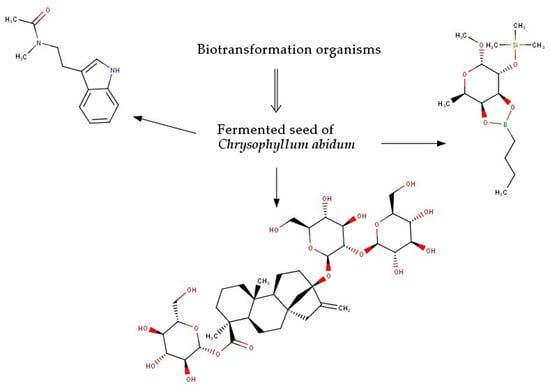
Fermentation Enhanced Biotransformation of Compounds in the Kernel of Chrysophyllum albidum
Abstract
1. Introduction
2. Results
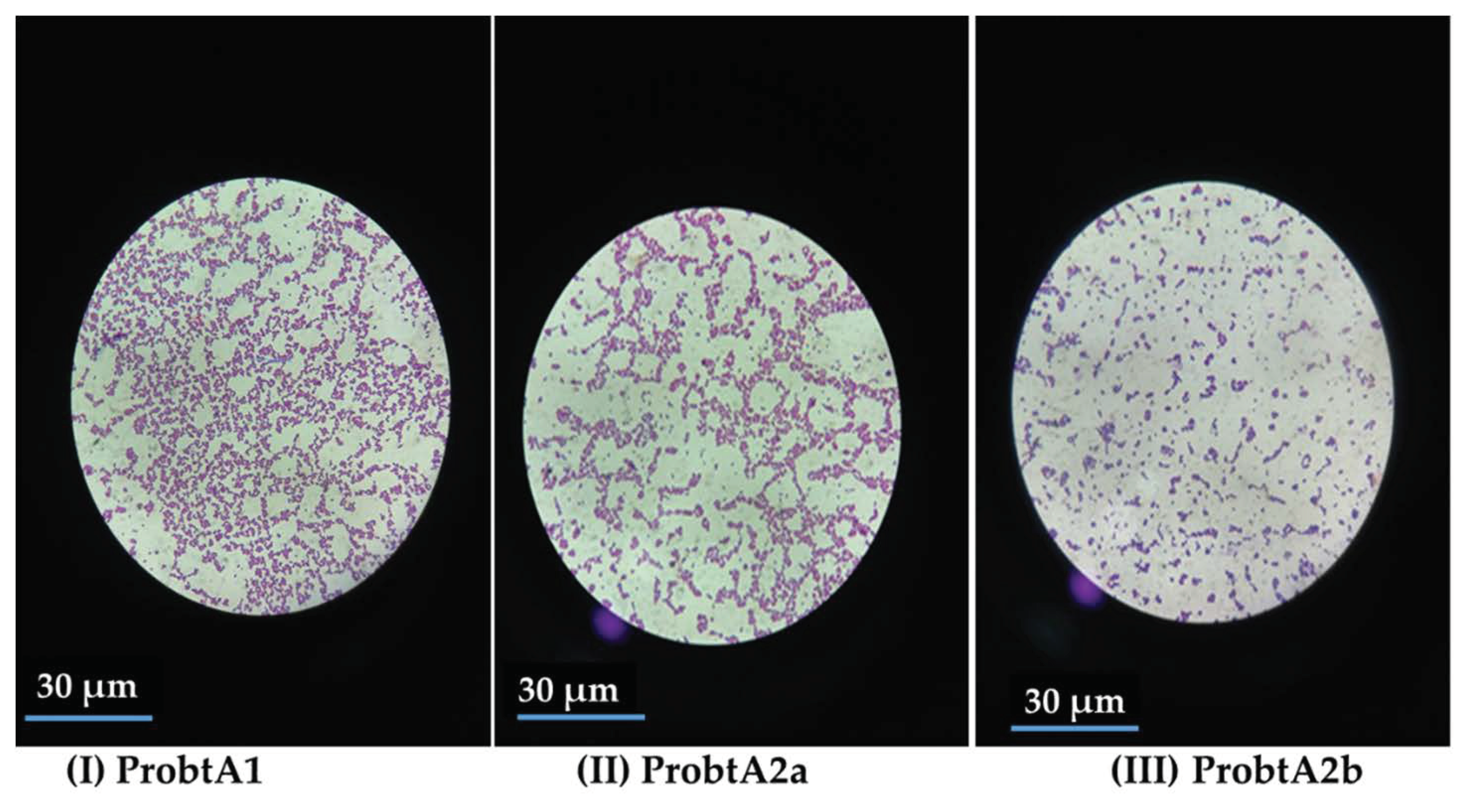
2.1. Identification of the Isolated Organisms Facilitating the Fermentation
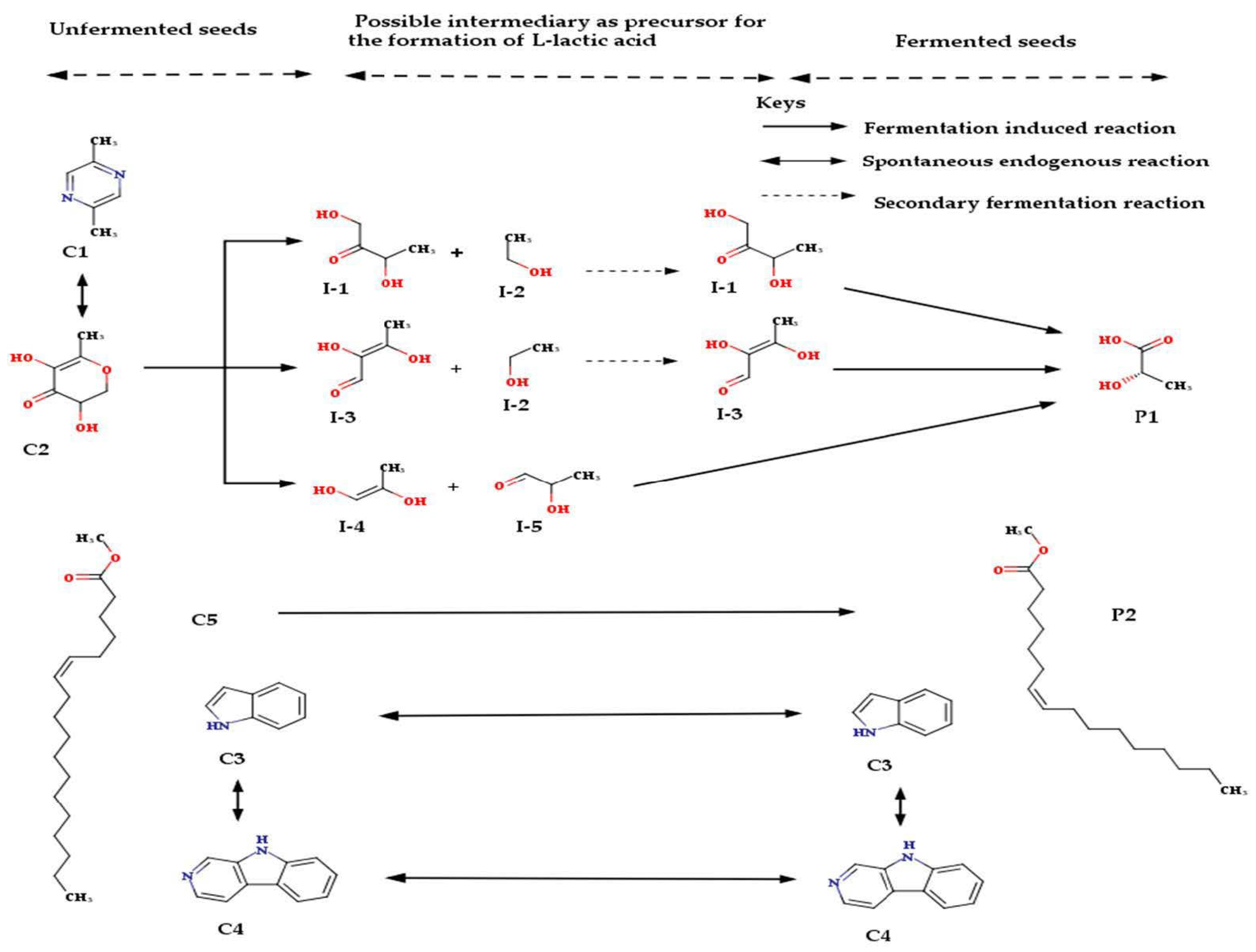
2.2. Biotransformation of Molecules by the Fermenting Organisms
3. Discussion
3.1. Identification of the Isolated Organisms Facilitating the Fermentation
3.2. Biotransformation of Molecules by the Fermenting Organisms
4. Materials and Methods
4.1. Plant Collection and Identification
4.2. Preparation of Flour from Kernel
4.3. Production of Both the Unfermented and the Fermented Aqueous Extracts
4.4. Identification of the Isolated Organisms Facilitating the Fermentation
4.4.1. Isolation of Lactic Acid Bacteria from the Fermented C. albidum Seeds
4.4.2. Gram Staining and Catalase Test
4.4.3. Biochemical Characterization of the Isolated Organisms
4.5. Sample Extract Concentration for Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
Procedure for GC-MS Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elemo, G.N.; Elemo, B.O.; Oladunmoye, O.O.; Erukainure, O.L. Comprehensive investigation into the nutritional composition of dehulled and defatted African locust bean seed (Parkia biglobosa). Afr. J. Plant Sci. 2011, 5, 291–295. [Google Scholar]
- Apata, D.F.; Babalola, T.O. The use of cassava, sweet potato and cocoyam, and their by-products by non-ruminants. Int. J. Food Sci. Nutr. Eng. 2012, 2, 54–62. [Google Scholar] [CrossRef]
- Afolabi, I.S.; Nwachukwu, I.C.; Ezeoke, C.S.; Woke, R.C.; Adegbite, O.A.; Olawole, T.D.; Martins, O.C. Production of a new plant-based milk from Adenanthera pavonina seed and evaluation of its nutritional and health benefits. Front. Nutr. 2018, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, I.S.; Marcus, G.D.; Olanrewaju, T.O.; Chizea, V. Biochemical effect of some food processing methods on the health promoting properties of under-utilized Carica papaya seed. J. Nat. Prod. 2011, 4, 17–24. [Google Scholar]
- Olawole, T.D.; Olalere, A.T.; Adeyemi, O.A.; Okwumabua, O.; Afolabi, I.S. Tannin and antioxidant status of fermented and dried Sorghum bicolor. Rasāyan J. Chem. 2019, 12, 523–530. [Google Scholar] [CrossRef]
- Behera, S.S.; El Sheikha, A.F.; Hammami, R.; Kumar, A. Traditionally fermented pickles: How the microbial diversity associated with their nutritional and health benefits? J. Funct. Foods 2020, 70, 103971. [Google Scholar] [CrossRef]
