Organo-Inorganic Hybrid Intumescent Fire Retardant Coatings for Thermoplastics Based on Poly(Vinylphosphonic Acid)
Abstract
1. Introduction
2. Results
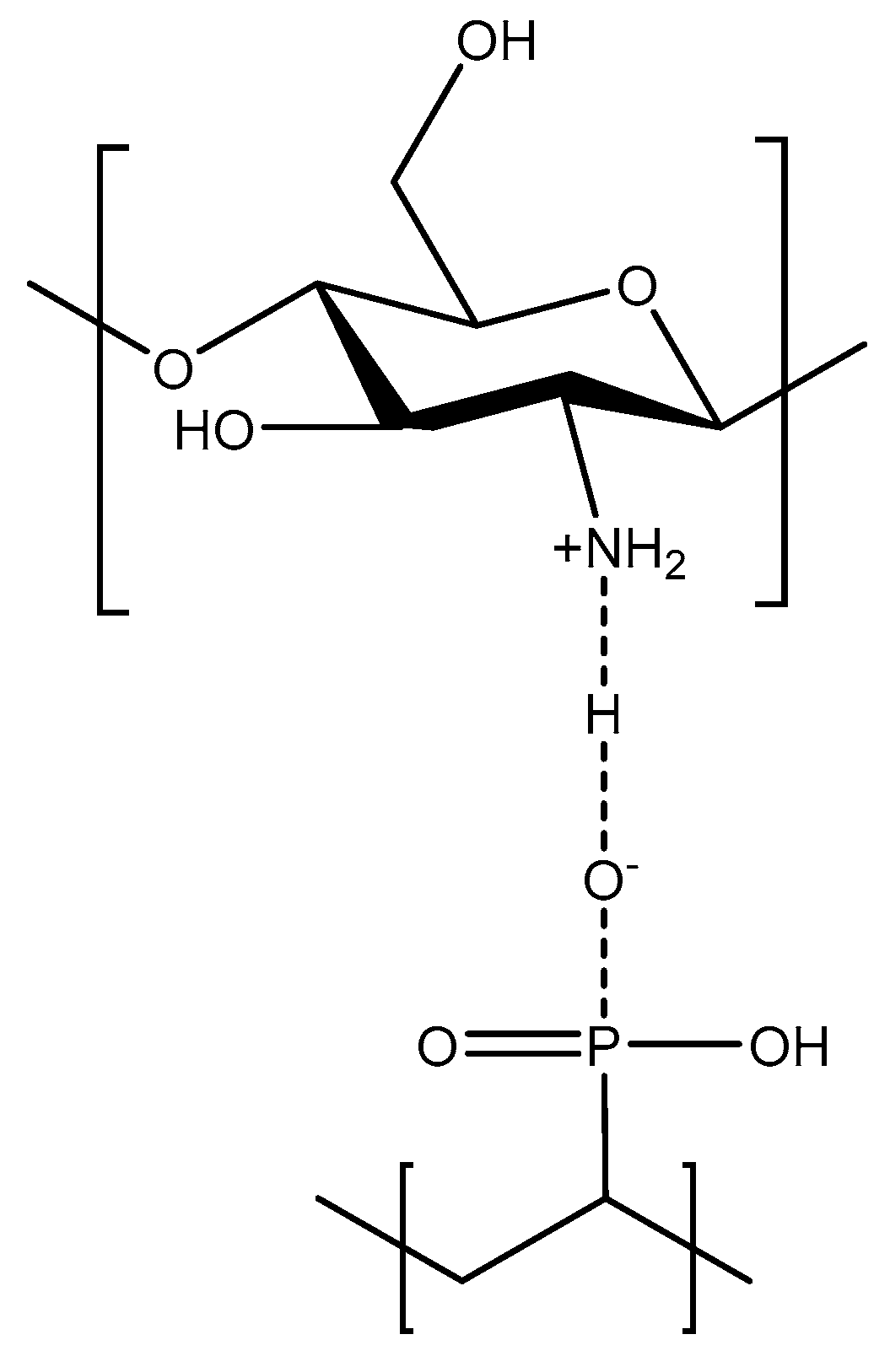
2.1. PVPA/Inorganic Hybrid Coating
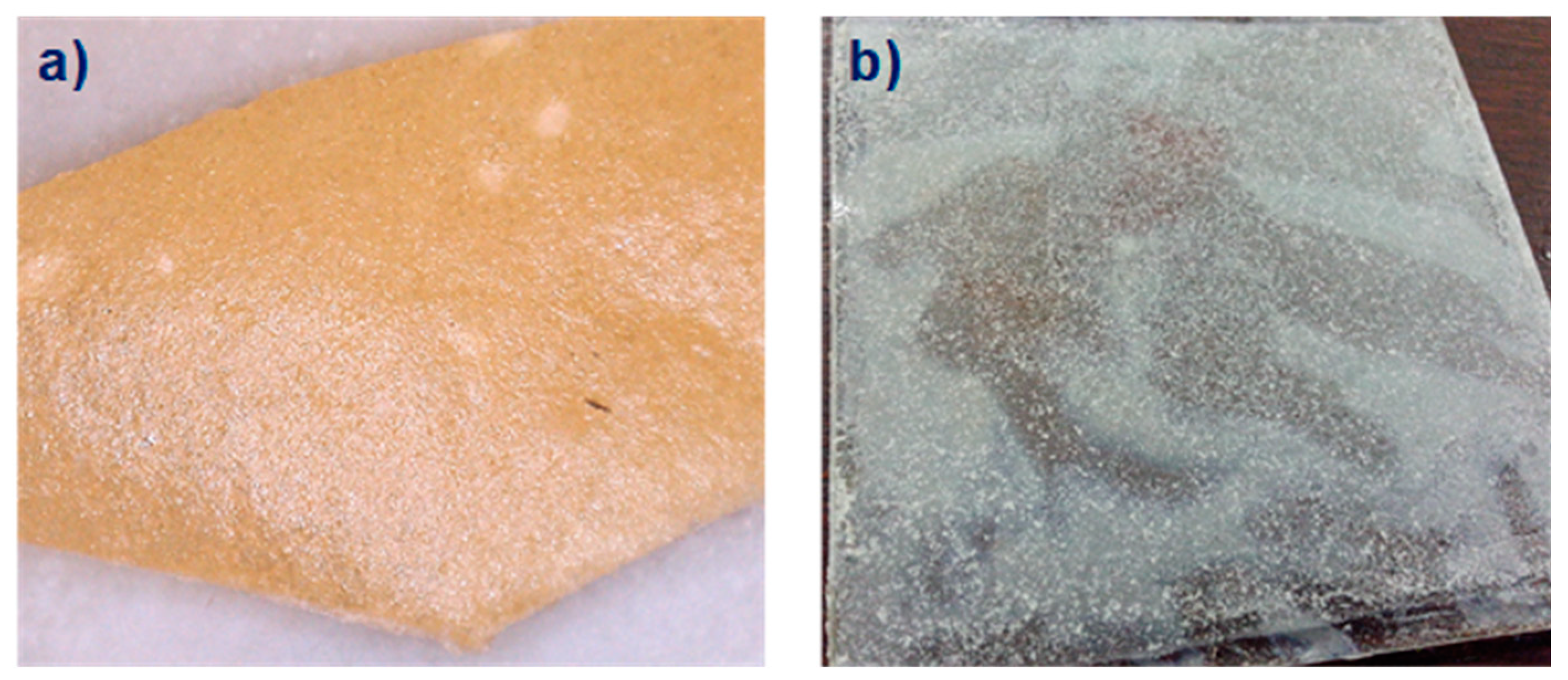
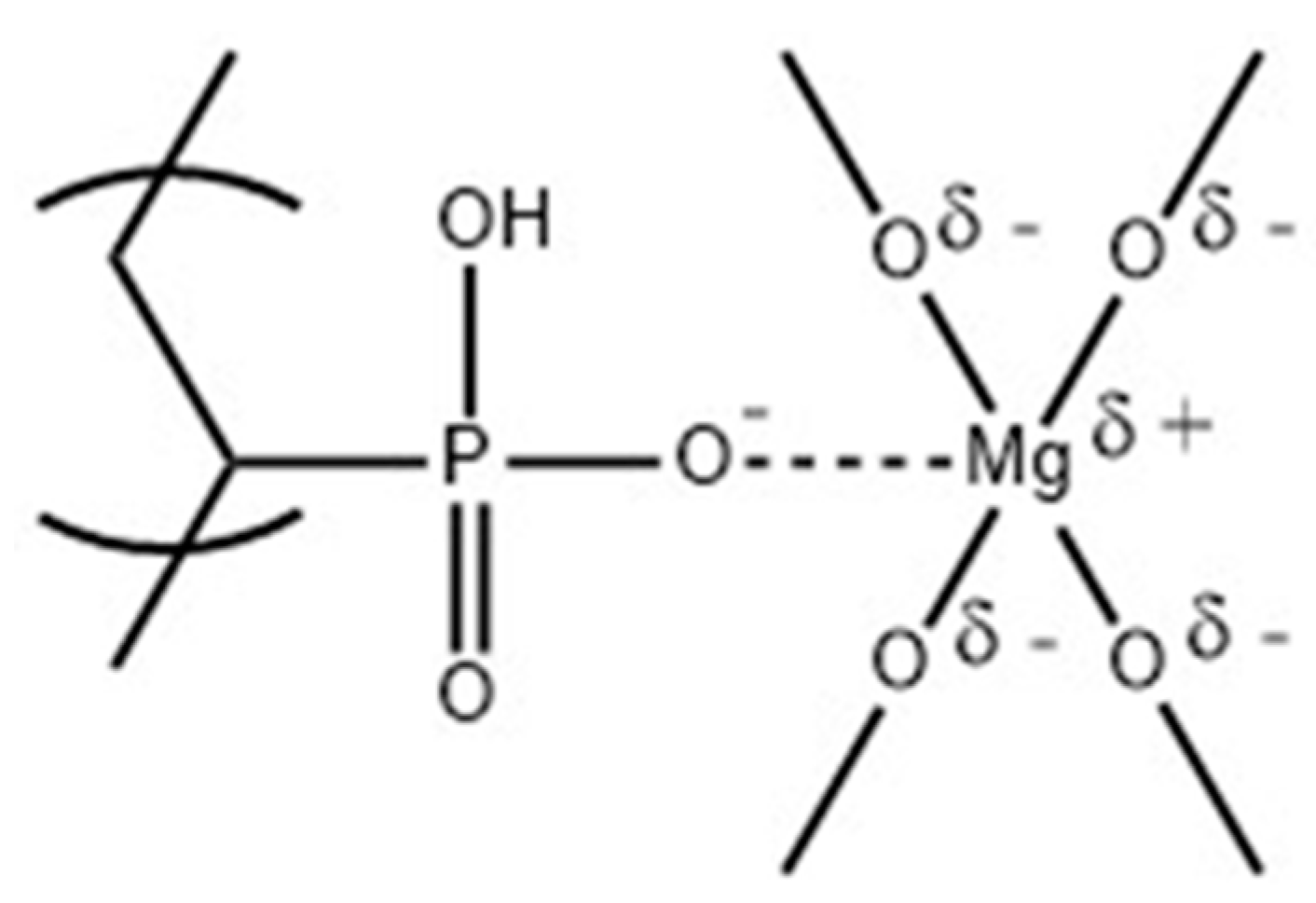
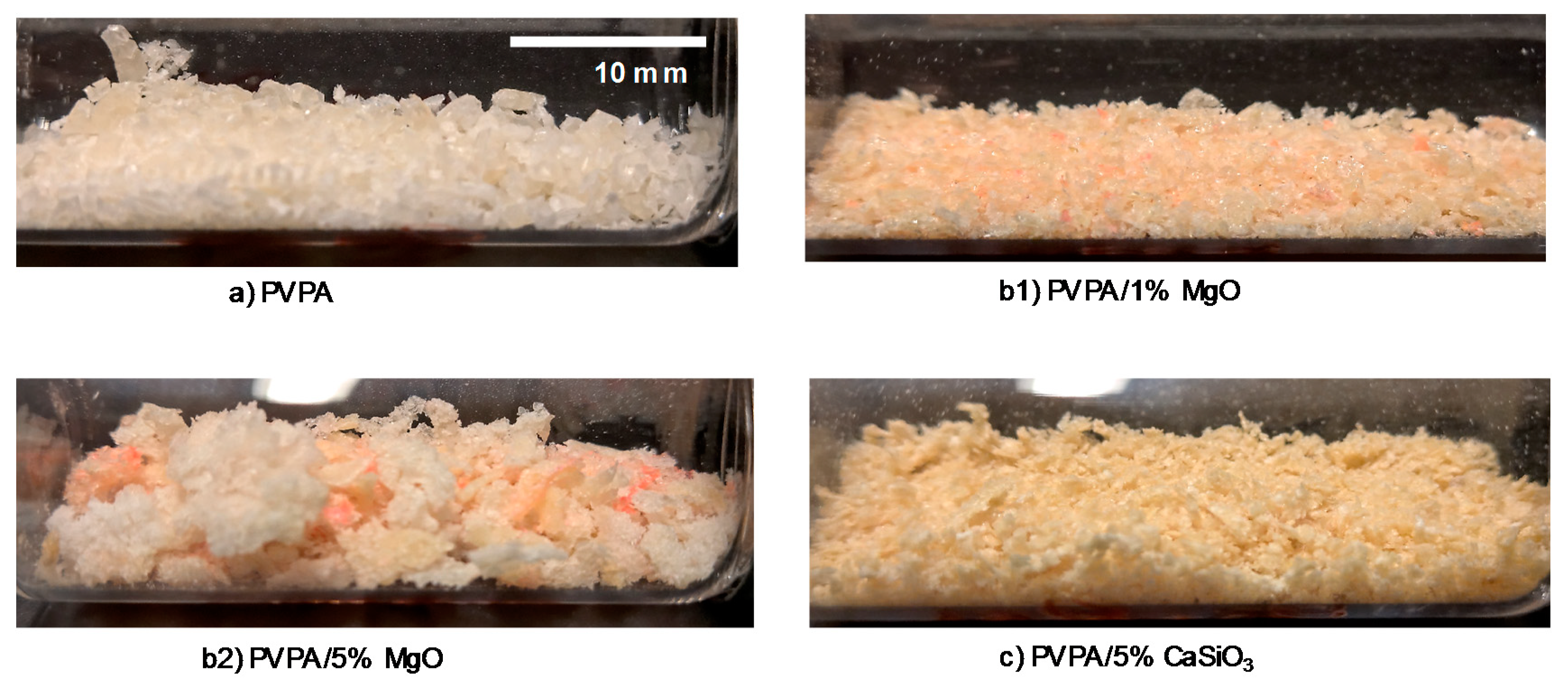
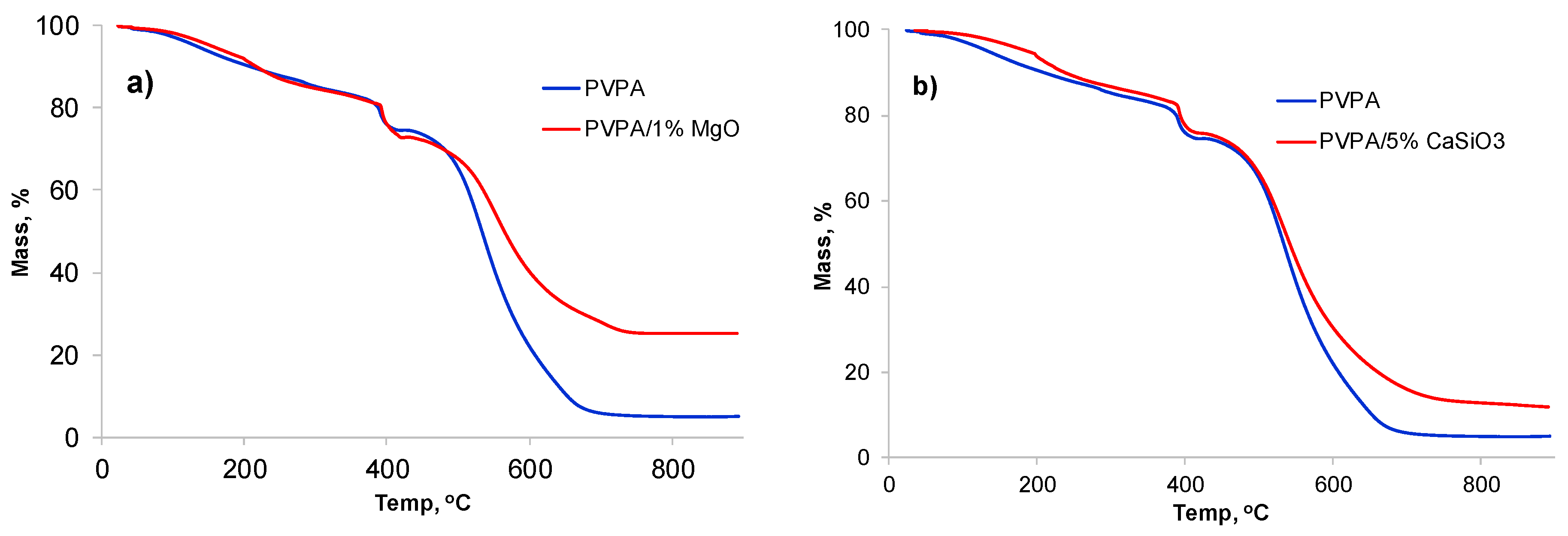
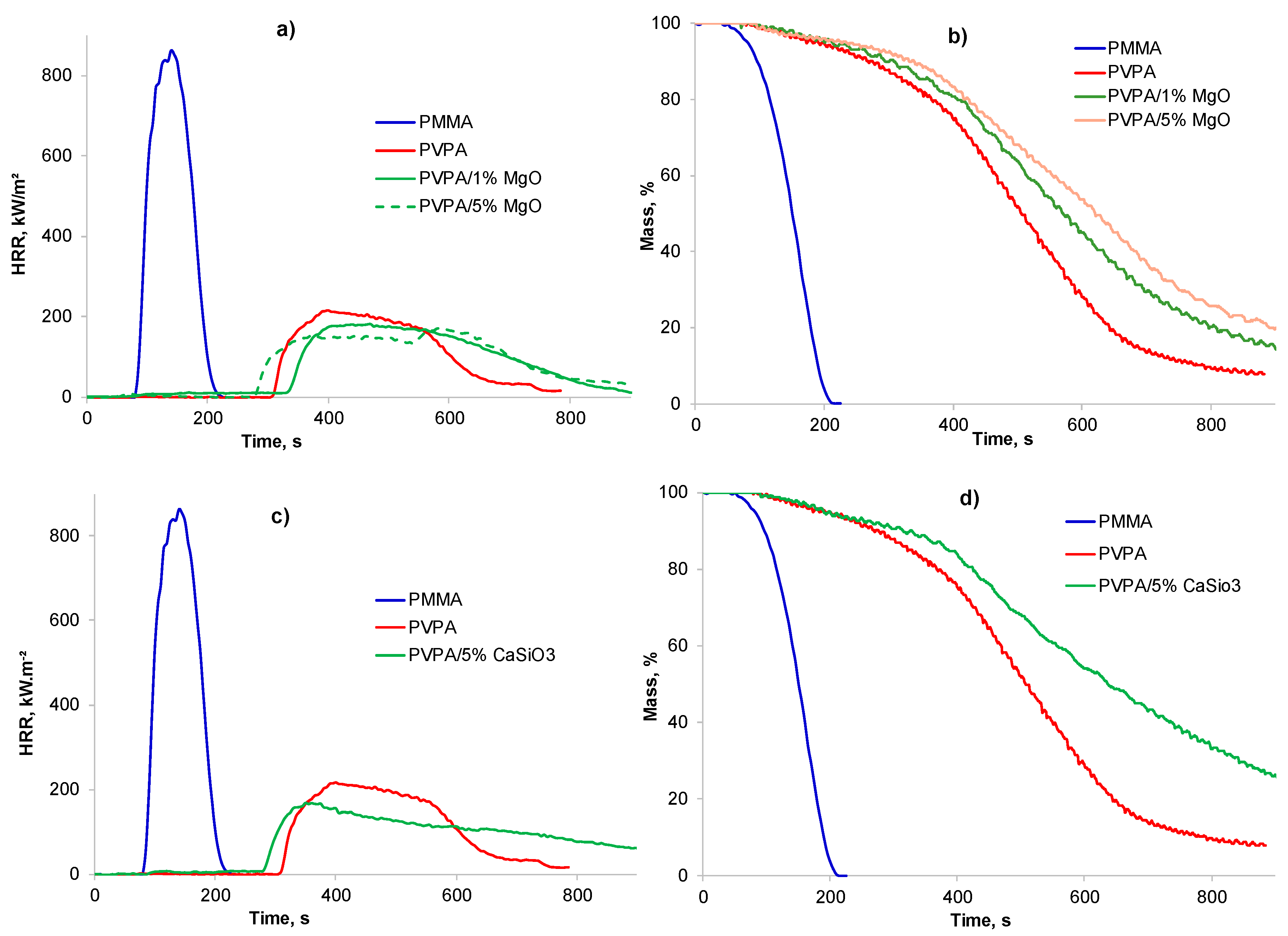
2.1.1. PVPA/MgO Coatings
2.1.2. PVPA/CaSiO3 Coatings
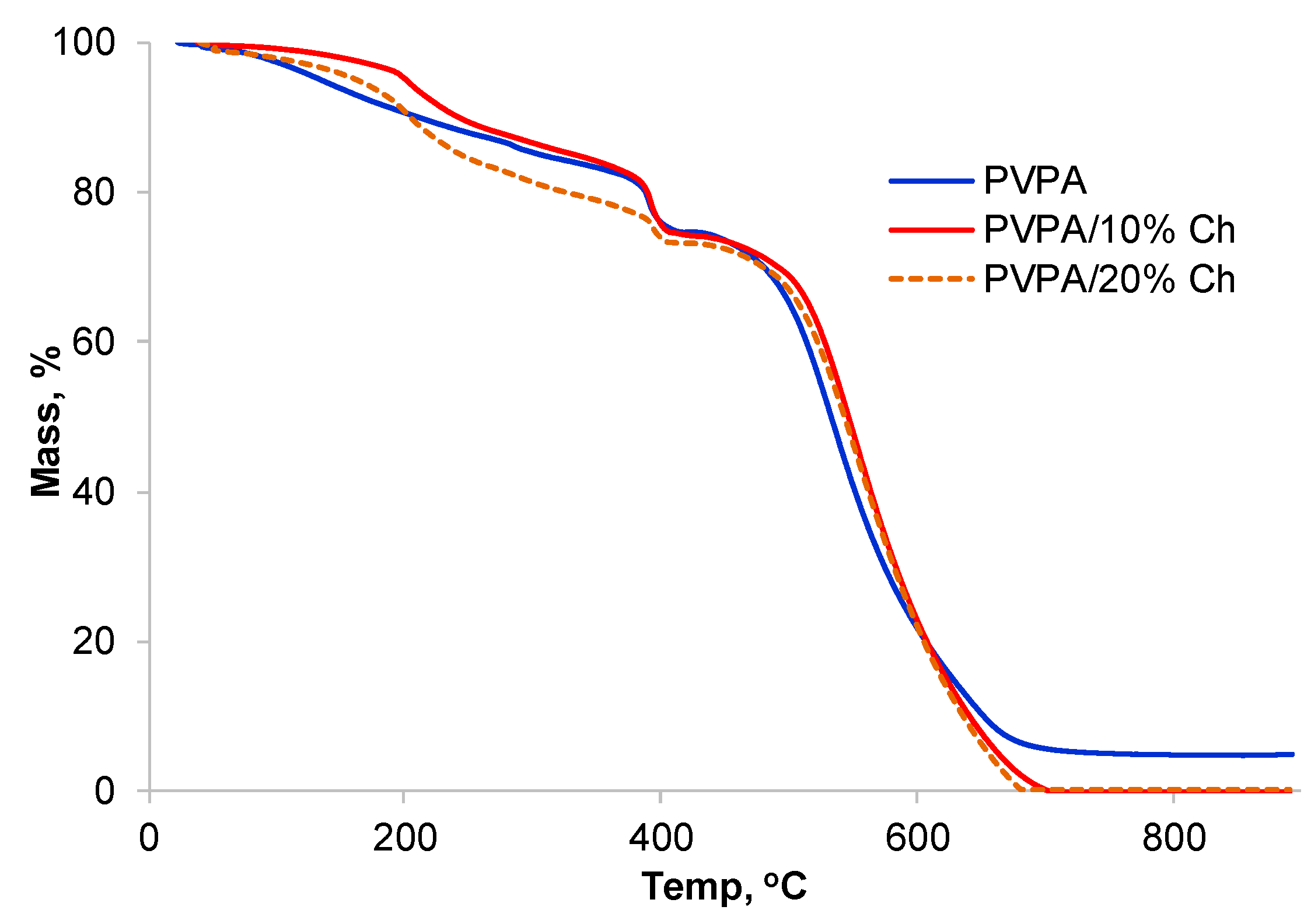
2.2. PVPA/Chitosan Coatings
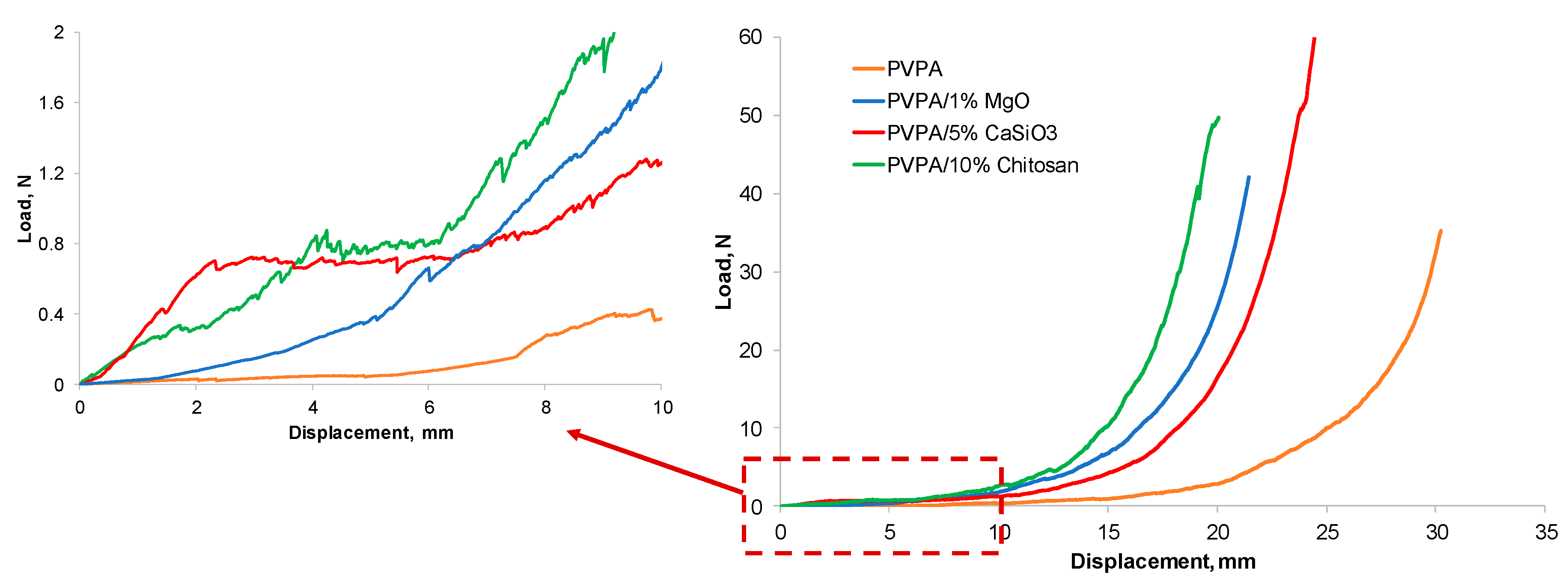
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. PVPA Coatings and Discs
4.2.2. PVPA/Inorganic Hybrid Coatings
4.2.3. PVPA/Chitosan Hybrid Coatings
