Versatile Nutraceutical Potentials of Watermelon—A Modest Fruit Loaded with Pharmaceutically Valuable Phytochemicals
Abstract
1. Introduction
2. Cardiovascular Protection by Watermelon
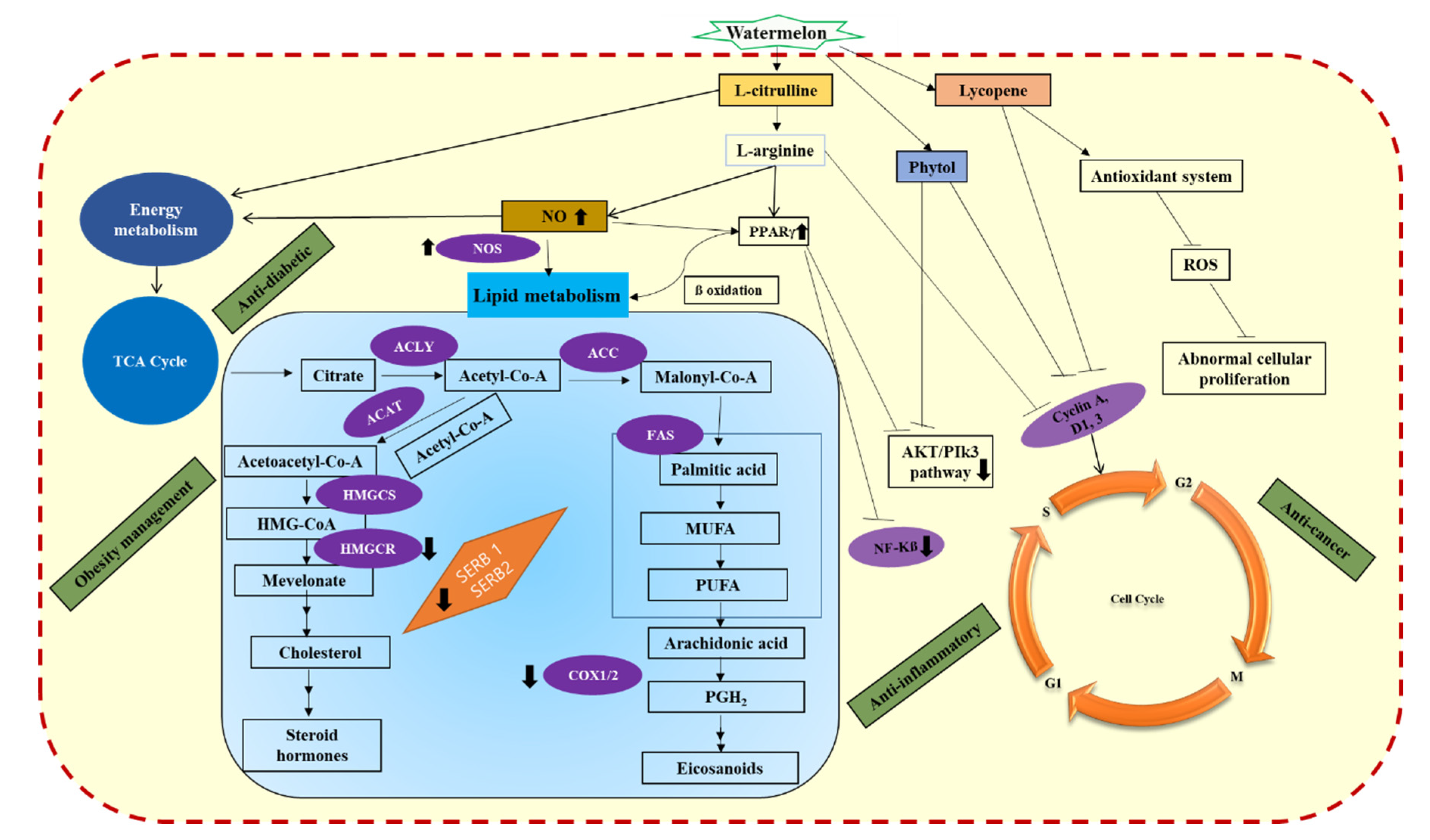
3. Watermelon as a Functional Food in Obesity Management and Anti-Diabetic Snack
4. Anti-Ulcerative Colitis Property of Watermelon
5. Anti-Oxidant Properties of Watermelon
6. Anticancer Properties of Watermelon
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Assefa, A.D.; Hur, O.S.; Ro, N.Y.; Lee, J.E.; Hwang, A.J.; Kim, B.S.; Rhee, J.H.; Yi, J.Y.; Kim, J.H.; Lee, H.S.; et al. Fruit Morphology, Citrulline, and Arginine Levels in Diverse Watermelon (Citrullus lanatus) Germplasm Collections. Plants 2020, 9, 1054. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.; Davis, A.; Collins, J.K. Watermelon: From dessert to functional food. Isr. J. Plant Sci. 2012, 60, 395–402. [Google Scholar]
- U.S. Department of Agriculture ARS. USDA National Nutrient Database for Standard Reference, Release 27; Service, A.R., Ed.; Department of Agriculture: Washington, DC, USA, 2015.
