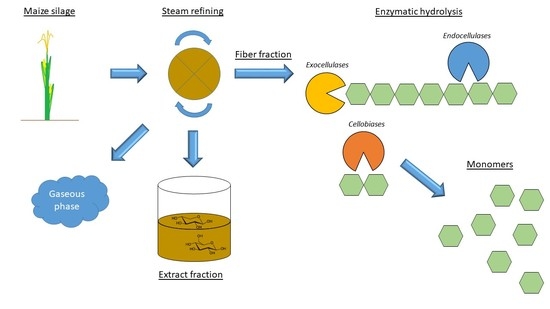
Maize Silage Pretreatment via Steam Refining and Subsequent Enzymatic Hydrolysis for the Production of Fermentable Carbohydrates
Abstract
1. Introduction
2. Results and Discussion
2.1. Raw Material Characterization
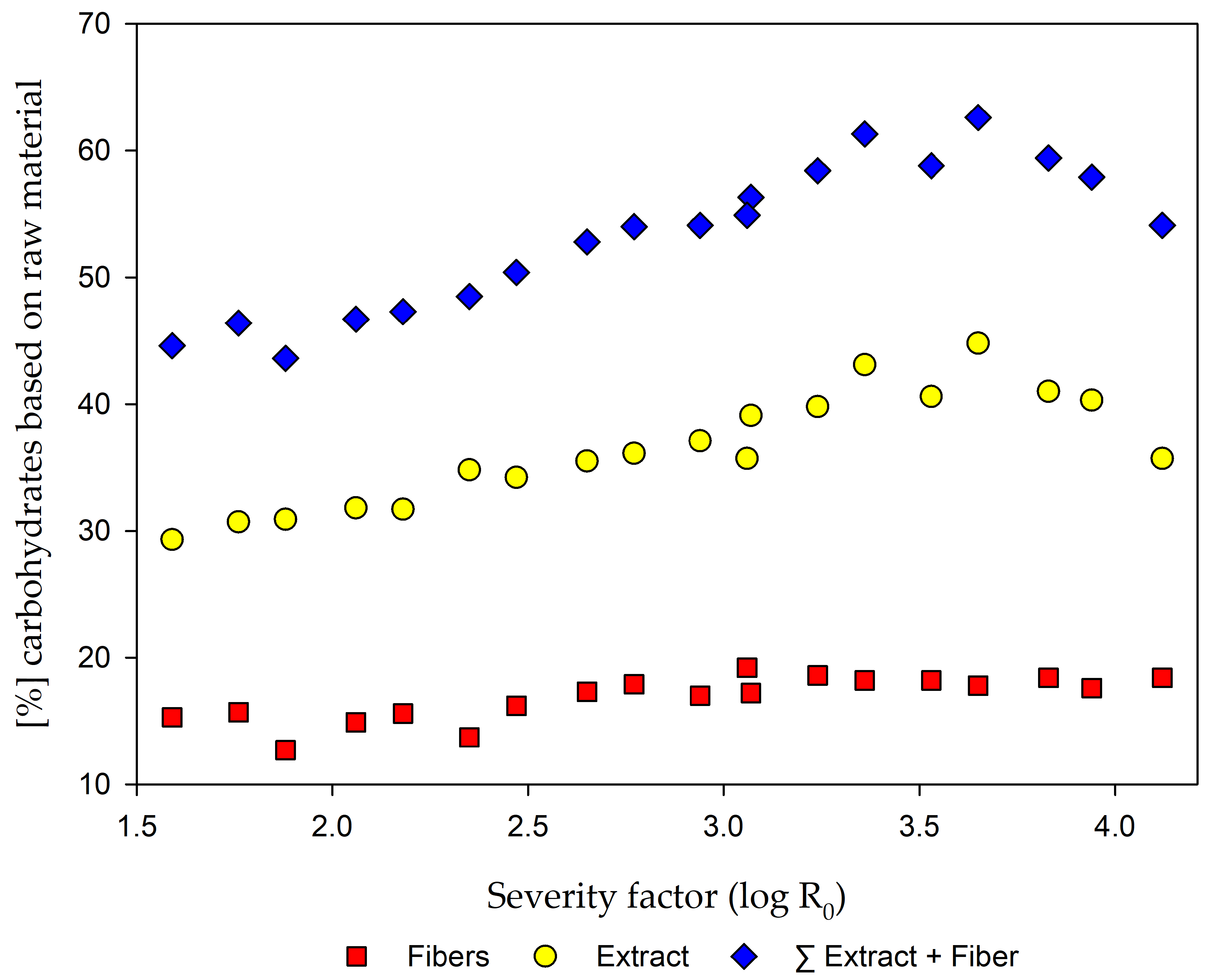
2.2. Fiber and Extract Yields after Steam Refining
2.3. Chemical Characterization of Fibers and Extracts
2.4. Degradation Products, Organic Acids, and pH Value
2.5. EH of Pretreated Fiber Fraction
2.6. Mass and Carbon Balance
3. Materials and Methods
3.1. Raw Material
3.2. Pretreatment Process
3.3. Acid Extract and Fiber Fraction Hydrolysis
3.4. Chromatographic Methods
3.5. Enzymatic Hydrolysis (EH)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jäger, G.; Büchs, J. Biocatalytic conversion of lignocellulose to platform chemicals. Biotechnol. J. 2012, 7, 1122–1136. [Google Scholar] [CrossRef]
- Becker, J.; Lange, A.; Fabarius, J.; Wittmann, C. Top value platform chemicals: Bio-based production of organic acids. Curr. Opin. Biotechnol. 2015, 36, 168–175. [Google Scholar] [CrossRef]
- Menon, V.; Rao, M. Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept. Prog. Energy Combust. Sci. 2012, 38, 522–550. [Google Scholar] [CrossRef]
- Valdivia, M.; Galan, J.L.; Laffarga, J.; Ramos, J.-L. Biofuels 2020: Biorefineries based on lignocellulosic materials. Microb. Biotechnol. 2016, 9, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Lucia, L.A. Lignocellulosic Biomass: A Potential Feedstock to Replace Petroleum. BioResources 2008, 3, 981–982. [Google Scholar]