- López-López, A.; Moreno-Baquero, J.M.; Rodríguez-Gómez, F.; García-García, P.; Garrido-Fernández, A. Sensory assessment by consumers of traditional and potentially probiotic green Spanish-style table olives. Front. Nutr. 2018, 5. [Google Scholar] [CrossRef]
- Adebayo, A.H.; Abolaji, A.O.; Kela, R.; Ayepola, O.O.; Olorunfemi, T.B.; Taiwo, O.S. Antioxidant activities of the leaves of Chrysophyllum albidum G. Pak. J. Pharm. Sci. 2011, 24, 545–551. [Google Scholar]
- Audu, S.S.; Beetseh, C.I.; Edward-Ekpu, D.U.; Ewuga, A.A. Proximate, mineral contents and physicochemical properties of Chrysophyllum albidum (African star apple) kernel flour and oil. J. Appl. Sci. Environ. Manag. 2019, 23, 1245. [Google Scholar] [CrossRef]
- Santos, D.I.; Saraiva, J.M.A.; Vicente, A.A.; Moldão-Martins, M. Methods for determining bioavailability and bioaccessibility of bioactive compounds and nutrients. In Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Barba, F.J., Saraiva, J.M.A., Cravotto, G., Lorenzo, J.M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 23–54. [Google Scholar] [CrossRef]
- Hughes, R.L. A review of the role of the gut microbiome in personalized sports nutrition. Front. Nutr. 2019, 6, 191. [Google Scholar] [CrossRef]
- Badri, D.V.; Chaparro, J.M.; Zhang, R.; Shen, Q.; Vivanco, J.M. Application of natural blends of phytochemicals derived from the root exudates of Arabidopsis to the soil reveal that phenolic-related compounds predominantly modulate the soil microbiome. J. Biol. Chem. 2013, 288, 4502–4512. [Google Scholar] [CrossRef]
- Besle, J.M.; Viala, D.; Martin, B.; Pradel, P.; Meunier, B.; Berdague, J.L.; Fraisse, D.; Lamaison, J.L.; Coulon, J.B. Ultraviolet-absorbing compounds in milk are related to forage polyphenols. J. Dairy Sci. 2010, 93, 2846–2856. [Google Scholar] [CrossRef] [PubMed]
- Motta, F.L.; Santana, M.H. Solid-state fermentation for humic acids production by a Trichoderma reesei strain using an oil palm empty fruit bunch as the substrate. Appl. Biochem. Biotechnol. 2014, 172, 2205–2217. [Google Scholar] [CrossRef] [PubMed]
- Olawole, T.D.; Okundigie, M.I.; Rotimi, S.O.; Okwumabua, O.; Afolabi, I.S. Preadministration of fermented sorghum diet provides protection against hyperglycemia-induced oxidative stress and suppressed glucose utilization in alloxan-induced diabetic rats. Front. Nutr. 2018, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Bolanle, O.O.; Olajumoke, O.; Pheabean, I.O.; Samuel, O.; Li, C.; Shen, G. Proximate composition, phytonutrients and antioxidant properties of oven dried and vacuum dried African star apple (Chrysophyllum albidum) Products. Int. J. Nutr. Food Sci. 2017, 66, 22–25. [Google Scholar] [CrossRef]