- Rimando, A.M.; Perkins-Veazie, P.M. Determination of citrulline in watermelon rind. J. Chromatogr. A 2005, 1078, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Agarwal, S. Role of antioxidant lycopene in cancer and heart disease. J. Am. Coll. Nutr. 2000, 19, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.J.; Platt, D.H.; Caldwell, R.B.; Caldwell, R.W. Therapeutic use of citrulline in cardiovascular disease. Cardiovasc. Drug Rev. 2006, 24, 275–290. [Google Scholar] [CrossRef]
- Tlili, I.; Hdider, C.; Lenucci, M.S.; Riadh, I.; Jebari, H.; Dalessandro, G. Bioactive compounds and antioxidant activities of different watermelon (Citrullus lanatus (Thunb.) Mansfeld) cultivars as affected by fruit sampling area. J. Food Compos. Anal. 2011, 24, 307–314. [Google Scholar] [CrossRef]
- Tomes, M.L.; Johnson, K.W.; Hess, M. The carotene pigment content of certain red fleshed watermelons. Inproc. Am. Soc. Hortic. Sci. 1963, 82, 460–464. [Google Scholar]
- Wan, X.; Liu, W.; Yan, Z.; Zhao, S.; He, N.; Liu, P. Changes of the contents of functional substances including lycopene, citrulline and ascorbic acid during watermelon fruits development. Sci. Agric. Sin. 2011, 44, 2738–2747. [Google Scholar]
- Tarazona-Díaz, M.P.; Viegas, J.; Moldao-Martins, M.; Aguayo, E. Bioactive compounds from flesh and by-product of fresh-cut watermelon cultivars. J. Sci. Food Agric. 2011, 91, 805–812. [Google Scholar] [CrossRef]
- Wada, M. Uber Citrullin, eine neue Aminosaure im PreBsaft der Wassermelone, Citrullus vulgaris schrad. Biochemische Zeitschrift 1930, 224, 420–429. [Google Scholar]
- Wu, G.; Collins, J.K.; Perkins-Veazie, P.; Siddiq, M.; Dolan, K.D.; Kelly, K.A.; Heaps, C.L.; Meininger, C.J. Dietary supplementation with watermelon pomace juice enhances arginine availability and ameliorates the metabolic syndrome in Zucker diabetic fatty rats. J. Nutr. 2007, 137, 2680–2685. [Google Scholar] [CrossRef] [PubMed]
- Yokota, A.; Kawasaki, S.; Iwano, M.; Nakamura, C.; Miyake, C.; Akashi, K. Citrulline and DRIP-1 protein (ArgE homologue) in drought tolerance of wild watermelon. Ann. Bot. 2002, 89, 825–832. [Google Scholar] [CrossRef] [PubMed]
- El-Adawy, T.A.; Taha, K.M. Characteristics and composition of different seed oils and flours. Food Chem. 2001, 74, 47–54. [Google Scholar] [CrossRef]
- Stafford, W.; Oke, O.L. Protein isolate from lesser known oilseeds from Nigeria. Nutr. Rep. Int. 1977, 16, 813–820. [Google Scholar]
- King, R.D.; Onuora, J.O. Aspects of melon seed protein characteristics. Food Chem. 1984, 14, 65–77. [Google Scholar] [CrossRef]
- Kaul, P. Nutritional potential, bioaccessibility of minerals and functionality of watermelon (Citrullus vulgaris) seeds. LWT-Food Sci. Technol. 2011, 44, 1821–1826. [Google Scholar]
- Sola, A.O.; Temitayo, O.O.; Olufunke, A.; Shittu, F. Chemical composition, nutritional values and antibacterial activities of watermelon seed (Citrullus lanatus). Int. J. Biochem. Res. Rev. 2019, 27. [Google Scholar] [CrossRef]
- Suliburska, J.; Bogdanski, P.; Krejpcio, Z.; Pupek-Musialik, D.; Jablecka, A. The effects of l-arginine, alone and combined with vitamin C, on mineral status in relation to its antidiabetic, anti-inflammatory, and antioxidant properties in male rats on a high-fat diet. Biol. Trace Elem. Res. 2014, 157, 67–74. [Google Scholar] [CrossRef]
- Alam, M.A.; Kauter, K.; Withers, K.; Sernia, C.; Brown, L. Chronic l-arginine treatment improves metabolic, cardiovascular and liver complications in diet-induced obesity in rats. Food Funct. 2013, 4, 83–91. [Google Scholar] [CrossRef]
- Evans, R.W.; Fernstrom, J.D.; Thompson, J.; Morris, S.M., Jr.; Kuller, L.H. Biochemical responses of healthy subjects during dietary supplementation with l-arginine. J. Nutr. Biochem. 2004, 15, 534–539. [Google Scholar] [CrossRef]
