Pomegranate Seed Oil and Bitter Melon Extract Affect Fatty Acids Composition and Metabolism in Hepatic Tissue in Rats
Abstract
1. Introduction
2. Results
2.1. Fatty Acids Profile and Characteristics of Applied Dietary Supplements
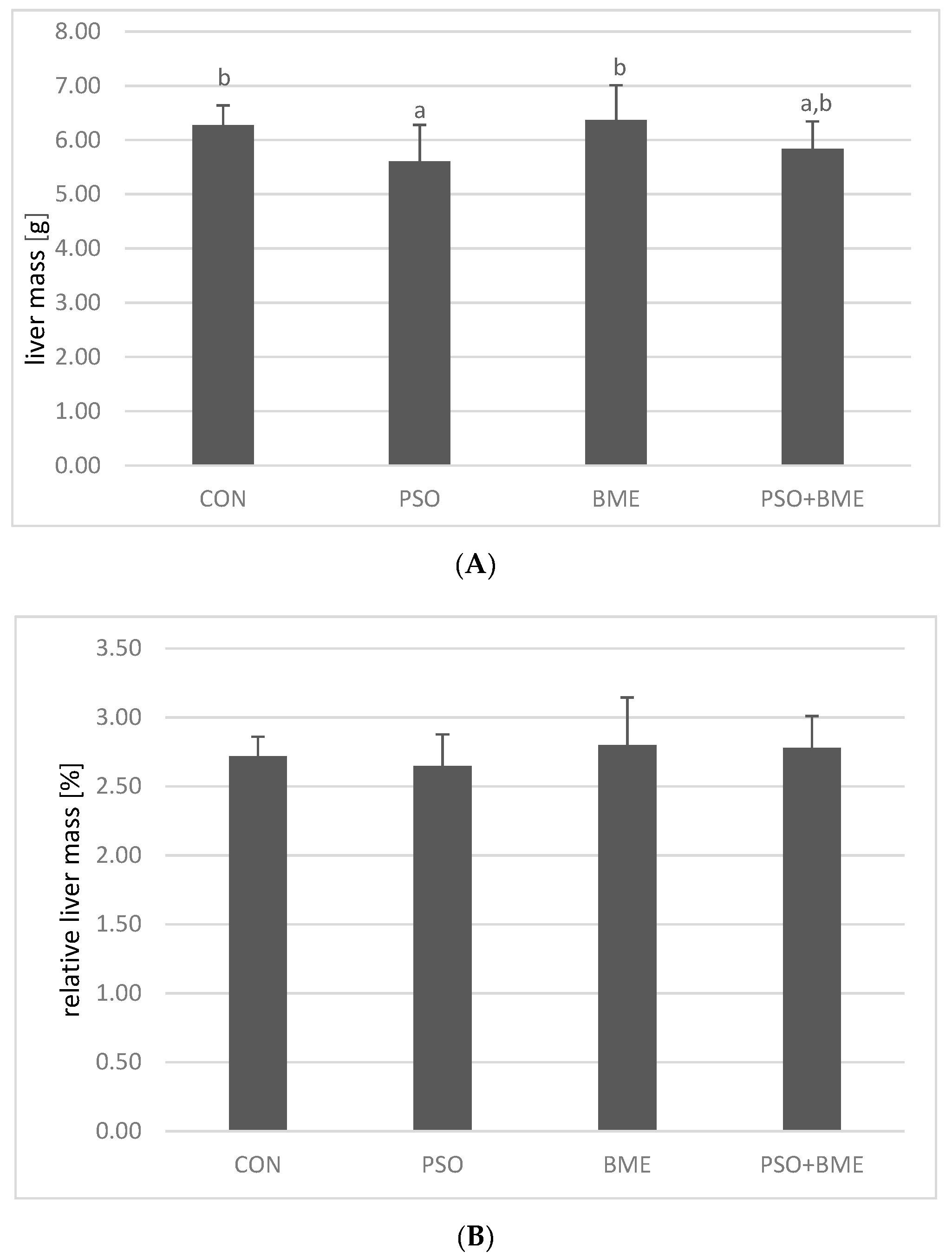
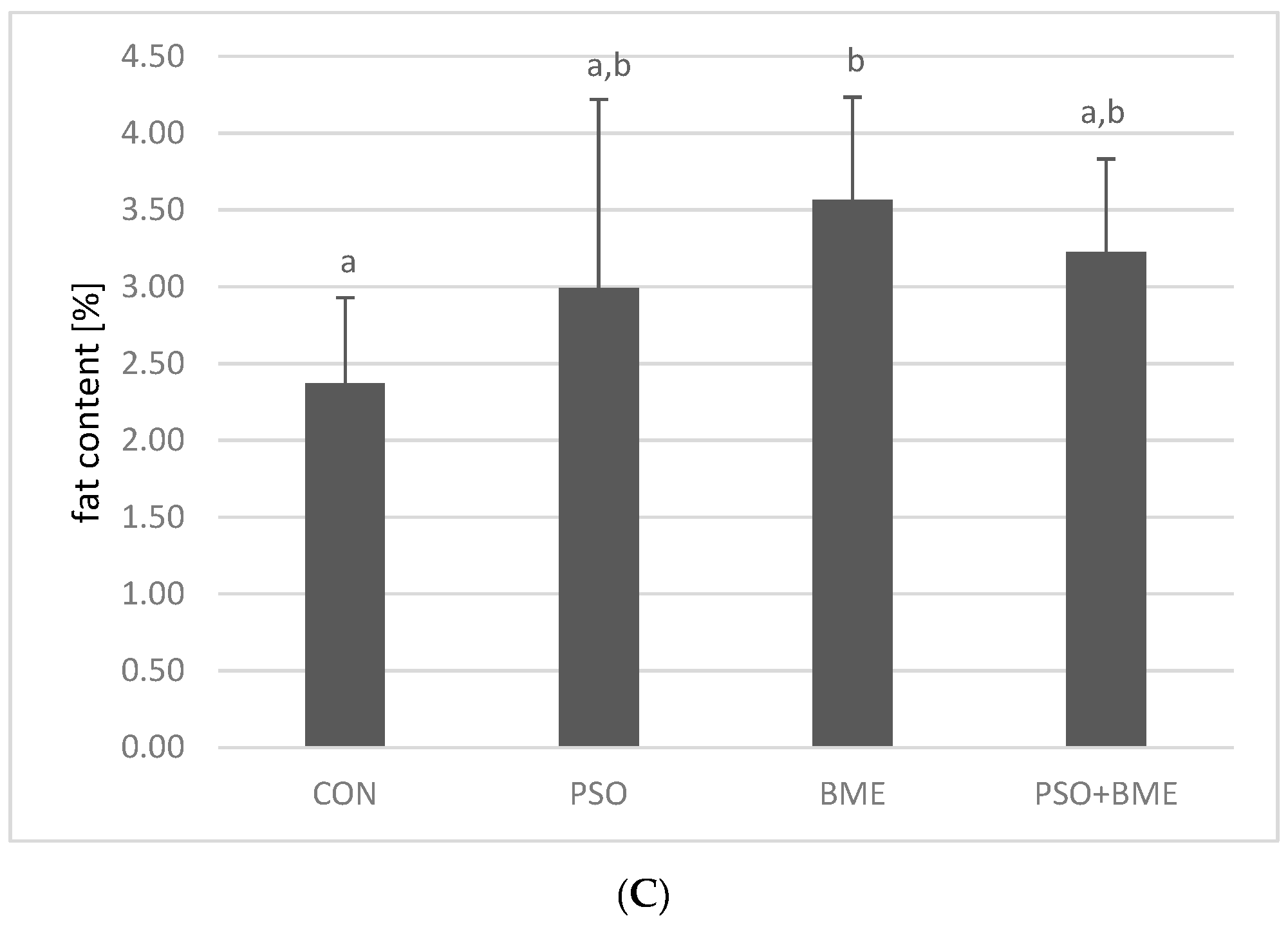
2.2. Liver Weight and Fat Content in Experimental Groups
2.3. Fatty Acids Profile in Liver