- Goswami, R.P.; Jayaprakasha, G.K.; Shetty, K.; Patil, B.S. Lactobacillus plantarum and natural fermentation-mediated biotransformation of flavor and aromatic compounds in horse gram sprouts. Process Biochem. 2018, 66, 7–18. [Google Scholar] [CrossRef]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented foods as a dietary source of live organisms. Front. Microbiol. 2018, 9, 1785. [Google Scholar] [CrossRef]
- De Vuyst, L.; Weckx, S. The cocoa bean fermentation process: From ecosystem analysis to starter culture development. J. Appl. Microbiol. 2016, 121, 5–17. [Google Scholar] [CrossRef]
- Capozzi, V.; Fragasso, M.; Romaniello, R.; Berbegal, C.; Russo, P.; Spano, G. Spontaneous food fermentations and potential risks for human health. Fermentation 2017, 3, 49. [Google Scholar] [CrossRef]
- Frias, J.; Martinez-Villaluenga, C.; Peñas, E. Fermented Foods in Health and Disease Prevention; Frias, J., Martinez-Villaluenga, C., Peñas, E., Eds.; Elsevier Incorporation: Amsterdam, The Netherlands, 2017; p. 760. [Google Scholar]
- Padmavathi, T.; Bhargavi, R.; Priyanka, P.R.; Niranjan, N.R.; Pavitra, P.V. Screening of potential probiotic lactic acid bacteria and production of amylase and its partial purification. J. Genet. Eng. Biotechol. 2018, 16, 357–362. [Google Scholar] [CrossRef]
- Ismail, Y.S.; Yulvizar, C.; Mazhitov, B. Characterization of lactic acid bacteria from local cow’s milk kefir. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Medan, Indonesia, 23–24 August 2017; p. 012019. [Google Scholar]
- Li, H.; Tang, X.-Y.; Wu, C.-J.; Yu, S.-J. Formation of 2,3-dihydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one (DDMP) in glucose-amino acids Maillard reaction by dry-heating in comparison to wet-heating. LWT 2019, 105, 156–163. [Google Scholar] [CrossRef]
- Preininger, M.; Gimelfarb, L.; Li, H.C.; Dias, B.E.; Fahmy, F.; White, J. Identification of dihydromaltol (2,3-dihydro-5-hydroxy-6-methyl-4H-pyran-4-one) in Ryazhenka Kefir and comparative sensory impact assessment of related cycloenolones. J. Agric. Food Chem. 2009, 57, 9902–9908. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, M.; Paraskevopoulou, A.; Pantazi, F.; Skendi, A. Cake perception, texture and aroma profile as affected by wheat flour and cocoa replacement with carob flour. Foods 2020, 9, 1586. [Google Scholar] [CrossRef] [PubMed]