- Wu, G.; Meininger, C.J. Arginine nutrition and cardiovascular function. J. Nutr. 2000, 130, 2626–2629. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.Y.; Hartig, N.; Kaufman, K.; Hooshmand, S.; Figueroa, A.; Kern, M. Watermelon consumption improves inflammation and antioxidant capacity in rats fed an atherogenic diet. Nutr. Res. 2015, 35, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Jobgen, W.S.; Fried, S.K.; Fu, W.J.; Meininger, C.J.; Wu, G. Regulatory role for the arginine–nitric oxide pathway in metabolism of energy substrates. J. Nutr. Biochem. 2006, 17, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Jobgen, W.; Fu, W.J.; Gao, H.; Li, P.; Meininger, C.J.; Smith, S.B.; Spencer, T.E.; Wu, G. High fat feeding and dietary l-arginine supplementation differentially regulate gene expression in rat white adipose tissue. Amino Acids 2009, 37, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Jensen-Urstad, A.P.; Semenkovich, C.F. Fatty acid synthase and liver triglyceride metabolism: Housekeeper or messenger? Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2012, 1821, 747–753. [Google Scholar] [CrossRef]
- Baenke, F.; Peck, B.; Miess, H.; Schulze, A. Hooked on fat: The role of lipid synthesis in cancer metabolism and tumour development. Dis. Models Mech. 2013, 6, 1353–1363. [Google Scholar] [CrossRef]
- Iantorno, M.; Campia, U.; Di Daniele, N.; Nistico, S.; Forleo, G.B.; Cardillo, C.; Tesauro, M. Obesity, inflammation and endothelial dysfunction. J. Biol. Regul. Homeost. Agents 2014, 28, 169–176. [Google Scholar]
- Zaiss, A.K.; Zuber, J.; Chu, C.; Machado, H.B.; Jiao, J.; Catapang, A.B.; Ishikawa, T.O.; Gil, J.S.; Lowe, S.W.; Herschman, H.R. Reversible suppression of cyclooxygenase 2 (COX-2) expression in vivo by inducible RNA interference. PLoS ONE 2014, 9, e101263. [Google Scholar] [CrossRef]
- Hong, M.Y.; Tseng, Y.T.; Kalaba, M.; Beidler, J. Effects of watermelon powder supplementation on colitis in high-fat diet-fed and dextran sodium sulfate-treated rats. J. Funct. Foods 2019, 54, 520–528. [Google Scholar] [CrossRef]
- Shanely, R.A.; Zwetsloot, J.J.; Jurrissen, T.J.; Hannan, L.C.; Zwetsloot, K.A.; Needle, A.R.; Bishop, A.E.; Wu, G.; Perkins-Veazie, P. Daily watermelon consumption decreases plasma sVCAM-1 levels in overweight and obese postmenopausal women. Nutr. Res. 2020, 76, 9–19. [Google Scholar] [CrossRef]
- Connolly, M.; Lum, T.; Marx, A.; Hooshmand, S.; Kern, M.; Liu, C.; Hong, M.Y. Effect of Fresh Watermelon Consumption on Risk Factors for Cardiovascular Disease in Overweight and Obese Adults (P06-102-19). Curr. Dev. Nutr. 2019, 3. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention. National Diabetes Statistics Report; Centers for disease control and prevention, US Department of Health and Human Services: Atlanta, GA, USA, 2020.
- Johnson, J.A.; Pohar, S.L.; Majumdar, S.R. Health care use and costs in the decade after identification of type 1 and type 2 diabetes: A population-based study. Diabetes Care 2006, 29, 2403–2408. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R.; Buse, J.; Ferrannini, E.; Stern, M. The metabolic syndrome: Time for a critical appraisal. Diabetologia 2005, 148, 1684–1699. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Matthews, D.R.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Response to Comments on Inzucchi et al. Management of Hyperglycemia in Type 2 Diabetes, 2015: A Patient-Centered Approach. Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2005, 28, 2289–2304. [Google Scholar]
- Marliss, E.B.; Chevalier, S.; Gougeon, R.; Morais, J.A.; Lamarche, M.; Adegoke, O.A.; Wu, G. Elevations of plasma methylarginines in obesity and ageing are related to insulin sensitivity and rates of protein turnover. Diabetologia 2006, 49, 351–359. [Google Scholar] [CrossRef]
- Pieper, G.M. Review of alterations in endothelial nitric oxide production in diabetes: Protective role of arginine on endothelial dysfunction. Hypertension 1998, 31, 1047–1060. [Google Scholar] [CrossRef]
- Míguez, L.; Marino, G.; Rodriguez, B.; Taboada, C. Effects of dietary l-arginine supplementation on serum lipids and intestinal enzyme activities in diabetic rats. J. Physiol. Biochem. 2004, 60, 31–37. [Google Scholar] [CrossRef]
- Mendez, J.D.; Balderas, F. Regulation of hyperglycemia and dyslipidemia by exogenous l-arginine in diabetic rats. Biochimie 2001, 83, 453–458. [Google Scholar] [CrossRef]