2.4. Fatty Acids Profile in Hepatic Microsomes
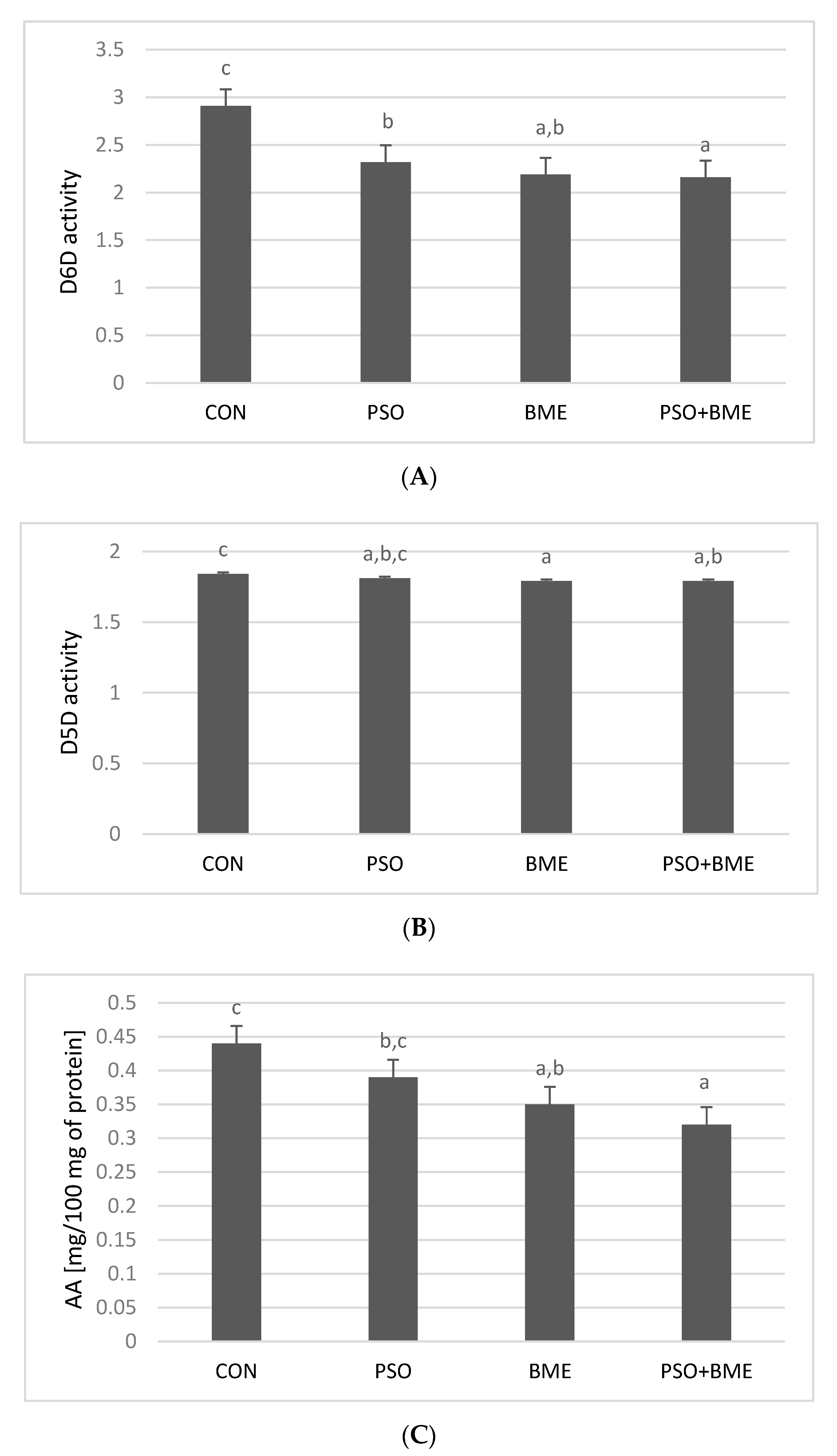
2.5. Analysis of Δ6- (D6D) and Δ5-Desaturase (D5D) Activities
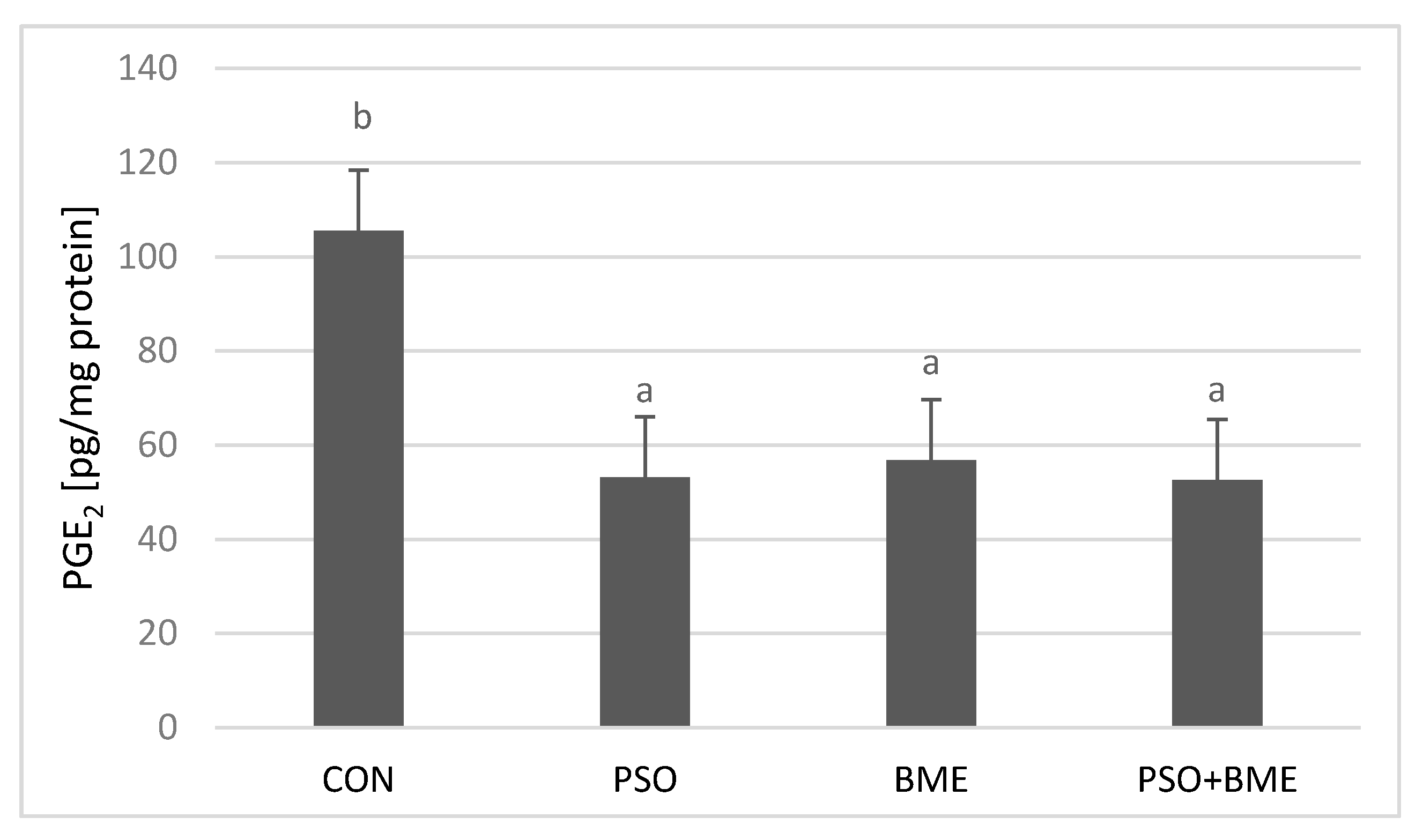
2.6. Liver PGE2 Levels
3. Discussion
4. Materials and Methods
4.1. Pomegranate Seed Oil
4.2. Bitter Melon Aqueous Extract
4.3. Animals
- CON—rats of the control group fed standard laboratory fodder and water ad libitum only,
- PSO—rats with unlimited access to the standard laboratory fodder and water receiving PSO in the amount of 0.15 mL given via a gavage daily,
- BME—rats with unlimited access to the standard laboratory fodder and 2% (w/v) BME as the only drinking liquid,
- PSO + BME—rats fed a standard laboratory fodder and 2% (w/v) BME as the only drinking liquid and receiving PSO in the amount of 0.15 mL daily given via a gavage daily.
