Review of Ginseng Anti-Diabetic Studies
Abstract
:1. Introduction
2. Anti-Diabetic Effects of Ginseng in Human Trials
3. Potential Mechanisms of the Anti-Diabetic Effect of Ginseng
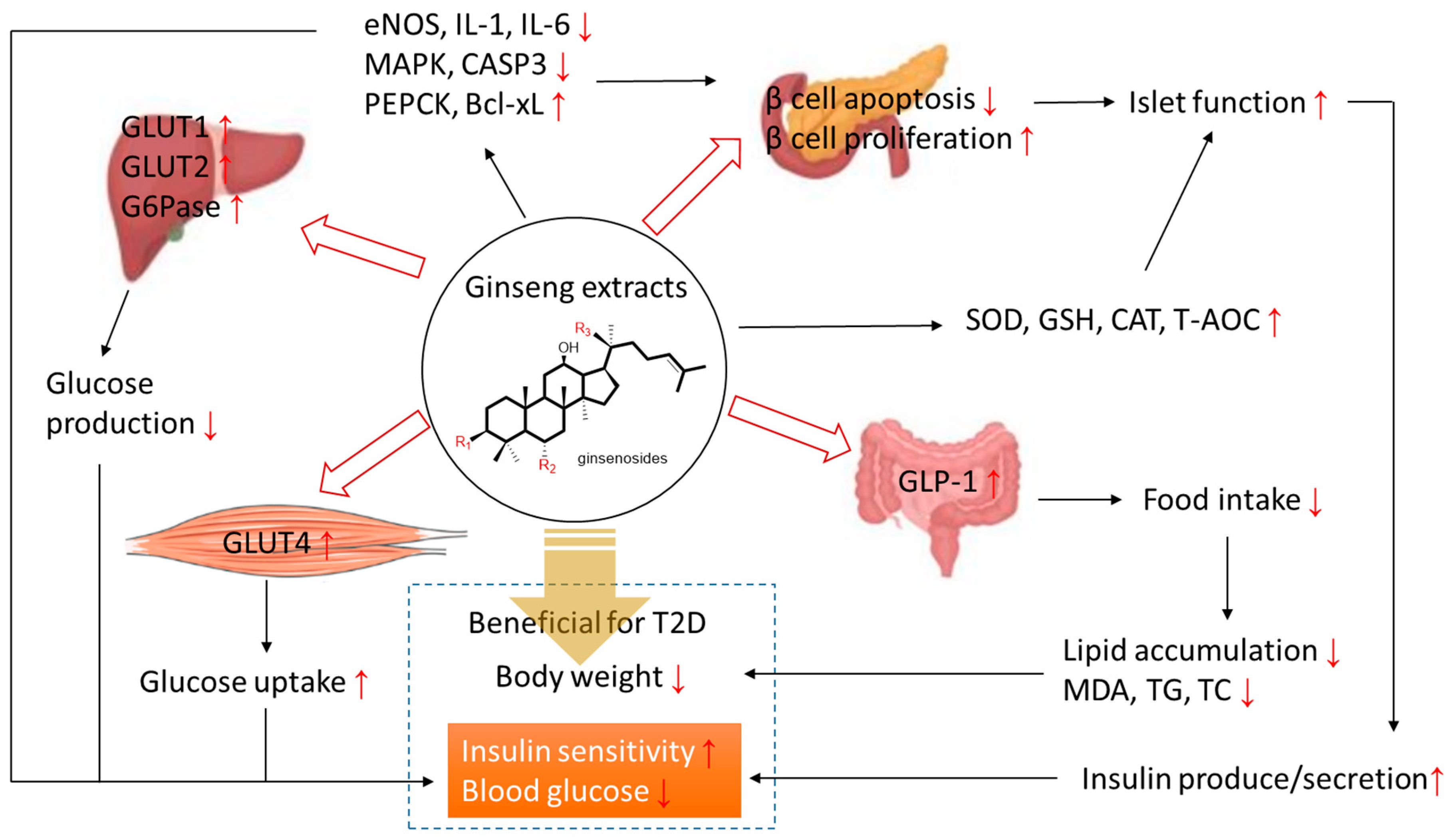
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Q.; Zhu, L.; Tang, Y.; Zhao, Z.; Yi, T.; Chen, H. Preparation-related structural diversity and medical potential in the treatment of diabetes mellitus with ginseng pectins. Ann. N. Y. Acad. Sci. 2017, 1401, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Aurora, R.N.; Punjabi, N.M. Obstructive sleep apnoea and type 2 diabetes mellitus: A bidirectional association. Lancet Respir. Med. 2013, 1, 329–338. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; IDF: Brussels, Belgium, 2019. [Google Scholar]
- Li, Z.; Jin, H.; Chen, W.; Sun, Z.; Jing, L.; Zhao, X.; Zhu, S.; Guo, X.; Study Group, C.N. Influencing Factors of Knowledge, Attitude, and Practice regarding Medical Nutrition Therapy in Patients with Diabetes: A National Cross-Sectional Study in Urban China. J. Diabetes Res. 2017, 2017, 8948452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef] [PubMed]
- Warren, R.E. The stepwise approach to the management of type 2 diabetes. Diabetes Res. Clin. Pr. 2004, 65S, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Shin, B.C.; Lee, M.S.; Lee, H.; Ernst, E. Red ginseng for type 2 diabetes mellitus: A systematic review of randomized controlled trials. Chin. J. Integr. Med. 2011, 17, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Che, J.Y.; Lu, D. Rethink Of Diabetes Treatment and Drug Development. Cell Dev. Biol. 2014, 3, e125. [Google Scholar]
- Tu, Y. Artemisinin-A Gift from Traditional Chinese Medicine to the World (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2016, 55, 10210–10226. [Google Scholar] [CrossRef]
- Bai, L.; Gao, J.; Wei, F.; Zhao, J.; Wang, D.; Wei, J. Therapeutic Potential of Ginsenosides as an Adjuvant Treatment for Diabetes. Front. Pharm. 2018, 9, 423. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Balan, P.; Popovich, D.G. Chapter 6-Comparison of the ginsenoside composition of Asian ginseng (Panax ginseng) and American ginseng (Panax quinquefolius L.) and their transformation pathways. Stud. Nat. Prod. Chem. 2019, 63, 161–195. [Google Scholar]
- Yuan, H.D.; Kim, J.T.; Kim, S.H.; Chung, S.H. Ginseng and diabetes: The evidences from in vitro, animal and human studies. J. Ginseng Res. 2012, 36, 27–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shishtar, E.; Sievenpiper, J.L.; Djedovic, V.; Cozma, A.I.; Ha, V.; Jayalath, V.H.; Jenkins, D.J.; Meija, S.B.; de Souza, R.J.; Jovanovski, E.; et al. The effect of ginseng (the genus panax) on glycemic control: A systematic review and meta-analysis of randomized controlled clinical trials. PLoS ONE 2014, 9, e107391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gui, Q.F.; Xu, Z.R.; Xu, K.Y.; Yang, Y.M. The Efficacy of Ginseng-Related Therapies in Type 2 Diabetes Mellitus: An Updated Systematic Review and Meta-analysis. Medicine (Baltim.) 2016, 95, e2584. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.W.; Kang, S.M.; Vassy, J.L.; Shin, H.; Lee, Y.H.; Ahn, H.Y.; Choi, S.H.; Park, K.S.; Jang, H.C.; Lim, S. Efficacy and safety of ginsam, a vinegar extract from Panax ginseng, in type 2 diabetic patients: Results of a double-blind, placebo-controlled study. J. Diabetes Investig. 2012, 3, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Oh, M.R.; Choi, E.K.; Kim, M.G.; Ha, K.C.; Lee, S.K.; Kim, Y.G.; Park, B.H.; Kim, D.S.; Chae, S.W. An 8-wk, randomized, double-blind, placebo-controlled clinical trial for the antidiabetic effects of hydrolyzed ginseng extract. J. Ginseng Res. 2014, 38, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.K.; Yoo, D.S.; Xu, H.; Park, N.I.; Kim, H.H.; Choi, J.E.; Park, S.U. Ginsenoside content of berries and roots of three typical Korean ginseng (Panax ginseng) cultivars. Nat. Prod. Commun. 2009, 4, 903–906. [Google Scholar] [CrossRef] [Green Version]