- Kohli, R.; Meininger, C.J.; Haynes, T.E.; Yan, W.; Self, J.T.; Wu, G. Dietary l-arginine supplementation enhances endothelial nitric oxide synthesis in streptozotocin-induced diabetic rats. J. Nutr. 2004, 134, 600–608. [Google Scholar] [CrossRef]
- Fu, W.J.; Haynes, T.E.; Kohli, R.; Hu, J.; Shi, W.; Spencer, T.E.; Carroll, R.J.; Meininger, C.J.; Wu, G. Dietary l-arginine supplementation reduces fat mass in Zucker diabetic fatty rats. J. Nutr. 2005, 135, 714–721. [Google Scholar] [CrossRef]
- Popov, D.; Costache, G.; Georgescu, A.; Enache, M. Beneficial effects of l-arginine supplementation in experimental hyperlipemia-hyperglycemia in the hamster. Cell Tissue Res. 2002, 308, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Creager, M.A.; Gallagher, S.J.; Girerd, X.J.; Coleman, S.M.; Dzau, V.J.; Cooke, J.P. l-arginine improves endothelium-dependent vasodilation in hypercholesterolemic humans. J. Clin. Investig. 1992, 90, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Sanchez-Gonzalez, M.A.; Perkins-Veazie, P.M.; Arjmandi, B.H. Effects of watermelon supplementation on aortic blood pressure and wave reflection in individuals with prehypertension: A pilot study. Am. J. Hypertens. 2011, 24, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Lum, T.; Connolly, M.; Marx, A.; Beidler, J.; Hooshmand, S.; Kern, M.; Liu, C.; Hong, M.Y. Effects of fresh watermelon consumption on the acute satiety response and cardiometabolic risk factors in overweight and obese adults. Nutrients 2019, 11, 595. [Google Scholar] [CrossRef]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Ballet, V.; Claes, K.; Verhaegen, J.; Van Assche, G.; Rutgeerts, P.J.; et al. 187 Bacterial Dysbiosis in Ulcerative Colitis Patients Differs From Crohn’s Disease Patients. Gastroenterology 2012, 142, S-46. [Google Scholar] [CrossRef]
- Danese, S.; Fiocchi, C. Ulcerative colitis. N. Engl. J. Med. 2011, 365, 1713–1725. [Google Scholar] [CrossRef]
- Clapper, M.L.; Cooper, H.S.; Chang, W.-C.L. Dextran sulfate sodium-induced colitis-associated neoplasia: A promising model for the development of chemopreventive interventions 1. Acta Pharm. Sin. 2007, 28, 1450–1459. [Google Scholar] [CrossRef]
- Ghosh, S.; Mitchell, R. Impact of inflammatory bowel disease on quality of life: Results of the European Federation of Crohn’s and Ulcerative Colitis Associations (EFCCA) patient survey. J. Crohn’s Colitis 2007, 1, 10–20. [Google Scholar] [CrossRef]
- Hatoum, O.A.; Binion, D.G.; Otterson, M.F.; Gutterman, D.D. Acquired microvascular dysfunction in inflammatory bowel disease: Loss of nitric oxide-mediated vasodilation. Gastroenterology 2003, 125, 58–69. [Google Scholar] [CrossRef]
- Hong, S.K.; Maltz, B.E.; Coburn, L.A.; Slaughter, J.C.; Chaturvedi, R.; Schwartz, D.A.; Wilson, K.T. Increased serum levels of l-arginine in ulcerative colitis and correlation with disease severity. Inflamm. Bowel Dis. 2010, 16, 105–111. [Google Scholar] [CrossRef]
- Coburn, L.A.; Horst, S.N.; Allaman, M.M.; Brown, C.T.; Williams, C.S.; Hodges, M.E.; Druce, J.P.; Beaulieu, D.B.; Schwartz, D.A.; Wilson, K.T. l-arginine availability and metabolism is altered in ulcerative colitis. Inflamm. Bowel Dis. 2016, 22, 1847–1858. [Google Scholar] [CrossRef]
- Ren, W.; Yin, J.; Wu, M.; Liu, G.; Yang, G.; Xion, Y.; Su, D.; Wu, L.; Li, T.; Chen, S.; et al. Serum amino acids profile and the beneficial effects of l-arginine or L-glutamine supplementation in dextran sulfate sodium colitis. PLoS ONE 2014, 9, e88335. [Google Scholar] [CrossRef]
- Coburn, L.A.; Gong, X.; Singh, K.; Asim, M.; Scull, B.P.; Allaman, M.M.; Williams, C.S.; Rosen, M.J.; Washington, M.K.; Barry, D.P.; et al. l-arginine supplementation improves responses to injury and inflammation in dextran sulfate sodium colitis. PLoS ONE 2012, 7, e33546. [Google Scholar] [CrossRef]
