1. Introduction
According to the Globocan database, 1.09 million new cases of colon and rectal cancer were diagnosed in 2018, and 551,000 died from the disease [
1]. This type of tumor is thus generally third in frequency but second in mortality. The highest incidence of colon cancer has been recorded in parts of Europe (e.g., Hungary, Slovenia, Slovakia, the Netherlands and Norway), Australia and New Zealand, and in the Far East (Japan, Korea, Singapore). The incidence of colorectal cancer in various regions of the world varies six to eight times, and is significantly higher in countries with higher socioeconomic development [
2,
3]. Economic development in the last decade has been found to increase incidence and mortality in the Baltic countries, Russia, China and Brazil, increase in incidence but decrease in mortality in Canada, the United Kingdom, Denmark and Singapore, and decrease in incidence and mortality in the United States, Japan and France [
4]. The reduction in mortality in developed countries is due to more advanced treatments as well as the introduction of prevention programs from the 1990s which result in earlier disease detection [
5].
The causes of colorectal cancer (CRC) are multifactorial, and include genetic and environmental factors. Most cases of colorectal cancer are sporadic, while the hereditary component is estimated at 18–35% [
6]. Among the environmental factors that can contribute to the development of the disease, the most important are high-calorie diet rich in animal fats, increased alcohol consumption, smoking as well as reduced intake of fruits, vegetables and fibers. Other risk factors include old age, male gender, insufficient physical activity as well as obesity, and inflammatory bowel diseases such as ulcerative colitis and Crohn’s disease [
7]. Additionally, it is known that patients who have undergone surgery for colorectal cancer have a three times higher risk of developing new, i.e., metachronous colon cancer. The presence of adenomas is also a recognized risk factor for colorectal cancer, and tubular adenomas pose low, tubulovillous medium, and villous adenomas a high risk for CRC. Genetic syndromes, such as genetic syndromes of familial adenomatous polyposis (FAP) and Lynch syndrome (hereditary nonpolyposis colorectal cancer, HNPCC) also cause an increased CRC incidence [
8]. Despite advancements in screening, approximately 35% of colorectal cancer patients present with stage IV metastatic disease at the time of diagnosis, and 20–50% with stage II or III will progress to stage IV at some point during the course of their disease [
9]. Despite advancements in treatment, five-year survival rate in patients with distant metastases is only about 10–15%. Colorectal cancer is a prototypical example of a multi-stage cancer development and progression by accumulation of various mutations and epigenetic changes [
10]. Current treatment of CRC includes surgery, radiation, chemotherapy and targeted therapy.
Medicinal mushroom research has identified about 800 mushroom species with significant pharmacological properties [
11,
12]. While more than 130 therapeutic effects of various mushroom species have been discovered, most of the research is focused on immunomodulatory and antitumor properties of mushrooms [
13,
14,
15,
16]. Antitumor immunological responses are primarily due to high-molecular weight compounds such as polysaccharides and polysaccharopeptide complexes, out of which various β-D-glucans are most well-known [
17]. Direct antitumor effects are the result of dysregulation of important pathways, such as nuclear factor-kappa B (NF-κB), mitogen activated protein kinase pathway (MAPK), Akt, Wnt, Notch, p53, TGFβ, MMPs, VEGF and others, involved in tumor development and progression [
18]. While most research focuses on various individual isolates from well-known mushroom species, some research indicates that combination of several mushroom species cause better anticancer effects [
18,
19,
20]. This is the consequence of combinatorial effects of polysaccharides on the immune system, as well as of various small-molecular weight compounds (terpenoids, lactones, alkaloids, sterols and phenolic substances) on cancer-promoting pathways and processes.
Besides its known pharmacological role as a antimetabolite and pyrimidine analog, 5-fluorouracil has been shown to have immunological antitumor effects in lower doses. These effects are primarily the consequence of increase in tumor antigenicity by stimulating the production of mutational antigens [
21]. Stress cell death caused by cytotoxic drugs through the activation of the DNA damage response (DDR), endoplasmic reticulum stress response and autophagy results in the release of DAMPs (danger associated molecular patterns) which in turn stimulate the activation of dendritic cells and presentation of tumor antigens to T lymphocytes [
22]. Cell death associated with the formation of DAMP is called immunogenic cell death (ICD) [
23]. Molecules that are ligands for NK receptors such as NKG2D and cell death receptors Fas/CD95 as well as adhesion molecules that enhance the recognition and killing of tumor cells by cytotoxic T lymphocytes are expressed on cancer cells that survive chemotherapy treatment [
24]. Non-targeted immune effects of chemotherapy include the elimination of many tumor-activated immunosuppressive mechanisms mediated by immunosuppressive cytokines such as TGFβ1, inhibitory receptors such as CTLA-4, PD-1, and immune cells with immunosuppressive or tolerogenic functions such as Treg, MDSC, and M2 polarized macrophages [
25]. Therefore, the rationale of using chemotherapeutics in lower, immune-stimulating doses with well-defined medicinal mushroom mixtures lays in the possible synergism between these two therapeutic approaches. This approach is of particular interest in the immunosuppressive environment of advanced tumors.
To date, there has been a limited amount of studies addressing antitumor effects of standardized medicinal mushroom blended extracts alone or in combination with standard chemotherapies. Recent meta-study of 213 studies, including 77 clinical studies that reported the use of various cytotoxic drugs, including 5-fluorouracil with
Ganoderma lucidum or
Trametes versicolor reported that these medicinal mushrooms enhance the efficacy and ameliorate their adverse effects, which leads to improved quality of life in cancer patients, without significant changes in pharmacokinetics of 5-fluorouracil [
26]. Hence, to better understand the mechanisms of antitumor action of Agarikon.1 and Agarikon Plus preparations, with or without 5-fluorouracil in a late colorectal cancer model, we have performed in vitro and in vivo studies presented herein.
