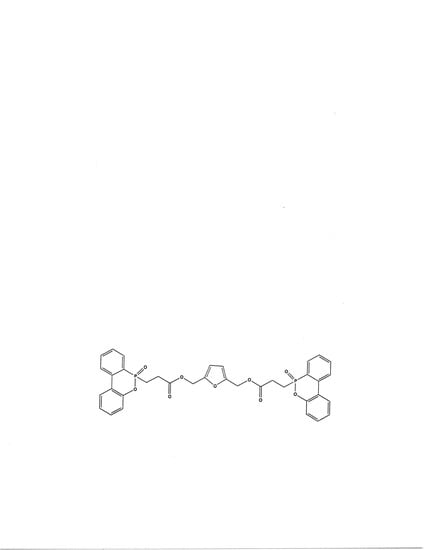
Effective Biobased Phosphorus Flame Retardants from Starch-Derived bis-2,5-(Hydroxymethyl)Furan
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Methods and Instrumentation
3.3. Test Specimen
3.4. Flammability Testing
3.5. Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability: Samples of the compounds are available from the authors. |
References
- Halary, J.-L.; Laupretre, F.; Monnerie, L. Polymer Materials; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Binder, W.H. The Past 40 years of Macromolecular Sciences: Reflections on Challenges in Synthetic and Material Science. Macrol. Rapid Commun. 2019, 40, e1800610. [Google Scholar] [CrossRef] [PubMed]
- Lecommandoux, S.; Klok, H.-A.; Zhong, Z.; Deming, T.J. Future Directions at the Frontier of Polymer Science and Biology. Biomacromolecules 2019, 20, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Buekens, A.; Li, X. Brominated Flame Retardants and the Formation of Dioxins and Furans in Fires and Combustion. J. Hazard. Mater. 2016, 304, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Cristale, J.; Belé, T.G.A.; Lacorte, S.; de Marchi, M.R.R. Occurrence of Flame Retardants in Landfills: A Case Study in Brazil. Environ. Res. 2019, 168, 420–427. [Google Scholar] [CrossRef]
- Darnerud, P.O. Brominated Flame Retardants as Possible Endocrine Disrupters. Int. J. Androl. 2008, 31, 152–160. [Google Scholar] [CrossRef]
- Legler, J.; Brouwer, A. Are Brominated Flame Retardants Endocrine Disruptors? Environ. Int. 2003, 29, 879–885. [Google Scholar] [CrossRef]
- Sjödin, A.; Patterson, D.G.; Bergman, A. A Review of Human Exposure to Brominated Flame Retardants—Particularly Polybrominated Diphenyl Ethers. Environ. Int. 2003, 29, 829–839. [Google Scholar] [CrossRef]
- Alace, M.; Wenning, R.J. The Significance of Brominated Flame Retardants in the Environment: Current Understanding, Issues and Challenges. Chemosphere 2002, 5, 579–582. [Google Scholar]
- Axelrad, D. Brominated Flame Retardants: Regulatory Actions and EPA Activities; U.S. Environmental Protection Agency: Washington, DC, USA, 2009.