- Ptasinska, A.; Wang, S.; Zhang, J.; Wesley, R.A.; Danner, R.L. Nitric oxide activation of peroxisome proliferator-activated receptor gamma through a p38 MAPK signaling pathway. FASEB J. 2007, 21, 950–961. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Hou, Y.; Zhu, H.; Zhao, S.; Ding, B.; Yin, Y.; Yi, G.; Shi, J.; Fan, W. Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Br. J. Nutr. 2008, 100, 552–560. [Google Scholar] [CrossRef]
- Cai, W.; Yang, T.; Liu, H.; Han, L.; Zhang, K.; Hu, X.; Zhang, X.; Yin, K.J.; Gao, Y.; Bennett, M.V.; et al. Peroxisome proliferator-activated receptor γ (PPARγ): A master gatekeeper in CNS injury and repair. Prog. Neurobiol. 2018, 163, 27–58. [Google Scholar] [CrossRef]
- Hulit, J.; Wang, C.; Li, Z.; Albanese, C.; Rao, M.; Di Vizio, D.; Shah, S.; Byers, S.W.; Mahmood, R.; Augenlicht, L.H.; et al. Cyclin D1 genetic heterozygosity regulates colonic epithelial cell differentiation and tumor number in ApcMin mice. Mol. Cell. Biol. 2004, 24, 7598–7611. [Google Scholar] [CrossRef]
- Warboys, C.M.; Chen, N.; Zhang, Q.; Shaifta, Y.; Vanderslott, G.; Passacquale, G.; Hu, Y.; Xu, Q.; Ward, J.P.; Ferro, A. Bidirectional cross-regulation between the endothelial nitric oxide synthase and β-catenin signalling pathways. Cardiovasc. Res. 2014, 104, 116–126. [Google Scholar] [CrossRef]
- Roessner, A.; Kuester, D.; Malfertheiner, P.; Schneider-Stock, R. Oxidative stress in ulcerative colitis-associated carcinogenesis. Pathol. Res. Pract. 2008, 204, 511–524. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Yasui, M.; Kanemaru, Y.; Kamoshita, N.; Suzuki, T.; Arakawa, T.; Honma, M. Tracing the fates of site-specifically introduced DNA adducts in the human genome. DNA Repair 2014, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ijah, U.J.; Ayodele, H.S.; Aransiola, S.A. Microbiological and some sensory attributes of water melon juice and watermelon-orange juice mix. J. Food Resour. Sci. 2015, 4, 49–61. [Google Scholar]
- Luckner, M. Secondary Metabolism in Microorganisms, Plants and Animals; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Rhodes, M.J. Physiological roles for secondary metabolites in plants: Some progress, many outstanding problems. Plant Mol. Biol. 1994, 24, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kulczyński, B.; Gramza-Michałowska, A.; Kobus-Cisowska, J.; Kmiecik, D. The role of carotenoids in the prevention and treatment of cardiovascular disease–Current state of knowledge. J. Funct. Foods 2017, 38, 45–65. [Google Scholar] [CrossRef]
- Lemos, Á.T.; Ribeiro, A.C.; Fidalgo, L.G.; Delgadillo, I.; Saraiva, J.A. Extension of raw watermelon juice shelf-life up to 58 days by hyperbaric storage. Food Chem. 2017, 231, 61–69. [Google Scholar] [CrossRef]
- Kehili, M.; Kammlott, M.; Choura, S.; Zammel, A.; Zetzl, C.; Smirnova, I.; Allouche, N.; Sayadi, S. Supercritical CO2 extraction and antioxidant activity of lycopene and β-carotene-enriched oleoresin from tomato (Lycopersicum esculentum L.) peels by-product of a Tunisian industry. Food Bioprod. Process. 2017, 102, 340–349. [Google Scholar] [CrossRef]
- Oberoi, D.P.; Sogi, D.S. Utilization of watermelon pulp for lycopene extraction by response surface methodology. Food Chem. 2017, 232, 316–321. [Google Scholar] [CrossRef]
- Naz, A.; Butt, M.S.; Sultan, M.T.; Qayyum, M.M.; Niaz, R.S. Watermelon lycopene and allied health claims. EXCLI J. 2014, 13, 650. [Google Scholar]
- Kyriacou, M.C.; Leskovar, D.I.; Colla, G.; Rouphael, Y. Watermelon and melon fruit quality: The genotypic and agro-environmental factors implicated. Sci. Hortic. 2018, 234, 393–408. [Google Scholar] [CrossRef]
- Soteriou, G.A.; Kyriacou, M.C.; Siomos, A.S.; Gerasopoulos, D. Evolution of watermelon fruit physicochemical and phytochemical composition during ripening as affected by grafting. Food Chem. 2014, 165, 282–289. [Google Scholar] [CrossRef]
- Oberoi, D.P.; Sogi, D.S. Prediction of lycopene degradation during dehydration of watermelon pomace (cv Sugar Baby). J. Saudi Soc. Agric. Sci. 2017, 16, 97–103. [Google Scholar] [CrossRef]
- Srivastava, S.; Srivastava, A.K. Lycopene; chemistry, biosynthesis, metabolism and degradation under various abiotic parameters. J. Food Sci. Technol. 2015, 52, 41–53. [Google Scholar] [CrossRef]