4.4. Lipid Extraction and Sample Preparation for Fatty Acids Analysis in Liver
4.5. Preparation of the Microsomal Fraction from Rat Liver
4.6. Sample Preparation for Fatty Acids Analysis in Hepatic Microsomes
4.7. Instrumental Analysis
4.8. Analysis of Δ6- and Δ5-Desaturase Activities
4.9. Measurement of Liver PGE2
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jurenka, J. Therapeutic applications of pomegranate (Punica granatum L.): A review. Altern. Med. Rev. 2008, 13, 128–144. [Google Scholar]
- Banihani, S.; Swedan, S.; Alguraan, Z. Pomegranate and type 2 diabetes. Nutr. Res. 2013, 33, 341–348. [Google Scholar] [CrossRef]
- Estrada-Luna, D.; Martinez-Hinojosa, E.; Cancino-Diaz, J.C.; Belefant-Miller, H.; Lopez-Rodriguez, G.; Betanzos-Cabrera, G. Daily supplementation with fresh pomegranate juice increases paraoxonase 1 expression and activity in mice fed a high-fat diet. Eur. J. Nutr. 2018, 57, 383–389. [Google Scholar] [CrossRef]
- Lansky, E.P.; Newman, R.A. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 2007, 109, 177–206. [Google Scholar] [CrossRef]
- Aviram, M.; Dornfeld, L.; Kaplan, M.; Coleman, R.; Gaitini, D.; Nitecki, S.; Hofman, A.; Rosenblat, M.; Volkova, N.; Presser, D.; et al. Pomegranate juice flavonoids inhibit LDL oxidation and cardiovascular disease: Studies in atherosclerotic mice and in humans. Drugs Exp. Clin. Res. 2002, 28, 49–62. [Google Scholar]
- Borowczyk, K.; Shih, D.M.; Jakubowski, H. Metabolism and neurotoxicity of homocysteine thiolactone in mice: Evidence for a protective role of paraoxonase 1. J. Alzheimers. Dis. 2012, 30, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, M.; Hayek, T.; Aviram, M. Anti-oxidative effects of pomegranate juice (PJ) consumption by diabetic patients on serum and on macrophages. Atherosclerosis 2006, 187, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Orgil, O.; Spector, L.; Holland, D.; Mahajna, J.; Amir, R. The anti-proliferative and anti-androgenic activity of different pomegranate accessions. J. Funct. Foods 2016, 26, 517–528. [Google Scholar] [CrossRef]
- Białek, A.; Białek, M.; Lepionka, T.; Tober, E.; Czuderna, M. The quality determination of selected commercial online purchased edible pomegranate seed oils with new argentometric liquid chromatography method. J. Diet. Suppl. 2020. [Google Scholar] [CrossRef]
- Krawinkel, M.B.; Keding, G.B. Bitter gourd (Momordica charantia): A dietary approach to hyperglycemia. Nutr. Rev. 2006, 64, 331–337. [Google Scholar] [CrossRef]
- Ray, R.B.; Raychoudhuri, A.; Steele, R.; Nerurkar, P. Bitter melon (Momordica charantia) extract inhibits breast cancer cell proliferation by modulating cell cycle regulatory genes and promotes apoptosis. Cancer Res. 2010, 70, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Anila, L.; Vijayalakshmi, N.R. Beneficial effects of flavonoids from Sesamum indicum, Emblica officinalis and Momordica charantia. Phytother. Res. 2000, 14, 592–595. [Google Scholar] [CrossRef]
- Raj, S.K.; Khan, M.S.; Singh, R.; Kumari, N.; Prakash, D. Occurrence of yellow mosaic geminiviral disease on bitter gourd (Momordica charantia) and its impact on phytochemical contents. Int. J. Food Sci. Nutr. 2005, 56, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Lepionka, T.; Białek, A.; Białek, M.; Czauderna, M.; Stawarska, A.; Wrzesień, R.; Bielecki, W.; Paśko, P.; Galanty, A.; Bobrowska-Korczak, B. Mammary cancer risk and serum lipid profile of rats supplemented with pomegranate seed oil and bitter melon extract. Prostaglandins Other Lipid Mediat. 2019, 142, 33–45. [Google Scholar] [CrossRef]
- Białek, A.; Stawarska, A.; Bodecka, J.; Białek, M.; Tokarz, A. Pomegranate seed oil influences the fatty acids profile and reduces the activity of desaturases in livers of Sprague-Dawley rats. Prostaglandins Other Lipid Mediat. 2017, 131, 9–16. [Google Scholar] [CrossRef]
- Meerts, I.A.T.M.; Verspeek-Rip, C.M.; Buskens, C.A.F.; Keizer, H.G.; Bassaganya-Riera, J.; Jouni, Z.E.; van Huygevoort, A.H.B.M.; van Otterdijk, F.M.; van de Waart, E.J. Toxicological evaluation of pomegranate seed oil. Food Chem. Toxicol. 2009, 47, 1085–1092. [Google Scholar] [CrossRef]