- Hogue, C. EU Authorization Said to be Leading to Safer Substitutes. Chem. Eng. News 2017, 95, 15. [Google Scholar]
- Hess, G. Industry Drops Flame Retardant. Chem. Eng. News 2010, 88, 10. [Google Scholar] [CrossRef]
- Velencoso, M.M.; Battig, A.; Markwart, J.C.; Schartel, B.; Wurm, F.R. Molecular Firefighting—How Modern Phosphorus Chemistry Can Help Solve the Challenge of Flame Retardancy. Angew. Chem. Int. Ed. 2018, 57, 10450–10467. [Google Scholar] [CrossRef]
- Morgan, A.B. The Future of Flame Retardant Polymers–Unmet Needs and Likely New Approaches. Polym. Rev. 2019, 59, 25–54. [Google Scholar] [CrossRef]
- Van der Veen, I.; de Boer, J. Phosphorus Flame Retardants: Properties, Production, Environmental Occurrence, Toxicity and Analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, C.; Striegl, R.; Mathes, S.; Adhart, C.; Edelmann, M.; Bono, E.; Gaan, S.; Salmeia, K.A.; Hoelting, L.; Krebs, A.; et al. Multiparameter Toxicity Assessment of Novel DOPO-derived Organophosphorus Flame Retardants. Arch. Toxicol. 2017, 91, 407–425. [Google Scholar] [CrossRef]
- Liu, M.; Yin, H.; Chen, X.; Yang, J.; Liang, Y.; Zhang, J.; Yang, F.; Deng, Y.; Lu, S. Preliminary Ecotoxicity Hazard Evaluation of DOPO-HQ as a Potential Alternative to Halogenated Flame Retardants. Chemosphere 2018, 193, 126–133. [Google Scholar] [CrossRef]
- Zhao, W.; Li, B.; Xu, M.; Zhang, L.; Liu, F.; Guan, L. Synthesis of a Novel Flame Retardant Containing Phosphorus and Sulfur and Its Application in Polycarbonate. Polym. Eng. Sci. 2012, 52, 2327–2335. [Google Scholar] [CrossRef]
- Braun, U.; Knoll, U.; Schartel, B.; Hoffmann, T.; Pospiech, D.; Artner, J.; Ciesielski, M.; Doring, M.; Perez-Graterol, R.; Sandler, J.K.W.; et al. Novel Phosphorus-containing Poly(ether sulfone)s and Their Blends with an Epoxy Resin: Thermal Decomposition and Fire Retardancy. Macromol. Chem. Phys. 2006, 207, 1501–1514. [Google Scholar] [CrossRef]
- Wagner, J.; Deglmann, P.; Fuchs, S.; Ciesielski, M.; Fleckenstein, C.A.; Döring, M. A Flame Retardant Synergism of Organic Disulfides and Phosphorous Compounds. Polym. Degrad. Stab. 2016, 129, 63–76. [Google Scholar] [CrossRef]
- Howell, B.A.; Daniel, Y.G. The Impact of Sulfur Oxidation Level on Flame Retardancy. J. Fire Sci. 2018, 36, 518–534. [Google Scholar] [CrossRef]
- Neisius, N.M.; Lutz, M.; Rentsch, D.; Hemberger, P.; Gaan, S. Synthesis of DOPO-based Phosphonamidates and Their Thermal Properties. Ind. Eng. Chem. Res. 2014, 53, 2889–2896. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Gaan, S. An Overview of Some Recent Advances in DOPO-Derivatives: Chemistry and Flame Retardant Applications. Polym. Degrad. Stab. 2015, 113, 119–134. [Google Scholar] [CrossRef]
- Dumitrascu, A.; Howell, B.A. Flame Retardant Polymeric Materials Achieved by Incorporation of Styrene Monomers Containing Both Nitrogen and Phosphorus. Polym. Degrad. Stab. 2012, 97, 2611–2618. [Google Scholar] [CrossRef]
- Yan, Y.; Liang, B. Flame-retardant Behavior and Mechanism of a DOPO-based Phosphorus–nitrogen Flame Retardant in Epoxy Resin. High Per. Polym. 2019, 31, 885–892. [Google Scholar] [CrossRef]