4.3. Characterization of Coatings
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weil, E.D. Fire-protective and flame-retardant coatings—A state of the art review. J. Fire Sci. 2011, 29, 259–296. [Google Scholar] [CrossRef]
- Qui, X.; Li, Z.; Li, X.; Zhang, Z. Flame retardant coatings prepared using layer by layer assembly: A review. Chem. Eng. J. 2018, 334, 108–122. [Google Scholar]
- Malucelli, G. Surface-engineered fire protective coatings for fabrics through sol-gel and layer-by-layer methods: An overview. Coatings 2016, 6, 33. [Google Scholar] [CrossRef]
- Yang, H.T.; Yu, B.; Song, P.G.; Maluk, C.; Wang, H. Surface-coating engineering for flame retardant flexible polyurethane foams: A critical review. Comp. Part B Eng. 2019, 176, 107185. [Google Scholar] [CrossRef]
- Alongi, J.; Han, Z.; Bourbigot, S. Intumescence: Tradition versus novelty. A comprehensive review. Progress Poly. Sci. 2015, 51, 28–73. [Google Scholar] [CrossRef]
- Opwis, K.; Wego, A.; Bahners, T.; Schollmeyer, E. Permanent flame retardant finishing of textile materials by a photochemical immobilization of vinyl phosphonic acid. Polym. Degrad. Stab. 2011, 96, 393–395. [Google Scholar] [CrossRef]
- Luangtriratana, P.; Kandola, B.K.; Ebdon, J.R. UV-polymerisable, phosphorus-containing, flame-retardant surface coatings for glass fiber-reinforced epoxy composites. Prog. Org. Coat. 2015, 78, 73–82. [Google Scholar] [CrossRef]