3. Discussion
Medicinal mushrooms have a large number of bioactive substances, and their indirect antitumor effects are primarily attributed to enhanced activation of the host immune system, which results from high content of various complex polysaccharides, of which β-glucans are the most studied. More recently, it has been found that various classes of compounds from medicinal mushrooms have significant direct antiproliferative or cytotoxic effects on tumors, mediated by inhibition of protein kinases and other signaling pathways (Wnt, EGFR, VEGFR, Jak/Stat, mTOR, Shh, TGF/Smad and others), DNA polymerases and DNA topoisomerases, as well as by modulation of G1/S and G2/M cell cycle checkpoints [
18,
40]). Our recent study has shown that the formulation Agarikon.1 can downregulate proteins essential in ribosome biogenesis and translation, which represents a fundamental process which is ubiquitously dysregulated during colorectal cancer progression [
41].
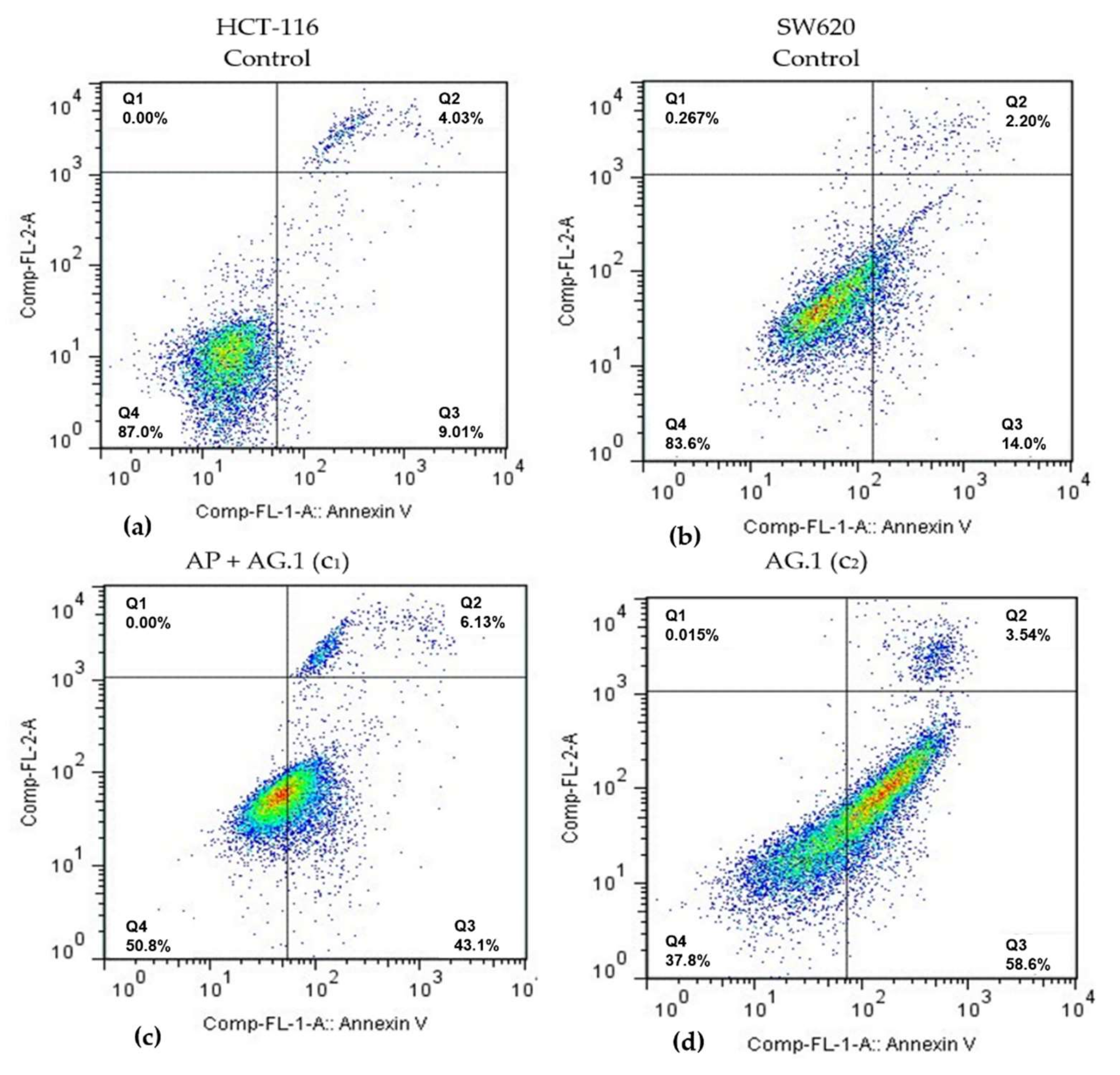
Significant dose-dependent cytotoxicity observed in SW620 and HCT-116 cells is in line with a large body of research documenting antiproliferative effects of various mushroom species used in formulations Agarikon.1 and Agarikon Plus. Cytotoxic effects of many mushroom species are the result of apoptosis induction. Dose-dependent cytotoxicity of
Trametes versicolor extract was established on four human breast cancer cell lines, including MCF-7, where the main mechanism is an induction of cell death (apoptosis) due to increased p53 expression paralleled by decreased expression of antiapoptotic Bcl-2 protein [
42]. Polysaccharopeptide PSP is the most well-studied substance from
Trametes versicolor, and has been confirmed in several studies to act selectively in cancer cells, with minimal effects on healthy cells [
43]. An extract of the same species dose-dependently inhibits the proliferation of HeLa and HepG2 cells [
44]. The activity of the enzymes laccase, peroxidase and glutathione reductase was determined in the extract, and the antitumor activity is a consequence of the presence of natural quinone substances in the extract which are formed by the activity of these enzymes on the lignin substrate. Tumor cells, unlike normal ones, have a high content of NAD(P)H-quinone oxidoreductase (DT-diaphorase), which converts quinones into substances that are toxic to the cell. This is why the extracts of these species are considered to be toxic specifically to tumor cells.