- Wang, P.; Chen, L.; Xiao, H. Flame Retardant Effect and Mechanism of a Novel DOPO Based Tetrazole Derivative on Epoxy Resin. J. Anal. Appl. Pyrolysis 2019, 139, 104–113. [Google Scholar] [CrossRef]
- Nazir, R.; Gaan, S. Recent Developments in P (O/S)–N Containing Flame Retardants. J. Appl. Polym. Sci. 2019, 137, 47910. [Google Scholar] [CrossRef]
- Tang, S.; Qian, L.J.; Qiu, Y.; Dong, Y.P. Synergistic Flame-retardant Effect and Mechanisms of Boron/phosphorus Compounds on Epoxy Resins. Polym. Advan. Technol. 2018, 29, 641–648. [Google Scholar] [CrossRef]
- Tang, S.; Qian, L.J.; Qiu, Y.; Dong, Y.P. High-performance Flame Retardant Epoxy Resin Based on a Bi-Group Molecule Containing Phosphaphenanthrene and Borate Groups. Polym. Degrad. Stab. 2018, 153, 210–219. [Google Scholar] [CrossRef]
- Zhang, W.C.; Li, X.M.; Yang, R.J. Pyrolysis and Fire Behavior of Epoxy Resin Composites Based on a Phosphorus-containing Polyhedral Oligomeric Silsesquioxane (DOPO-POSS). Polym. Degrad. Stab. 2011, 96, 1821–1832. [Google Scholar] [CrossRef]
- Qian, Y.; Wei, P.; Hao, J.W. Preparation of Hybrid Phosphamide Containing Polysilsesquioxane and Its Effect on Flame Retardancy and Mechanical Properties of Polypropylene Composites. Compos. B. Eng. 2013, 45, 1541–1547. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Li, L.; Yang, R. Study of the Synergistic Effect of Silicon and Phosphorus on the Blowing-out Effect of Epoxy Resin Composites. Polym. Degrad. Stab. 2012, 97, 1041–1048. [Google Scholar] [CrossRef]
- Sag, J.; Goedderz, D.; Kubla, P.; Griener, L.; Schonberger, F.; Doring, M. Phosphorus-containing Flame Retardants from Biobased Chemicals and their Application in Polyesters and Epoxy Resins. Molecules 2019, 24, 3746. [Google Scholar] [CrossRef]
- Howell, B.A.; Daniel, Y.G.; Ostrander, E.A. Flame Retardants from Renewable Sources: Food Waste, Plant Oils, and Starch. In Green Polymer Chemistry: New Products, Processes, and Applications; Cheng, H.N., Smith, P.B., Eds.; American Chemical Society: Washington, DC, USA, 2018; pp. 405–421. [Google Scholar]
- Sonnier, R.; Taguet, A.; Ferry, L.; Lopez-Cuesta, J.M. Towards Biobased Flame Retardant Polymers; Springer International Publishing AG: Cham, Switzerland, 2018. [Google Scholar]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Biobased Flame Retardants: When Nature Meets Fire Protection. Mater. Sci. Eng. R 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Xia, Z.; Kiratitanavit, W.; Facendola, P.; Thota, S.; Yu, S.; Kumar, J.; Mosurkal, R.; Nagarajan, R. Fire Resistant Polyphenols Based on Chemical Modification of Bioderived Tannic Acid. Polym. Degrad. Stab. 2018, 153, 227–243. [Google Scholar] [CrossRef]
- Howell, B.A.; Daniel, Y.G. Thermal Degradation of Phosphorus Esters Derived From Isosorbide and 10-Undecenoic Acid. J. Therm. Anal. Calorim. 2015, 121, 411–419. [Google Scholar] [CrossRef]
- Daniel, Y.G.; Howell, B.A. Flame Retardant Properties of Isosorbide bis-Phosphorus Esters. Polym. Degrad. Stab. 2017, 140, 25–31. [Google Scholar] [CrossRef]
