1H-NMR Determination of Organic Compounds in Municipal Wastewaters and the Receiving Surface Waters in Eastern Cape Province of South Africa
Abstract
1. Introduction
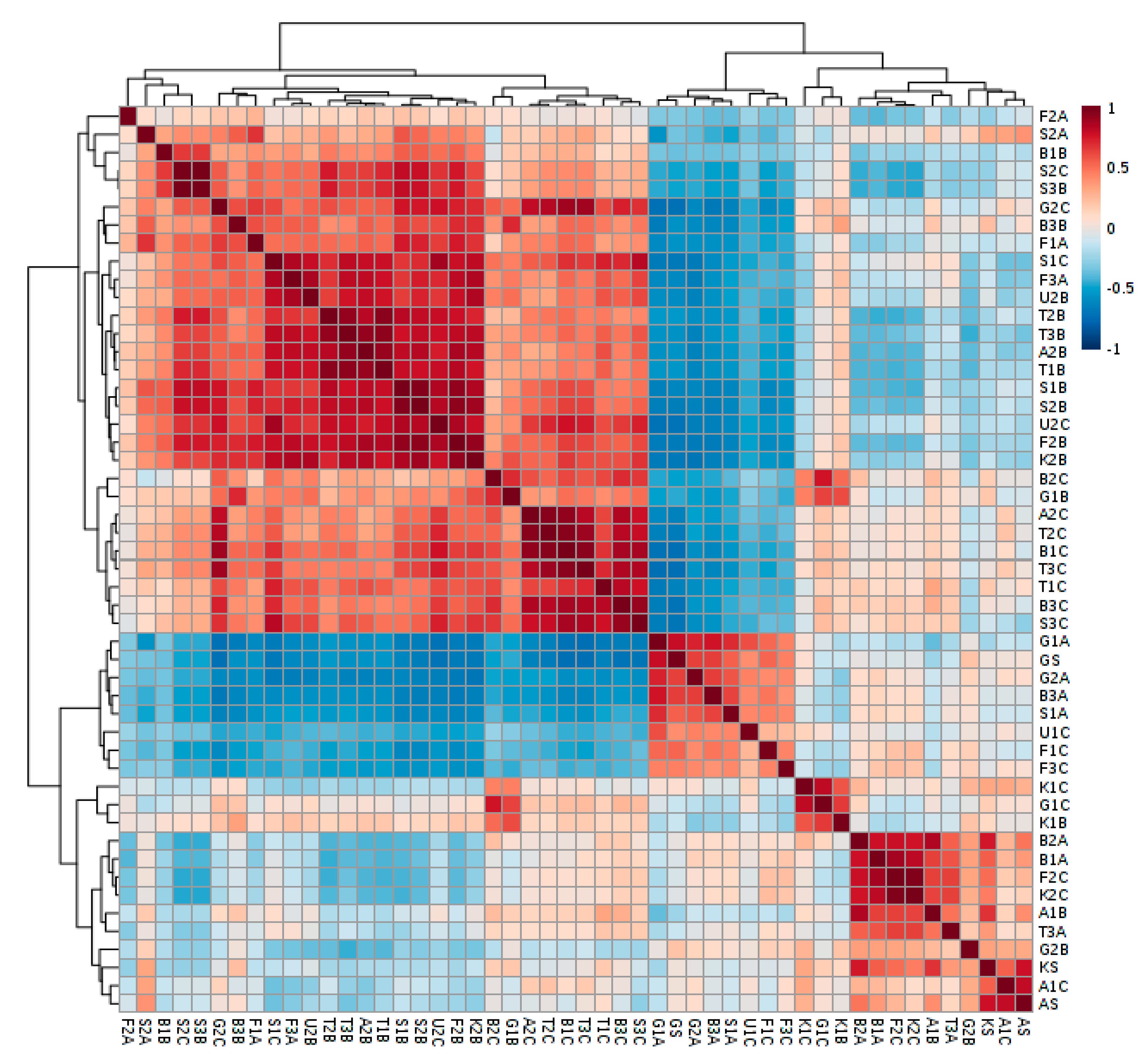
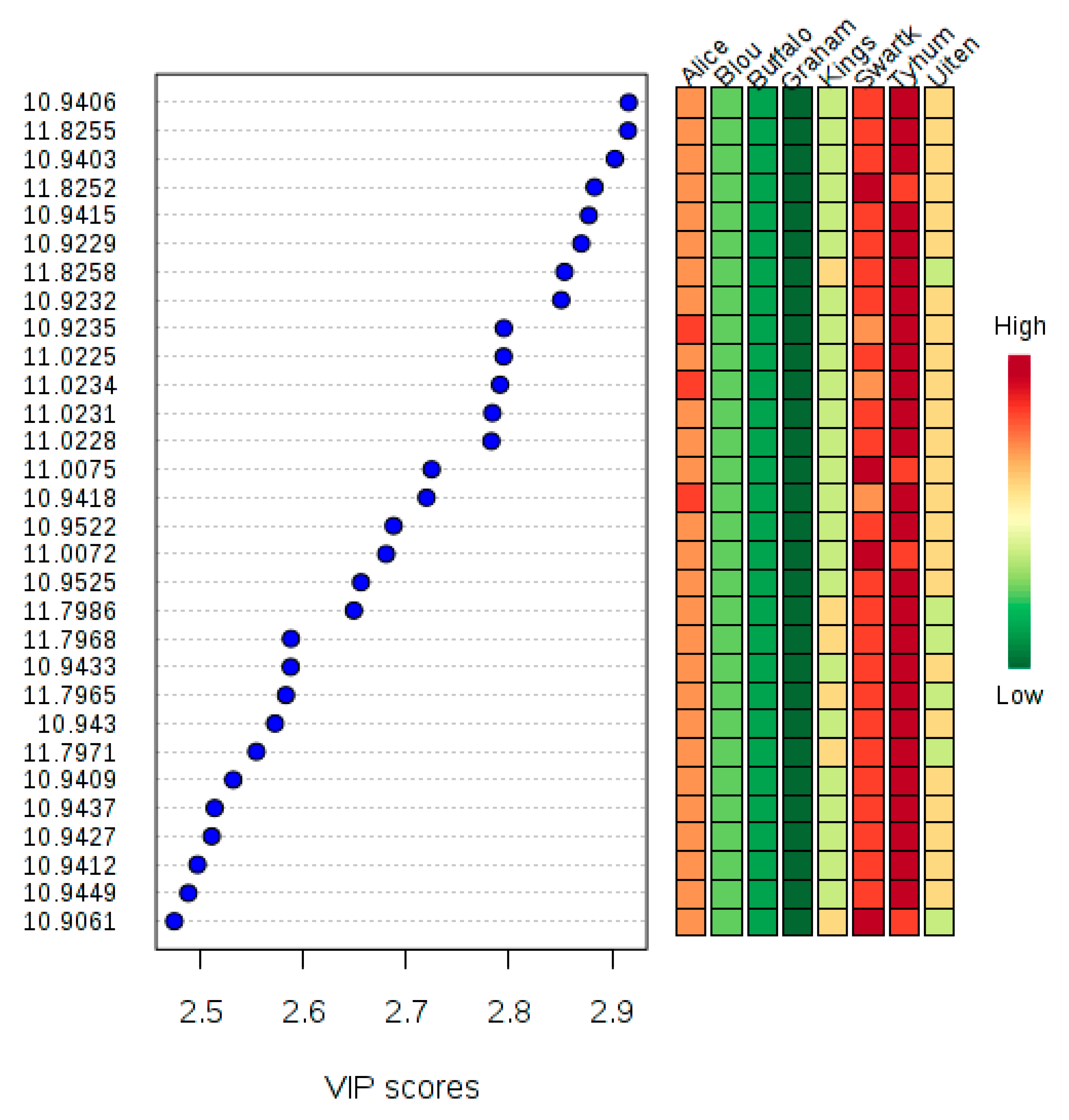
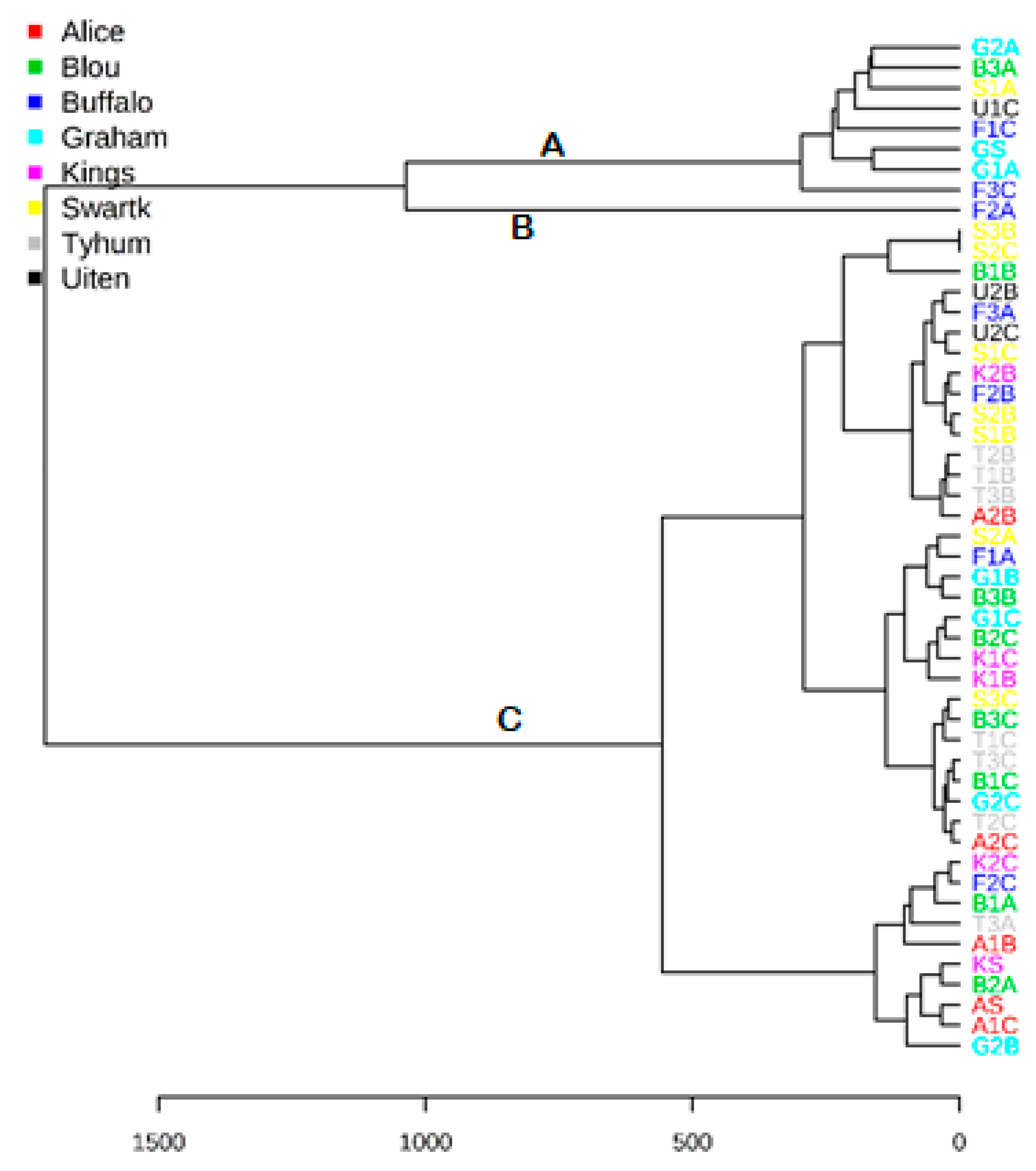
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Materials
4.3. Procedure
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tu, J. Spatial variations in the relationship between land use and water quality across an urbanisation gradient in the watersheds of northern Georgia, USA. Environ. Manag. 