 3.72 br s |
| 8 | 45.2 | - | 39.1 | - |
| 9 | 43.2 | 2.06 m | 44.1 | 1.39 m |
| 10 | 38.8 | - | 37.2 | - |
| 11 | 17.5 | 1.69 m, 1.54 m | 16.1 | 1.32 m |
| 12 | 34.4 | 1.87 m, 1.49 m | 25.4 | 1.87 m |
| 13 | 48.1 | - | 28.4 | - |
| 14 | 162.7 | - | 36.1 | - |
| 15 | 120.3 | 5.44 br s | 25.6 | 1.92 m, 1.55 m |
| 16 | 35.9 | 2.16 m | 25.7 | 1.67 m |
| 17 | 59.3 | 1.71 m | 48.6 | 1.99 m |
| 18 | 19.6 | 1.11 s | 13.5 | 0.72 m, 0.51 m |
| 19 | 15.8 | 0.98 s | 14.7 | 0.95 overlap |
| 20 | 47.5 | 2.33 m | 49.1 | 2.06 m |
| 21 | 109.7 | 4.92 d (3.3) | 109.1 | 4.96 m |
| 22 | 36.1 | 1.94 m, 1.65 m | 31.7 | 1.59 m, 1.51 m |
| 23 | 77.1 | 4.27 m | 75.9 | 4.24 m |
| 24 | 78.4 | 3.24 m | 77.1 | 3.22 m |
| 25 | 73.9 | - | 72.5 | - |
| 26 | 27.0 | 1.26 s | 24.1 | 1.20 s |
| 27 | 25.3 | 1.20 s | 25.9 | 1.25 s |
| 28 | 27.5 | 0.86 s | 26.8 | 0.83 s |
| 29 | 28.6 | 1.09 s | 20.9 | 0.91 overlap |
| 30 | 22.3 | 0.93 s | 18.8 | 1.05 s |
| 1′ | 174.6 | - | 166.7 | - |
| 2′ | 25.3 | 1.19 m | 116.2 | 5.77 s |
| 3′ | 44.7 | 2.23 m | 156.2 | - |
| 4′ | 22.8 | 0.96 s | 26.0 | 1.92 s |
| 5′ | 22.8 | 0.96 s | 19.1 | 2.17 s |
| 21-OCH2CH3 | 64.8 | 3.76 m, 3.46 m | 67.7 | 3.68 m, 3.40 m |
| 21-OCH2CH3 | 15.8 | 1.20 s | 12.8 | 0.92 overlap |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Wang, R.-s.; Xuan, L.-l.; Wang, X.-h.; Li, W.-z. Two New Apotirucallane-Type Triterpenoids from the Pericarp of Toona sinensis and Their Ability to Reduce Oxidative Stress in Rat Glomerular Mesangial Cells Cultured under High-Glucose Conditions. Molecules 2020, 25, 801. https://doi.org/10.3390/molecules25040801
Liu D, Wang R-s, Xuan L-l, Wang X-h, Li W-z. Two New Apotirucallane-Type Triterpenoids from the Pericarp of Toona sinensis and Their Ability to Reduce Oxidative Stress in Rat Glomerular Mesangial Cells Cultured under High-Glucose Conditions. Molecules. 2020; 25(4):801. https://doi.org/10.3390/molecules25040801
Chicago/Turabian StyleLiu, Di, Rong-shen Wang, Lu-lu Xuan, Xiao-hong Wang, and Wan-zhong Li. 2020. "Two New Apotirucallane-Type Triterpenoids from the Pericarp of Toona sinensis and Their Ability to Reduce Oxidative Stress in Rat Glomerular Mesangial Cells Cultured under High-Glucose Conditions" Molecules 25, no. 4: 801. https://doi.org/10.3390/molecules25040801
APA StyleLiu, D., Wang, R.-s., Xuan, L.-l., Wang, X.-h., & Li, W.-z. (2020). Two New Apotirucallane-Type Triterpenoids from the Pericarp of Toona sinensis and Their Ability to Reduce Oxidative Stress in Rat Glomerular Mesangial Cells Cultured under High-Glucose Conditions. Molecules, 25(4), 801. https://doi.org/10.3390/molecules25040801




