Identification of 1-Butyl-Lysergic Acid Diethylamide (1B-LSD) in Seized Blotter Paper Using an Integrated Workflow of Analytical Techniques and Chemo-Informatics
Abstract
1. Introduction
2. Results
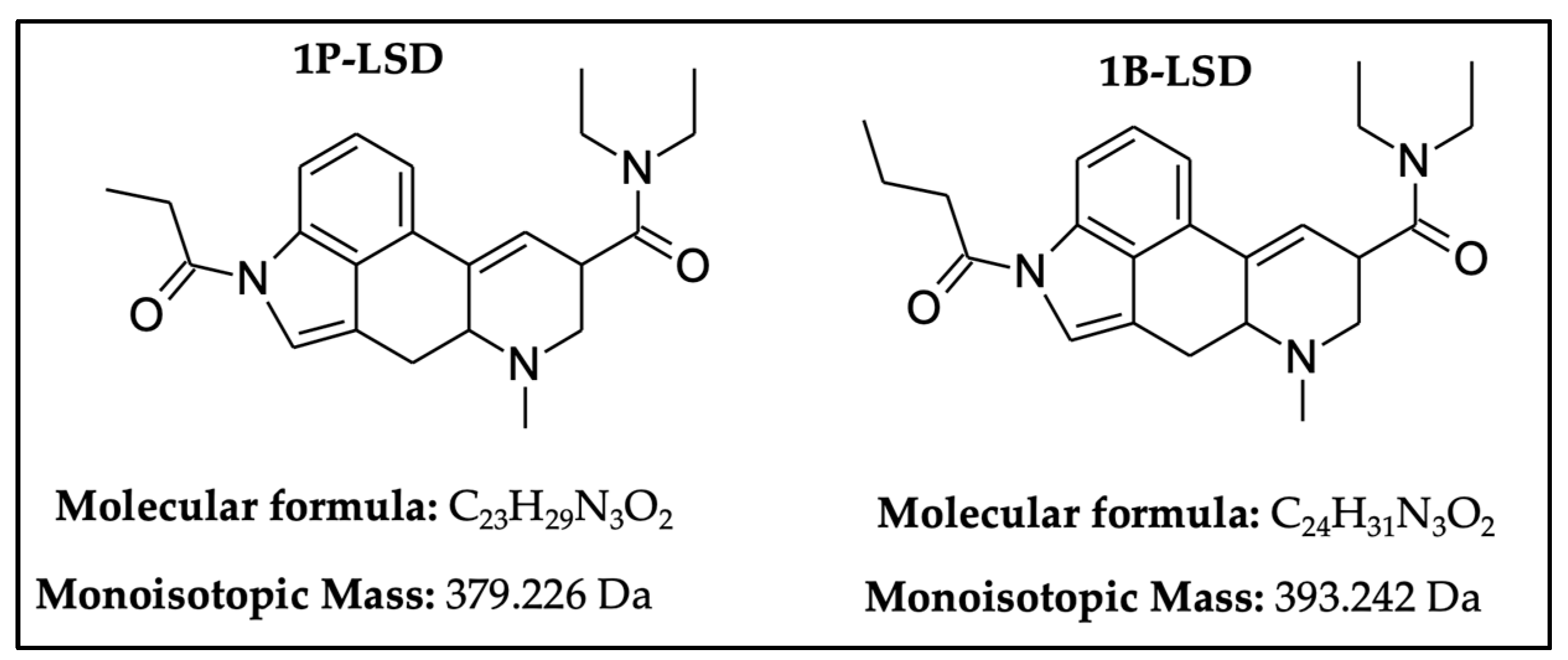
2.1. GC-MS Analysis
2.2. UHPLC–HR-MS Experiment
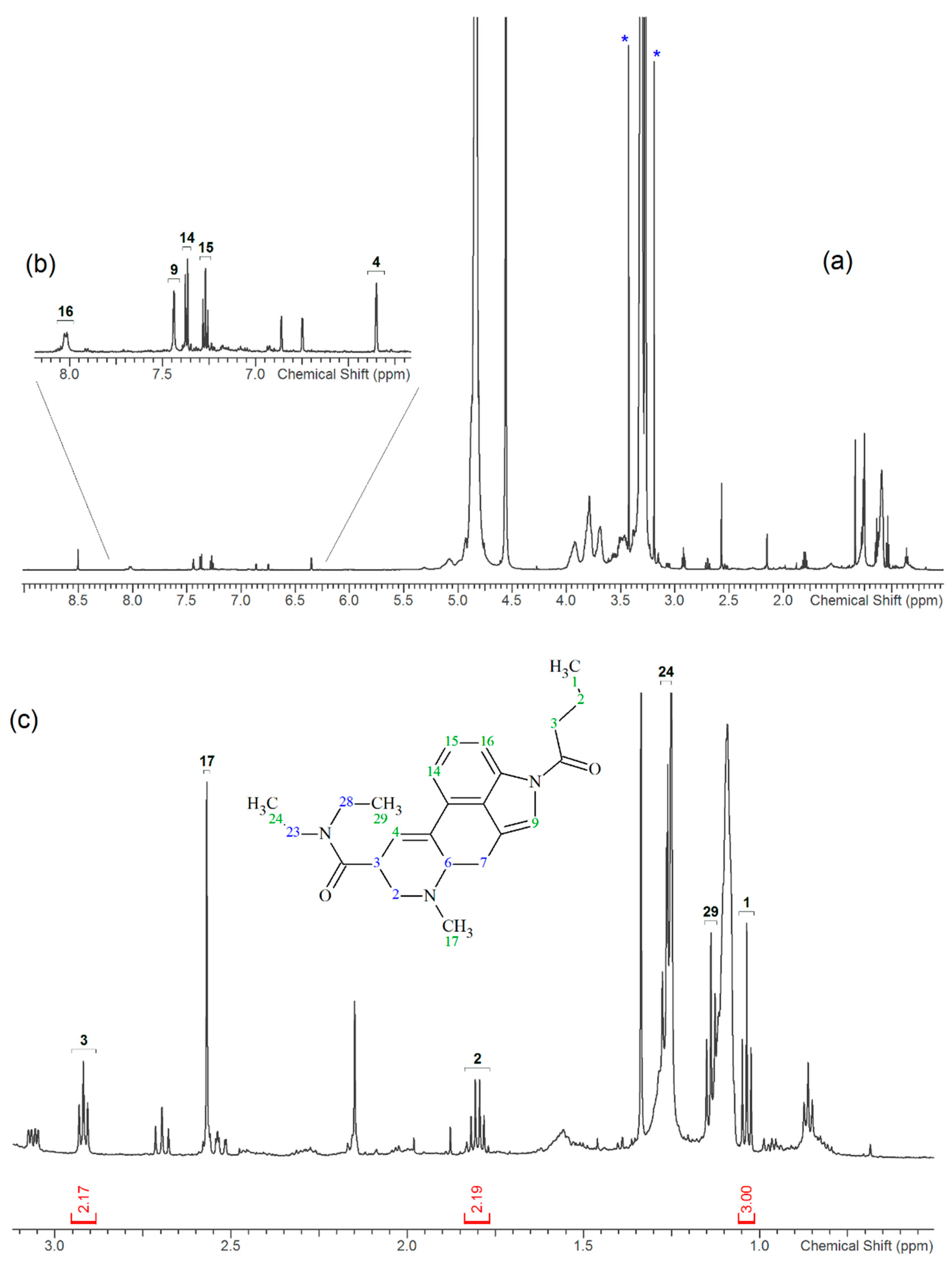
2.3. NMR
3. Discussion
3.1. GC–MS and HR-MS
3.2. NMR
4. Materials and Methods
4.1. Chemical and Reagents
4.2. Seized Blotter Sample
4.3. Sample Preparation
4.4. Instrumental Analysis
4.4.1. GC–MS
4.4.2. NMR
4.4.3. HR-MS/MS
4.5. Subsection Chemoinformatics Tools
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- EMCDDA. European Drug Report 2017: Trends and Developments. Publications of of the European Union 2017. Available online: http://www.emcdda.europa.eu/system/files/publications/4541/TDAT17001ENN.pdf (accessed on 27 December 2019).