- Cutzach, I.; Chatonnet, P.; Henry, R.; Dubourdieu, D. Identification of volatile compounds with a “toasty” aroma in heated oak used in barrelmaking. J. Agric. Food Chem. 1997, 45, 2217–2224. [Google Scholar] [CrossRef]
- Asikin, Y.; Hirose, N.; Tamaki, H.; Ito, S.; Oku, H.; Wada, K. Effects of different drying-solidification processes on physical properties, volatile fraction, and antioxidant activity of non-centrifugal cane brown sugar. LWT-Food Sci. Technol. 2016, 66, 340–347. [Google Scholar] [CrossRef]
- Qin, P.; Ma, T.; Wu, L.; Shan, F.; Ren, G. Identification of tartary buckwheat tea aroma compounds with gas chromatography-mass spectrometry. J. Food Sci. 2011, 76, S401–S407. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Sun, B.; Ren, F.; Chen, H.; Zhang, N.; Zhang, Y. Influence of different frying processes on the flavor characteristics and sensory profile of garlic oil. Molecules 2019, 24, 4456. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.C.; Hammer, E.; Hanschke, R.; Arambarri, A.M.; Schauer, F. Biotransformation of biphenyl by the filamentous fungus Talaromyces helicus. World J. Microbiol. Biotechnol. 2005, 21, 101–106. [Google Scholar] [CrossRef]
- Zheng, L.; Yan, X.; Han, X.; Chen, H.; Lin, W.; Lee, F.S.; Wang, X. Identification of norharman as the cytotoxic compound produced by the sponge (Hymeniacidon perleve)-associated marine bacterium Pseudoalteromonas piscicida and its apoptotic effect on cancer cells. Biotechnol. Appl. Biochem. 2006, 44, 135–142. [Google Scholar] [CrossRef]
- Casal, S. Neuroactive β-carbolines norharman and harman in coffee. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: London, UK, 2015; pp. 737–743. [Google Scholar] [CrossRef]
- Piechowska, P.; Zawirska-Wojtasiak, R.; Mildner-Szkudlarz, S. Bioactive beta-carbolines in food: A review. Nutrients 2019, 11, 814. [Google Scholar] [CrossRef]
- Pfau, W.; Skog, K. Exposure to beta-carbolines norharman and harman. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 802, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Draxler, S.; Lippitsch, M.E. Excited-state acid-base kinetics and equilibria in norharman. J. Phys. Chem. 1993, 97, 11493–11496. [Google Scholar] [CrossRef]
- Wronska, A.K.; Bogus, M.I.; Kaczmarek, A.; Kazek, M. Harman and norharman, metabolites of entomopathogenic fungus Conidiobolus coronatus (Entomopthorales), disorganize development of Galleria mellonella (Lepidoptera) and affect serotonin-regulating enzymes. PLoS ONE 2018, 13, e0204828. [Google Scholar] [CrossRef] [PubMed]