- Williams, K.V.; Ebdon, J.R.; Kandola, B.K. Intumescent fire-retardant coatings for plastics based on poly(vinylphosphonic acid): Improving water resistance with comonomers. J. Appl. Poly. Sci. 2020, 137, 47601. [Google Scholar] [CrossRef]
- Ohlemiller, T.J.; Shields, J.R. The effect of surface coatings on fire growth over composite materials in a corner configuration. Fire Saf. J. 1999, 32, 173–193. [Google Scholar] [CrossRef]
- Luangtriratana, P.; Kandola, B.K.; Duquesne, S.; Bourbigot, S. Quantification of thermal barrier efficiency of intumescent coatings on glass fiber-reinforced epoxy composites. Coatings 2018, 8, 347. [Google Scholar] [CrossRef]
- Wilson, A.D.; Ellis, J. Poly-Vinylphosphonic Acid and Metal Oxide or Cermet or Glass Ionomer Cement. U.S. Patent 5,079,277, 7 January 1992. [Google Scholar]
- Mauritz, K.A. Review and Critical Analyses of Theories of Aggregation in Ionomers. J. Macromol. Sci. Part C. Polym. Rev. 1988, 88, 65–98. [Google Scholar] [CrossRef]
- Laufer, G.; Kirkland, C.; Cain, A.A.; Grunlan, J.C. Clay–Chitosan Nanobrick Walls: Completely Renewable Gas Barrier and Flame-Retardant Nanocoatings. ACS Appl. Mater. Interfaces 2012, 4, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Laufer, G.; Kirkland, C.; Morgan, A.B.; Grunlan, J.C. Intumescent multilayer nanocoating, made with renewable polyelectrolytes, for flame-retardant cotton. Biomacromolecules 2012, 13, 2843–2848. [Google Scholar] [CrossRef] [PubMed]
- Laufer, G.; Morgan, A.; Priolo, M.A.; Kirkland, C.; Grunlan, J.C. High oxygen barrier, clay and chitosan-based multilayer thin films: An environmentally friendly foil replacement. Green Mater. 2013, 1, 4–10. [Google Scholar] [CrossRef]