- Dey, L.; Xie, J.T.; Wang, A.; Wu, J.; Maleckar, S.A.; Yuan, C.S. Anti-hyperglycemic effects of ginseng: Comparison between root and berry. Phytomedicine 2003, 10, 600–605. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, S.; Kim, M.J.; Kim, M.S.; Kim, J.; Park, C.W.; Seo, D.; Shin, S.S.; Oh, S.W. Efficacy and safety of Panax ginseng berry extract on glycemic control: A 12-wk randomized, double-blind, and placebo-controlled clinical trial. J. Ginseng Res. 2018, 42, 90–97. [Google Scholar] [CrossRef]
- Bang, H.; Kwak, J.H.; Ahn, H.Y.; Shin, D.Y.; Lee, J.H. Korean red ginseng improves glucose control in subjects with impaired fasting glucose, impaired glucose tolerance, or newly diagnosed type 2 diabetes mellitus. J. Med. Food 2014, 17, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Oh, M.R.; Park, S.H.; Kim, S.Y.; Back, H.I.; Kim, M.G.; Jeon, J.Y.; Ha, K.C.; Na, W.T.; Cha, Y.S.; Park, B.H.; et al. Postprandial glucose-lowering effects of fermented red ginseng in subjects with impaired fasting glucose or type 2 diabetes: A randomized, double-blind, placebo-controlled clinical trial. BMC Complement. Altern. Med. 2014, 14, 237. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.H.; Ahn, S.C.; Lee, S.Y.; Jeong, D.W.; Choi, E.J.; Kim, Y.J.; Lee, J.G.; Lee, Y.H.; Shin, B.C. Effect of Korean red ginseng on insulin sensitivity in non-diabetic healthy overweight and obese adults. Asia Pac. J. Clin. Nutr. 2013, 22, 365–371. [Google Scholar] [PubMed]
- Kwon, D.H.; Bose, S.; Song, M.Y.; Lee, M.J.; Lim, C.Y.; Kwon, B.S.; Kim, H.J. Efficacy of Korean Red Ginseng by Single Nucleotide Polymorphism in Obese Women: Randomized, Double-blind, Placebo-controlled Trial. J. Ginseng Res. 2012, 36, 176–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.W.; Kim, H.N.; Shim, J.Y.; Yoo, B.Y.; Kim, D.H.; Lee, S.H.; Park, J.S.; Kim, M.J.; Yoo, J.H.; Cho, B.; et al. Safety and tolerability of Korean Red Ginseng in healthy adults: A multicenter, double-blind, randomized, placebo-controlled trial. J. Ginseng Res. 2018, 42, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.L.; Morgan, L.M.; Bishop, J.; Jovanovski, E.; Jenkins, D.J.A.; Vuksan, V. Co-administration of a konjac-based fibre blend and American ginseng (Panax quinquefolius L.) on glycaemic control and serum lipids in type 2 diabetes: A randomized controlled, cross-over clinical trial. Eur. J. Nutr. 2018, 57, 2217–2225. [Google Scholar] [CrossRef]
- Vuksan, V.; Xu, Z.Z.; Jovanovski, E.; Jenkins, A.L.; Beljan-Zdravkovic, U.; Sievenpiper, J.L.; Mark Stavro, P.; Zurbau, A.; Duvnjak, L.; Li, M.Z.C. Efficacy and safety of American ginseng (Panax quinquefolius L.) extract on glycemic control and cardiovascular risk factors in individuals with type 2 diabetes: A double-blind, randomized, cross-over clinical trial. Eur. J. Nutr. 2019, 58, 1237–1245. [Google Scholar] [CrossRef]
- Mucalo, I.; Jovanovski, E.; Vuksan, V.; Bozikov, V.; Romic, Z.; Rahelic, D. American Ginseng Extract (Panax quinquefolius L.) Is Safe in Long-Term Use in Type 2 Diabetic Patients. Evid. Based Complement. Altern. Med. 2014, 2014, 9168. [Google Scholar] [CrossRef] [Green Version]
- Park, E.Y.; Kim, H.J.; Kim, Y.K.; Park, S.U.; Choi, J.E.; Cha, J.Y.; Jun, H.S. Increase in Insulin Secretion Induced by Panax ginseng Berry Extracts Contributes to the Amelioration of Hyperglycemia in Streptozotocininduced Diabetic Mice. J. Ginseng Res. 2012, 36, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Seo, E.; Kim, S.; Lee, S.J.; Oh, B.C.; Jun, H.S. Ginseng berry extract supplementation improves age-related decline of insulin signaling in mice. Nutrients 2015, 7, 3038–3053. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Pan, J.H.; Cho, H.T.; Kim, Y.J. Black Ginseng Extract Counteracts Streptozotocin-Induced Diabetes in Mice. PLoS ONE 2016, 11, e0146843. [Google Scholar] [CrossRef] [Green Version]
- Song, B.; Ding, L.; Zhang, H.; Chu, Y.; Chang, Z.; Yu, Y.; Guo, D.; Zhang, S.; Liu, X. Ginsenoside Rb1 increases insulin sensitivity through suppressing 11beta-hydroxysteroid dehydrogenase type I. Am. J. Transl. Res. 2017, 9, 1049–1057. [Google Scholar]