- Wronska, A.K.; Bogus, M.I. Harman and norharman, metabolites of the entomopathogenic fungus Conidiobolus coronatus (Entomophthorales), affect the serotonin levels and phagocytic activity of hemocytes, insect immunocompetent cells, in Galleria mellonella (Lepidoptera). Cell Biosci. 2019, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Teizeira de Mattos, M.J.; Tempest, D.W. Metabolic and energetic aspects of the growth of Klebsiella aerogenes NCTC 418 on glucose in anaerobic chemostat culture. Arch. Microbiol. 1983, 134, 80–85. [Google Scholar] [CrossRef]
- Toraya, T.; Honda, S.; Fukui, S. Fermentation of 1,2-propanediol and 1,2-ethanediol by some genera of Enterobacteriaceae, Involving Coenzyme B12-dependent diol dehydratase. J. Bacteriol. 1979, 139, 39–47. [Google Scholar] [CrossRef]
- Park, Y.S.; Choi, U.J.; Nam, N.H.; Choi, S.J.; Nasir, A.; Lee, S.G.; Kim, K.J.; Jung, G.Y.; Choi, S.; Shim, J.Y.; et al. Engineering an aldehyde dehydrogenase toward its substrates, 3-hydroxypropanal and NAD+, for enhancing the production of 3-hydroxypropionic acid. Sci. Rep. 2017, 7, 17155. [Google Scholar] [CrossRef]
- Niesbach, A.; Lutze, P.; Górak, A. Reactive distillation for production of n-butyl acrylate from bio-based raw materials. In Proceedings of the European Symposium on Computer Aided Process Engineering–ESCAPE 23, Lappeenranta, Finland, 9–12 June 2013; pp. 223–228. [Google Scholar]
- Chandrasekhar, K.; Lee, Y.J.; Lee, D.W. Biohydrogen production: Strategies to improve process efficiency through microbial routes. Int. J. Mol. Sci. 2015, 16, 8266–8293. [Google Scholar] [CrossRef]
- Park, M.K.; Choi, H.S.; Kim, Y.S.; Cho, I.H. Change in profiles of volatile compounds from two types of Fagopyrum esculentum (buckwheat) soksungjang during fermentation. Food Sci. Biotechnol. 2017, 26, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Deuscher, Z.; Gourrat, K.; Repoux, M.; Boulanger, R.; Laboure, H.; Le Quere, J.L. Key aroma compounds of dark chocolates differing in organoleptic properties: A GC-O comparative study. Molecules 2020, 25, 1809. [Google Scholar] [CrossRef] [PubMed]