- Elumalai, M.; Karthika, B.; Usha, V. Lycopene-role in cancer prevention. Int. J. Pharma Bio Sci. 2013, 4, 371–378. [Google Scholar]
- Sharma, S.; Sarvesh, P.; Dwivedi, J.; Amita, T. First report on laxative activity of Citrullus lanatus. Pharmacologyonline 2011, 2, 790–797. [Google Scholar]
- Abu-Reidah, I.M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Profiling of phenolic and other polar constituents from hydro-methanolic extract of watermelon (Citrullus lanatus) by means of accurate-mass spectrometry (HPLC–ESI–QTOF–MS). Food Res. Int. 2013, 51, 354–362. [Google Scholar] [CrossRef]
- Al-Sayed, H.M.; Ahmed, A.R. Utilization of watermelon rinds and sharlyn melon peels as a natural source of dietary fiber and antioxidants in cake. Ann. Agric. Sci. 2013, 58, 83–95. [Google Scholar] [CrossRef]
- Tița, B.; Statti, G.A. Nutraceutical properties and health-promoting biological activities of fruits of watermelon cultivars with different origins. Farmacia 2020, 68, 4. [Google Scholar]
- Choudhary, B.R.; Haldhar, S.M.; Maheshwari, S.K.; Bhargava, R.; Sharma, S.K. Phytochemicals and antioxidants in watermelon (Citrullus lanatus) genotypes under hot arid region. Indian J. Agric. Sci. 2015, 85, 414–417. [Google Scholar]
- Choo, W.S.; Sin, W.Y. Ascorbic acid, lycopene and antioxidant activities of red-fleshed and yellow-fleshed watermelons. Adv. Appl. Sci. Res. 2012, 3, 2779–2784. [Google Scholar]
- Feizy, J.; Jahani, M.; Ahmadi, S. Antioxidant activity and mineral content of watermelon peel. J. Food Bioprocess Eng. 2020. [Google Scholar] [CrossRef]
- Kujala, T.S.; Vienola, M.S.; Klika, K.D.; Loponen, J.M.; Pihlaja, K. Betalain and phenolic compositions of four beetroot (Beta vulgaris) cultivars. Eur. Food Res. Technol. 2002, 214, 505–510. [Google Scholar] [CrossRef]
- Gouveia, S.; Castilho, P.C. Characterisation of phenolic acid derivatives and flavonoids from different morphological parts of Helichrysum obconicum by a RP-HPLC–DAD-(−)–ESI-MSn method. Food Chem. 2011, 129, 333–344. [Google Scholar] [CrossRef]
- Bellés, J.M.; López-Gresa, M.P.; Fayos, J.; Pallás, V.; Rodrigo, I.; Conejero, V. Induction of cinnamate 4-hydroxylase and phenylpropanoids in virus-infected cucumber and melon plants. Plant Sci. 2008, 174, 524–533. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M.; Cisowski, W. High-performance liquid chromatographic determination of flavone C-glycosides in some species of the Cucurbitaceae family. J. Chromatogr. A. 1994, 675, 240–243. [Google Scholar] [CrossRef]
- Pikulski, M.; Brodbelt, J.S. Differentiation of flavonoid glycoside isomers by using metal complexation and electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2003, 14, 1437–1453. [Google Scholar] [CrossRef]
- Siciliano, T.; De Tommasi, N.; Morelli, I.; Braca, A. Study of flavonoids of Sechium edule (Jacq) Swartz (Cucurbitaceae) different edible organs by liquid chromatography photodiode array mass spectrometry. J. Agric. Food Chem. 2004, 52, 6510–6515. [Google Scholar] [CrossRef]
- Billett, E.E.; Grayer-Barkmeijer, R.J.; Johnson, C.B.; Harborne, J.B. The effect of blue light on free and esterified phenolic acids in etiolated gherkin tissues. Phytochemistry 1981, 20, 1259–1263. [Google Scholar] [CrossRef]
- Hong, J.L.; Qin, X.Y.; Shu, P.; Wu, G.; Wang, Q.; Qin, M.J. Analysis of catalpol derivatives by characteristic neutral losses using liquid chromatography combined with electrospray ionization multistage and time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 2680–2686. [Google Scholar] [CrossRef]
- Rodríguez-Medina, I.C.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Use of high-performance liquid chromatography with diode array detection coupled to electrospray-Qq-time-of-flight mass spectrometry for the direct characterization of the phenolic fraction in organic commercial juices. J. Chromatogr. A 2009, 1216, 4736–4744. [Google Scholar] [CrossRef]
- Cai, J.; Wang, W. Study on content of total flavonoids in leaves of cucumber. Shipin Kexue (Beijing, China). Food Sci. 2005, 8, 194–197. [Google Scholar]