Ganoderma lucidum is one of the most studied species of medicinal mushrooms, and its dose-dependent induction of apoptosis in SW620 metastatic colorectal cancer cells is the consequence of caspase-3, Bax and p53 activation, with simultaneous inhibition of Bcl-2 expression at mRNA and XIAP at the protein level [
45]. The bioactive protein C91-3 apoptotic protein 24,414 was isolated from
Lentinus edodes, which has a dose-dependent effect on reducing proliferation and increasing the proportion of A549 lung adenocarcinoma cells in apoptosis [
46]. Antiproliferative and proapoptotic activity of
Agaricus blazei extract results from strong inhibition of telomerase activity, induction of cytochrome release, caspase activation, and regulation of Bcl-2 synthesis [
47].
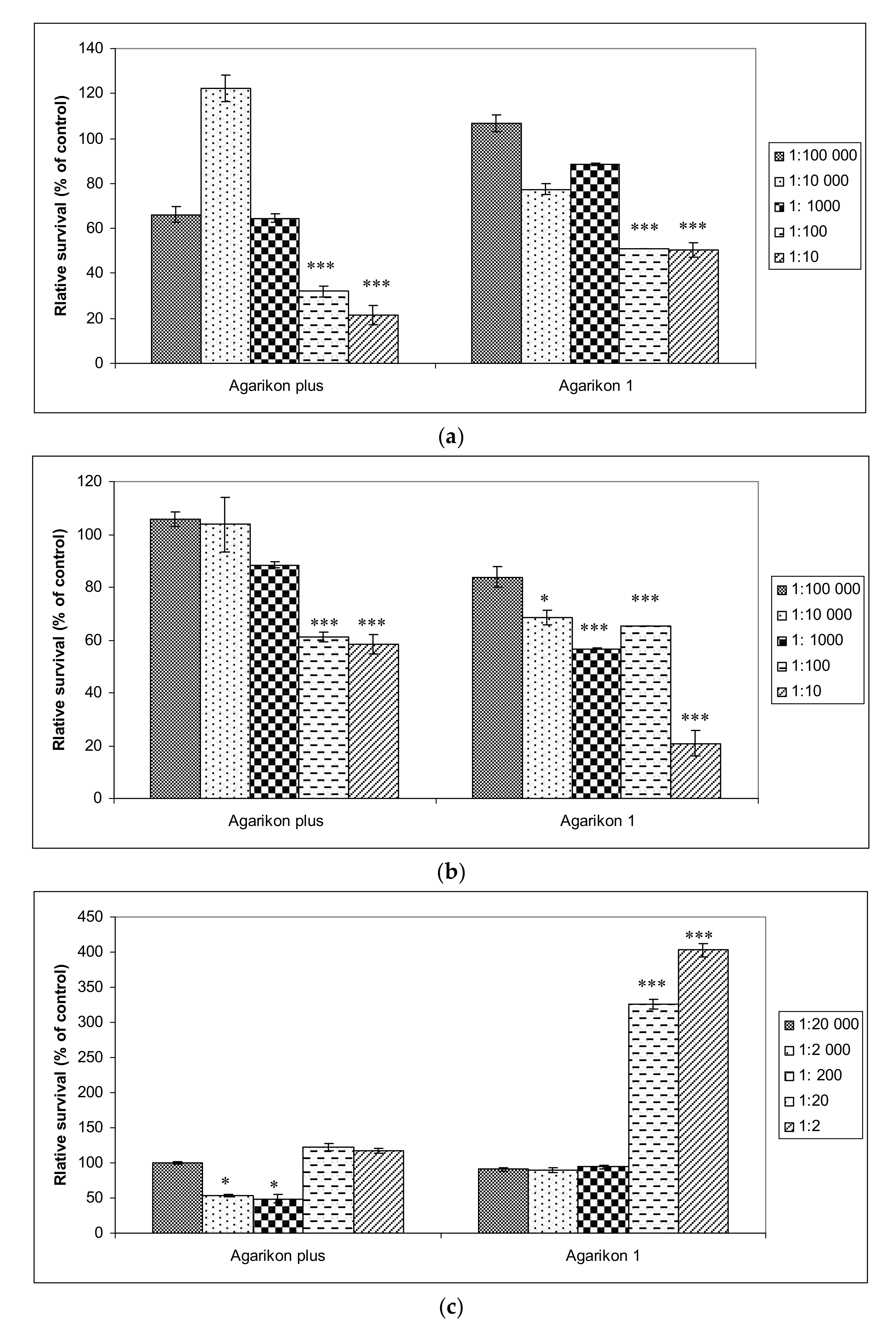
Mild (AP) to no cytotoxicity (AG.1) was observed in healthy human fibroblast cells WI-38, and regenerative activity was also observed with higher concentrations of Agarikon Plus and Agarikon.1 (
Figure 1). Such results are in agreement with some studies that have shown the proliferative activity of certain medicinal mushroom extracts on fibroblasts, which can result in faster wound healing [
48]. Certain components of extracts, such as caffeic acid, can have an antioxidant effect on healthy cells and a prooxidative effect on tumor cells, leading to oxidative DNA damage and induction of cell death (apoptosis) in tumor cells [
49].