- Tye, Y.Y.; Lee, K.T.; Wan Abdullah, W.N.; Leh, C.P. The world availability of non-wood lignocellulosic biomass for the production of cellulosic ethanol and potential pretreatments for the enhancement of enzymatic saccharification. Renew. Sust. Energ. Rev. 2016, 60, 155–172. [Google Scholar] [CrossRef]
- U.S. Department of Energy. 2016 Billion-Ton Report: Advancing Domestic Resources for a Thriving Bioeconomy. Volume 1: Economic Availability of Feedstocks; U.S. Department of Energy: Washington, DC, USA, 2016.
- U.S. Department of Energy. U.S. Billion-Ton Update: Biomass Supply for a Bioenergy and Bioproducts Industry; U.S. Department of Energy: Washington, DC, USA, 2011.
- U.S. Department of Energy. Biomass as a Feedstock for a Bioenergy and Bioproducts Industry: The Technical Feasibility of a Billion-Ton Annual Supply; U.S. Department of Energy: Washington, DC, USA, 2005.
- Yousuf, A.; Pirozzi, D.; Sannino, F. Fundamentals of lignocellulosic biomass. In Lignocellulosic Biomass to Liquid Biofuels; Elsevier: Cambridge, MA, USA, 2020; pp. 1–15. ISBN 9780128159361. [Google Scholar]
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global maize production, utilization, and consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef]
- FAOSTAT. World Production Item Maize. 2018. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 18 December 2020).