- Tian, W.; Chen, L.; Zhang, L.; Wang, B.; Li, X.B.; Fan, K.R.; Ai, C.H.; Xia, X.; Li, S.D.; Li, Y. Effects of ginsenoside Rg1 on glucose metabolism and liver injury in streptozotocin-induced type 2 diabetic rats. Genet. Mol. Res. 2017, 16, gmr16019463. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Jung Yang, H.; Lee, I.S.; Kim, K.H.; Park, J.; Jeong, H.S.; Kim, Y.; Ahn, K.S.; Na, Y.C.; Jang, H.J. The aglycone of ginsenoside Rg3 enables glucagon-like peptide-1 secretion in enteroendocrine cells and alleviates hyperglycemia in type 2 diabetic mice. Sci. Rep. 2015, 5, 18325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.S.; Jang, H.J.; Oh, M.Y.; Eom, D.W.; Kang, K.S.; Kim, Y.J.; Lee, J.H.; Ham, J.Y.; Choi, S.Y.; Wee, Y.M.; et al. Ginsenoside Rg3 enhances islet cell function and attenuates apoptosis in mouse islets. Transpl. Proc. 2014, 46, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Karikura, M.; Miyase, T.; Tanizawa, H.; Taniyama, T. Studies on Absorption, Distribution, Excretion and Metabolism of Ginseng Saponins. VII. Comparison of the Decomposition Modes of Ginsenoside-Rb1 and -Rb2 in the Digestive Tract of Rats. Chem. Pharm. Bull. 1991, 39, 2357–2361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, H.-K.; Kim, K.-S.; Chung, S.-K.; Kim, J.-K. Effect of wild Korean ginseng (Panax ginseng) extract on blood glucose and serum lipid contents in rats with multiple low-dose streptozotocin-induced diabetes. Food Sci. Biotechnol. 2015, 24, 1505–1511. [Google Scholar] [CrossRef]
- Shishtar, E.; Jovanovski, E.; Jenkins, A.; Vuksan, V. Effects of Korean White Ginseng (Panax Ginseng C.A. Meyer) on Vascular and Glycemic Health in Type 2 Diabetes: Results of a Randomized, Double Blind, Placebo-controlled, Multiple-crossover, Acute Dose Escalation Trial. Clin. Nutr. Res. 2014, 3, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Murthy, H.N.; Dandin, V.S.; Lee, E.J.; Paek, K.Y. Efficacy of ginseng adventitious root extract on hyperglycemia in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2014, 153, 917–921. [Google Scholar] [CrossRef]
- Nam, S.J.; Han, Y.J.; Lee, W.; Kang, B.; Choi, M.K.; Han, Y.H.; Song, I.S. Effect of Red Ginseng Extract on the Pharmacokinetics and Efficacy of Metformin in Streptozotocin-Induced Diabetic Rats. Pharmaceutics 2018, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.Y.; Kim, K. Regulation of signaling molecules associated with insulin action, insulin secretion and pancreatic beta-cell mass in the hypoglycemic effects of Korean red ginseng in Goto-Kakizaki rats. J. Ethnopharmacol. 2012, 142, 53–58. [Google Scholar] [CrossRef]
- Hong, B.N.; Ji, M.G.; Kang, T.H. The efficacy of red ginseng in type 1 and type 2 diabetes in animals. Evid. Based Complement. Altern. Med. 2013, 2013, 593181. [Google Scholar] [CrossRef]
- Yoo, K.M.; Lee, C.; Lo, Y.M.; Moon, B. The hypoglycemic effects of American red ginseng (Panax quinquefolius L.) on a diabetic mouse model. J. Food Sci 2012, 77, H147–H152. [Google Scholar] [CrossRef] [PubMed]
- Kang, O.H.; Shon, M.Y.; Kong, R.; Seo, Y.S.; Zhou, T.; Kim, D.Y.; Kim, Y.S.; Kwon, D.Y. Anti-diabetic effect of black ginseng extract by augmentation of AMPK protein activity and upregulation of GLUT2 and GLUT4 expression in db/db mice. BMC Complement. Altern. Med. 2017, 17, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheon, J.M.; Kim, D.I.; Kim, K.S. Insulin sensitivity improvement of fermented Korean Red Ginseng (Panax ginseng) mediated by insulin resistance hallmarks in old-aged ob/ob mice. J. Ginseng Res. 2015, 39, 331–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.T.; Kim, H.B.; Lee, K.H.; Choi, Y.R.; Kim, H.J.; Shin, I.S.; Gyoung, Y.S.; Joo, S.S. Steam-dried ginseng berry fermented with Lactobacillus plantarum controls the increase of blood glucose and body weight in type 2 obese diabetic db/db mice. J. Agric. Food Chem. 2012, 60, 5438–5445. [Google Scholar] [CrossRef]
- Jeon, W.J.; Oh, J.S.; Park, M.S.; Ji, G.E. Anti-hyperglycemic effect of fermented ginseng in type 2 diabetes mellitus mouse model. Phytother. Res. 2013, 27, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Jo, K.; Kim, J.S.; Pyo, M.K.; Kim, J. GS-E3D, a new pectin lyase-modified red ginseng extract, inhibited diabetes-related renal dysfunction in streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2017, 17, 430. [Google Scholar] [CrossRef]