- Zhang, P.Y.; Wang, J.C.; Liu, S.H.; Chen, K.S. A novel burdock fructo oligosaccharide induces changes in the production of salicylates, activates defence enzymes and induces systemic acquired resistance to Colletotrichum orbiculare in cucumber seedlings. J. Phytopathol. 2009, 157, 201–207. [Google Scholar] [CrossRef]
- Nahum, A.; Hirsch, K.; Danilenko, M.; Watts, C.K.; Prall, O.W.; Levy, J.; Sharoni, Y. Lycopene inhibition of cell cycle progression in breast and endometrial cancer cells is associated with reduction in cyclin D levels and retention of p27 Kip1 in the cyclin E–cdk2 complexes. Oncogene 2001, 20, 3428–3436. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E. Tomatoes, tomato-based products, lycopene, and cancer: Review of the epidemiologic literature. J. Natl. Cancer Inst. 1999, 91, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Fesseha, M.; Hong, M.Y. Effects of Watermelon Consumption on Cellular Proliferation, and Apoptosis in Rat Colon (P05-019-19). Curr. Dev. Nutr. 2019, 3, 3–5. [Google Scholar] [CrossRef]
- Glenn, K.; Klarich, D.S.; Kalaba, M.; Figueroa, A.; Hooshmand, S.; Kern, M.; Hong, M.Y. Effects of Watermelon Powder and l-arginine Supplementation on Azoxymethane-Induced Colon Carcinogenesis in Rats. Nutr. Cancer 2018, 70, 938–945. [Google Scholar] [CrossRef]
- Sueakham, T.; Chantaramanee, C.; Iawsipo, P. Anti-proliferative effect of Thai watermelon leaf extracts on cervical and breast cancer cells. NU. Int. J. Sci. 2018, 15, 89–95. [Google Scholar]
- Itoh, T.; Ono, A.; Kawaguchi, K.; Teraoka, S.; Harada, M.; Sumi, K.; Ando, M.; Tsukamasa, Y.; Ninomiya, M.; Koketsu, M.; et al. Phytol isolated from watermelon (Citrullus lanatus) sprouts induces cell death in human T-lymphoid cell line Jurkat cells via S-phase cell cycle arrest. Food Chem. Toxicol. 2018, 115, 425–435. [Google Scholar] [CrossRef]
- Pagano, M.; Pepperkok, R.; Verde, F.; Ansorge, W.; Draetta, G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992, 11, 961–971. [Google Scholar] [CrossRef]
- Yam, C.H.; Fung, T.K.; Poon, R.Y. Cyclin A in cell cycle control and cancer. Cell. Mol. Life Sci. 2002, 59, 1317–1326. [Google Scholar] [CrossRef]
- Diehl, J.A. Cycling to cancer with cyclin D1. Cancer Biol. Ther. 2002, 1, 226–231. [Google Scholar] [CrossRef]
- Giacinti, C.; Giordano, A. RB and cell cycle progression. Oncogene 2006, 25, 5220–5227. [Google Scholar] [CrossRef] [PubMed]
- Cuenda, A.; Rousseau, S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta Mol. Cell Res. 2007, 1773, 1358–1375. [Google Scholar] [CrossRef] [PubMed]
- Maddika, S.; Ande, S.R.; Wiechec, E.; Hansen, L.L.; Wesselborg, S.; Los, M. Akt-mediated phosphorylation of CDK2 regulates its dual role in cell cycle progression and apoptosis. J. Cell Sci. 2008, 121, 979–988. [Google Scholar] [CrossRef]
- Seifrtova, M.; Havelek, R.; Chmelarova, M.; Cmielova, J.; Muthna, D.; Stoklasova, A.; Zemankova, S.; Rezacova, M. The effect of ATM and ERK1/2 inhibition on mitoxantrone-induced cell death of leukaemic cells. Folia Biol. 2011, 57, 74–81. [Google Scholar]
- Yang, T.Y.; Chang, G.C.; Chen, K.C.; Hung, H.W.; Hsu, K.H.; Sheu, G.T.; Hsu, S.L. Sustained activation of ERK and Cdk2/cyclin-A signaling pathway by pemetrexed leading to S-phase arrest and apoptosis in human non-small cell lung cancer A549 cells. Eur. J. pharmacol. 2011, 663, 17–26. [Google Scholar] [CrossRef]
- Liu, P.; Begley, M.; Michowski, W.; Inuzuka, H.; Ginzberg, M.; Gao, D.; Tsou, P.; Gan, W.; Papa, A.; Kim, B.M.; et al. Cell-cycle-regulated activation of Akt kinase by phosphorylation at its carboxyl terminus. Nature 2014, 508, 541–545. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, N.; Hadden, T.J.; Rishi, A.K. Akt, FoxO and regulation of apoptosis. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 1978–1986. [Google Scholar] [CrossRef]
- Rastogi, N.; Gara, R.K.; Trivedi, R.; Singh, A.; Dixit, P.; Maurya, R.; Duggal, S.; Bhatt, M.L.; Singh, S.; Mishra, D.P. (6)-Gingerolinduced myeloid leukemia cell death is initiated by reactive oxygen species and activation of miR-27b expression. Free Radic. Biol. Med. 2014, 68, 288–301. [Google Scholar] [CrossRef]