- Thurner, S.; Fleschhut, M.; Eder, J. Verfahrenstechnik zum Einsatz von Körnermaisstroh in der Biogaserzeugung. In Ackerbau—Technische Lösungen für die Zukunft. Landtechnische Jahrestagung; Wendl, G., Ed.; Bavarian State Research Center for Agriculture: Freising, Germany, 2017; pp. 51–65. [Google Scholar]
- Glassner, D.; Hettenhaus, J.; Schechinger, T. Corn Stover Potential: Recasting the Corn Sweetener Industry. In Perspectives on new crops and new uses. In Proceedings of the Fourth National Symposium New Crops and New Uses, Biodiversity and Agricultural Sustainability, Phoenix, AZ, USA, 8–11 November 1998; Janick, J., Ed.; ASHS Press: Alexandria, VA, USA, 1999; pp. 74–82, ISBN 0-9615027-0-3. [Google Scholar]
- Eisenhuber, K.; Jäger, A.; Wimberger, J.; Kahr, H. Comparison of different pretreatment methods for straw for lignocellulosic bioethanol production. Agron. Res. 2013, 11, 173–182. [Google Scholar]
- Thrän, D.; Arendt, O.; Adwiraah, H.; Kaltschmitt, M. Nebenprodukte, Rückstände und Abfälle. In Energie aus Biomasse: Grundlagen, Techniken und Verfahren, 3rd ed.; Kaltschmitt, M., Hartmann, H., Hofbauer, H., Eds.; Springer Vieweg: Berlin/Heidelberg, Germany, 2016; pp. 273–323. ISBN 978-3-662-47437-2. [Google Scholar]
- Himmel, M.E.; Ding, S.-Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuel Bioprod. Biorefin. 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. Pretreatment: The key to unlocking low-cost cellulosic ethanol. Biofuel Bioprod. Biorefin. 2008, 2, 26–40. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.E.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part II: Fundamentals of different pre-treatments to increase the enzymatic digestibility of lignocellulose. Biofuel Bioprod. Biorefin. 2012, 6, 561–579. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Yang, B.; Dai, Z.; Ding, S.-Y.; Wyman, C.E. Enzymatic hydrolysis of cellulosic biomass. Biofuels 2011, 2, 421–449. [Google Scholar] [CrossRef]
- Maitan-Alfenas, G.P.; Visser, E.M.; Guimarães, V.M. Enzymatic hydrolysis of lignocellulosic biomass: Converting food waste in valuable products. Curr. Opin. Food Sci. 2015, 1, 44–49. [Google Scholar] [CrossRef]
- Cok, B.; Tsiropoulos, I.; Roes, A.L.; Patel, M.K. Succinic acid production derived from carbohydrates: An energy and greenhouse gas assessment of a platform chemical toward a bio-based economy. Biofuel Bioprod. Biorefin. 2014, 8, 16–29. [Google Scholar] [CrossRef]
- Takkellapati, S.; Li, T.; Gonzalez, M.A. An Overview of Biorefinery Derived Platform Chemicals from a Cellulose and Hemicellulose Biorefinery. Clean Technol. Environ. Policy 2018, 20, 1615–1630. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G.R.; Aden, A.; Bozell, J.J.; Holladay, J.; White, J.; Manheim, A.; Eliot, D.; Lasure, L.; Jones, S.; et al. Top Value Added Chemicals from Biomass. Volume I: Results of Screening for Potential Candidates from Sugars and Synthesis Gas; US Department of Energy: Washington, DC, USA, 2004.
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Choi, S.; Song, C.W.; Shin, J.H.; Lee, S.Y. Biorefineries for the production of top building block chemicals and their derivatives. Metab. Eng. 2015, 28, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Schober, C.M. Untersuchungen Zum Einsatz Technischer und Mikrobiell Hergestellter Enzymsysteme zur Hydrolyse der Lignocellulose in Maissilage. Ph.D. Thesis, Universität Hohenheim, Hohenjeim, Stuttgart, Germany, 2008. [Google Scholar]