2013, 51, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cobbing, J.E.; de Wit, M. The Grootfontein aquifer: Governance of a hydrosocial system at Nash equilibrium. S. Afr. J. Sci. 2018, 114, 53–59. [Google Scholar] [CrossRef]
- Iyinbor, A.A.; Adebesin, B.O.; Abimbola, O.; Adelani-Akande, A. Water pollution: Effects, prevention and climatic impact. Water Chall. Urban. World 2018, 33. [Google Scholar] [CrossRef]
- United Nations Development Programme (UNDP). Sustainable Development Goals 6: Clean Water and Sanitation. 2019. Available online: https://www.undp.org/content/undp/en/home/sustainable-development-goals/goal-6-clean-water-and-sanitation.html (accessed on 15 October 2019).
- Rafi, S.; Niaz, O.; Naseem, S.; Majeed, U.; Naz, H. Natural and Anthropogenic Sources of Groundwater Salinization in Parts of Karachi, Pakistan. Int. J. Econ. Environ. Geol. 2019, 10, 22–28. [Google Scholar]
- American Chemical Society (ACS). National Historic Chemical Landmarks. NMR and MRI: Applications in Chemistry and Medicine. 2011. Available online: http://www.acs.org/content/acs/en/education/whatischemistry/landmarks/mri.html (accessed on 11 September 2019).