| S. NO | Compound Name | Class | Reference |
|---|---|---|---|
| 1. | 3-O-Feruloylsucrose | Hydroxycinnamic acid derivatives | [84] |
| 2. | Ajugol | Iridoid | [78] |
| 3. | Apigenin-O-hexoside I | Flavonoid | [78] |
| 4. | Apigenin-O-hexoside II | Flavonoid | [78] |
| 5. | Aviprin I | Coumarin | [78] |
| 6. | β-carotene | Carotenoid | [2] |
| 7. | Caffeoylhexose I | Hydroxycinnamic acid derivatives | [78] |
| 8. | Catalposide | Iridoid | [78] |
| 9. | Chrysoeriol-O-hexoside I | Flavonoid | [78] |
| 10. | Chrysoeriol-O-hexoside II | Flavonoid | [78] |
| 11. | Cimifugin | Phenol | [78] |
| 12. | Citrulline | Amino acid | [4] |
| 13. | Coumarin | Coumarin | [78] |
| 14. | Decaffeoylacetoside | Hydroxycinnamic acid derivatives | [78] |
| 15. | Dicaffeoylshikimic acid II | Hydroxycinnamic acid derivatives | [85] |
| 16. | Dihydrophilonotisflavone | Flavonoid | [78] |
| 17. | Eriodictyol 7-glucoside | Flavonoid | [78] |
| 18. | Ferulic acid hexoside I | Hydroxycinnamic acid derivatives | [78,86] |
| 19. | Glehlinoside C | Phenol | [78] |
| 20. | Hydroquinone glucuronide | Phenol | [78] |
| 21. | Isolariciresinol 9′-β-d-glucopyranoside I | Lignan | [78] |
| 22. | Isoorientin | Flavonoid | [78] |
| 23. | Isovitexin | Flavonoid | [87,88] |
| 24. | Kaempferol rhamnoside–hexoside I | Flavonoid | [78] |
| 25. | Leachianol G | Phenol | [78] |
| 26. | Lucenin-2-methyl ether | Flavonoid | [78] |
| 27. | Luteolin-O-hexoside I | Flavonoid | [89] |
| 28. | Lycopene | Carotenoid | [2] |
| 29. | Naringenin 7-rutinoside I | Flavonoid | [78] |
| 30. | O-Feruloylquinide | Hydroxycinnamic acid derivatives | [86] |
| 31. | Obtusoside | Coumarin | [78] |
| 32. | p-Coumaric acid glucoside I | Hydroxycinnamic acid derivatives | [78,90] |
| 33. | p-Coumaric acid glucoside II | Hydroxycinnamic acid derivatives | [78,90] |
| 34. | Phloroglucinol glucuronide | Hydroxybenzoic acid derivative | [78] |
| 35. | Picroside | Iridoid | [91] |
| 36. | Protocatechuic acid glucoside I | Hydroxybenzoic acid derivative | [92] |
| 37. | Protocatechuic acid glucoside II | Hydroxybenzoic acid derivative | [92] |
| 38. | Quercitin | Flavonoid | [78] |
| 39. | Rutin | Flavonoid | [78,93] |
| 40. | Salicylic acid-O-hexoside I | Hydroxybenzoic acid derivatives | [94] |
| 41. | Salicylic acid-O-hexoside II | Hydroxybenzoic acid derivatives | [94] |
| 42. | Saligenin glucopyranoside | Phenol | [78] |
| 43. | Shikonine | Phenol | [78] |
| 44. | Sinapic acid glucoside | Hydroxycinnamic acid derivatives | [78] |
| 45. | Taxifolin-O-hexoside I | Flavonoid | [78] |
| 46. | Tri-O-caffeoylshikimic acid I | Hydroxybenzoic acid derivatives | [85] |
| 47. | Vanillic acid hexoside | Hydroxybenzoic acid derivatives | [78] |
| 48. | Vanillin hexoside I | Hydroxybenzoic acid derivatives | [78] |
| 49. | Vanillin hexoside II | Hydroxybenzoic acid derivatives | [78] |
| 50. | Vanillin hexoside III | Hydroxybenzoic acid derivatives | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manivannan, A.; Lee, E.-S.; Han, K.; Lee, H.-E.; Kim, D.-S. Versatile Nutraceutical Potentials of Watermelon—A Modest Fruit Loaded with Pharmaceutically Valuable Phytochemicals. Molecules 2020, 25, 5258. https://doi.org/10.3390/molecules25225258
Manivannan A, Lee E-S, Han K, Lee H-E, Kim D-S. Versatile Nutraceutical Potentials of Watermelon—A Modest Fruit Loaded with Pharmaceutically Valuable Phytochemicals. Molecules. 2020; 25(22):5258. https://doi.org/10.3390/molecules25225258
Chicago/Turabian StyleManivannan, Abinaya, Eun-Su Lee, Koeun Han, Hye-Eun Lee, and Do-Sun Kim. 2020. "Versatile Nutraceutical Potentials of Watermelon—A Modest Fruit Loaded with Pharmaceutically Valuable Phytochemicals" Molecules 25, no. 22: 5258. https://doi.org/10.3390/molecules25225258
APA StyleManivannan, A., Lee, E.-S., Han, K., Lee, H.-E., & Kim, D.-S. (2020). Versatile Nutraceutical Potentials of Watermelon—A Modest Fruit Loaded with Pharmaceutically Valuable Phytochemicals. Molecules, 25(22), 5258. https://doi.org/10.3390/molecules25225258