- Thomsen, M.H.; Holm-Nielsen, J.B.; Oleskowicz-Popiel, P.; Thomsen, A.B. Pretreatment of whole-crop harvested, ensiled maize for ethanol production. Appl. Biochem. Biotech. 2008, 148, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Koponen, H. Biogas Production from Ensiled Maize with and without Hydrothermal Pretreatment. Master’s Thesis, University of Jyväskylä, Jyväskylä, Finland, 2010. [Google Scholar]
- Galbe, M.; Zacchi, G. A review of the production of ethanol from softwood. Appl. Microbiol. Biotech. 2002, 59, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Capolupo, L.; Faraco, V. Green methods of lignocellulose pretreatment for biorefinery development. Appl. Microbiol. Biotech. 2016, 100, 9451–9467. [Google Scholar] [CrossRef]
- del Río, P.G.; Gullón, P.; Rebelo, F.R.; Romaní, A.; Garrote, G.; Gullón, B. A Whole-Slurry Fermentation Approach to High-Solid Loading for Bioethanol Production from Corn Stover. Agronomy 2020, 10, 1790. [Google Scholar] [CrossRef]
- Klupsch, R. Untersuchungen zur Herstellung von Chemiezellsto aus Aspen-und Buchenholz nach dem Dampfdruck-Extraktionsverfahren. Ph.D. Thesis, University of Hamburg, Hamburg, Germany, 2000. [Google Scholar]
- Klupsch, R.; Kordsachia, O.; Puls, J.; Karstens, T. Herstellung von Chemiezellstoffen nach dem Dampfdruck-Extraktionsverfahren. Ipw Int. Pap. Das Pap. 2001, 55, 73–79. [Google Scholar]
- Schütt, F.; Haas, N.P.; Dehne, L.; Koch, G.; Janzon, R.; Saake, B. Steam pretreatment for enzymatic hydrolysis of poplar wood: Comparison of optimal conditions with and without SO2 impregnation. Holzforschung 2013, 67, 9–17. [Google Scholar] [CrossRef]
- Schütt, F.; Puls, J.; Saake, B. Optimization of steam pretreatment conditions for enzymatic hydrolysis of poplar wood. Holzforschung 2011, 65, 453–459. [Google Scholar] [CrossRef]
- Krafft, M.J.; Bendler, M.; Schreiber, A.; Saake, B. Steam Refining with Subsequent Alkaline Lignin Extraction as an Alternative Pretreatment Method to Enhance the Enzymatic Digestibility of Corn Stover. Agronomy 2020, 10, 811. [Google Scholar] [CrossRef]
- Hagel, S.; Saake, B. Fractionation of Waste MDF by Steam Refining. Molecules 2020, 25, 2165. [Google Scholar] [CrossRef]
- Janzon, R.; Schütt, F.; Oldenburg, S.; Fischer, E.; Körner, I.; Saake, B. Steam pretreatment of spruce forest residues: Optimal conditions for biogas production and enzymatic hydrolysis. Carbohydr. Polym. 2014, 100, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Stücker, A.; Schütt, F.; Saake, B.; Lehnen, R. Lignins from enzymatic hydrolysis and alkaline extraction of steam refined poplar wood: Utilization in lignin-phenol-formaldehyde resins. Ind. Crop. Prod. 2016, 85, 300–308. [Google Scholar] [CrossRef]
- Schütt, F.; Westereng, B.; Horn, S.J.; Puls, J.; Saake, B. Steam refining as an alternative to steam explosion. Bioresour. Technol. 2012, 111, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, F.; Strømman, A.H. Chemicals from lignocellulosic biomass: Opportunities, perspectives, and potential of biorefinery systems. Biofuel Bioprod. Biorefin. 2011, 5, 548–561. [Google Scholar] [CrossRef]
- Giuliano, A.; Poletto, M.; Barletta, D. Process optimization of a multi-product biorefinery: The effect of biomass seasonality. Chem. Eng. Res. Des. 2016, 107, 236–252. [Google Scholar] [CrossRef]
- Miao, Z.; Shastri, Y.; Grift, T.E.; Hansen, A.C.; Ting, K.C. Lignocellulosic biomass feedstock transportation alternatives, logistics, equipment configurations, and modeling. Biofuel Bioprod. Biorefin. 2012, 6, 351–362. [Google Scholar] [CrossRef]