- Newton, M.; Breeds, E.; Morris, R. Advances in electronics prompt a fresh look at continuous wave (CW) nuclear magnetic resonance (NMR). Electronics 2017, 6, 89. [Google Scholar] [CrossRef]
- Klinowski, J.; Hennel, J.W. Fundamentals of Nuclear Magnetic Resonance; Wiley: New York, NY, USA; Longman Scientific & Technical: Essex, UK, 1993. [Google Scholar]
- Rahman, A.; Choudhary, M.I.; Wahab, A. Solving Problems with NMR Spectroscopy, 2nd ed.; Academic Press: London, UK, 2016. [Google Scholar]
- Pan, B.; Yang, F.; Ye, Y.; Wu, Q.; Li, C.; Huber, T.; Su, X. 3D structure determination of a protein in living cells using paramagnetic NMR spectroscopy. Chem. Commun. 2016, 52, 10237–10240. [Google Scholar] [CrossRef]
- Bieri, M.; Kwan, A.H.; Mobli, M.; King, G.F.; Mackay, J.P.; Gooley, P.R. Macromolecular NMR spectroscopy for the non-spectroscopist: Beyond macromolecular solution structure determination. FEBS J. 2011, 278, 704–715. [Google Scholar] [CrossRef]
- Lisi, G.P.; Loria, J.P. Using NMR spectroscopy to elucidate the role of molecular motions in enzyme function. Prog. Nucl. Magn. Res. Spec. 2016, 92, 1–17. [Google Scholar] [CrossRef]
- Aznauryan, M.; Delgado, L.; Soranno, A.; Nettels, D.; Huang, J.R.; Labhardt, A.M.; Schuler, B. Comprehensive structural and dynamical view of an unfolded protein from the combination of single-molecule FRET, NMR, and SAXS. Proc. Nat. Acad. Sci. USA 2016, 113, E5389–E5398. [Google Scholar] [CrossRef]
- Mandala, V.S.; Williams, J.K.; Hong, M. Structure and dynamics of membrane proteins from solid-state NMR. Ann. Rev. Biophys. 2018, 47, 201–222. [Google Scholar] [CrossRef]
- Dalvit, C.; Vulpetti, A. Ligand-based fluorine NMR screening: Principles and applications in drug discovery projects. J. Med. Chem. 2018, 62, 2218–2244. [Google Scholar] [CrossRef] [PubMed]
- Luzarowski, M.; Skirycz, A. Emerging strategies for the identification of protein–metabolite interactions. J. Exp. Bot. 2019, 70, 4605–4618. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.G., III. Enzyme dynamics from NMR spectroscopy. Account. Chem. Res. 2015, 48, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.J.; Simpson, M.J.; Soong, R. Environmental nuclear magnetic resonance spectroscopy: An overview and a primer. Anal. Chem. 2017, 90, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.S.; Beyer, F.L.; Schmidt-Rohr, K. High-sensitivity multinuclear NMR spectroscopy of a smectite clay and clay-intercalated polymer. Solid State Nucl. Magn. Res. 2002, 22, 110–127. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kogel-Knabner, I.; Lehmann, J.; Manning, D.A.C. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Feng, X.; Simpson, A.J.; Schlesinger, W.H.; Simpson, M.J. Altered microbial community structure and organic matter composition under elevated CO2 and N fertilisation in Duke Forest. Glob. Chang. Biol. 2010, 16, 2104–2116. [Google Scholar] [CrossRef]
- Bundy, J.G.; Davey, M.P.; Viant, M.R. Environmental metabolomics: A critical review and future perspectives. Metabolomics 2009, 5, 3. [Google Scholar] [CrossRef]
- Sardans, J.; Penuelas, J.; Rivas-Ubach, A. Ecological metabolomics: Overview of current developments and future challenges. Chemoecology 2011, 21, 191–225. [Google Scholar] [CrossRef]