- Yoshime, L.T.; Melo, I.L.P.; Sattler, J.A.G.; Torres, R.P.; Mancini-Filho, J. Bioactive compounds and the antioxidant capacities of seed oils from pomegranate (Punica granatum L.) and bitter gourd (Momordica charantia L.). Food Sci. Technol. 2019, 39 (Suppl. 2), 571–580. [Google Scholar] [CrossRef]
- Enwerem, N.; Okunji, P.; Oyonumo, N.; Samson, A. Momordica Charantia (Bitter Melon): Safety and Efficacy During Pregnancy and Lactation. Int. J. Stud. Nurs. 2018, 3, 140–145. [Google Scholar] [CrossRef][Green Version]
- Verardo, V.; Garcia-Salas, P.; Baldi, E.; Segura-Carretero, A.; Fernandez-Gutierrez, A.; Caboni, M.F. Pomegranate seeds as a source of nutraceutical oil naturally rich in bioactive lipids. Food Res. Int. 2014, 65, 445–452. [Google Scholar] [CrossRef]
- Sreekumar, S.; Sithul, H.; Muraleedharan, P.; Azeez, J.M.; Sreeharshan, S. Pomegranate fruit as a rich source of biologically active compounds. BioMed. Res. Int. 2014. [Google Scholar] [CrossRef]
- Białek, A.; Jelińska, M.; Tokarz, A. Influence of maternal diet enrichment with conjugated linoleic acids on lipoxygenase metabolites of polyunsaturated fatty acids in serum of their offspring with 7,12-dimethylbenz[a]anthracene induced mammary tumors. Prostaglandins Other Lipid Mediat. 2015, 116–117, 10–18. [Google Scholar]
- Jelińska, M.; Białek, A.; Mojska, H.; Gielecińska, I.; Tokarz, A. Effect of conjugated linoleic acid mixture supplemented daily after carcinogen application on linoleic and arachidonic acid metabolites in rat serum and induced tumours. Biochim. Biophy. Acta 2014, 1842, 2230–2236. [Google Scholar] [CrossRef] [PubMed]
- Białek, A.; Tokarz, A.; Zagrodzki, P. Conjugated linoleic acids in diet of female rats inhibit the breats cancer formation in their offspring. J. Food Nutr. Res. 2014, 53, 39–50. [Google Scholar]
- Stawarska, A.; Białek, A.; Tokarz, A. Heating of vegetable oils influences the activity of enzymes participating in arachidonic acid formation in Wistar rats. Nutr. Res. 2015, 35, 930–938. [Google Scholar] [CrossRef]
- Stawarska, A.; Białek, A.; Stanimirova, I.; Stawarski, T.; Tokarz, A. The effect of conjugated linoleic acids (CLA) supplementation on the activity of enzymes participating in the formation of arachidonic acid in liver microsomes of rats—Probable mechanism of CLA anticancer activity. Nutr. Cancer 2015, 67, 145–155. [Google Scholar] [PubMed]
- Białek, A.; Jelińska, M.; Tokarz, A.; Pergół, A.; Pinkiewicz, K. Influence of pomegranate seed oil and bitter melon aqueous extract on polyunsaturated fatty acids and their lipoxygenase metabolites concentration in serum of rats. Prostaglandins Other Lipid Mediat. 2016, 126, 29–37. [Google Scholar] [CrossRef]
- Yamasaki, M.; Kitagawa, T.; Koyanagi, N.; Chujo, H.; Maeda, H.; Kohno-Murase, J.; Imamura, J.; Tachibana, H.; Yamada, K. Dietary effect of pomegranate seed oil on immune function and lipid metabolism in mice. Nutrition 2006, 22, 54–59. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Kawakami, Y.; Abe, R.; Nakagawa, K.; Koba, K.; Imamura, J.; Iwata, T.; Ikeda, I.; Miyazawa, T. Conjugated linolenic acid is slowly absorbed in rat intestine, but quickly converted to conjugated linoleic acid. J. Nutr. 2006, 136, 2153–2159. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Tokuyama, Y.; Igarashi, M.; Nakagawa, K.; Ohsaki, Y.; Komai, M. Alpha-eleostearic acid (9Z11E13E-18:3) is quickly converted to conjugated linoleic acid (9Z11E-18:2) in rats. J. Nutr. 2004, 134, 2634–2639. [Google Scholar] [CrossRef]
- Pereira de Melo, I.L.; de Oliveira e Silva, A.M.; Teixeira de Carvalho, E.B.; Yoshime, L.T.; Sattler, J.A.G.; Mancini-Filho, J. Incorporation and effects of punicic acid on muscle and adipose tissues of rats. Lipids Health Dis. 2016, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Czumaj, A.; Śledziński, T. Biological Role of Unsaturated Fatty Acid Desaturases in Health and Disease. Nutrients 2020, 12, 356. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.; Arias, N.; Fernández-Quintela, A.; del Puy Portillo, M. Are conjugated linolenic acid isomers an alternative to conjugated linoleic acid isomers in obesity prevention? Endocrinol. Nutr. 2014, 61, 209–219. [Google Scholar] [CrossRef]