Some medicinal mushrooms and their components are an example of potential drugs or medical products with a pleiotropic action, i.e., simultaneously affect multiple pharmacological targets or causing their inhibition (multitarget agents or multitarget inhibitors), which is also a property of many other natural products and their isolates, such as soy isoflavones and other legumes, turmeric from various species of the genus
Curcuma from the ginger family, resveratrol and many other natural (poly)phenols and their subgroups, such as epigallocatechin-3-gallate (EGCG) from green tea. In the treatment of complex pathologies such as cancer, such an approach of multiple pharmacological targets is necessary, which is further emphasized by the fact that pharmacological approaches to cancer treatment which involve one or several targets are usually very short-lived and yield weaker treatment outcomes [
50]. In this regard, the use of combination of several medicinal mushroom species is also based on the observation that mixtures of polysaccharides from several species of certain medicinal mushrooms can in some cases achieve both cumulative immunomodulatory and other antitumor effects. Synergistic immunostimulatory effects may result from a cumulative activation of multiple receptor types on immune cells, such as dectin receptor-1 and various Toll-like receptors, which can lead to stronger expression and synthesis of different immunostimulatory cytokines, while synergistic direct antitumor effects can result from the activation of multiple cancer-inhibitory pathways and effects [
18,
51].
Recently, new pharmacological mechanisms of action of various chemotherapeutics that have been used for a long time in standard therapies for various forms of cancer have been discovered. One of the main mechanisms observed in certain cytotoxic chemotherapies is their immunostimulatory effect. Although chemotherapeutics, when administered in higher doses, with the main goal of reducing tumor mass (cytotoxic effect) have primarily an immunosuppressive effect due to their toxicity to the bone marrow, their use in lower doses may lead to the opposite effect [
22,
52]. Therefore, in our study, 5-fluorouracil was dosed metronomically (metronomic scheduling protocol), which implies lower doses than those used during the classic dosing of this chemotherapeutic. Such dosing, in addition to immunostimulatory, also reduces the toxic effects of chemotherapeutics, significantly affects the inhibition of angiogenesis and reduces the possibility of developing drug resistance [
53]. For this purpose, such dosing is often used in the clinic where a higher dose is given for the first four days, after which half of that dose every other day for four days is administered, and we also applied this scheme in our study [
54]. By using chemotherapeutics in such so-called suboptimal doses, it is assumed that immunosuppressive cells are preferentially destroyed over effector immune cells. In this way, the last step of immune editing of the tumor, which includes immune escape, could potentially be prevented, and more significant effects of various tumor immunotherapies can be achieved, where the use of fungal substances is conceptually closest to active nonspecific immunotherapy according to standard classification.
The significance of the CT26.WT syngeneic model in tumor immunology research was also confirmed by the fact that the increased activity of tumor associated macrophages (TAMs) in Balb/c mice resulted in increased tumor growth, angiogenesis and metastasis [
55]. In addition to the use of a very aggressive and undifferentiated colon cancer cell line CT26.WT, treatment with Agarikon.1 and Agarikon Plus, alone or in combination with 5-fluorouracil was started only when the tumor volume averaged 800–1000 mm
3, which is a very late disease model [
56]. For comparison, early models are defined by the start of treatment often as soon as on the next or only a few days after tumor inoculation, when the average values of tumor volumes are 40–60 mm
3.
The idea that the immune system models tumor immunogenicity is the basis of the cancer immunoediting hypothesis, which means that the host’s immune system can have a protective or promoting effect on the tumor [