- Gaspar, H.; Bronze, S.; Oliveira, C.; Victor, B.L.; Machuqueiro, M.; Pacheco, R.; Caldeira, M.J.; Santos, S. Proactive response to tackle the threat of emerging drugs: Synthesis and toxicity evaluation of new cathinones. Forens. Sci. Int. 2018, 290, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Lobo Vicente, J.; Chassaigne, H.; Holland, M.V.; Reniero, F.; Kolář, K.; Tirendi, S.; Vandecasteele, I.; Vinckier, I.; Guillou, C. Systematic analytical characterization of new psychoactive substances: A case study. Forens. Sci. Int. 2016, 265, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Hida, M.; Mitsui, T. Rapid identification of lysergic acid diethylamide in blotter paper by microscope FT-IR. Anal. Sci. 1999, 15, 289–291. [Google Scholar] [CrossRef]
- Brandt, S.D.; Kavanagh, P.; Westphal, F.; Stratford, A.; Elliott, S.P.; Hoang, K.; Wallach, J.; Halberstadt, A.L. Return of the lysergamides. Part I: Analytical and behavioural characterization of 1-propionyl-D-lysergic acid diethylamide (1P-LSD): Analytical and behavioural characterization of 1-propionyl-LSD. Drug Test. Anal. 2016, 8, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.D.; Kavanagh, P.V.; Westphal, F.C.; Elliott, S.P.D.; Wallach, J.E.; Stratford, A.F.; Nichols, D.E.; Halberstadt, A.L. Return of the lysergamides. Part III: Analytical characterization of N6-ethyl-6-norlysergic acid diethylamide (ETH-LAD) and 1-propionyl ETH-LAD (1P–ETH-LAD). Drug Test. Anal. 2017, 9, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.D.; Kavanagh, P.V.; Westphal, F.; Elliott, S.P.; Wallach, J.; Colestock, T.; Burrow, T.E.; Chapman, S.J.; Stratford, A.; Nichols, D.E.; et al. Return of the lysergamides. Part II: Analytical and behavioural characterization of N6-allyl-6-norlysergic acid diethylamide (AL-LAD) and (2’S,4’S)-lysergic acid 2,4-dimethylazetidide (LSZ). Drug Test. Anal. 2017, 9, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.D.; Kavanagh, P.V.; Twamley, B.; Westphal, F.; Elliott, S.P.; Wallach, J.; Stratford, A.; Klein, L.M.; McCorvy, J.D.; Nichols, D.E.; et al. Return of the lysergamides. Part IV: Analytical and pharmacological characterization of lysergic acid morpholide (LSM-775). Drug Test. Anal. 2018, 10, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.D.; Kavanagh, P.V.B.; Westphal, F.C.; Stratford, A.D.; Elliott, S.P.E.; Dowling, G.B.F.; Wallach, J.G.; Halberstadt, A.L. Return of the lysergamides. Part V: Analytical and behavioural characterization of 1-butanoyl-d-lysergic acid diethylamide (1B-LSD). Drug Test. Anal. 2019, 11, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Wagmann, L.; Richter, L.H.J.; Kehl, T.; Wack, F.; Bergstrand, M.P.; Brandt, S.D.; Stratford, A.; Maurer, H.H.; Meyer, M.R. In Vitro metabolic fate of nine LSD-based new psychoactive substances and their analytical detectability in different urinary screening procedures. Anal. Bioanal. Chem. 2019, 411, 4751–4763. [Google Scholar] [CrossRef] [PubMed]