- Grisso, R.D.; McCullough, D.; Cundiff, J.S.; Judd, J.D. Harvest schedule to fill storage for year-round delivery of grasses to biorefinery. Biomass Bioenerg. 2013, 55, 331–338. [Google Scholar] [CrossRef]
- Sahoo, K.; Mani, S. Techno-economic assessment of biomass bales storage systems for a large-scale biorefinery. Biofuel Bioprod Biorefin. 2017, 11, 417–429. [Google Scholar] [CrossRef]
- Tanjore, D.; Richard, T.L.; Marshall, M.N. Experimental methods for laboratory-scale ensilage of lignocellulosic biomass. Biomass Bioenerg. 2012, 47, 125–133. [Google Scholar] [CrossRef]
- Haag, N.L.; Grumaz, C.; Wiese, F.; Kirstahler, P.; Merkle, W.; Nägele, H.-J.; Sohn, K.; Jungbluth, T.; Oechsner, H. Advanced green biorefining: Effects of ensiling treatments on lactic acid production, microbial activity and supplementary methane formation of grass and rye. Biomass Conv. Biorefin. 2016, 6, 197–208. [Google Scholar] [CrossRef]
- Haag, N.L.; Nägele, H.-J.; Fritz, T.; Oechsner, H. Effects of ensiling treatments on lactic acid production and supplementary methane formation of maize and amaranth-an advanced green biorefining approach. Bioresour. Technol. 2015, 178, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Vervaeren, H.; Hostyn, K.; Ghekiere, G.; Willems, B. Biological ensilage additives as pretreatment for maize to increase the biogas production. Renew. Energ. 2010, 35, 2089–2093. [Google Scholar] [CrossRef]
- Russell, J.R. Influence of harvest date on the nutritive value and ensiling characteristics of maize stover. Anim. Feed Sci. Tech. 1986, 14, 11–27. [Google Scholar] [CrossRef]
- Oleskowicz-Popiel, P.; Thomsen, A.B.; Schmidt, J.E. Ensiling—Wet-storage method for lignocellulosic biomass for bioethanol production. Biomass Bioenerg. 2011, 35, 2087–2092. [Google Scholar] [CrossRef]
- Arrigo, Y. Silomais anreichern: Mehr Energie aber Verschwendung von Biomasse: Merkblatt für die Praxis. ALP aktuell 2012, 45, 1–4. [Google Scholar]
- Daccord, R.; Arrigo, Y.; Vogel, R. Nährwert von Maissilage. Agrarforschung 1995, 2, 397–400. [Google Scholar]
- Arrigo, Y.; Stoll, P. Schätzung des Nährwerts von Maissilage. Agrar. Schweiz 2012, 3, 442–449. [Google Scholar]
- Bruni, E.; Jensen, A.P.; Pedersen, E.S.; Angelidaki, I. Anaerobic digestion of maize focusing on variety, harvest time and pretreatment. Appl. Energ. 2010, 87, 2212–2217. [Google Scholar] [CrossRef]
- Xu, J.; Thomsen, M.H.; Thomsen, A.B. Feasibility of hydrothermal pretreatment on maize silage for bioethanol production. Appl. Biochem. Biotech. 2010, 162, 33–42. [Google Scholar] [CrossRef]
- Deutschle, A.L. Charakterisierung und Anwendung von Kationischen Arabinoxylanen. Ph.D. Thesis, University of Hamburg, Hamburg, Germany, 2015. [Google Scholar]
- Baar, J.; Paschová, Z.; Hofmann, T.; Kolář, T.; Koch, G.; Saake, B.; Rademacher, P. Natural durability of subfossil oak: Wood chemical composition changes through the ages. Holzforschung 2019, 74, 47–59. [Google Scholar] [CrossRef]
- Hames, B.R. Biomass compositional analysis for energy applications. Methods Mol. Biol. 2009, 581, 145–167. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, C.K.; Qin, F.; Mussatto, S.I. Advances and opportunities in biomass conversion technologies and biorefineries for the development of a bio-based economy. Biomass Bioenerg. 2018, 119, 54–60. [Google Scholar] [CrossRef]