57]. Immune editing is a process that occurs only after malignant transformation has already occurred and is an extrinsic tumor suppressor mechanism that is activated after the failure of intrinsic tumor suppressor mechanisms, such as DNA repair. Because various groups of medicinal fungal substances, primarily polysaccharides and polysaccharide and protein complexes (polysaccharopeptides), have numerous immunostimulatory effects, this may also explain 100% survival of animals up to 45 days after tumor cell inoculation in a preventive subsection treated with Agarikon.1 or Agarikon Plus (
Table 3). Since we used an induced rather than a spontaneous tumor, it can be assumed that treatment with Agarikon.1 or Agarikon Plus leads to increased immune cell activation, which can therefore prolong the equilibrium phase, which is the second phase of the tumor immune response according to cancer immunoediting hypothesis. Namely, it is known that the role of the acquired immune system is particularly important in the equilibrium phase, with key roles of interleukin-12 (IL-12), IFN-γ and CD4
+ and CD8
+ T lymphocytes [
58]. Accordingly, studies confirm that in the advanced stages of the tumor, the number of neutrophils and macrophages is increased, and the number of T and B lymphocytes is reduced [
59]. Therefore, one of the key immunotherapeutic goals in advanced tumors could be to maintain immune balance for as long as possible. In solid tumors, one of the key obstacles, therefore, in addition to the reduced effectiveness of antitumor effector cells, is the effect of the tumor microenvironment, which inhibits the effect of activated cells in situ, i.e., in the tumor tissue itself. Although a study of 6 tumor models showed that Ki-67 proliferation indices in weakly immunogenic and highly immunogenic (including CT26) tumors were similar, more immunogenic tumors showed slower growth in immunocompetent mice than less immunogenic ones [
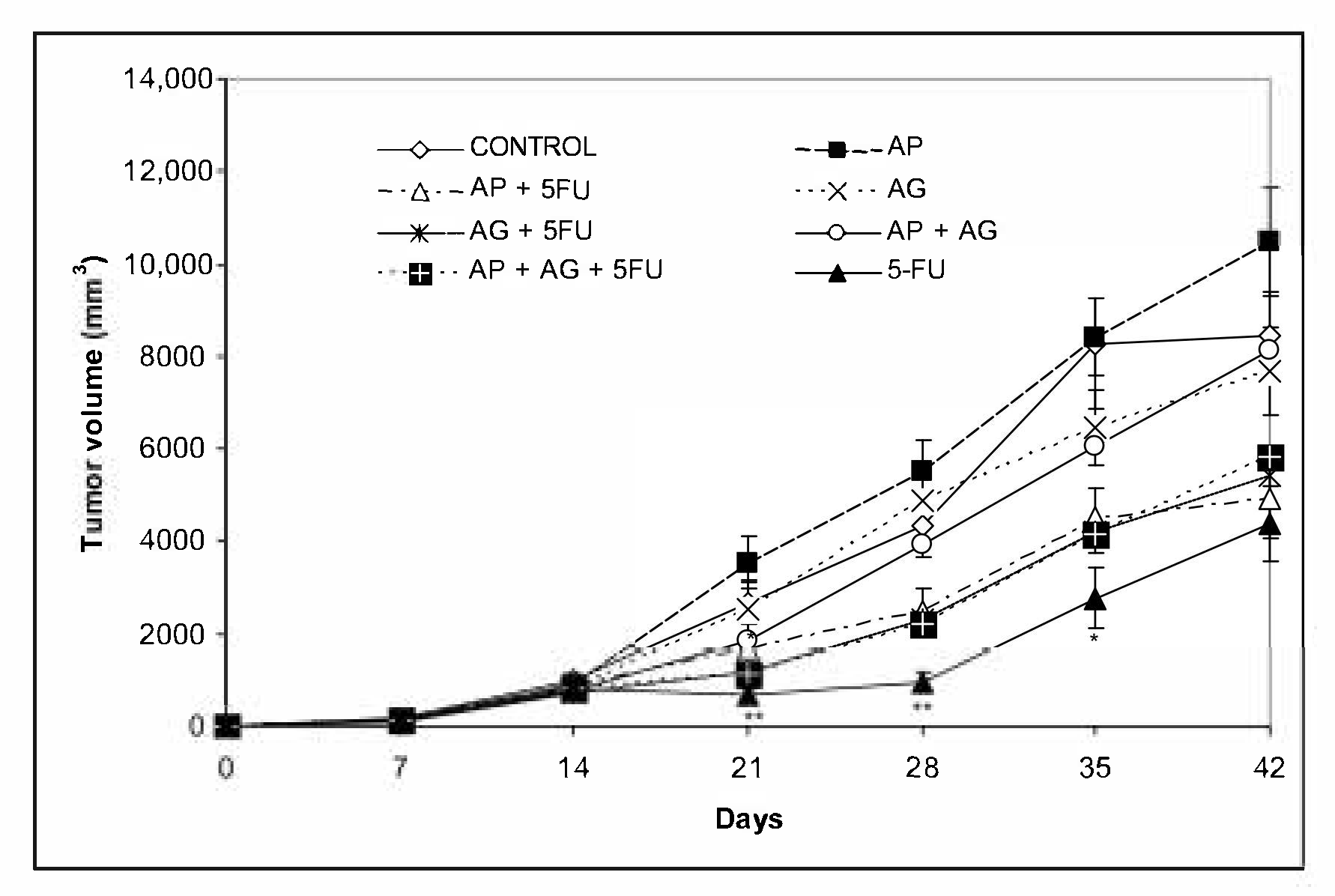
56]. This indicates the differential significance of potential inhibition of tumor growth by activating the immune system versus classical cytotoxic chemotherapy, which has the most pronounced effect on rapidly proliferating tumor cells. In our study, inhibition of tumor growth was most pronounced with the use of 5-FU, while the reduction was less pronounced with the use of AP, AG.1 alone or in combination (
Figure 3). In none of the groups was a synergistic effect of 5-FU with AG.1 or AP on a more pronounced reduction in tumor volume observed, compared to 5-FU alone. However, the best survival was recorded in the group treated with AP + 5FU, although the tumor volume was higher in this group compared to the treatment with 5-FU (
Figure 3,
Table 3), which is consistent with the data that survival in CRC does not show a correlation with volume, namely the size of the primary tumor [
60,
61]. Additionally, survival was better in the AG.1-treated group alone (OS = 50%; OS, overall survival) compared to the AG + 5FU-treated group (OS = 33%), although tumor volume was lower in the latter group. Altogether, Pearson’s correlation coefficient between final tumor volume and overall survival (OS) was
r = −0.533, indicating a moderate negative correlation. In a study on the CT26.WT model, Wu et al. (2016) noted a significant difference in survival between untreated and Balb/c mice treated with 5-fluorouracil (40 mg/kg), starting at day 12 after tumor cell inoculation [
62]. However, although multiple cycles of chemotherapy resulted in a reduced increase in tumor volume, compared to one cycle of chemotherapy, no significant difference in improvement in overall survival (OS) was observed between mice that underwent one (C1) compared to mice that underwent two (C2), three (C3), or four (C4) cycles of 5-fluorouracil chemotherapy. This was associated with only a short-term improved immune response after a single cycle of chemotherapy, as the percentage of tumor infiltrating CD8
+ lymphocytes was increased after cycle 1 of 5-FU (C1) but not after cycle 3 (C3). Repeated cycles of chemotherapy further reduced the number of CD4
+ lymphocytes as well as B-lymphocytes. The same study also noted that tumor growth was inhibited during chemotherapy itself, and accelerated between chemotherapy cycles, which is also consistent with the observed dynamics of tumor growth in the groups that included 5-FU administration in our study (
Figure 3). Finally, this study confirms the independence of survival parameters relative to tumor volume on the CT26 model, which is consistent with clinical data.