- Mashhadi, Z.; Boeglin, W.E.; Brash, A.R. Robust inhibitory effects of conjugated linolenic acids on a cyclooxygenase-related linoleate 10S-dioxygenase: Comparison with COX-1 and COX-2. Biochim. Biophys. Acta 2015, 1851, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Huang, Y.W.; Liu, S.; Chang, H.L.; Ye, W.; Shu, S.; Sugimoto, Y.; Funk, J.A.; Smeak, D.D.; Hill, L.; et al. Conjugated Linoleic Acid (CLA) Modulates Prostaglandin E2 (PGE2) Signaling in Canine Mammary Cells. Anticancer Res. 2006, 26, 889–898. [Google Scholar]
- Białek, A.; Stawarska, A.; Tokarz, A.; Czuba, K.; Konarska, A.; Mazurkiewicz., M. Enrichment of maternal diet with conjugated linoleic acids influences desaturases activity and fatty acids profile in livers and hepatic microsomes of the offspring with 7,12-dimethylbenz[a]anthracene-induced mammary tumors. Acta Pol. Pharm. 2014, 71, 747–761. [Google Scholar]
- Bondia-Pons, I.; Molto-Puigmarti, C.; Castellote, A.I.; Lopez-Sabater, M.C. Determination of conjugated linoleic acid in human plasma by gas chromatography. J. Chromat. A 2007, 1157, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.C.; Lee, Y.T.; Chen, M.F. Effects of fish oil and vitamin E on the antioxidant defense system in diet-induced hypercholesterolemic rabbits. Prostaglandins Other Lipid Mediat 2001, 66, 99–108. [Google Scholar] [CrossRef]
- Keelan, M.; Clandinin, M.T.; Thomson, A.B.R. Dietary lipids influence the activity of Δ5-desaturase and phospholipid fatty acids in rat enterocyte microsomal membranes. Can. J. Physiol. Pharm. 1997, 75, 1009–1014. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animals. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Raclot, T.; Groscolas, R. Differential mobilization of white adipose tissue fatty acids according to chain length, unsaturation, and positional isomerism. J. Lipid Res. 1993, 34, 1515–1526. [Google Scholar] [PubMed]
- Lowry, D.H.; Rosenbrough, J.J.; Farr, A.A.; Randal, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Fatty Acids [%]: | BME | PSO |
|---|---|---|
| Lauric acid (C12:0) | nd | <0.1 |
| Tridecanoic acid (C13:0) | 1.3 ± 0.9 | nd |
| Myristic acid (C14:0) | nd | 0.3 ± 0.2 |
| Pentadecanoic acid (C15:0) | 0.4 ± 0.4 | nd |
| Palmitic acid (C16:0) | 17.4 ± 3.0 | 21.5 ± 0.3 |
| Palmitoleic acid (C16:1) | nd | 0.2 ± 0.0 |
| Heptadecanoic acid (C17:0) | 0.3 ± 0.1 | 0.4 ± 0.0 |
| Heptadecenoic acid (C17:1) | nd | 0.1 ± 0.0 |
| Stearic acid (C18:0) | 11.2 ± 2.6 | 17.7 ± 0.2 |
| Oleic acid (C18:1 n-9 cis) | 13.2 ± 3.1 | 10.9 ± 0.4 |
| Linolelaidic acid (C18:2 n-6 trans) | nd | 0.1 ± 0.0 |
| Linoleic acid (C18:2 n-6 cis) | 29.5 ± 7.5 | 20.0 ± 0.0 |
| γ-linolenic acid (C18:3 n-6) | nd | 0.1 ± 0.0 |
| α-linolenic acid (C18:3 n-3) | 11.2 ± 2.8 | 7.5 ± 0.1 |
| Rumenic acid (C18:2 cis-9, trans-11) | nd | nd |
| Eicosenoic acid (C20:1) | nd | 0.4 ± 0.0 |
| Eicosadienoic acid (C20:2) | nd | 0.3 ± 0.0 |
| Docosanoic acid (C22:0) | nd | 0.1 ± 0.0 |
| Punicic acid (cis-9, trans-11, cis-13 C18:3) | nd | 6.6 ± 0.2 |
| cis-13,16-docosadienoic acid (C22:2) | nd | 1.4 ± 0.2 |
| Tricosylic Acid (C23:0) | nd | 0.3 ± 0.0 |
| Lignoceric acid (C24:0) | nd | 0.1 ± 0.0 |
| Fatty Acids [%] | CON (n = 12) | PSO (n = 12) | BME (n = 12) | PSO + BME (n = 12) | p Value |
|---|---|---|---|---|---|
| SFA | |||||
| Lauric acid (C12:0) | 0.10 ± 0.10 a,b | 0.09 ± 0.06 b | 0.03 ± 0.01 a | 0.04 ± 0.01 a,b | 0.0052 # |
| Tridecanoic acid (C13:0) | 0.01 ± 0.01 | 0.05 ± 0.05 | 0.03 ± 0.03 | 0.01 ± 0.00 | n.s. |
| Myristic acid (C14:0) | 0.21 ± 0.11 b | 0.20 ± 0.07 b | 0.13 ± 0.02 a | 0.13 ± 0.01 a | 0.0017 # |
| Pentadecanoic acid (C15:0) | 0.22 ± 0.06 c | 0.15 ± 0.02 a | 0.17 ± 0.01 a,b,c | 0.17 ± 0.02 a,b | 0.0003 # |
| Palmitic acid (C16:0) | 17.8 ± 0.8 | 17.7 ± 0.9 | 17.6 ± 0.7 | 18.1 ± 0.8 | n.s. |
| Heptadecanoic acid (C17:0) | 0.70 ± 0.07 c | 0.56 ± 0.06 a | 0.64 ± 0.05 b,c | 0.61 ± 0.07 a,b | <0.0001 * |
| Stearic acid (C18:0) | 22.0 ± 1.7 | 22.7 ± 1.3 | 22.6 ± 1.4 | 23.0 ± 1.3 | n.s. |