- Harle, O.; Falentin, H.; Niay, J.; Valence, F.; Courselaud, C.; Chuat, V.; Maillard, M.B.; Guedon, E.; Deutsch, S.M.; Thierry, A. Diversity of the metabolic profiles of a broad range of lactic acid bacteria in soy juice fermentation. Food Microbiol. 2020, 89, 103410. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, W.; Wang, Z.; Chen, T. Production of riboflavin and related cofactors by biotechnological processes. Microb. Cell Fact. 2020, 19, 31. [Google Scholar] [CrossRef]
- Kondybayev, A.; Zhakupbekova, A.; Amutova, F.; Omarova, A.; Nurseitova, M.; Akhmetsadykova, S.; Akhmetsadykov, N.; Konuspayeva, G.; Faye, B. Volatile organic compounds profiles in milk fermented by lactic bacteria. Int. J. Biol. Chem. 2018, 11, 57–67. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, Y.; Nakai, S.; Hosomi, M.; Zhang, H.; Kronzucker, H.J.; Shi, W. Stimulation of nitrogen removal in the rhizosphere of aquatic duckweed by root exudate components. Planta 2014, 239, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Dadwal, V.; Agrawal, H.; Sonkhla, K.; Joshi, R.; Gupta, M. Characterization of phenolics, amino acids, fatty acids and antioxidant activity in pulp and seeds of high altitude Himalayan crab apple fruits (Malus baccata). J. Food Sci. Technol. 2018, 55, 2160–2169. [Google Scholar] [CrossRef] [PubMed]
- Al Bratty, M.; Makeen, H.A.; Alhazmi, H.A.; Syame, S.M.; Abdalla, A.N.; Homeida, H.E.; Sultana, S.; Ahsan, W.; Khalid, A. Phytochemical, cytotoxic, and antimicrobial evaluation of the fruits of miswak plant, Salvadora persica L. J. Chem. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Satoh, S.; Ozaki, M.; Matsumoto, S.; Nabatame, T.; Kaku, M.; Shudo, T.; Asayama, M.; Chohnan, S. Enhancement of fatty acid biosynthesis by exogenous acetyl-CoA carboxylase and pantothenate kinase in Escherichia coli. Biotechnol. Lett. 2020, 42, 2595–2605. [Google Scholar] [CrossRef]
- Wang, H.L.; Brattstrom, O.; Brakefield, P.M.; Francke, W.; Lofstedt, C. Identification and biosynthesis of novel male specific esters in the wings of the tropical butterfly, Bicyclus martius sanaos. J. Chem. Ecol. 2014, 40, 549–559. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, C.; Gibson, T.; Jauregi, P. Novel probiotic-fermented milk with angiotensin I-converting enzyme inhibitory peptides produced by Bifidobacterium bifidum MF 20/5. Int. J. Food Microbiol. 2013, 167, 131–137. [Google Scholar] [CrossRef]
- Devi, S.M.; Archer, A.C.; Halami, P.M. Screening, characterization and In vitro evaluation of probiotic properties among lactic acid bacteria through comparative analysis. Probiotics Antimicrob. Proteins 2015, 7, 181–192. [Google Scholar] [CrossRef]