- Saba, E.; Kim, S.H.; Kim, S.D.; Park, S.J.; Kwak, D.; Oh, J.H.; Park, C.K.; Rhee, M.H. Alleviation of diabetic complications by ginsenoside Rg3-enriched red ginseng extract in western diet-fed LDL(-/-) mice. J. Ginseng Res. 2018, 42, 352–355. [Google Scholar] [CrossRef]
- Yang, H.; Son, G.W.; Park, H.R.; Lee, S.E.; Park, Y.S. Effect of Korean Red Ginseng treatment on the gene expression profile of diabetic rat retina. J. Ginseng Res. 2016, 40, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Chen, Y.; Li, X.; Tai, G.; Fan, Y.; Zhou, Y. Anti-hyperglycemic and anti-oxidative activities of ginseng polysaccharides in STZ-induced diabetic mice. Food Funct. 2014, 5, 845–848. [Google Scholar] [CrossRef]
- Tsai, C.C.; Chan, P.; Chen, L.J.; Chang, C.K.; Liu, Z.; Lin, J.W. Merit of ginseng in the treatment of heart failure in type 1-like diabetic rats. Biomed. Res. Int. 2014, 2014, 484161. [Google Scholar] [CrossRef]
- Sen, S.; Chen, S.; Wu, Y.; Feng, B.; Lui, E.K.; Chakrabarti, S. Preventive effects of North American ginseng (Panax quinquefolius) on diabetic retinopathy and cardiomyopathy. Phytother. Res. 2013, 27, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Yin, H.J.; Guo, C.Y.; Huang, Y.; Xia, C.D.; Liu, Q. Influence of high blood glucose fluctuation on endothelial function of type 2 diabetes mellitus rats and effects of Panax Quinquefolius Saponin of stem and leaf. Chin. J. Integr. Med. 2013, 19, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Takamura, Y.; Nomura, M.; Uchiyama, A.; Fujita, S. Effects of Aerobic Exercise Combined with Panaxatriol Derived from Ginseng on Insulin Resistance and Skeletal Muscle Mass in Type 2 Diabetic Mice. J. Nutr. Sci. Vitam. 2017, 63, 339–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.M.; Park, C.H.; Park, S.K.; Seung, T.W.; Kang, J.Y.; Ha, J.S.; Lee, D.S.; Lee, U.; Kim, D.O.; Heo, H.J. Ginsenoside Re Ameliorates Brain Insulin Resistance and Cognitive Dysfunction in High Fat Diet-Induced C57BL/6 Mice. J. Agric. Food Chem. 2017, 65, 2719–2729. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Chen, P.; Tao, Z.; Zhou, N.; Gong, X.; Xu, Z.; Zhang, M.; Zhang, D.; Chen, B.; Tao, Z.; et al. Beneficial effects of ginsenoside-Rg1 on ischemia-induced angiogenesis in diabetic mice. Acta Biochim. Biophys. Sin. (Shanghai) 2012, 44, 999–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.T.; Zhen, J.; Pang, B.; Gu, J.N.; Wu, S.S. Ginsenoside Rg1 ameliorates oxidative stress and myocardial apoptosis in streptozotocin-induced diabetic rats. J. Zhejiang Univ. Sci. B 2015, 16, 344–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Zhen, J.; Yang, Y.; Gu, J.; Wu, S.; Liu, Q. Ginsenoside Rg1 ameliorates diabetic cardiomyopathy by inhibiting endoplasmic reticulum stress-induced apoptosis in a streptozotocin-induced diabetes rat model. J. Cell Mol. Med. 2016, 20, 623–631. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Zhao, Z.; Shang, W.; Liu, C.; Zhang, B.; Zhao, L.; Cai, H. Ginsenoside Rg1 nanoparticle penetrating the blood-brain barrier to improve the cerebral function of diabetic rats complicated with cerebral infarction. Int. J. Nanomed. 2017, 12, 6477–6486. [Google Scholar] [CrossRef] [Green Version]
- Lo, S.H.; Hsu, C.T.; Niu, H.S.; Niu, C.S.; Cheng, J.T.; Chen, Z.C. Ginsenoside Rh2 Improves Cardiac Fibrosis via PPARdelta-STAT3 Signaling in Type 1-Like Diabetic Rats. Int. J. Mol. Sci. 2017, 18, 1364. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Li, W.; Li, X.; Zhang, M.; Chen, L.; Zheng, Y.N.; Sun, G.Z.; Ruan, C.C. Antidiabetic effects of malonyl ginsenosides from Panax ginseng on type 2 diabetic rats induced by high-fat diet and streptozotocin. J. Ethnopharmacol. 2013, 145, 233–240. [Google Scholar] [CrossRef]