- Romão, W.; Sabino, B.D.; Bueno, M.I.M.; Vaz, B.G.; Júnior, A.C.; Maldaner, A.O.; de Castro, E.V.; Lordeiro, R.A.; Nascentes, C.C.; Eberlin, M.N.; et al. LSD and 9,10-dihydro-LSD Analyses in Street Drug Blotter Samples via Easy Ambient Sonic-Spray Ionization MassSpectrometry (EASI-MS). J. Forensic Sci. 2012, 57, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.C. Differentiation of lysergic acid diethylamide (LSD) from N-methyl-N-propyl and N-butyl amides of lysergic acid. J. Forensic Sci. 1989, 34, 532–546. [Google Scholar] [CrossRef]
- Laing, R.R. (Ed.) Hallucinogens. In A Forensic Drug Handbook; Elsevier Science Ltd.: London, UK, 2003. [Google Scholar]
- Siegel, J.A. Part II: Mass Spectrometry of Lysergic Acid Diethylamide (LSD). In Handbook of Forensic Drug Analysis; Smith, F.P., Ed.; Elsevier Academic Press: San Diego, CA, USA, 2004; pp. 186–187. [Google Scholar]
Sample Availability: No sample is available. |
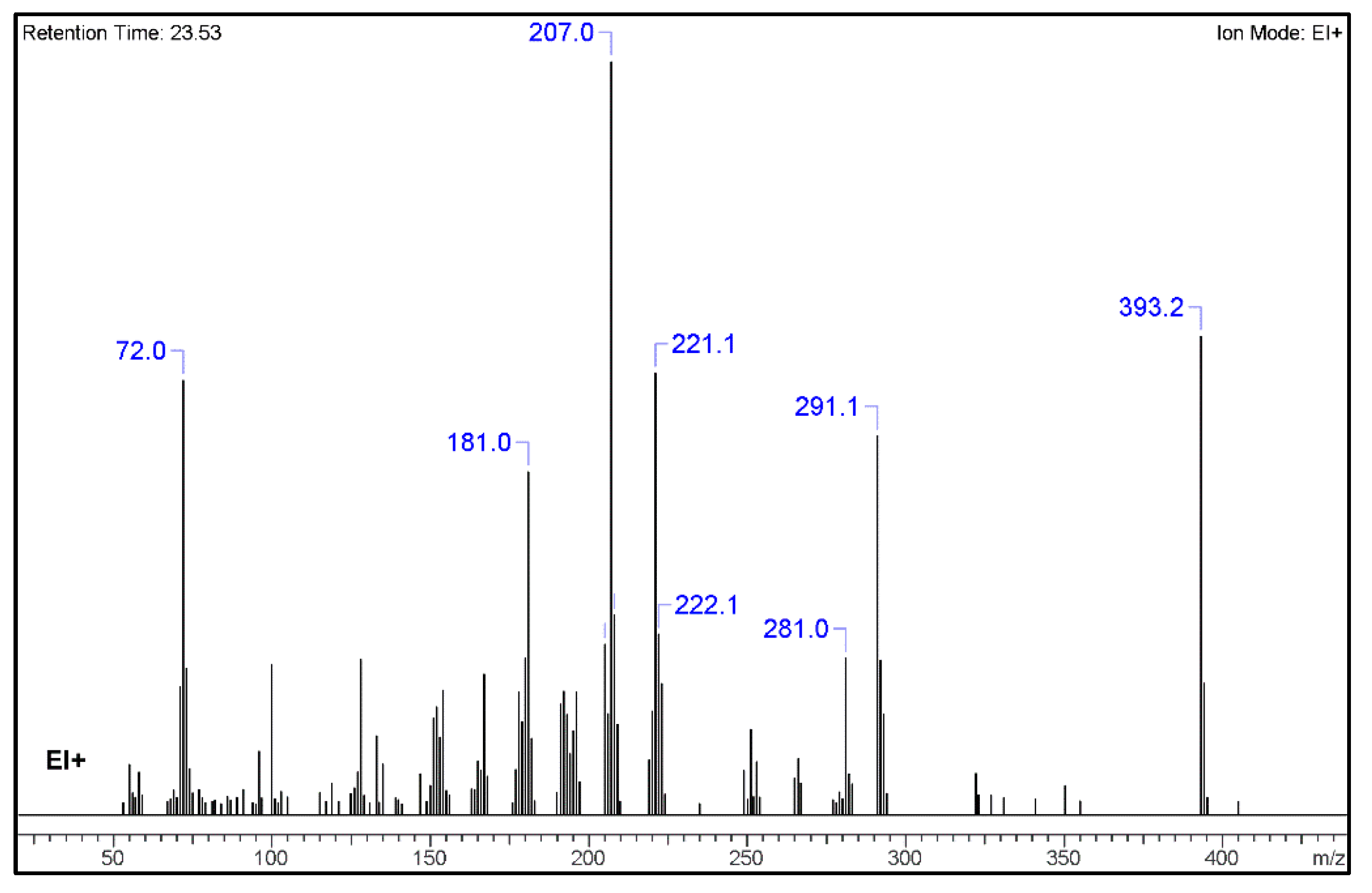
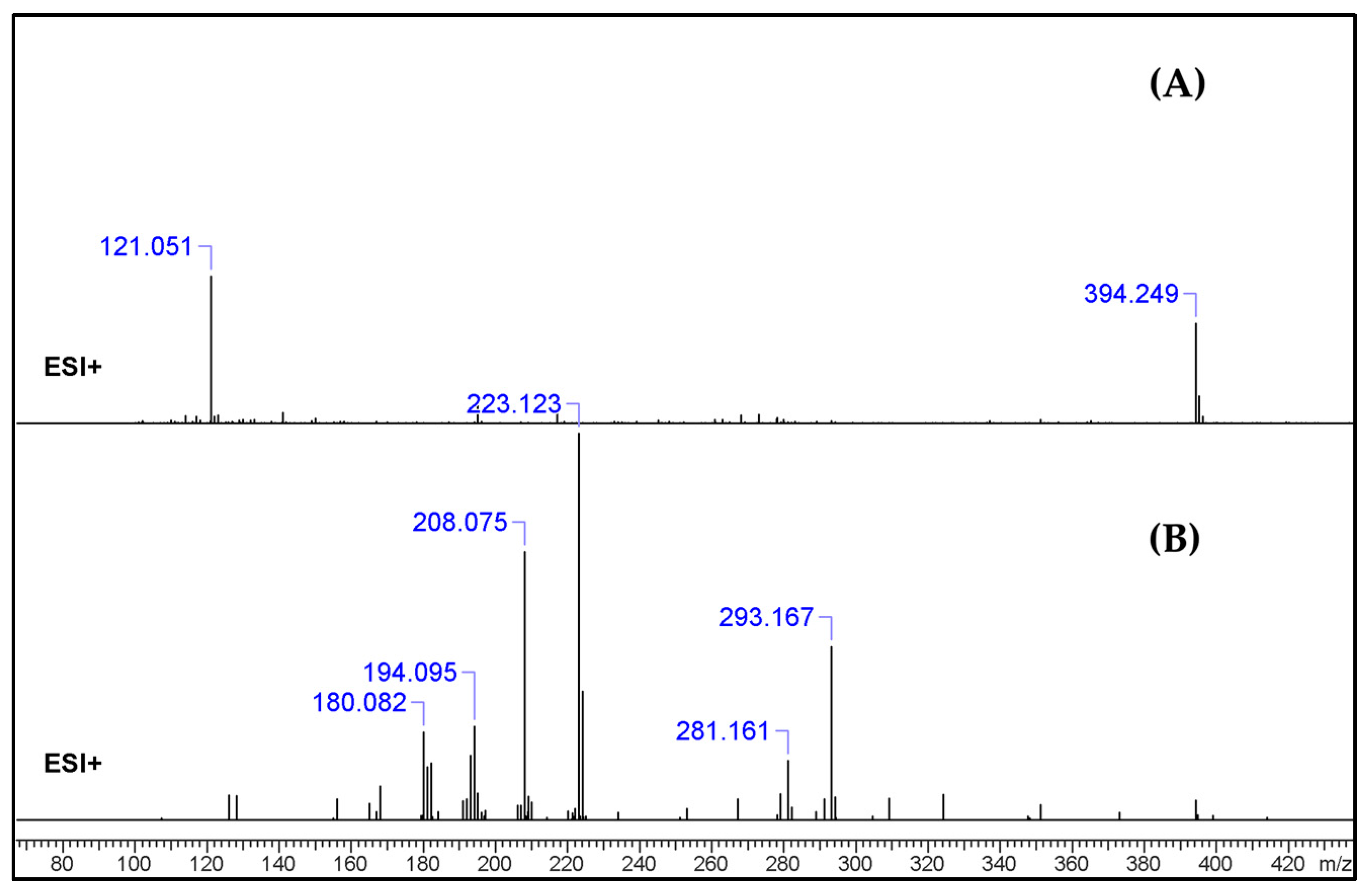
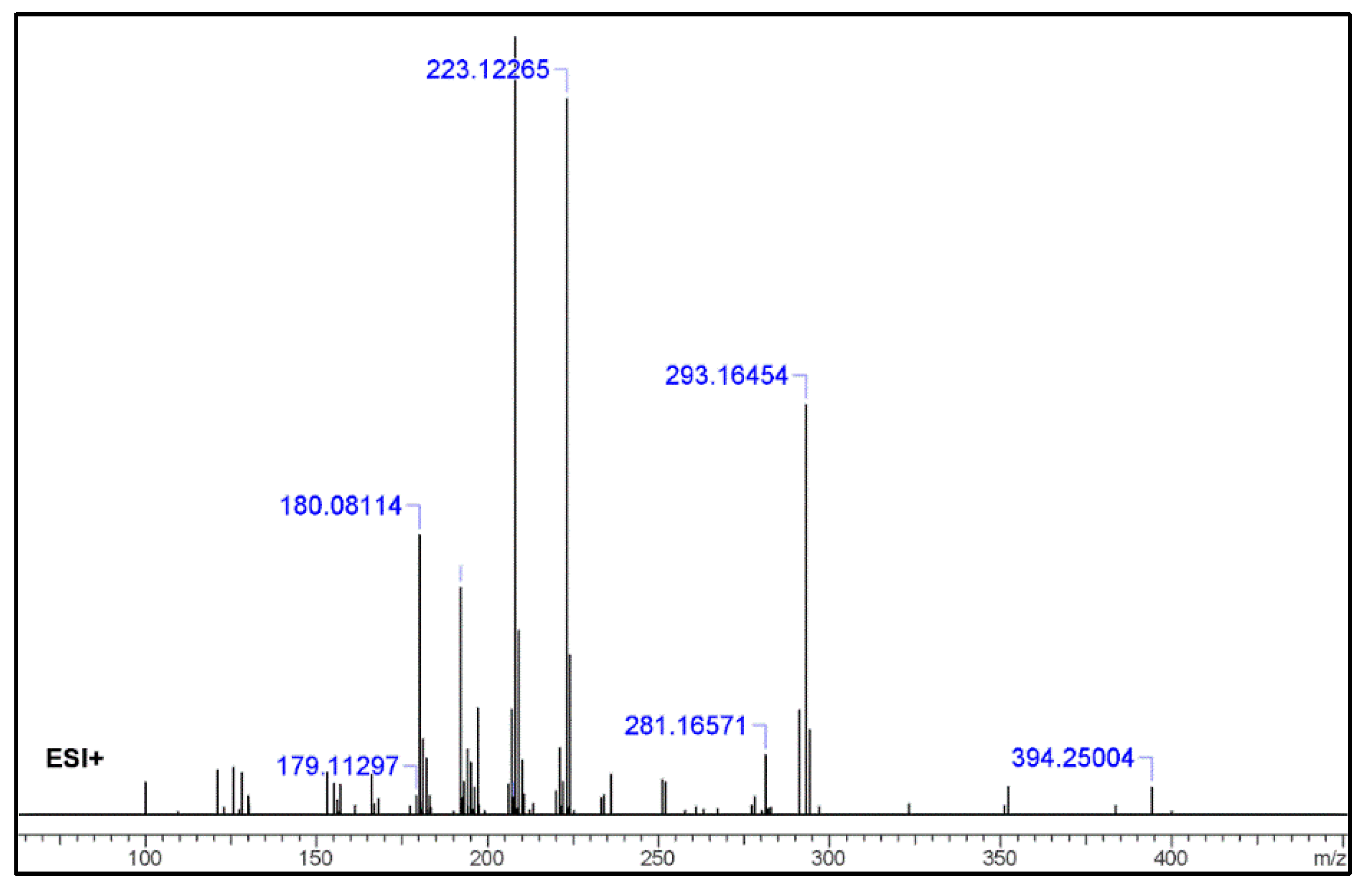
| No. | Fragment New Structure | Formula | Label | m/z Exp. 1 | RI Exp. (%) 2 |
|---|---|---|---|---|---|