- Mosier, N.S.; Hendrickson, R.; Brewer, M.; Ho, N.; Sedlak, M.; Dreshel, R.; Welch, G.; Dien, B.S.; Aden, A.; Ladisch, M.R. Industrial Scale-Up of pH-Controlled Liquid Hot Water Pretreatment of Corn Fiber for Fuel Ethanol Production. Appl. Biochem. Biotech. 2005, 125, 77–98. [Google Scholar] [CrossRef]
- Lynd, L.R.; Elander, R.T.; Wyman, C.E. Likely Features and Costs of Mature Biomass Ethanol Technology. In Seventeenth Symposium on Biotechnology for Fuels and Chemicals; Wyman, C.E., Davison, B.H., Eds.; Humana Press: Totowa, NJ, USA, 1996; pp. 741–761. ISBN 978-1-4612-6669-3. [Google Scholar]
- Frey, O. Analytik und Bilanzierung von Mais-Hydrolysaten. Master’s Thesis, University of Hamburg, Hamburg, Germany, 2020. [Google Scholar]
- Palmqvist, E.; Hahn-Hägerdal, B.; Galbe, M.; Zacchi, G. The effect of water-soluble inhibitors from steam-pretreated willow on enzymatic hydrolysis and ethanol fermentation. Enzyme Microb. Tech. 1996, 19, 470–476. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Gurram, R.N.; Datta, S.; Lin, Y.J.; Snyder, S.W.; Menkhaus, T.J. Removal of enzymatic and fermentation inhibitory compounds from biomass slurries for enhanced biorefinery process efficiencies. Bioresour. Technol. 2011, 102, 7850–7859. [Google Scholar] [CrossRef]
- Martín, C.; Galbe, M.; Nilvebrant, N.-O.; Jönsson, L.J. Comparison of the Fermentability of Enzymatic Hydrolyzates of Sugarcane Bagasse Pretreated by Steam Explosion Using Different Impregnating Agents. Appl. Biochem. Biotech. 2002, 98–100, 699–716. [Google Scholar] [CrossRef]
- Han, G.; Deng, J.; Zhang, S.; Bicho, P.; Wu, Q. Effect of steam explosion treatment on characteristics of wheat straw. Ind. Crops Prod. 2010, 31, 28–33. [Google Scholar] [CrossRef]
- Jacquet, N.; Quiévy, N.; Vanderghem, C.; Janas, S.; Blecker, C.; Wathelet, B.; Devaux, J.; Paquot, M. Influence of steam explosion on the thermal stability of cellulose fibres. Polym. Degrad. Stabil. 2011, 96, 1582–1588. [Google Scholar] [CrossRef]
- Kaar, W.E.; Gutierrez, C.V.; Kinoshita, C.M. Steam explosion of sugarcane bagasse as a pretreatment for conversion to ethanol. Biomass Bioenerg. 1998, 14, 277–287. [Google Scholar] [CrossRef]
- Li, H.; Chen, H. Detoxification of steam-exploded corn straw produced by an industrial-scale reactor. Process. Biochem. 2008, 43, 1447–1451. [Google Scholar] [CrossRef]
- Ruiz, E.; Cara, C.; Manzanares, P.; Ballesteros, M.; Castro, E. Evaluation of steam explosion pre-treatment for enzymatic hydrolysis of sunflower stalks. Enzyme Microb. Tech. 2008, 42, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Garrote, G.; Domínguez, H.; Parajó, J.C. Manufacture of Xylose-Based Fermentation Media from Corncobs by Posthydrolysis of Autohydrolysis Liquors. Appl. Biochem. Biotech. 2001, 95, 195–208. [Google Scholar] [CrossRef]
- Schütt, F. Dampfdruckaufschluss und enzymatische Hydrolyse von Pappelholz. Ph.D. Thesis, University of Hamburg, Hamburg, Germany, 2012. [Google Scholar]
- Zubr, J. Methanogenic fermentation of fresh and ensiled plant materials. Biomass 1986, 11, 159–171. [Google Scholar] [CrossRef]
- Sørensen, J.L.; Nielsen, K.F.; Rasmussen, P.H.; Thrane, U. Development of a LC-MS/MS method for the analysis of enniatins and beauvericin in whole fresh and ensiled maize. J. Agric. Food Chem. 2008, 56, 10439–10443. [Google Scholar] [CrossRef]
- Stelte, W. Steam Explosion for Biomass Pre-Treatment; Resultat Kontrakt (RK) Report; Danish Technological Institute: Taastrup, Danemark, 2013. [Google Scholar]