- Du, N.; Xu, Z.; Gao, M.; Liu, P.; Sun, B.; Cao, X. Combination of Ginsenoside Rg1 and Astragaloside IV reduces oxidative stress and inhibits TGF-beta1/Smads signaling cascade on renal fibrosis in rats with diabetic nephropathy. Drug Des. Dev. Ther. 2018, 12, 3517–3524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, M.; Guo, G.; Xiao, J.; Sheng, X.; Zhang, X.; Tie, Y.; Cheng, Y.K.; Ji, X. Ginsenoside Rg3 stereoisomers differentially inhibit vascular smooth muscle cell proliferation and migration in diabetic atherosclerosis. J. Cell Mol. Med. 2018, 22, 3202–3214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Wu, W.; Wang, G.; Xu, W.; Zhang, F.; Wu, B.; Tian, Y. Protective effect of ginsenoside Rg3 on lung injury in diabetic rats. J. Cell Biochem. 2019, 120, 3323–3330. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.S.; Lee, J.S.; Choi, J.Y.; Park, H.Y. Ginseng total saponin modulates podocyte p130Cas in diabetic condition. J. Ginseng Res. 2013, 37, 94–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.M.; Lee, O.H.; Kim, K.J.; Lee, B.Y. Ginsenoside Rg1 promotes glucose uptake through activated AMPK pathway in insulin-resistant muscle cells. Phytother. Res. 2012, 26, 1017–1022. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, M.F.; Su, Y.P.; Jiang, H.M.; You, X.J.; Yang, Y.J.; Zhang, H.L. Ginsenoside Re reduces insulin resistance through activation of PPAR-gamma pathway and inhibition of TNF-alpha production. J. Ethnopharmacol 2013, 147, 509–516. [Google Scholar] [CrossRef]
- Siraj, F.M.; SathishKumar, N.; Kim, Y.J.; Kim, S.Y.; Yang, D.C. Ginsenoside F2 possesses anti-obesity activity via binding with PPARgamma and inhibiting adipocyte differentiation in the 3T3-L1 cell line. J. Enzym. Inhib. Med. Chem. 2015, 30, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Yesmin Simu, S.; Ahn, S.; Castro-Aceituno, V.; Yang, D.C. Ginsenoside Rg5: Rk1 Exerts an Anti-obesity Effect on 3T3-L1 Cell Line by the Downregulation of PPARgamma and CEBPalpha. Iran. J. Biotechnol. 2017, 15, 252–259. [Google Scholar] [CrossRef] [Green Version]
- Reeds, D.N.; Patterson, B.W.; Okunade, A.; Holloszy, J.O.; Polonsky, K.S.; Klein, S. Ginseng and Ginsenoside Re Do Not Improve β-Cell Function or Insulin Sensitivity in Overweight and Obese Subjects With Impaired Glucose Tolerance or Diabetes. Diabetes Care 2011, 34, 1071–1076. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Huang, J.; Hu, X.; Li, K.; Sun, C. Simultaneous determination of ginsenoside (G-Re, G-Rg1, G-Rg2, G-F1, G-Rh1) and protopanaxatriol in human plasma and urine by LC-MS/MS and its application in a pharmacokinetics study of G-Re in volunteers. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 2011–2017. [Google Scholar] [CrossRef]

| Types | Samples | Processing | Main Components (mg/g Dry Weight) | Ref. |
|---|---|---|---|---|
| White ginseng | WKGE | Shade dried, Soxhlet extracted by water | 1.93 Re, 1.91 Rc, 1.81 Rb2, 1.56 Rb1, 1.24 Rg1, 0.6 Rf, (×10−3) | [36] |
| KWG | Air dried | 9.1 Re, 3.0 Rg1, 2.4 Rb1, 1.3 Rc, 0.7 Rb2 | [37] | |
| TCMGAR | Air dried | 11.2 Rg3, 4.2 Rd, 4.1 Rb1, 3.8 Rh2, 2.3 Rb2, 2.0 Rc | [38] | |
| AG | Air dried | 96.7 total ginsenosides (PPD:PPT = 3.03:1) | [26] | |
| Red ginseng | KRG | Steamed and air dried | 1.93 Rb1, 1.3 Rg3, 1.0 Rd, 0.93 Rb2 | [39] |
| KRG | Steamed at 90–100 °C; for 3 h and air dried | 51.6 Rb1, 28.9 Rg1, 22.2 Rc, 21.6 Re, 18.2 Rb2 | [22] | |
| KRG | Steamed and air dried | 2.43 Rb1, 1.58 Rg1, 0.95 Rc, 0.89 Rb1, 0.62 Re | [40] | |
| KRG | Steamed and air dried | 4.6 Rb1, 2.8 Rc, 2.3 Rb2, 1.4 Rg2, 1.2 Rg3, 1.2 Re | [41] | |
| KRG | Steamed and air dried | 8.03 Rb1, 3.29 Rc, 2.80 Rb2, 2.50 Rg3, 1.47 Rf, 1.29 Re, 1.18 Rg1, 1.0 Rd | [24] | |
| KRG | Steamed and air dried | 16.58 total ginsenosides (PPD:PPT = 1.65:1) | [20] | |
| ARG | Steamed in autoclave and dried | Related fatty acids, 58.1 cinnamic acid, 50.1 ferulic acid | [42] | |
| Black ginseng | BGE | Steamed and dried, repeat several cycles, extracted by 70% ethanol at 70 °C for 12 h | 5.6 C-K, 4.7 Rg5, 1.7 Rg3, 1.5 Rb1, 0.8 Rg2, 0.7 Rc | [30] |
| GBG05-FF | Repeated steaming at 95 °C for 6 h and drying at 60 °C, extracted by 70% ethanol at 80 °C for 8 h | 11.7 Rg5, 6.9 Rk1, 5.2 Rg3, 1.9 Rh4 | [43] | |