In addition to the Th1 and Th2 immune responses which are analogous to those mediated by M1 and M2 macrophages, respectively, Th17 are the third major subtype of Th cells that produce IL-17, IL-6, IL-8, and IL-26 and promote inflammation and granulocyte activity [
63]. IL-23 is a cytokine that is essential as a key regulator of IL-17 expression, and is necessary for the induction, survival, and expansion of Th17 cells [
64]. Th17 cells are characterized by their plasticity, and can, depending on the tumor microenvironment, have protumor effects, largely by stimulating tumor angiogenesis, while in other cases IL-17 stimulates IFN-γ
+ effector T lymphocytes and NK cells in the tumor microenvironment, leading to increased antitumor immunity [
65]. Adaptive transmission has shown that Th17 cells have a superior antitumor effect over Th1 and Th2 cells, and are considered long-term memory cells that mediate long-term antitumor immunity due to their longer retention in the tumor microenvironment. Due to the above, it has been suggested that Th17 and Th1 cells are phenotypically and functionally related in the tumor microenvironment [
66]. Macrophage Toll-like receptors (TLRs) are one of several groups of receptors involved in promoting non-specific immunity. Fungal polysaccharides can stimulate TLR signaling and lymphocytes and macrophages via TLR4 receptors, while TLR2, which is thought to be primarily a receptor for bacterial lipoproteins, activates dendritic cells, T lymphocytes, and NK cells [
67]. Induction of cytokines such as TNF-α and MIP-2 (CXCL2) requires signals from TLR2 and TLR6 [
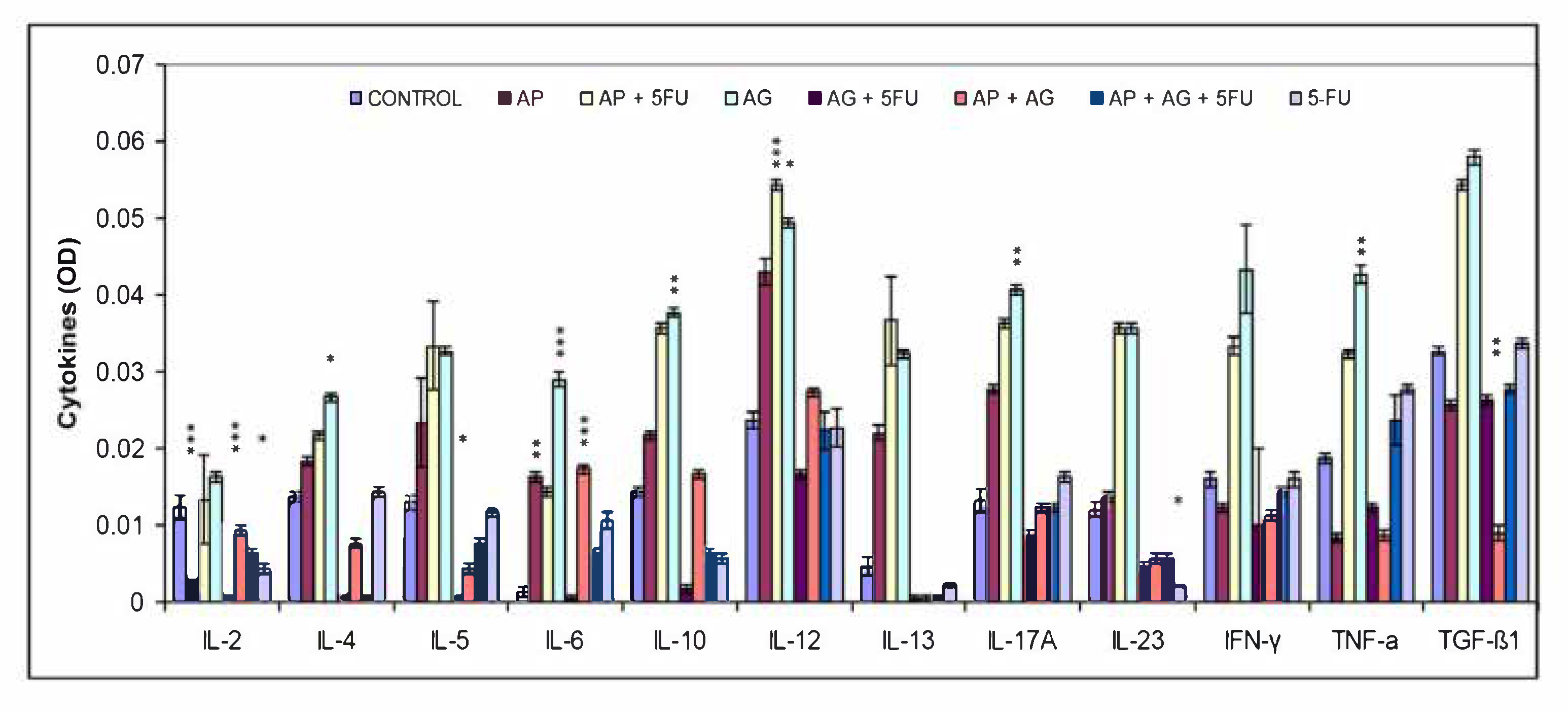
68]. In order to assess the polarization of the immune response, the proportions of Th1/Th2/Th17 cytokines in the serum of Balb/c mice were quantified after treatment with AP, AG.1, 5-FU and their mutual combinations (
Figure 4). An increase in Th1 cytokines compared to the control group was observed especially in the groups treated with AP (IL-6, IL-12), AP + 5FU and AG.1 (IL-6, IL-12, IFN-γ, TNF-α). A significant increase in the Th17 cytokines IL-17α and IL-23, as well as IL-6 in AG.1, was also observed in the groups treated with AP + 5FU and AG.1. IL-12, the main mediator and activator of innate immune cells, in addition to macrophages, also stimulates NK cells, which leads to the synthesis of IFN-γ, which has a strong antiproliferative role. Cytokines have pleiotropic properties and often, depending on the (microenvironmental) context, can act to stimulate or suppress tumor development. Thus, TNF-α is known to be a proinflammatory cytokine that can stimulate tumor growth, angiogenesis and metastasis, through activation of NF-κB, VEGF and MMPs, while in other cases its antitumor role mediated by tumor stroma destruction predominates by stimulating cytotoxic T lymphocyte and macrophage tumor infiltration and dendritic cell activation, which can lead to strong regression of malignant tumors [
69,
70]. TGF-β is also an example of a differentiating cytokine, which has contextually different roles, and most often in the early tumor stages has proapoptotic or antitumor, while in the later tumor stages it can stimulate epithelial-mesenchymal transition [
71]. Given that a significant increase in TGF-β1 was also observed in the groups treated with AP + 5FU and AG.1 which also have a significant increase in Th1 cytokine levels, it can be concluded that TGF-β had antiproliferative properties [
72]. In the same groups, the highest increase in IL-10 was observed, which is important for the normal function of helper CD4
+ lymphocytes, CD4
+ lymphocyte-mediated immune response, and suppression of tumor-mediated inflammation. Thus IL-10 represents a key antitumor cytokine [
73]. Although AP or AG.1 alone showed significant effects on the secretion of different cytokine subgroups, no cumulative effect on elevated cytokine levels was observed in the group treated with AP + AG.1, which may indicate a competition of AP and AG.1 components for immune cell receptors.
In addition to the cytokine profile, the key difference between M1 and M2 macrophages is in their amino acid metabolism, which plays key roles in their functions. M1 macrophages are characterized by increased expression of iNOS (NOS2) and production of NO, which is an important effector of their microbiocidal and tumoricidal activity. The roles of NO in tumor development and progression are multiple; at low concentrations (<100 nM) it stimulates proliferation and angiogenesis, at medium (100–500 nM) increased invasiveness, metastasis, and suppression of apoptosis, while at higher (>500 nM) it stimulates DNA damage, oxidative stress, cytotoxicity due to peroxynitrite production and apoptosis [