| 1 | 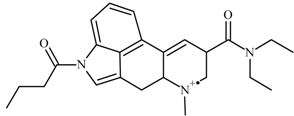 | C24H31N3O2(+) | M | 393.2 | 100.0 |
| 2 |  | C18H15Ν2O2(+) | M-C6H16N | 291.1 | 79.3 |
| 3 | 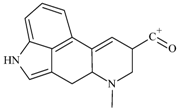 | C16H15N2O(+) | M-C8H16NO | 251.1 | 15.5 |
| 4 | 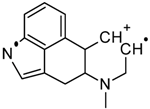 | C15H13N2(+) | M-C9H18NO2 | 221.1 | 58.665 |
| 5 | 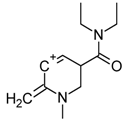 | C12H19N2O(+) | M-C12H12NO | 207.0 | 69.3 |
| 6 |  | C12H9N2(+) | M-C12H11NO | 181.0 | 45.6 |
| 7 | 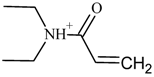 | C7H14NO(+) | M-C17H20N2O | 128.1 | 20.6 |
| 8 | 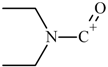 | C5H10NO(+) | M-C19H21N2O | 100.0 | 31.4 |
| 9 |  | C4H10N(+) | M-C20H21N2O2 | 72.0 | 90.8 |
| No. | Fragment New Structure | Formula | Label | m/z Exp. 1 | RI Exp. (%) 2 |
|---|---|---|---|---|---|
| 1 | 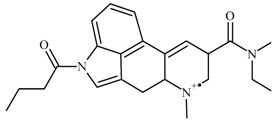 | C24H31N3O2(+) | M | 394.24890 | 3.5 |
| 2 |  | C21H26N3O2(+) | M + H-C3H6 | 352.20195 | 3.6 |
| 3 |  | C19H21N2O(+) | M + H-C5H11NO | 293.16454 | 52.7 |
| 4 |  | C15H15N2(+) | M-C9H16NO2 | 223.12265 | 27.5 |
| 5 |  | C12H20N2O(+) | M-C12H12NO | 208.07528 | 69.3 |
| 6 | 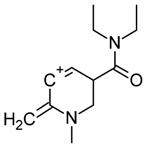 | C12H19N2O(+) | M + H-C12H13NO | 207.14919 | 13.5 |
| 7 |  | C12H8N2(+) | M-C12H11NO | 180.06820 | 36.0 |
| 8 | 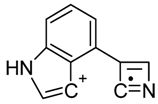 | C11H6N2(+) | M-C11H9NO | 166.05255 | 5.1 |