| Fermented ginseng | FRG | RGE incubated with yeast at 40 °C for 12 h | 4.9 Rg3, 4.8 Rb1, 3.4 Rb2, 2.9 Rg2, 1.8 Re, 1.4 C-K, 0.8 Rg1 | [44] |
| FRG | RGE incubated with L. plantarum at 35–40 °C for 15 d | 4.9 C-K, 3.5 Re, 3.3 Rb1, 3 Rb2, 2.4 Rd, 2 Rc | [21] | |
| FSGB | SGB incubated with L. plantarum at 30 °C for 72 h | Quinic acid, linoleic acid, palmitic acid | [45] | |
| FGE | GE incubated with microorganism | 61.0 C-K, 27.7 Rg3, 12.1 Rh1, 9.5 Rd, 8.2 Rg2, 3.1 Rh2, 2.3 Rb2 | [46] | |
| GS-E3D | RGE incubated with 10% pectin lyase at 50 °C for 5 d | 30.2 Rb1, 17.6 Rb2, 14.0 Rc, 12.6 Re, 5.9 Rg1, 4.7 Rf, 2.7 Rg3, 1.5 Rg5 | [47] | |
| VEG | GE incubated with vinegar (pH 2.3) at 90 °C for 6 h | 40.5 Rg3, 4.1 Rc, 4.0 Rd, 1.9 Rb2, 1.1 Rf | [15] | |
| HGE | GE incubated with an enzyme solution | 7.54 Rg1, 6.3 C-K, 5.42 Rb1, 1.87 Re, 0.70 Rd | [16] | |
| Fractioned ginseng | Rg3-RGE | RG multiple extracted by 55% ethanol | 51.7 Rg3, 3.86 Rb1, 3.71 Rh1, 3.55 Rg2, 1.6 Rd, 1.53 Rb2 | [48] |
| GB | Removed seeds and air dried, refluxed with 70% ethanol for 10 h | 110.6 Re, 21.1 Rc, 19.0 Rb2, 16.6 Rg1, 16.5 Rd, 8.4 Rg2, 7.7 Rb1 | [19] |
| Material | Design (Sample Size and Subjects) | Drug Treatment and Duration | Results | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BW | HbA1c | FBG | FI | PG | PI | HOMA-IR | Safety | ||||
| Vinegar extract of ginseng (VEG) | RCT (72 type 2 diabetic patients) | Four group (n = 18/group): 1500, 2000, 3000 mg of VEG, or placebo daily; 8 weeks | # | + | + | + | + | # | # | * | [15] |
| Korean red ginseng (KRG) | RCT (50 obese women) | 6 g/d of KRG n = 24, or placebo n = 26; 8 weeks | + | # | - | # | # | # | # | # | [23] |
| Korean red ginseng (KRG) | Multicenter, RCT (1000 healthy adults) | 2 g/d of KRG n = 495, or placebo n = 505; 24 weeks. | - | # | # | # | # | # | # | * | [24] |
| Korean red ginseng (KRG) | RCT (68 obese participants without diabetes) | 6 g/d of KRG n = 34, or placebo n = 34; 12 weeks | - | # | - | - | # | # | - | # | [22] |
| Korean red ginseng (KRG) | RCT (41 type 2 diabetic patients) | 5 g/d of KRG n = 21, placebo n = 20; 12 weeks | - | - | + | + | + | + | + | # | [20] |
| Fermented red ginseng (FRG) | RCT (42 impaired fasting glucose or type 2 diabetic patients) | 2.7 g/d of FRG n = 21, placebo n = 21; 4 weeks | # | # | - | - | + | + | # | * | [21] |
| Hydrolyzed ginseng extract (HGE) | RCT (23 impaired fasting glucose participants) | 960 mg/d of HGE n = 12, placebo n = 11; 8 weeks | - | # | + | - | + | - | - | * | [16] |
| Korean white ginseng (KWG) | RCT crossover trial (25 type 2 diabetic patients) | 1 g, 3 g, 6 g KWG, or 3 g placebo together with 50 g glucose-load, acute test | # | # | - | # | - | # | # | * | [37] |
| Ginseng berry extract (GBE) | RCT (72 participants) | 1 g/d GBE n = 34, placebo n = 38; 12 weeks | - | - | + | - | + | - | - | * | [19] |
| American ginseng (AG) | RCT parallel trial (74 type 2 diabetic patients) | 3 g/d of AG n = 35, placebo n = 39; 12 weeks | # | # | # | # | # | # | # | * | [27] |
| American ginseng (AG) | RCT crossover trial (39 type 2 diabetic patients) | 6 g/d of fiber from KGB together with 3 g AG; 12 weeks | - | + | - | - | # | # | # | * | [25] |
| American ginseng (AG) | RCT crossover trial (24 type 2 diabetic patients) | 3 g/d AG or placebo with original treatment; 8 weeks | - | + | + | - | # | # | # | * | [26] |
| Material | Dose [Route of Administration] | Duration | Animal | Molecular Mechanism | Ref. |
|---|---|---|---|---|---|
| Ginseng extracts | |||||
| Fermented steam-dried ginseng berry (FSGB) | 0.5 g/kg [ig] | 7 wk | db/db mice | Decreased the blood glucose and body weight; increased the immune cell population and GLUT1 expression. | [45] |
| Ginseng berry (GB) | 100, 200 mg/kg [ig] | 10 wk | STZ-induced mice | Enhanced beta-cell proliferation and glucose tolerance, decreased blood glucose. | [28] |
| Tissue culture raised mountain ginseng adventitious root (TCMGARs) | 125, 250, 500 mg/kg [diet] | 4 wk | STZ-induced rats | Significantly reduced the blood glucose, TC, and TG levels. | [38] |