74]. Arginase 1, which in addition to M2 macrophages in the tumor microenvironment is classically secreted by myeloid-derived suppressor cells (MDSC), has various immunosuppressive roles. In addition to its suppressive effect on NO, arginase 1 has been shown to inhibit T lymphocyte receptor (TCR) expression in the tumor microenvironment and thus obstruct specific antigen-dependent responses. Arginase 1, like arginase 2, hydrolyzes L-arginine to urea and L-ornithine, which is the main substrate for polyamine formation. Polyamines are necessary for cell cycle and tumor progression [
75]. High arginase activity has been reported in patients with gastric, colon, breast, and lung cancers. By analyzing the obtained results of NO and arginase-1 concentrations in the samples, it has been proven that the tested preparations and their combinations have a significant effect on macrophage polarization. Thus, in all treated groups, an increased concentration of NO was recorded compared to control, and is present in cytotoxic concentrations (higher than 500 nM) (
Table 4). The most significant increase in NO concentration was recorded in the groups treated with AP + 5-FU, AP + AG.1 and 5-FU, which are also the three groups with the best survival. On the other hand, the activity of arginase-1, one of the main markers of M2, as well as TAM macrophages, was significantly reduced in all treated groups compared to the untreated group, and has reached statistical significance in the groups treated with AP + 5-FU, AG.1 and 5-FU, which showed the best survival. Based on the above data, we conclude that macrophage polarization from tumor (M2) to antitumor (M1) phenotype occurs in all treated groups. Furthermore, repolarization of TAM in M1 macrophages results in other, non-immune effects mediated by macrophages, such as a reduction in levels of proangiogenic factors such as VEGF, MMP-2, and MMP-9. The effect of polarization of tumor-associated macrophages (TAM) from immunosuppressive to activated i.e., tumoricidal M1 macrophage phenotype has been recognized as one of the main antitumor effects of β-glucans [
76]. Bioactive polysaccharides from medicinal mushrooms have previously been thought to stimulate an immune response independent of T lymphocytes. It was subsequently found that β-glucans, after being processed into low molecular weight carbohydrates, bind to MHC-II (major histocompatibility complex class II) within dendritic cells, which can lead to direct activation of CD4
+ lymphocytes and Th1 responses [
77]. Furthermore, it is known that when administered at certain doses and intervals (chemotherapeutic protocol), some chemotherapeutics may have targeted (acting on tumor cells) and non-targeted (acting on immune cells) immunostimulatory effects [
22].
Proteolytic degradation of the basement membrane and surrounding extracellular matrix (ECM) is a necessary process in tumor metastasis and spread. Matrix metalloproteinases, such as MMP-2 and MMP-9, in addition to degrading most ECM proteins, also affect the activity of other proteases, growth factors, cytokines, and various cell surface ligands and receptors [
78]. Although in most tumors reduced levels of MMP-2 and MMP-9 correlate with better prognosis, and reduced invasiveness and metastasis, the role of matrix metalloproteinases is pleiotropic [
79]. This also explains the fact that MMP inhibitors developed in the clinic have proved unsuccessful [
80]. MMP-3, -7, -9, and -12 in addition to plasmin have been found to participate in the release of angiostatin from plasminogen thereby reducing endothelial cell proliferation and migration [
81]. MMP-9 cleaves and activates antiangiogenic precursors angiostatin, endostatin, and tumstatin by its protease activity, which all have an inhibitory effect on tumor growth and vascular tumor density [
82]. Tumor stage also has a significant effect on the effects of matrix metalloproteinases. Thus, in advanced colon cancer (CRC), high levels of MMP-12 have been associated with better prognosis, while increased levels of TIMP, the negative tissue regulator of MMP, correlated with a negative prognosis in that cancer [
83]. This dual role of matrix metalloproteinases is also visible in our results, where in all treated groups, except those treated with AP, an increased concentration of MMP-2 was found compared to the control (
Table 6). The concentration of MMP-9, on the other hand, was increased compared to the untreated group in the groups treated with AP or AG.1, and slightly decreased in the groups treated with 5-FU alone or in combination with AP or AG.1 (
Table 6). In general, a very weak correlation was found between MMP-2 and MMP-9 levels and survival and tumor volume.