- Huang, F.; Wang, X.; Li, S. The Structures and ionic-conductivity of complexes formed by poly(tetramethylene succinate) and alkali-metal salts. J. Macromol. Sci. Chem. 1991, A28, 175–196. [Google Scholar] [CrossRef]
- Cain, A.A.; Murray, S.; Holder, K.M.; Nolen, C.R.; Grunlan, J.C. Intumescent nanocoating extinguishes flame on fabric using aqueous polyelectrolyte complex deposited in a single step. Macromol. Mater. Eng. 2014, 299, 1180–1187. [Google Scholar] [CrossRef]
- Yasmeen, S.; Kabiraz, M.K.; Saha, B.; Qadir, M.R.; Gafur, M.A.; Masum, S.H. Chromium (VI) ions removal from tannery effluent using chitosan-microcrystalline cellulose composite as adsorbent. Int. Res. J. Pure Appl. Chem. 2016, 10, 1–14. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structures and Properties; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Reaction to Fire Tests for Building Products–Building Products Excluding Floorings Exposed to the Thermal Attack by a Single Burning Item; EN 13823; European Committee for Standardization: Brussels, Belgium, 2002.
- Dursch, W.; Herwing, W.; Engelhardt, F. Process for the Preparation of Polymers of Vinylphosphonic Acid in Protic Solvents. U.S. Patent 4,696,987, 29 September 1987. [Google Scholar]
- Reaction-to-Fire Tests–Heat Release, Smoke Production and Mass Loss Rate–Part 1: Heat Release Rate (Cone Calorimeter Method); BS EN ISO 5660-1; British Standards Institute: London, UK, 2015.