| Heneicosanoic acid (C21:0) | 0.16 ± 0.03 c | 0.14 ± 0.02 a | 0.14 ± 0.01 a,b | 0.15 ± 0.03 a,b,c | 0.0133 # |
| Docosanoic acid (C22:0) | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | n.s. |
| Tricosylic Acid (C23:0) | 0.05 ± 0.03 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.02 | n.s. |
| Lignoceric acid (C24:0) | 0.15 ± 0.03 | 0.09 ± 0.07 | 0.17 ± 0.03 | 0.16 ± 0.02 | n.s. |
| Σ SFA | 41.3 ± 1.2 | 41.8 ± 0.7 | 41.6 ± 1.3 | 42.4 ± 0.9 | n.s. |
| MUFA | |||||
| Pentadecenoic acid (C15:1) | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.00 | n.s. |
| Palmitoleic acid (C16:1) | 0.72 ± 0.23 | 0.68 ± 0.10 | 0.69 ± 0.17 | 0.64 ± 0.13 | n.s. |
| Heptadecenoic acid (C17:1) | 0.13 ± 0.01 b | 0.11 ± 0.01 a,b | 0.12 ± 0.02 a,b | 0.11 ± 0.02 a | 0.0242 * |
| Elaidic acid (C18:1 n-9 trans) | 0.20 ± 0.07 b | 0.10 ± 0.00 a | 0.18 ± 0.05 b | 0.18 ± 0.04 b | 0.0156 # |
| Oleic acid (C18:1 n-9 cis) | 7.75 ± 2.05 | 6.65 ± 0.69 | 7.17 ± 1.24 | 6.1 ± 1.0 | n.s. |
| Eicosenoic acid (C20:1) | 0.08 ± 0.01 | 0.09 ± 0.02 | 0.08 ± 0.03 | 0.07 ± 0.04 | n.s. |
| Docosenoic acid (C22:1) | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.01 | n.s. |
| Σ MUFA | 8.88 ± 2.25 | 7.59 ± 0.76 | 8.27 ± 1.43 | 7.08 ± 1.16 | n.s. |
| PUFA | |||||
| Linolelaidic acid (C18:2 n-6 trans) | 0.15 ± 0.08 c | 0.11 ± 0.06 a,b,c | 0.07 ± 0.02 a,b | 0.06 ± 0.02 a | 0.0228 # |
| Linoleic acid (C18:2 n-6 cis) | 15.0 ± 1.3 | 15.5 ± 0.9 | 15.5 ± 0.9 | 15.0 ± 1.4 | n.s. |
| γ-linolenic acid (C18:3 n-6) | 0.22 ± 0.06 a | 0.31 ± 0.06 c | 0.28 ± 0.09 a,b,c | 0.23 ± 0.04 a,b | 0.0013 * |
| α-linolenic acid (C18:3 n-3) | 1.16 ± 0.24 | 1.24 ± 0.18 | 1.17 ± 0.22 | 1.09 ± 0.22 | n.s. |
| Rumenic acid (C18:2 cis-9, trans-11) | 0.037 ± 0.007 a | 0.34 ± 0.08 b | 0.041 ± 0.008 a | 0.41 ± 0.14 b | <0.0001 # |
| Eicosadienoic acid (C20:2) | 0.11 ± 0.06 b | 0.07 ± 0.03 a,b | 0.07 ± 0.02 a,b | 0.05 ± 0.02 a | 0.0224 # |
| Dihomo-γ-linolenic acid (C20:3 n-6) | 0.47 ± 0.04 a,b | 0.32 ± 0.22 a | 0.52 ± 0.08 b | 0.53 ± 0.09 b | 0.0188 # |
| Arachidonic acid (C20:4 n-6) | 16.2 ± 1.4 a | 18.5 ± 0.8 b | 17.8 ± 1.4 b | 18.3 ± 1.1 b | <0.0001 * |
| Eicosatrienoic Acid (C20:3 n-3) | 0.16 ± 0.03 a | 0.16 ± 0.02 a,b | 0.18 ± 0.04 a,b,c | 0.21 ± 0.07 c | 0.0066 # |
| Eicosapentaenoic acid (C20:5 n-3) | 0.82 ± 0.14 | 0.87 ± 0.15 | 0.89 ± 0.17 | 0.84 ± 0.19 | n.s. |
| cis-13,16-docosadienoic acid (C22:2) | 0.03 ± 0.00 | 0.04 ± 0.01 | 0.03 ± 0.02 | 0.05 ± 0.02 | n.s. |
| Docosahexaenoic acid (C22:6 n-3) | 9.41 ± 1.29 b | 8.08 ± 0.88 a | 8.35 ± 1.04 a,b | 8.85 ± 0.84 a,b | 0.0152 * |
| Σ PUFA | 43.5 ± 2.1 a | 45.5 ± 1.0 b | 44.9 ± 0.9 a,b | 45.5 ± 0.62 b | 0.0072 # |
| n-3 | 11.5 ± 1.1 b | 10.3 ± 0.8 a | 10.6 ± 0.9 a,b | 11.0 ± 0.6 a,b | 0.0102 * |
| n-6 | 31.8 ± 1.5 a | 34.7 ± 0.8 b | 34.2 ± 1.2 b | 34.0 ± 0.6 b | 0.0001 # |
| n-6/n-3 | 2.78 ± 0.25 a | 3.37 ± 0.28 b | 3.26 ± 0.39 b | 3.10 ± 0.2 b | <0.0001 * |
| (MUFA + PUFA)/SFA | 1.27 ± 0.07 | 1.27 ± 0.04 | 1.28 ± 0.06 | 1.24 ± 0.05 | n.s. |
| PUFA/SFA | 1.05 ± 0.06 | 1.09 ± 0.03 | 1.08 ± 0.03 | 1.07 ± 0.03 | n.s. |
| PI | 163.9 ± 13.8 | 164.1 ± 6.8 | 163.6 ± 7.9 | 168.7 ± 7.3 | n.s. |
| Fatty Acids [%]: | CON (n = 12) | PSO (n = 12) | BME (n = 12) | PSO + BME (n = 12) | p Value |
|---|---|---|---|---|---|
| SFA | |||||
| Lauric acid (C12:0) | 0.07 ± 0.04 a,b | 0.07 ± 0.01 b | 0.09 ± 0.04 b | 0.05 ± 0.02 a | 0.0058 # |
| Tridecanoic acid (C13:0) | 0.03 ± 0.02 | 0.03 ± 0.01 | 0.03 ± 0.02 | 0.04 ± 0.02 | n.s. |
| Myristic acid (C14:0) | 0.24 ± 0.06 | 0.21 ± 0.03 | 0.20 ± 0.03 | 0.20 ± 0.04 | n.s. |
| Pentadecanoic acid (C15:0) | 0.23 ± 0.05 b | 0.17 ± 0.02 a | 0.19 ± 0.02 a,b | 0.19 ± 0.03 a,b | 0.0036 # |
| Palmitic acid (C16:0) | 18.2 ± 0.8 | 18.2 ± 1.6 | 17.5 ± 0.9 | 18.0 ± 1.0 | n.s. |
| Heptadecanoic acid (C17:0) | 0.72 ± 0.06 c | 0.56 ± 0.06 a | 0.64 ± 0.05 b | 0.58 ± 0.06 a,b | <0.0001 * |
| Stearic acid (C18:0) | 24.4 ± 1.9 | 24.8 ± 1.6 | 24.2 ± 1.9 | 23.7 ± 0.9 | n.s. |
| Heneicosanoic acid (C21:0) | 0.10 ± 0.02 | 0.08 ± 0.01 | 0.10 ± 0.02 | 0.09 ± 0.02 | n.s. |
| Docosanoic acid (C22:0) | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | n.s. |