- Hu, C.; Bourbigot, S.; Delaunay, T.; Collinet, M.; Marcille, S.; Fontaine, G. Synthesis of Isosorbide Based Flame Retardants: Application for Polybutylene Succinate. Polym. Degrad. Stab. 2019, 164, 9–17. [Google Scholar] [CrossRef]
- Howell, B.A.; Sun, W. Biobased Flame Retardants From Tartaric Acid and Derivatives. Polym. Degrad. Stab. 2018, 157, 199–211. [Google Scholar] [CrossRef]
- Howell, B.A.; Oberdorfer, K.L.; Ostrander, E.A. Phosphorus Flame Retardants for Polymeric Materials from Gallic Acid and Other Naturally Occurring Multihydroxybenzoic Acids. Int. J. Polym. Sci. 2018, 2018, 7237236. [Google Scholar] [CrossRef]
- Howell, B.A.; Alrubayyi, A. 2-Dopyl-1,4-di(2-dopylpropanoyl)benzene, An Effective Phosphorus Flame Retardant. Polym. Degrad. Stab. 2019, 162, 196–200. [Google Scholar] [CrossRef]
- Ménard, R.; Negrell, C.; Ferry, L.; Sonnier, R.; David, G. Synthesis of Biobased Phosphorus-containing Flame Retardants for Epoxy Thermosets Comparison of Additive and Reactive Approaches. Polym. Degrad. Stab. 2015, 120, 300–312. [Google Scholar] [CrossRef]
- Ménard, R.; Negrell, C.; Fache, M.; Ferry, L.; Sonnier, R.; David, G. From a Biobased Phosphorus-containing Epoxy Monomer to Fully Biobased Flame-retardant Thermosets. RSC Adv. 2015, 5, 70856–70867. [Google Scholar] [CrossRef]
- Princen, L.H. Alternate Industrial Feedstocks From Agriculture. Econ. Bot. 1982, 36, 302–312. [Google Scholar] [CrossRef]
- Miller, D. The Bioharvest Is Here. The Progressive Farmer. 2018, pp. 22–25. Available online: http://dtnpf-digital.com/publication/?i=495985&article_id=3086440&view=articleBrowser&ver=html5#{%22issue_id%22:495985,%22view%22:%22articleBrowser%22,%22publication_id%22:%2227946%22,%22article_id%22:%223086440%22} (accessed on 24 January 2020).
- Hong, J.; Radojčić, D.; Ionescu, M.; Petrović, Z.S.; Eastwood, E. Advanced Materials From Corn: Isosorbide-based Epoxy Resins. Polym. Chem. 2014, 5, 5360–5368. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Van Putten, R.J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made From Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Teong, S.P.; Yi, G.; Zhang, Y. Hydroxymethylfurfural Production From Bioresources: Past, Present and Future. Green Chem. 2014, 16, 2015–2026. [Google Scholar] [CrossRef]
- Wang, T.; Nolte, M.W.; Shanks, B.H. Catalytic Dehydration of C6 Carbohydrates for the Production of Hydroxymethylfurfural (HMF) as a Versatile Platform Chemical. Green Chem. 2014, 16, 548–572. [Google Scholar] [CrossRef]
- Jiang, Y.; Woortman, A.J.J.; Alberda van Ekenstein, G.O.R.; Petrović, D.M.; Loos, K. Enzymatic Synthesis of Biobased Polyesters Using 2,5-bis(Hydroxymethyl)furan as the Building Block. Biomacromolecules 2014, 15, 2482–2493. [Google Scholar] [CrossRef]
- Zeng, C.; Seino, H.; Ren, J.; Hatanaka, K.; Yoshie, N. Biobased Furan Polymers with Self-healing Ability. Macromolecules 2013, 46, 1794–1802. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.; Xie, Z.; Han, J.; Xu, J.; Guo, B. Synthesis and Properties of Biobased Multiblock Polyesters Containing Poly(2,5-furandimethylene succinate) and Poly (butylene succinate) Blocks. Ind. Eng. Chem. Res. 2017, 56, 3937–3946. [Google Scholar] [CrossRef]