- Cappello, T.; Mauceri, A.; Corsaro, C.; Maisano, M.; Parrino, V.; Paro, G.L.; & Fasulo, S. Impact of environmental pollution on caged mussels Mytilus galloprovincialis using NMR-based metabolomics. Mar. Pollut. Bull. 2013, 77, 132–139. [Google Scholar] [CrossRef]
- Ahlgren, J.; Tranvik, L.; Gogoll, A.; Waldebäck, M.; Markides, K.; Rydin, E. Sediment depth attenuation of biogenic phosphorus compounds measured by 31P NMR. Environ. Sci. Technol. 2005, 39, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Navalon, S.; Alvaro, M.; Garcia, H. Analysis of organic compounds in an urban wastewater treatment plant effluent. Environ. Technol. 2011, 32, 295–306. [Google Scholar] [CrossRef]
- Filho, A.E.G.; Alexandre e Silva, L.M.; Ferreira, A.G. Advancements in wastewater characterisation through NMR spectroscopy. Magn. Reson. Chem. 2015, 53, 648–657. [Google Scholar]
- Wagner, N.D.; Helm, P.A.; Simpson, A.J.; Simpson, M.J. Metabolomic responses to pre-chlorinated and final effluent wastewater with the addition of a sub-lethal persistent contaminant in Daphnia magna. Environ. Sci. Pollut. Res. 2019, 26, 9014–9026. [Google Scholar] [CrossRef] [PubMed]
- Emwas, A.H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.A.; Wishart, D.S. NMR spectroscopy for metabolomics research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Dayrit, F.M.; de Dios Angel, C. 1H and 13C NMR for the Profiling of Natural. Product Extracts. IntechOpen 2017, 71040. Available online: https://www.intechopen.com/books/spectroscopic-analyses-developments-and-applications/1h-and-13c-nmr-for-the-profiling-of-natural-product-extracts-theory-and-applications (accessed on 15 November 2019).
- Reich, H.J. Structural Determination Using NMR. 2019. Available online: https://www.chem.wisc.edu/areas/reich/nmr/ (accessed on 9 September 2019).
- Kennpohl, D.; Farmer, S.; Spinney, R. Nuclear Magnetic Resonance Spectroscopy. Chem. Libretexts 2016. Available online: https://chem.libretexts.org/Courses/Athab (accessed on 13 November 2019).
- Silverstein, R.M.; Bassler, G.C.; Morrill, T.C. Characteristic group absorptions of organic molecules. In Spectrometric Identification of Organic Compounds, 5th ed.; Wiley: New York, NY, USA, 1991; p. 103. [Google Scholar]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic quotient normalisation as a robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef]
- Messai, H.; Farman, M.; Sarraj-Laabidi, A.; Hammami-Semmar, A.; Semmar, N. Chemometrics methods for specificity, authenticity and traceability analysis of olive oils: Principles, classifications and applications. Foods 2016, 5, 77. [Google Scholar] [CrossRef]
- Stuhl, C.; Anwander, R. Dimethylmagnesium revisited. Dalton Trans. 2018, 47, 12546–12552. [Google Scholar] [CrossRef]
- Nalepa, C.J.; Shelton, D.L. The use of bromine in water treatment as well as future applications. Chim. Oggi 2003, 21, 43–45. [Google Scholar]
- World Health Organization. Bromine as a Drinking Water Disinfectant. Available online: http://www.who.int/water_sanitation_health/publications/bromine-02032018?ua=1 (accessed on 14 November 2019).