- Biswas, B.; Kandola, B.K. The effect of chemically reactive type flame retardant additives on flammability of PES toughened epoxy resin and carbon fiber-reinforced composites. Polym. Adv. Technol. 2011, 22, 1192–1204. [Google Scholar] [CrossRef]
- Paints and Varnishes–Cross Cut Test; BS EN ISO 2409; British Standards Institute: London, UK, 2007.
- Paints and Varnishes–Determination of Resistance to Liquids–Water Immersion Method; BS EN ISO 2812-2; British Standards Institute: London, UK, 2007.
Sample Availability: Not available. |






| Sample * | CT/mm | MT/wt% | MS/wt% | MD/wt% |
|---|---|---|---|---|
| PVPA | 0.42 ± 0.12 | 0.04 | 89 | 16 |
| PVPA/1% MgO | 0.40 ± 0.03 | 0.19 | 54 | 18 |
| PVPA/5% MgO | 0.53 | 0.34 | 52 | 19 |
| PVPA/10% MgO | 0.67 | 0.09 | 40 | 23 |
| PVPA/5% CaSiO3 | 0.63 ± 0.04 | 0.17 | 97 | 15 |
| PVPA ƚ/chitosan (gel) | - | 96 | - | 37 |
| PVPA/10% chitosan | 0.26 ± 0.01 | 0.67 | 24 | 16 |
| PVPA/20% chitosan | 0.48 ± 0.02 | 0.25 | 30 | 29 |
| Coating on PMMA * | CT/mm | TTI/s | TTFO/s | PHRR/kW m−2 | TTPHR/s | THR/MJ m−2 | CH/mm |
|---|---|---|---|---|---|---|---|
| None | - | 89 ± 3 | 275 ± 37 | 900 ± 44 | 150 ± 5 | 78 ± 1 | - |
| PVPA | 0.28 ± 0.02 | 338 ± 62 | 810 ± 20 | 190 ± 10 | 450 ± 70 | 60 ± 5 | 21 ± 1 |
| 0.42 ± 0.9 | No ignition | 32 ± 6 | |||||
| 321 | 774 | 205 | 445 | 63 | 22 | ||
| PVPA/1% MgO | 0.37 | No ignition | 27 | ||||
| 0.44 | 723 | 1028 | 257 | 765 | 38 | 36 | |
| PVPA/5% MgO | 0.53 | 33.7 | 378 | 659 | 343 | 425 | 62.3 |
| PVPA/5% CaSiO3 | 0.60 ± 0.04 | 460 ± 170 | 1150 ± 40 | 154 ± 15 | 535 ± 185 | 48 ± 28 | 28 ± 3 |
| PVPA/10% Ch | 0.27 | No ignition | 13 | ||||
| 0.25 | 600 | 1490 | 115 | 765 | 44 | 16 | |
| PVPA/20% Ch | 0.50 | 730 | 2015 | 70 | 1000 | 64 | 13 |
| 0.47 | 390 | 1510 | 66 | 700 | 47 | 15 | |
| Sample * | CT/mm | MT/wt% | MS/wt% | TTI/s | TTFO/s | PHRR/kW m−2 | TTPHR/s | THR/MJ m−2 | CH/mm |
|---|---|---|---|---|---|---|---|---|---|
| PVPA/chitosan | 0.5 | 0.25 | 30 | 730 | 2015 | 70 | 1000 | 64 | 13 |
| P(VPA-co-AN) | 0.5 | 0.9 | 47 | 385 | 675 | 247 | 440 | 43 | 28 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandola, B.K.; Williams, K.V.; Ebdon, J.R. Organo-Inorganic Hybrid Intumescent Fire Retardant Coatings for Thermoplastics Based on Poly(Vinylphosphonic Acid). Molecules 2020, 25, 688. https://doi.org/10.3390/molecules25030688
Kandola BK, Williams KV, Ebdon JR. Organo-Inorganic Hybrid Intumescent Fire Retardant Coatings for Thermoplastics Based on Poly(Vinylphosphonic Acid). Molecules. 2020; 25(3):688. https://doi.org/10.3390/molecules25030688
Chicago/Turabian StyleKandola, Baljinder K., Katherine V. Williams, and John R. Ebdon. 2020. "Organo-Inorganic Hybrid Intumescent Fire Retardant Coatings for Thermoplastics Based on Poly(Vinylphosphonic Acid)" Molecules 25, no. 3: 688. https://doi.org/10.3390/molecules25030688
APA StyleKandola, B. K., Williams, K. V., & Ebdon, J. R. (2020). Organo-Inorganic Hybrid Intumescent Fire Retardant Coatings for Thermoplastics Based on Poly(Vinylphosphonic Acid). Molecules, 25(3), 688. https://doi.org/10.3390/molecules25030688