Angiogenesis is a key process necessary for tumor growth and metastasis, and is defined as formation of new blood vessels from the existing microvasculature [
84]. Because VEGF-VEGFR is the major signaling pathway for tumor vascular formation and remodeling, monitoring changes in VEGF levels is one of the standard methods for assessing antiangiogenic therapies [
38]. In a number of tumor types, including CRC, VEGF has been recognized as a negative prognostic factor [
85]. The use of antiangiogenic therapies may improve the outcomes of conventional antitumor therapies due to increased tumor specificity and reduced development of tumor cell resistance [
86]. The obtained results show a significant decrease in VEGF concentration in all treated groups compared to the control (
Table 5). These results are in line with previous research on the effects of certain types of medicinal mushrooms. Cao and Lin (2006) found that the Gl-PP polysaccharopeptide isolated from
Ganoderma lucidum has antiangiogenic properties resulting from direct apoptosis of HUVEC (human umbilical vein endothelial cells) cells, as well as a decrease in antiapoptotic Bcl-2 expression and an increase in Bax expression in these cells [
87]. Since VEGF levels were also reduced in the groups where elevated concentrations of MMP-2 and MMP-9 were recorded, this further indicates the previously mentioned dual role of matrix metalloproteinases, which likely leads to increased production of antiangiogenic factors that contribute to reduced tumor aggressiveness and an increased survival. Our results of the cumulative effect of metronomically dosed administration of 5-fluorouracil and immunotherapeutic action of AG and AP.1 on the reduction of VEGF levels also confirm the results of previous studies. Wada et al. (2007) confirmed that the combination of interferon-α immunotherapy and metronomically dosed 5-fluorouracil has a cumulative effect on reducing angiogenesis and tumor growth in a mouse model [
88].
Due to its sensitivity, the comet test or single cell gel electrophoresis (SCGE) in alkaline conditions is a suitable method for assessing different types of DNA damage, such as single-stranded and double-stranded DNA breaks, apurine and apyrimidine sites, and DNA repair [
89]. Thus, by measuring cytotoxic or genotoxic effects using a comet test on tumor cells, it is possible to determine the therapeutic effectiveness, while on healthy cells it is a standard method of determining the potential adverse effects of therapies. In our research, we performed comet assay analysis on peripheral leukocytes and hepatocytes, in order to establish potential genotoxic effects of treatment on healthy tissues. Our results of the alkaline comet test on peripheral blood leukocytes and hepatocytes indicate a slight increase in all parameters of the comet test, which is statistically significant almost everywhere due to the large number of samples (
Table 7 and
Table 8). Since a large number of samples are used in the analysis of comet test results, where each cell represents one statistical sample, the possibility of exaggerating statistical significance as a measure of biological effect is particularly emphasized. Therefore, in order to facilitate the assessment of the biological effect, i.e., the significance of comet test results as a measure of cytotoxicity or genotoxicity, we compared absolute values between individual parameters of a particular treated group in comparison with negative control, as described by [
90]. A single substance may have cytotoxic or genotoxic effects depending on the dose. A genotoxic substance can also have mutagenic effects, making it a carcinogenic substance. As a reliable method of distinguishing the cytotoxicity and genotoxicity of an individual substance using a comet test, the relative ratio (FC, fold change) of individual parameters is used, most simply the tail moment, which is the product of the remaining two parameters [
91]. Daza et al. (2004) compared the tail moment relative ratios of cytotoxic (cordycepin, fluorodeoxyuridine, puromycin) and known genotoxic (camptothecin, actinomycin C) substances on leukocyte cells. Mean tail moment parameter of cytotoxic substances ranged from 0.8–1.07, while genotoxic substances were characterized by significantly higher tail moments, which were 5.16–8.27 for actinomycin C and 6.30–12.13 for camptothecin, depending on the applied concentration of the substance [
92]. In our study, we found that in all treated groups, the obtained tail moment ratios exclude genotoxic effect of the tested preparations Agarikon Plus, Agarikon.1, 5-fluorouracil, and their mutual combinations. Our data for 5-fluorouracil are in agreement with existing data on its tail-moment ratios when this drug was used in various chemotherapeutic protocols [
93]. The non-genotoxic effects of tested mushroom extracts is likewise in line with previous data which confirm that several polysaccharides in clinical use have been shown to lack long-term toxicity, i.e., genotoxicity or mutagenicity, and can mitigate the genotoxic effects of chemotherapy and radiotherapy [
94].
In conclusion, our data confirms antitumor effectiveness of blended medicinal mushroom preparations Agarikon.1 and Agarikon Plus in the advanced colorectal cancer mouse model, which is a good basis for further translational research. Furthermore, our results support the usefulness of such preparations use in enhancement of the effectiveness of standard cytotoxic chemotherapies by improving their immunomodulating, antiangiogenic and antimetastatic properties.