- Gál, B.; Bucher, C.; Burns, N.Z. Chiral alkyl halides: Underexplored motifs in medicine. Mar. Drugs 2016, 14, 206. [Google Scholar] [CrossRef]
- Campestre, C.; Locatelli, M.; Guglielmi, P.; De Luca, E.; Bellagamba, G.; Menta, S.; Carradori, S. Analysis of imidazoles and triazoles in biological samples after Microextraction by packed sorbent. J. Enzym. Inhib. Med. Chem. 2017, 32, 1053–1063. [Google Scholar] [CrossRef]
- Olujimi, O.O.; Fatoki, O.S.; Odendaal, J.P.; Daso, A.P. Chemical monitoring and temporal variation in levels of endocrine-disrupting chemicals (priority phenols and phthalate esters) from selected wastewater treatment plant and freshwater systems in the Republic of South Africa. Microchem. J. 2012, 101, 11–23. [Google Scholar] [CrossRef]
- Adeniji, A.O.; Okoh, O.O.; Okoh, A.I. Analytical methods for the determination of the distribution of total petroleum hydrocarbons in the water and sediment of aquatic systems: A review. J. Chem. 2017. [Google Scholar] [CrossRef]
- Brutti, R.; Magu, M.M.; Agorku, E.S.; Govender, P.P. Alternative method for qualitative analysis of specific non-volatile organic compounds present in South African water systems. S. Afr. J. Chem. 2016, 69, 244–253. [Google Scholar] [CrossRef]
Sample Availability: Samples are not available from the authors. |

| δ (ppm) | Compound | Sample |
|---|---|---|
| −0.4 | (CH3)2Zn | F2A |
| 0.1 | Cyclopropane | B2A, B3A, F1A, F2A, S1A, S3B, T1A, T1B, T2B, T3A, T3B |
| 0.8–0.9 | (CH3)4C (CH3)3CH | B1A, B1B, B1C, B2A, B2B, B2C, B3A, B3C, F1A, F1C, F2A, F2B, F2C, F3A, F3C, S1A, S1B, S1C, S2A, S2B, S2C, S3B, S3C, T1A, T1B, T1C, T2B, T2C, T3A, T3B, T3C |
| 1.0–1.2 | CH3CH2OH (CH3CH2)2CO (CH3)2COH | B1A, B2C, F1A, F1C, F2C, F3C, S2A, T1C, T2B, T3A, T3C |
| 1.2–1.3 | CH3CH2CH3 | B1A, B1B, B1C, B2A, B2B, B2C, B3A, B3C, F1C, F2B, F2C, F3A, F3C, S1A, S1B, S1C, S2A, S2B, S2C, S3B, S2C, T1A, T1B, T1C, T2B, T2C, T3A, T3B, T3C |
| 1.3–1.4 | CH2P(CH3)3  | B1B, B1C, B2A, B2B, B2C, B3A, F2B, F2C, F3A, S1B, S1C S2A, S2B, S2C, S3B, T1A, T1B, T2B, T3A, T3B, T3C |
| 1.4–1.5 | 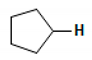 | B2B, B2C, B3A, B3C, F2B, F3A, S2A, S2B, S2C, S3B, S3C, T2B, T2C, T3B |
| 1.5–1.69 | Chlorinated alkane (CH3)3C-Cl | B2A, B2B, B2C, B3A, B3C, F1A, F2A, F2B, S2C, S3B, T1B, T2B, T3B, T3C |
| 1.7–1.8 | Brominated alkane BrC(CH3)3; BrCH2CH3, | B1A, B2A, F1A, F1C, S2C, S3C |
| 1.8–1.9 | CH3CH2I | B1A, B1B, B1C, B2C, B3A, B3C, F2C, F3C, S1C, T3A, T3C |
| 1.9–2 | Propyne HC≡C-Me (HC≡C)2CH | B1C, B2B, B2C, F1A, F2B, F3A, S1B, S1C, S2A, S2B, S2C, S3B, S3C, T1B, T1C, T2B, T2C, T3B |
| 1–4 | RNH2 amino | B2A, B2C, F2B, S2B |
| 2–2.05 | Acetonitrile, methacrylonitrile CH3-C≡N | B1A, B1C, B1B, B2B, B2C, B3C, F1A, F1C, F2A, F2B, F3A, F3C, S1A, S2A, S2B, S2C, S3B, S3C, T1A, T1B, T1C, T2B, T2C, T3A, T3B, T3C |