| Ginseng berry (GB) | 0.05% [diet] | 6 m | C57BL/6 mice (15 months old mice) | Increased the parameters of insulin sensitivity, IRS, AKT, and FOXO1; decreased PPAR-γ. | [29] |
| Wild Korean ginseng extract (WKGE) | 100, 200, 300 mg/kg [ig] | 8 wk | STZ-induced rats | Significantly reduced blood glucose, ALT and alkaline phosphatase levels. | [36] |
| Black ginseng extract (BGE) | 50, 100, 200 mg/kg [ig] | 5 wk | STZ-induced mice | Reduced hyperglycemia and NF-κB; increased the insulin/glucose ratio and β-cell function. | [30] |
| Black ginseng ethanol extract (GBG05-FF) | 300, 900 mg/kg [diet] | 4 wk | db/db mice | Reduced the parameters of fasting blood glucose, glucose tolerance, HbA1c, TG, TC levels, and lipid accumulation; enhanced the phosphorylation of the AMPK, and up-regulated the expressions of GLUT2 and GLUT4. | [43] |
| Red ginseng (RG) | 100, 200 mg/kg [ig] | 8 wk | ICR mice for type 1 (STZ induced) db/db mice for type 2 | Improved the threshold shift of hearing, delayed latencies, and signal intensity decrease in type 2 diabetic mice; changes with no significance in type 1 diabetic mice. | [41] |
| Korean red ginseng (KRG) | 200 mg/kg [ig] | 10 wk | STZ-induced rats | 39 genes were upregulated more than two-fold; 84 genes were down-regulated. | [49] |
| Pectin lyase-modified red ginseng extract (GS-E3D) | 25, 50, 100 mg/kg [ig] | 6 wk | STZ-induced rats | Decreased the urinary levels of albumin, 8-OHdG, and AGEs; suppressed oxidative stress. | [47] |
| Rg3-enriched red ginseng extract (Rg3-RGE) | 2.5, 5 mg/kg [ig] | 12 wk | LDL-/- mice | Reduced the levels of glucose, TG, LDL, AST, and AST; inhibited atheroma formation. | [48] |
| Korean red ginseng (KRG) | 200 mg/kg [diet] | 12 wk | Goto Kakizaki rats | Reduced blood glucose, PTP-1B, UCP-2, and PARP; enhanced the production of GLUT-4 and insulin. | [40] |
| Fermented red ginseng (FRG) | 0.5%, 1% [diet] | 16 wk | ob/ob mice | Decreased body weight, blood glucose, and hyperlipidemia; increased the expressions of IR, LPL, GLUT1, GLUT4, PPAR-γ, and PEPCK. | [44] |
| Red ginseng extract (RGE) | 2 g/kg [ig] | 5 wk | STZ-induced rats | Metformin or RGE administered alone can reduce the FBG, but co-administration recovered the FBG to the control level. | [39] |
| Fermented ginseng extract (FGE) | 0.1% w/w [diet] | 8 wk | db/db mice | Decreased blood glucose, HbA1c, TNF-α, and lymphocytes; increased adiponectin, serum insulin, PPAR-γ2, and GLUT-2. | [46] |
| Ginseng polysaccharides | 0, 12.5, 25, 50, 100, 200 mg/kg [ig] | 10 d | STZ-induced ICR mice | Increased the concentration of insulin, SOD, and glycogen; decreased the content of MDA. | [50] |
| Ginseng powder | 150 mg/kg [ig] | 7 d | STZ-induced rats | Enhanced the expression of PPAR-δ and the phosphorylation of troponin1. | [51] |
| American red ginseng (ARG) | 150 mg/kg [ip] | 30 d | db/db mice | Reduced blood glucose, plasma cholesterol and LDL; increased glycogen and HDL. | [42] |
| American ginseng | 200 mg/kg [ig] | 2 m (type 1), 4 m (type2) | C57BL/6 mice for type 1 (STZ- induced), db/db mice for type 2 | Increased stroke volume, ejection fraction, cardiac output, and left ventricle pressure; reduced oxidative stress. | [52] |
| American ginseng saponin (PQS) of stem and leaf | 30, 60 mg/kg [ig] | 8 wk | STZ-induced rats | Up-regulated the expressions of HGF, NO, ET-1, TNF-α, and slCAM-1. | [53] |
| Ginsenosides | |||||
| Panaxatriol | 11 mg/d + 45 min/d (15 m/min) exercise [diet] | 6 wk | KK-Ay/Ta Jcl (KKAy) mice | Significantly lowered blood glucose; no significant differences in body weight; significantly improved insulin resistance. | [54] |
| Rb1 | 10 mg/kg [ip] | 1 wk | C57BL/6 mice (high fat) | Decreased fasting blood glucose, glucose tolerance, and 11β-HSD1; increased insulin sensitivity. | [31] |
| Re | 5, 10, 20 mg/kg [ig] | 4 wk | C57BL/6 mice (high fat) | Decreased the parameters of TG, TC, LDL-C, GOT, and GPT; increased HDLC, regulating ACh, AChE, MDA, SOD, and GSH via JNK pathway. | [55] |
| Rg1 | 10 mg/kg [ip] | 4 wk | STZ-induced rats | Improved angiogenesis, increased eNOS activation, up-regulated VEGF expression, and inhibited apoptosis. | [56] |