- Osamudiamen, P.M.; Afolabi, L.O. Physicochemical characteristics, proximate and mineral compositions of the underutilized seed and oil of Chrysophyllum albidum from Ibadan, Nigeria. Electron. J. Environ. Agric. Food Chem. 2012, 11, 351–357. [Google Scholar]
- Omeje, K.O.; Iroha, O.K.; Edeke, A.A.; Omeje, H.C.; Apeh, V.O. Characterization and fatty acid profile analysis of oil extracted from unexploited seed of African star apple (Udara). OCL 2019, 26, 10. [Google Scholar] [CrossRef]
- Mazzocchi, A.; Venter, C.; Maslin, K.; Agostoni, C. The role of nutritional aspects in food allergy: Prevention and management. Nutrients 2017, 9, 850. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.-N.; Tan, Z.-W.; Wang, F.-S. Mechanistic studies on the formation of pyrazines by Maillard reaction between l-ascorbic acid and l-glutamic acid. Lwt-Food Sci. Technol. 2013, 50, 64–71. [Google Scholar] [CrossRef]
- Yu, A.-N.; Tan, Z.-W.; Shi, B.-A. Influence of the pH on the formation of pyrazine compounds by the Maillard reaction of l-ascorbic acid with acidic, basic and neutral amino acids. Asia-Pac. J. Chem. Eng. 2012, 7, 455–462. [Google Scholar] [CrossRef]
Sample Availability: The samples for generating the identified compounds and the isolated organisms in this study are available from the authors. |
| S/N | Peaks | tR | Area (%) | Similarity Index (%) | Class of Compound | IUPAC Name | Common Name |
|---|---|---|---|---|---|---|---|
| 1 | 4 | 5.74 | 1.27 | 89 | Pyrazine | 2,5-dimethylpyrazine | 2,5-dimethylpyrazine |
| 2 | 15 | 9.13 | 2.90 | 86 | Ketone | 3,5-Dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one | 2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one |
| 3 | 19 | 11.25 | 1.31 | 91 | Indole | 1H-indole | Indole |
| 4 | 41 | 17.69 | 3.01 | 87 | Beta-carboline | 9H-Pyrido[3,4-b]indole | Norharman |
| 5 | 43 | 18.04 | 4.33 | 85 | Fatty acid ester | Methyl cis-6-octadecenoate | Methyl Petroselinate |
| S/N | Peaks | Tr | Area (%) | Similarity Index (%) | Class of Compound | IUPAC Name | Common Name |
|---|---|---|---|---|---|---|---|
| 1 | 3 | 6.85 | 28.57 | 94 | Carboxylic acid | (2S)-2-hydroxypropanoic acid | l-Lactic acid |
| 2 | 6 | 11.21 | 1.51 | 92 | Indole | 1H-indole | Indole |
| 3 | 20 | 17.71 | 5.07 | 95 | Beta-carboline | 9H-Pyrido[3,4-b]indole | Norharman |
| 4 | 22 | 18.04 | 1.73 | 85 | Fatty acid esters | 7-Hexadecenoic acid, methyl ester, (Z)- | Formyl 7E-hexadecenoate |
| S/N | Substrates | ProbtA1 | ProbtA2a | ProbtA2b |
|---|---|---|---|---|
| 0 | Control | − | − | − |