- Choi, E.H.; Lee, J.; Son, S.U.; Song, C. Biomass-derived Furanic Polycarbonates: Mild Synthesis and Control of the Glass Transition Temperature. J. Polym. Sci. Polym. Chem. 2019, 57, 1796–1800. [Google Scholar] [CrossRef]
- Howell, B.A.; Lazar, S.T. Biobased Plasticizers From Carbohydrate-derived 2,5-bis (Hydroxymethyl) furan. Ind. Eng. Chem. Res. 2018, 58, 1222–1228. [Google Scholar] [CrossRef]
- Daniel, Y.G.; Howell, B.A. Phosphorus Flame Retardants From Isosorbide bis-Acrylate. Polym. Degrad. Stab. 2018, 156, 14–21. [Google Scholar] [CrossRef]
- Wendels, S.; Chavez, T.; Bonnet, M.; Salmeia, K.A.; Gaan, S. Recent Developments in Organophosphorus Flame Retardants Containing P-C Bond and their Application. Materials 2017, 10, 784. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Bykov, Y.; Döring, M. New Star-shaped Phosphorus-containing Flame Retardants Based on Acrylates for Epoxy Resins. Polym. Adv. Technol. 2013, 24, 834–840. [Google Scholar] [CrossRef]
- Rabe, S.; Chuenban, Y.; Schartel, B. Exploring the Modes of Action of Phosphorus-based Flame Retardants in Polymeric Systems. Materials 2017, 10, 455. [Google Scholar] [CrossRef]
- Schartel, B.; Perret, B.; Dittrich, B.; Ciesielski, M.; Krämer, J.; Müller, P.; Alstadt, V.; Zang, L.; Döring, M. Flame Retardancy of Polymers: the Role of Specific Reactions in the Condensed Phase. Macromol. Mater. Eng. 2016, 301, 9–35. [Google Scholar] [CrossRef]
- Braun, U.; Balabanovich, A.I.; Schartel, B.; Knoll, U.; Artner, J.; Ciesielski, M.; Doring, M.; Perez, R.; Sandler, J.K.W.; Altstadt, V.; et al. Influence of the Oxidation State of Phosphorus on the Decomposition and Fire Behavior of Flame-retarded Epoxy Resin Composites. Polymer 2006, 47, 8495–8508. [Google Scholar] [CrossRef]
- Balabanovich, A.I.; Pospiech, D.; Häußler, L.; Harnisch, C.; Döring, M. Pyrolysis Behavior of Phosphorus Polyesters. J. Anal. Appl. Pyrolysis 2009, 86, 99–107. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Fage, J.; Liang, S.; Gaan, S. An Overview of Mode of Action and Analytical Methods for Evaluation of Gas Phase Activities of Flame Retardants. Polymers 2015, 7, 504–526. [Google Scholar] [CrossRef]
- Liang, S.; Hemberger, P.; Neisius, N.M.; Bodi, A.; Grützmacher, H.; Levalois-Grützmacher, J.; Gaan, S. Elucidating the Thermal Decomposition of Dimethyl Methylphosphonate by Vacuum Ultraviolet (VUV) Photoionization: Pathways to the PO Radical, A Key Species in Flame-retardant Mechanisms. Chem. Eur. J. 2015, 21, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Howell, B.A.; Cho, Y.J. Brominated Aryl Phospholanes as Dual Functional Flame Retardants for Polymeric Materials. Polym. Mater. Sci. Eng. 2008, 98, 361. [Google Scholar]
- Howell, B.A. Development of Additives Possessing both Solid-phase and Gas-phase Flame Retardant Activities. Polym. Degrad. Stab. 2008, 93, 2052–2057. [Google Scholar] [CrossRef]

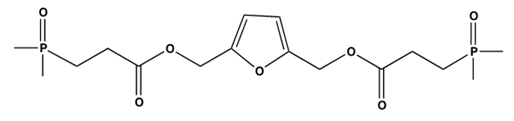 | |||||||