| Rg1 | 10,15, 20 mg/kg [ip] | 12 wk | STZ-induced rats | Decreased the parameters of cTnI, CK-MB, MDA, apoptosis, and CASP3; increased expressions of SOD, CAT, GSH, and Bcl-xL. | [57] |
| Rg1 | 10,15, 20 mg/kg [iv] | 12 wk | STZ-induced rats | Reduced the expressions of cTnI, GRP78, and CHOP; inhibited endoplasmic reticulum stress-induced apoptosis. | [58] |
| Rg1 | 56.25 μM/kg [iv] | 10 d | STZ-induced rats | Reduced the cerebral infarction volume; promoted neuronal recovery. | [59] |
| Rg1 | 25, 50 mg/kg [ig] | 8 wk | STZ-induced rats | Reduced blood glucose levels and insulin resistance index; increased the parameters of TC, TG, LDL-C, AST, and ALT. | [32] |
| Rg3 | 0.5 mg/kg [ig] | Once | db/db mice | Increased the production of GLP-1 and insulin; decreased blood glucose. | [33] |
| Rh2 | 5 mg/kg [iv] | 4 wk | STZ-induced rats | Decreased fasting blood glucose, ratio of heart weight/body weight, and PPAR-δ; increased STAT3, CCN2, and fibronectin. | [60] |
| Malonyl ginsenosides (MGR) | 50, 100 mg/kg [ip] | 3 wk | STZ-induced rats (high fat) | Significantly lowered FBG levels; reduced TG and TC; increased glucose disposal; no effect on body weight. | [61] |
| Ginsenoside Rg1 + Astragaloside IV | (50 Rg1 + 16 IV) mg/kg [ig] | 8 wk | STZ-induced rats | Lowered MDA level and increased levels of CAT, GSH-PX, and T-AOC. Significantly reduced the levels of BUN, SCr, β2-MG, and UCr. Diminished the mRNA overexpression of TGF-β1 and CTGF; increased Smad7 expression. | [62] |
| Rg3 | 10 mg/kg/2 days [ip] | 4 wk | STZ-induced ApoE-/- mice | 20(S)-Rg3 showed stronger anti-proliferative and anti-migratory effects than 20(R)-Rg3; (S)-isomer significantly reduced the plaque size of diabetic atherosclerosis. | [63] |
| Rg3 | 0.5 mg/kg [ig] | 3 m | STZ-induced rats (high fat) | Inhibited inflammatory response (inhibited the expression of TGF-β1, IL-1, IL-6, IL-12) and promoted the activation of PI3K and MAPK signaling pathways to prevent lung tissue damage induced by hyperglycemia. | [64] |
| Material | Cell Line | Drug Dose | Molecular Mechanism | Ref. |
|---|---|---|---|---|
| Ginseng total saponin | Mouse podocytes | 1 µg/mL for 6, 24, and 48 h | Ginseng total saponins improved p130Cas protein of podocytes. | [65] |
| Ginsenoside Rg3 | Human NCI-H716 | 10 µM | Enhanced secreting of GLP-1. | [33] |
| Ginsenoside Rg3 | Mouse islet cell | 4 µM | Increased glucose-induced insulin secretion (2.3-fold higher); enhanced islet function and attenuated apoptosis. | [34] |
| Ginsenoside Rg3 | Human pulmonary cell BEAS-2B | 25, 50, 75, 100 μg/mL | No effect on the viability of BEAS-2B cells; high concentration could induce apoptosis compared with the control group. | [64] |
| Ginsenoside Rg1 | C2C12 muscle cell | 10, 20, 40 µM | Enhanced glucose uptake and GLUT4 by AMPK pathways. | [66] |
| Ginsenoside Re | 3T3-L1 cell | 3, 10, 30, 60 µM | Increased glucose uptake and the expressions of PPAR-γ2, IRS-1, ap2, and adiponectin genes expressions; helped the translocation of GLUT4 to the membranes; inhibited the expression and production of TNF-α. | [67] |
| Ginsenoside F2 | 3T3-L1 cell | 10, 50, 100 µM | Reduced the content of lipid accumulation; down-regulated expression of PPAR-γ and perilipin. | [68] |
| Ginsenoside Rg5:Rk1 mixture | 3T3-L1 cell | 10, 100 µg/mL | Inhibited lipid accumulation and reduced TG level. Decreased the mRNA level of STAT3, PPAR-γ, CEBPα, and ap2; reduced protein expression of PPAR-γ and CEBPα. | [69] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Balan, P.; Popovich, D.G. Review of Ginseng Anti-Diabetic Studies. Molecules 2019, 24, 4501. https://doi.org/10.3390/molecules24244501
Chen W, Balan P, Popovich DG. Review of Ginseng Anti-Diabetic Studies. Molecules. 2019; 24(24):4501. https://doi.org/10.3390/molecules24244501
Chicago/Turabian StyleChen, Wei, Prabhu Balan, and David G. Popovich. 2019. "Review of Ginseng Anti-Diabetic Studies" Molecules 24, no. 24: 4501. https://doi.org/10.3390/molecules24244501
APA StyleChen, W., Balan, P., & Popovich, D. G. (2019). Review of Ginseng Anti-Diabetic Studies. Molecules, 24(24), 4501. https://doi.org/10.3390/molecules24244501