| 1 | Glycerol | − | − | − |
| 2 | Erythritol | − | − | − |
| 3 | d-Arabinose | − | − | − |
| 4 | l-Arabinose | + | + | + |
| 5 | d-Ribose | + | + | + |
| 6 | d-Xylose | − | − | − |
| 7 | l-Xylose | − | − | − |
| 8 | d-Adonitol | − | − | − |
| 9 | Methyl-βd-xylopyranoside | − | − | − |
| 11 | d-Galactose | + | + | + |
| 12 | d-Glucose | + | + | + |
| 13 | d-Fructose | + | + | + |
| 14 | d-Mannose | + | + | + |
| 15 | l-Sorbose | − | − | − |
| 16 | l-Rhamnose | − | − | − |
| 17 | Dulcitol | − | − | − |
| 18 | Inositol | − | − | − |
| 19 | d-Mannitol | − | + | + |
| 20 | d-Sorbitol | − | − | − |
| 21 | Methyl-αd-Mannopyranoside | − | − | − |
| 22 | Methyl-αd-Glucopyranoside | − | − | − |
| 23 | N-Acetylglucosamine | + | + | + |
| 24 | Amygdalin | − | − | − |
| 25 | Arbutin | + | + | + |
| 28 | Esculin ferric citrate | + | + | + |
| 29 | Salicin | + | + | + |
| 30 | d-Cellobiose | + | + | + |
| 31 | d-Maltose | + | + | + |
| 32 | d-Lactose | − | − | − |
| 33 | d-Mellibiose | + | + | + |
| 34 | d-Saccharose | + | + | + |
| 35 | d-Trehalose | + | + | + |
| 38 | Inulin | − | − | − |
| 39 | d-Melezitose | − | - | − |
| 40 | d-Raffinose | + | + | + |
| 41 | Amidon | + | − | − |
| 43 | Glycogen | − | − | − |
| 44 | Xylitol | − | − | − |
| 45 | Gentiobiose | + | − | + |
| 46 | d-Turanose | − | − | − |
| 47 | d-Lyxose | − | − | − |
| 48 | d-Tagatose | − | − | + |
| 49 | d-Fucose | − | − | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odutayo, O.E.; Omonigbehin, E.A.; Olawole, T.D.; Ogunlana, O.O.; Afolabi, I.S. Fermentation Enhanced Biotransformation of Compounds in the Kernel of Chrysophyllum albidum. Molecules 2020, 25, 6021. https://doi.org/10.3390/molecules25246021
Odutayo OE, Omonigbehin EA, Olawole TD, Ogunlana OO, Afolabi IS. Fermentation Enhanced Biotransformation of Compounds in the Kernel of Chrysophyllum albidum. Molecules. 2020; 25(24):6021. https://doi.org/10.3390/molecules25246021
Chicago/Turabian StyleOdutayo, Oluwatofunmi E., Emmanuel A. Omonigbehin, Tolulope D. Olawole, Olubanke O. Ogunlana, and Israel S. Afolabi. 2020. "Fermentation Enhanced Biotransformation of Compounds in the Kernel of Chrysophyllum albidum" Molecules 25, no. 24: 6021. https://doi.org/10.3390/molecules25246021
APA StyleOdutayo, O. E., Omonigbehin, E. A., Olawole, T. D., Ogunlana, O. O., & Afolabi, I. S. (2020). Fermentation Enhanced Biotransformation of Compounds in the Kernel of Chrysophyllum albidum. Molecules, 25(24), 6021. https://doi.org/10.3390/molecules25246021