|---|---|---|---|---|---|---|---|
| Phosphorus Substituent | Csp3–H | Csp2–H | C=O | Aromatic Nucleus | C–O–C | P=O | P–O–C |
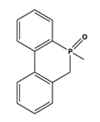 | 3071 | 2923 | 1732 | 1598 | 1235 | 1197 | 910, 757 |
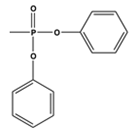 | 3065 | 2930 | 1740 | 1598 | 1186 | 1162 | 927, 762 |
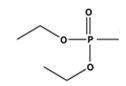 | 3066 | 2926, 2953 | 1737 | 1596 | 1232 | 1208 | 907, 754 |
 | ||||
|---|---|---|---|---|
| Phosphorus Substituent | Methylene Protons Adjacent to Phosphorus | Methylene Protons Adjacent to Carbonyl | Methylene Protons Adjacent to Furan Nucleus | Furan Protons |
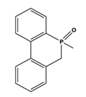 | 2.36 | 2.68 | 4.98 | 6.31 |
 | 2.46 | 2.85 | 5.02 | 6.37 |
 | 2.02 | 2.58 | 4.99 | 6.32 |
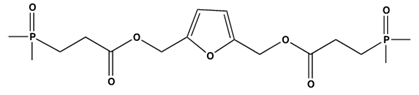 | |||||
|---|---|---|---|---|---|
| Phosphorus Substitutent | Carbon Atoms Adjacent to Phosphorus | Carbon Atoms Adjacent to Carbonyl | Carbon Atoms Adjacent to Oxygen and Furan Nucleus | Furan Carbon Atoms | Ester Carbonyl Carbon Atoms |
 | 23.2 | 26.3 | 58.1 | 111.9, 164.0 | 171.8 |
 | 21.4 | 27.4 | 58.6 | 111.9, 149.9 | 171.2 |
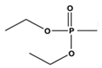 | 20.9 | 27.4 | 58.3 | 111.7, 149.9 | 171.6 |
| Flame Retardants Structure | Loading of Additive (wgt%) a | Loading of Phosphorus (wgt%) b | LOI c | UL-94 Designation | PHRR (W/g) d |
|---|---|---|---|---|---|
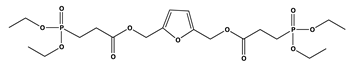 | 8 | 1 | 24.2 | - | 391 |
| 17 | 2 | 24.6 | - | 363 | |
| 41 | 5 | 27.0 | V1 | 350 | |
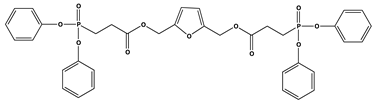 | 11 | 1 | 24.5 | - | 420 |
| 23 | 2 | 25.1 | - | 333 | |
| 57 | 5 | 26.9 | V0 | 246 | |
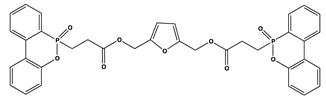 | 11 | 1 | 28.4 | V1 | 344 |
| 22 | 2 | 29.1 | V0 | 305 | |
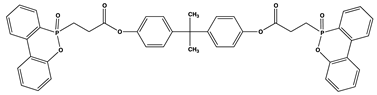 | 12 | 1 | 28.6 | V0 | 449 |
| 25 | 2 | 30.3 | V0 | 330 | |
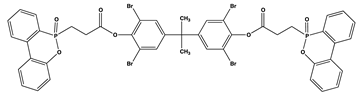 | 17 | 1 | 28.1 | V0 | 320 |
| 35 | 2 | 30.4 | V0 | 262 |
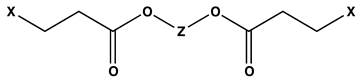 | ||||
|---|---|---|---|---|
| Z | X | LOI | UL-94 Designation | PHRR (W/g) |
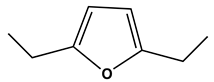 | 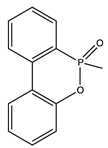 | 29.1 | V0 | 305 |
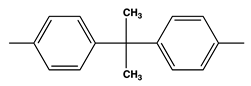 | 30.3 | V0 | 330 | |
 | 30.4 | V0 | 262 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howell, B.A.; Han, X. Effective Biobased Phosphorus Flame Retardants from Starch-Derived bis-2,5-(Hydroxymethyl)Furan. Molecules 2020, 25, 592. https://doi.org/10.3390/molecules25030592
Howell BA, Han X. Effective Biobased Phosphorus Flame Retardants from Starch-Derived bis-2,5-(Hydroxymethyl)Furan. Molecules. 2020; 25(3):592. https://doi.org/10.3390/molecules25030592
Chicago/Turabian StyleHowell, Bob A., and Xiaorui Han. 2020. "Effective Biobased Phosphorus Flame Retardants from Starch-Derived bis-2,5-(Hydroxymethyl)Furan" Molecules 25, no. 3: 592. https://doi.org/10.3390/molecules25030592
APA StyleHowell, B. A., & Han, X. (2020). Effective Biobased Phosphorus Flame Retardants from Starch-Derived bis-2,5-(Hydroxymethyl)Furan. Molecules, 25(3), 592. https://doi.org/10.3390/molecules25030592