- Anastas, P.T.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Anastas, P.T.; Kirchhoff, M.M. Origins, current status, and future challenges of green chemistry. Acc. Chem. Res. 2002, 35, 686–694. [Google Scholar] [CrossRef]
- Tang, S.L.Y.; Smith, R.L.; Poliakoff, M. Principles of green chemistry: PRODUCTIVELY. Green Chem. 2005, 7, 761. [Google Scholar] [CrossRef]
- Tang, S.L.Y.; Bourne, R.A.; Smith, R.L.; Poliakoff, M. The 24 Principles of Green Engineering and Green Chemistry: “IMPROVEMENTS PRODUCTIVELY”. Green Chem. 2008, 10, 268. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- He, M.; Sun, Y.; Han, B. Green carbon science: Scientific basis for integrating carbon resource processing, utilization, and recycling. Angew. Chem. Int. Ed. 2013, 52, 9620–9633. [Google Scholar] [CrossRef]
- Beltrame, P.L.; Carniti, P.; Visciglio, A.; Focher, B.; Marzetti, A. Fractionation and bioconversion of steam-exploded wheat straw. Bioresour. Technol. 1992, 39, 165–171. [Google Scholar] [CrossRef]
- Turn, S.Q.; Kinoshita, C.M.; Kaar, W.E.; Ishimura, D.M. Measurements of gas phase carbon in steam explosion of biomass. Bioresour. Technol. 1998, 64, 71–75. [Google Scholar] [CrossRef]
- Bendler, M. Dampfdruck-Refiner-Aufschluss und enzymatische Hydrolyse von Maisstroh. Bachelor’s Thesis, Hamburg University of Applied Sciences, Hamburg, Germany, 2018. [Google Scholar]
- Grimm, K. Techniques of whole crop harvesting and whole crop silage in cattle farming. Landtechnik 1983, 38, 138–140. [Google Scholar]
- Lorenz, D.; Erasmy, N.; Akil, Y.; Saake, B. A new method for the quantification of monosaccharides, uronic acids and oligosaccharides in partially hydrolyzed xylans by HPAEC-UV/VIS. Carbohydr. Polym. 2016, 140, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Overend, R.P.; Chornet, E.; Gascoigne, J.A. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. A 1987, 321, 523–536. [Google Scholar] [CrossRef]
- Herrmann, C.; Heiermann, M.; Idler, C. Effects of ensiling, silage additives and storage period on methane formation of biogas crops. Bioresour. Technol. 2011, 102, 5153–5161. [Google Scholar] [CrossRef]
- Misthilger, B.; Schneider, M.; Harms, K.; Burger, T.; Thurner, S. Silierung von Körnermaisstroh. In Körnermaisstroh als Biogassubstrat; Bavarian State Research Center for Agriculture: Poing/Freising, Germany, 2019. [Google Scholar]



| Raw Material % (w/w) | |
|---|---|
| Stalks | 15.8 |
| Kernels | 17.7 |
| Cobs | 14.0 |
| Leaves and Husks | 23.8 |
| Fines (≤ 4 mm) | 28.7 |
| Non-Extracted | Extracted | Starch Determination | ||
|---|---|---|---|---|
| % (w/w) | ||||
| Extraction | Petrol ether | n.d. | 2.5 | n.d. |
| Acetone/Water (9:1) | 6.6 | |||
| Hot water | 15.6 | |||
| ∑ | 24.7 | |||
| Starch | n.d. | n.d. | 38.6 | |
| Carbohydrates | Glucose | 51.9 | 43.8 | 18.3 |
| Xylose | 12.3 | 13.7 | 11.0 | |
| Arabinose | 2.2 | 2.5 | 2.0 | |
| Galactose | 0.8 | 0.8 | 0.6 | |
| Mannose | 0.2 | 0.3 | 0.1 | |
| Rhamnose | 0.1 | 0.1 | 0.1 | |
| ∑ | 66.0 | 61.2 | 70.7 | |
| Lignin | acid-insoluble | 11.1 | 9.3 | 11.7 |
| acid-soluble | 3.2 | 2.6 | 1.5 | |
| ∑ | 14.3 | 11.9 | 13.2 | |
| Ash | 3.49 | 3.52 | n.d. | |
| Organic acids * | Lactic acid | 4.9 | n.d. | n.d. |
| Acetic acid | 0.9 | |||
| Elements | C | n.d. | 46.8 | n.d. |
| H | 6.1 | |||
| S ** | 0.6 | |||
| Fiber Fraction | Extract Fraction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| log R0 | Gluc | Xyl | Arab | Others * | Residue ** | Gluc | Xyl | Arab | Others * | Residue |