| 2–2.2 |  carbonyl compounds | B1A, B1C, B2A, B2C, B3A, B3C, F1A, F1C, F2B, F2C, F3A, S1B, S2B, S1C, S2C, S3B, S3C, T1B, T3B, T3C |
| 2.3–2.4 | HC≡CH acetylenic | B1C, B2B, B2C, F1A, F2B, F2C, F3C, F2C, F3C, S2C, T1A, T1C, T2C, T3A |
| 2.4–2.5 | (CH3CH2)2CO (CH3CH2)3N | B1C, B3A, F1A, F3C, S1A |
| 2.2–3 | Ar–C–H benzylic | F1C, F2B, S1A, S1C, S2B, S2C, S3C, T1C, T3A |
| 2.7–2.8 | CH3Br bromides | B2C, B3A, F1C, S3C |
| 2.8–2.9 | (CH3)2SO2 | S3C |
| 3–3.1 | (CH3)2CHCl chlorides | B1A, B2A, F1A, F1C |
| 3.3–4 | HC–OH alcohols HC–OR ethers Alkyl halides | B1A, B1B, B1C, B2A, B2C, B3A, B3C, F1A, F2A, F2C, F3A, S1A, S2A, S2B, S2C, T1A, T1C, T2B, T3A, T3B, T3C |
| 3.5 | 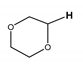 Dioxane Dioxane | B1C, F1A |
| 3.6–3.7 | BrCH2CH2Br | B1B, B2C, F1A, F2C, F3C, S1A, S2B, S3B, S3C, T1A, T1B, T3A |
| 3.7–4.1 | RCOO–CH esters | B1C, B2C, B3A, F1A, F3C, S1A, S1B, S1C, T1A, T2C, T3A |
| 4–4.5 | HC–F fluorides | B2A, B2B, B2C, B3A, F1C, F2B, F2C, S1A, S1C, S2C, S3C, T3A, T3C |
| 4.9–5 | CH2Br2 | F3A, S3C |
| 4.5–5.2 | ArOH phenolic | B1B, B1C, B2A, B2B, B3C, F2A, F2B, F3A, S1B, S2A, S2B, S2C, S3B, S3C, T1A, T1B, T1C, T2B, T2C, T3B, T3C |
| 4.6–5.5 | 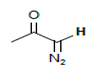 vinylic | B1B, B1C, B2A, B2B, B2C, B3A, B3C, F1A, F2B, F3A, S1A, S1B, S1C, S2A, S2B, S2C, S3B, S3C, T1A, T1B, T1C, T2B, T2C, T3A, T3B, T3C |
| 5.0–5.1 | PhCH2Cl chlorides | F3A, S2C, B1B, B1C, B2B, B2C, B3C, F1A, F2B, S1B, S1C, S2A, S2B, S3B, T2B, T3B, T3C |
| 6.9–8.5 | C=CH shift in heterocyclic compounds | B1B, B1C, B2A, B2C, B3A, B3C, F1A, F1A, F1C, F2A, F2B, F3A, S1B, S1C, S2B, S3B, S3C, T1B, T1C, T2B, T2C, T3A, T3B |
| δ, ppm | Description | Sample |
|---|---|---|
| 0.1 | Cyclopropane | KS, AS, A1C, A2C, G1A, G1B, G2A, K1B, K1C, K2B, K2C |
| 0.8–0.9 | (CH3)4C (CH3)3C | GS, KS, A1B, A1C, A2B, A2C, G1A, G1B, G1C, G2A, G2B, G2C, K1B, K1C, K2B, K2C, U1C, U2B, U2C |
| 1.0–1.2 | CH3CH2OH (CH3CH2)2CO (CH3)2COH | GS, KS, AS, A1C, G1A, G1C, G2A, G2B, G2C, K1B, K1C, K2C, U1C |
| 1.2–1.3 | CH3CH2CH3 | GS, KS, A1B, A1C, A2B, A2C, G1A, G1B, G1C, G2A, G2B, G2C, K1B, K1C, K2B, K2C, U1C, U2B, U2C |
| 1.3–1.4 | CH2P(CH3)3 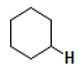 | AS, KS, A1B, A1C, A2B, A2C, G1A, G1B, G1C, G2A, G2B, G2C, K1B, K1C, K2B, U1C, U2B, U2C |
| 1.4–1.5 |  | AS, A1B, A1C, A2C, G2B, G2C, K1B, K1C, K2B, U1C, U2B, U2C |
| 1.5–1.69 | Chlorinated alkane (CH3)3C-Cl | GS, KS, A1B, A2B, A2C, G1A, G1B, G1C, G2A, G2B, G2C, K1B, K1C, K2B, U1C, U2B, U2C |
| 1.7–1.8 | Brominated alkane BrC(CH3)3; BrCH2CH3, | G2B, G2C |
| 1.8–1.9 | CH3CH2I | GS, G1A, G1C, G2A, K1C, K2C |
| 1.9–2 | Propyne HC≡C-Me (HC≡C)2CH | GS, KS, A2B, A2C, G1A, G1B, G1C, G2A, G2B, G2C, K1B, K1C, K2B, U1C, U2C |
| 1–4 | RNH2 Amine | KS, GS, A1B, A1C, G1A, G1B, G1C, G2B, G2C, U1C |