| 9 | 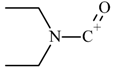 | C5H10NO(+) | M-C19H22N2O | 100.07569 | 4.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsochatzis, E.; Lopes, J.A.; Reniero, F.; Holland, M.; Åberg, J.; Guillou, C. Identification of 1-Butyl-Lysergic Acid Diethylamide (1B-LSD) in Seized Blotter Paper Using an Integrated Workflow of Analytical Techniques and Chemo-Informatics. Molecules 2020, 25, 712. https://doi.org/10.3390/molecules25030712
Tsochatzis E, Lopes JA, Reniero F, Holland M, Åberg J, Guillou C. Identification of 1-Butyl-Lysergic Acid Diethylamide (1B-LSD) in Seized Blotter Paper Using an Integrated Workflow of Analytical Techniques and Chemo-Informatics. Molecules. 2020; 25(3):712. https://doi.org/10.3390/molecules25030712
Chicago/Turabian StyleTsochatzis, Emmanouil, Joao Alberto Lopes, Fabiano Reniero, Margaret Holland, Jenny Åberg, and Claude Guillou. 2020. "Identification of 1-Butyl-Lysergic Acid Diethylamide (1B-LSD) in Seized Blotter Paper Using an Integrated Workflow of Analytical Techniques and Chemo-Informatics" Molecules 25, no. 3: 712. https://doi.org/10.3390/molecules25030712
APA StyleTsochatzis, E., Lopes, J. A., Reniero, F., Holland, M., Åberg, J., & Guillou, C. (2020). Identification of 1-Butyl-Lysergic Acid Diethylamide (1B-LSD) in Seized Blotter Paper Using an Integrated Workflow of Analytical Techniques and Chemo-Informatics. Molecules, 25(3), 712. https://doi.org/10.3390/molecules25030712