| [% w/w] | [% w/w] | |||||||||
| 1.59 | 21.4 | 9.7 | 1.5 | 0.7 | 12.4 | 28.6 | 0.7 | 0.3 | 0.2 | 1.5 |
| 1.76 | 22.7 | 11.3 | 1.8 | 0.7 | 12.3 | 30.0 | 0.7 | 0.3 | 0.2 | 2.3 |
| 1.88 | 20.2 | 10.5 | 1.7 | 0.7 | 12.2 | 30.2 | 0.7 | 0.3 | 0.2 | 1.6 |
| 2.06 | 20.1 | 10.6 | 1.6 | 0.6 | 12.4 | 31.0 | 0.8 | 0.5 | 0.3 | 3.3 |
| 2.18 | 19.4 | 9.5 | 1.4 | 0.6 | 12.4 | 30.9 | 0.8 | 0.5 | 0.3 | 1.5 |
| 2.35 | 17.6 | 8.8 | 1.2 | 0.6 | 12.1 | 33.9 | 0.9 | 0.6 | 0.2 | 1.7 |
| 2.47 | 19.3 | 10.1 | 1.3 | 0.6 | 11.4 | 32.9 | 1.3 | 0.9 | 0.3 | 1.3 |
| 2.65 | 19.6 | 10.5 | 1.2 | 0.5 | 11.4 | 34.2 | 1.3 | 0.8 | 0.4 | 2.5 |
| 2.77 | 19.5 | 9.8 | 1.0 | 0.5 | 12.0 | 34.2 | 1.9 | 1.1 | 0.3 | 1.3 |
| 2.94 | 17.1 | 7.7 | 0.7 | 0.4 | 11.2 | 34.7 | 2.4 | 1.0 | 0.4 | 2.5 |
| 3.07 | 17.4 | 7.5 | 0.6 | 0.3 | 10.9 | 35.7 | 3.4 | 1.2 | 0.6 | 0.6 |
| 3.06 | 17.3 | 7.0 | 0.5 | 0.3 | 11.0 | 32.0 | 3.7 | 1.2 | 0.4 | 1.8 |
| 3.24 | 17.0 | 6.2 | 0.4 | 0.3 | 9.8 | 35.1 | 4.7 | 1.2 | 0.8 | 3.2 |
| 3.36 | 16.9 | 4.8 | 0.2 | 0.2 | 9.3 | 36.4 | 6.7 | 1.5 | 0.8 | 3.9 |
| 3.53 | 16.4 | 3.7 | 0.2 | 0.2 | 9.8 | 34.0 | 6.6 | 1.1 | 0.9 | 2.8 |
| 3.65 | 16.7 | 3.3 | 0.2 | 0.1 | 9.4 | 37.8 | 7.0 | 1.3 | 0.8 | 4.2 |
| 3.83 | 16.4 | 2.4 | 0.1 | 0.1 | 9.9 | 34.1 | 6.9 | 1.0 | 1.0 | 2.9 |
| 3.94 | 16.0 | 1.8 | 0.1 | 0.1 | 9.3 | 33.9 | 6.4 | 1.0 | 0.8 | 5.2 |
| 4.12 | 16.7 | 1.6 | 0.1 | 0.1 | 11.5 | 30.6 | 5.1 | 0.7 | 0.8 | 0.9 |
| ∑ CH | Residue | ∑ Organic Acids | ∑ Furans | ∑ Carbon | |
|---|---|---|---|---|---|
| [%] Based on Raw Material | gC/100 g | ||||
| Extract | 46.9 | 4.2 | 6.17 | 0.15 | 32.0 |
| Fiber | 20.3 | 9.4 | - | - | 14.6 |
| ∑ | 67.2 | 13.6 | 6.17 | 0.15 | 46.6 |
| Raw Material | 70.7 | 13.2 | 5.8 | - | 46.8 |
| Run | Temperature | Time | Severity Factor | Run | Temperature | Time | Severity Factor |
|---|---|---|---|---|---|---|---|
| # | °C | min | log R0 | # | °C | min | log R0 |
| 1 | 0 | 0 | 0.00 | 11 | 160 | 15 | 2.94 |
| 2 | 120 | 10 | 1.59 | 12 | 160 | 20 | 3.07 |
| 3 | 120 | 15 | 1.76 | 13 | 170 | 10 | 3.06 |
| 4 | 130 | 10 | 1.88 | 14 | 170 | 15 | 3.24 |
| 5 | 130 | 15 | 2.06 | 15 | 180 | 10 | 3.36 |
| 6 | 140 | 10 | 2.18 | 16 | 180 | 15 | 3.53 |
| 7 | 140 | 15 | 2.35 | 17 | 190 | 10 | 3.65 |
| 8 | 150 | 10 | 2.47 | 18 | 190 | 15 | 3.83 |
| 9 | 150 | 15 | 2.65 | 19 | 200 | 10 | 3.94 |
| 10 | 160 | 10 | 2.77 | 20 | 200 | 15 | 4.12 |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krafft, M.J.; Frey, O.; Schwarz, K.U.; Saake, B. Maize Silage Pretreatment via Steam Refining and Subsequent Enzymatic Hydrolysis for the Production of Fermentable Carbohydrates. Molecules 2020, 25, 6022. https://doi.org/10.3390/molecules25246022
Krafft MJ, Frey O, Schwarz KU, Saake B. Maize Silage Pretreatment via Steam Refining and Subsequent Enzymatic Hydrolysis for the Production of Fermentable Carbohydrates. Molecules. 2020; 25(24):6022. https://doi.org/10.3390/molecules25246022
Chicago/Turabian StyleKrafft, Malte Jörn, Olga Frey, Katrin U. Schwarz, and Bodo Saake. 2020. "Maize Silage Pretreatment via Steam Refining and Subsequent Enzymatic Hydrolysis for the Production of Fermentable Carbohydrates" Molecules 25, no. 24: 6022. https://doi.org/10.3390/molecules25246022
APA StyleKrafft, M. J., Frey, O., Schwarz, K. U., & Saake, B. (2020). Maize Silage Pretreatment via Steam Refining and Subsequent Enzymatic Hydrolysis for the Production of Fermentable Carbohydrates. Molecules, 25(24), 6022. https://doi.org/10.3390/molecules25246022