| 2–2.05 | Acetonitrile, methacrylonitrile CH3-C≡N | GS, KS, AS, A1B, A1C, A2B, A2C, G1A, G1B, G1C, G2A, G2C, K1C, K2B, U1C, U2B, U2C |
| 2–2.2 |  carbonyl compounds | GS, AS, AIB, A2B, A2C, G1A, G1B, G1C, K1B, K1C, K2B, K2C, U1C, U2B |
| 2.3–2.4 | HC≡CH acetylenic | A1C, G1C, G2A, K2C |
| 2.4–2.5 | (CH3CH2)2CO (CH3CH2)3N | GS, AS, A1B, A1C, A2C, G1B, G2A, G2B, K1B, K1C, K2B, K2C, U1C, U2C |
| 2.2–3 | Ar–C–H benzylic | GS, KS, A1C, A2B, G1B, G2A, G2B, G2C |
| 2.7–2.8 | CH3Br | GS, G1A, G1C, K1B, K1C |
| 2.9–3 | HC≡C-Ph | K2C |
| 3.3–3.5 | PhCOC≡CH | U2B |
| 3.6–3.7 | BrCH2CH2Br | KS, G1A, G1C, G2A, G2C, K1C, K2B, K2C |
| 3.4–4 | CH2: Alkyl halides, Alcohols, Ethers | GS, KS, A1B, A1C, A2B, A2C, G1A, G1B, G2A, G2B, K1C, K2B, K2C, U1C, U2B, U2C |
| 4–4.1 | MeCOOCH2CH3 (CH3)2CHCl | GS, KS, A2C, G1A, G1B, G2A, G2B, K2C, U1C |
| 4–4.5 | RCH2OH | GS, KS, AS, A2C, G1B, G2A, G2B, G2C, K1B, U1C, U2C |
| 4.5–5 | PhCH2Cl CH2Br PhCH=CH2 | A1B, A2B, A2C, G1B, G2C, K1B, K2B, U2B, U2C |
| 5.0–5.1 | PhCH2Cl | A1B, A2B, A2C, G1B |
| 5.3–5.5 | RCH=CH2 (CH3O)2CH2 | GS, KS, AS, A1B, A1C, A2B, A2C, G1A, G1B, G1C, G2A, G2B, G2C, K1B, K1C, K2B, U1C, U2B, U2C |
| 5.32 | CH2Cl2 | GS, G1A, G1B |
| 6.2–6.7 | RCH=CH2 | A1B, A2B, G1A, G1B, G2A, K1B, K2B, K2C, U1C, U2B, U2C |
| 5.5–7.5 | Phenolic compounds | KSB, A1B, A2B, A2C, G1B, G2A, G2B, G2C, K2B, U1C |
| 7–7.2 | 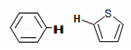 | A1B, A2B, G1A, G1B, G2B, G2C, KS, K1B, K1C, K2B, K2C, U1C, U2B, U2C |
| 7.4–7.9 | Furan Naphthalene Methenamine Imidazole | KS, A1B, A2B, G1A, G1B, G1C, G2B, K1B, K2B. U2C |
| 8.1–8.7 | 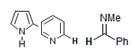 | G2A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farounbi, A.I.; Mensah, P.K.; Olawode, E.O.; Ngqwala, N.P. 1H-NMR Determination of Organic Compounds in Municipal Wastewaters and the Receiving Surface Waters in Eastern Cape Province of South Africa. Molecules 2020, 25, 713. https://doi.org/10.3390/molecules25030713
Farounbi AI, Mensah PK, Olawode EO, Ngqwala NP. 1H-NMR Determination of Organic Compounds in Municipal Wastewaters and the Receiving Surface Waters in Eastern Cape Province of South Africa. Molecules. 2020; 25(3):713. https://doi.org/10.3390/molecules25030713
Chicago/Turabian StyleFarounbi, Adebayo I., Paul K. Mensah, Emmanuel O. Olawode, and Nosiphiwe P. Ngqwala. 2020. "1H-NMR Determination of Organic Compounds in Municipal Wastewaters and the Receiving Surface Waters in Eastern Cape Province of South Africa" Molecules 25, no. 3: 713. https://doi.org/10.3390/molecules25030713
APA StyleFarounbi, A. I., Mensah, P. K., Olawode, E. O., & Ngqwala, N. P. (2020). 1H-NMR Determination of Organic Compounds in Municipal Wastewaters and the Receiving Surface Waters in Eastern Cape Province of South Africa. Molecules, 25(3), 713. https://doi.org/10.3390/molecules25030713