| Tricosylic Acid (C23:0) | 0.03 ± 0.00 a | 0.06 ± 0.03 a,b | 0.05 ± 0.02 a,b | 0.08 ± 0.04 b | 0.0059 # |
| Lignoceric acid (C24:0) | 0.19 ± 0.06 | 0.19 ± 0.03 | 0.18 ± 0.02 | 0.17 ± 0.03 | n.s. |
| Σ SFA | 44.2 ± 1.9 | 44.4 ± 1.6 | 43.2 ± 1.4 | 43.1 ± 1.1 | n.s. |
| MUFA | |||||
| Myristoleic acid (C14:1) | 0.03 ± 0.01 b | 0.01 ± 0.01 a,b | 0.01 ± 0.01 a | 0.02 ± 0.01 a,b | 0.0119 # |
| Pentadecenoic acid (C15:1) | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.03 ± 0.03 | n.s. |
| Palmitoleic acid (C16:1) | 0.65 ± 0.13 | 0.59 ± 0.13 | 0.58 ± 0.13 | 0.63 ± 0.13 | n.s. |
| Heptadecenoic acid (C17:1) | 0.12 ± 0.01 b | 0.10 ± 0.02 a | 0.12 ± 0.02 a,b | 0.11 ± 0.02 a,b | 0.0147 # |
| Elaidic acid (C18:1 n-9 trans) | 0.23 ± 0.08 b | 0.14 ± 0.07 a | 0.18 ± 0.04 a,b | 0.19 ± 0.04 a,b | 0.0080 * |
| Oleic acid (C18:1 n-9 cis) | 7.68 ± 1.25 c | 5.92 ± 0.80 a | 6.96 ± 0.83 a,b,c | 6.07 ± 0.92 a,b | 0.0007 # |
| Eicosenoic acid (C20:1) | 0.10 ± 0.01 b | 0.08 ± 0.02 a,b | 0.09 ± 0.03 b | 0.06 ± 0.02 a | 0.0010 # |
| Σ MUFA | 8.78 ± 1.32 c | 6.85 ± 0.92 a | 7.96 ± 0.97 a,b,c | 7.07 ± 1.05 a,b | 0.0009 # |
| PUFA | |||||
| Linolelaidic acid (C18:2 n-6 trans) | 0.07 ± 0.03 | 0.06 ± 0.02 | 0.07 ± 0.02 | 0.06 ± 0.02 | n.s. |
| Linoleic acid (C18:2 n-6 cis) | 13.1 ± 1.0 | 13.3 ± 0.9 | 13.2 ± 1.0 | 12.8 ± 1.5 | n.s. |
| γ-linolenic acid (C18:3 n-6) | 0.18 ± 0.03 a | 0.27 ± 0.08 c | 0.26 ± 0.07 b,c | 0.20 ± 0.04 a,b | 0.0002 # |
| α-linolenic acid (C18:3 n-3) | 1.09 ± 0.20 | 1.05 ± 0.20 | 1.03 ± 0.17 | 1.10 ± 0.21 | n.s. |
| Rumenic acid C18:2 (cis-9, trans-11) | 0.052 ± 0.011 a | 0.31 ± 0.05 b | 0.049 ± 0.009 a | 0.34 ± 0.09 b | <0.0001 # |
| Eicosadienoic acid (C20:2) | 0.12 ± 0.08 b | 0.06 ± 0.03 a,b | 0.08 ± 0.02 b | 0.05 ± 0.02 a | 0.0040 # |
| Dihomo-γ-linolenic acid (C20:3 n-6) | 0.46 ± 0.09 | 0.41 ± 0.11 | 0.48 ± 0.08 | 0.45 ± 0.09 | n.s. |
| Arachidonic acid (C20:4 n-6) | 16.9 ± 1.1 a | 20.4 ± 1.3 c | 19.8 ± 1.2 b,c | 19.0 ± 1.4 b | <0.0001 * |
| Eicosatrienoic Acid (C20:3 n-3) | 0.28 ± 0.11 | 0.28 ± 0.05 | 0.24 ± 0.03 | 0.28 ± 0.05 | n.s. |
| Eicosapentaenoic acid (C20:5 n-3) | 0.72 ± 0.15 | 0.80 ± 0.17 | 0.85 ± 0.16 | 0.74 ± 0.16 | n.s. |
| cis-13,16-docosadienoic acid (C22:2) | 0.05 ± 0.04 | 0.03 ± 0.02 | 0.04 ± 0.02 | 0.05 ± 0.03 | n.s. |
| Docosahexaenoic acid (C22:6 n-3) | 8.27 ± 0.91 | 7.73 ± 1.01 | 8.10 ± 1.25 | 8.14 ± 0.98 | n.s. |
| Σ PUFA | 41.2 ± 2.1 a | 44.7 ± 1.4 b | 44.1 ± 1.2 b | 43.2 ± 1.5 a,b | 0.0003 # |
| n-3 | 10.3 ± 1.0 | 9.85 ± 0.86 | 10.2 ± 1.2 | 10.2 ± 0.7 | n.s. |
| n-6 | 30.6 ± 1.5 a | 34.4 ± 1.3 c | 33.7 ± 0.9 b,c | 32.6 ± 1.2 b | <0.0001 * |
| n-6/n-3 | 2.98 ± 0.23 a | 3.52 ± 0.34 b | 3.35 ± 0.47 b | 3.19 ± 0.23 a,b | 0.0023 * |
| (MUFA + PUFA)/SFA | 1.13 ± 0.09 | 1.16 ± 0.08 | 1.21 ± 0.07 | 1.17 ± 0.05 | n.s. |
| PUFA/SFA | 0.93 ± 0.08 a | 1.01 ± 0.07 b | 1.02 ± 0.05 b | 1.00 ± 0.05 b | 0.0055 * |
| PI | 155.5 ± 10.1 a | 166.2 ± 9.2 b | 166.6 ± 8.9 b | 163.1 ± 10.7 a,b | 0.0271 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stawarska, A.; Lepionka, T.; Białek, A.; Gawryjołek, M.; Bobrowska-Korczak, B. Pomegranate Seed Oil and Bitter Melon Extract Affect Fatty Acids Composition and Metabolism in Hepatic Tissue in Rats. Molecules 2020, 25, 5232. https://doi.org/10.3390/molecules25225232
Stawarska A, Lepionka T, Białek A, Gawryjołek M, Bobrowska-Korczak B. Pomegranate Seed Oil and Bitter Melon Extract Affect Fatty Acids Composition and Metabolism in Hepatic Tissue in Rats. Molecules. 2020; 25(22):5232. https://doi.org/10.3390/molecules25225232
Chicago/Turabian StyleStawarska, Agnieszka, Tomasz Lepionka, Agnieszka Białek, Martyna Gawryjołek, and Barbara Bobrowska-Korczak. 2020. "Pomegranate Seed Oil and Bitter Melon Extract Affect Fatty Acids Composition and Metabolism in Hepatic Tissue in Rats" Molecules 25, no. 22: 5232. https://doi.org/10.3390/molecules25225232
APA StyleStawarska, A., Lepionka, T., Białek, A., Gawryjołek, M., & Bobrowska-Korczak, B. (2020). Pomegranate Seed Oil and Bitter Melon Extract Affect Fatty Acids Composition and Metabolism in Hepatic Tissue in Rats. Molecules, 25(22), 5232. https://doi.org/10.3390/molecules25225232









