Abstract
Most data published on curcumin and curcumin-based formulations are very promising. In cancer research, the majority of data has been obtained in vitro. Less frequently, researchers used experimental animals. The results of several clinical studies are conclusive, and these studies have established a good foundation for further research focusing on implementing curcumin in clinical oncology. However, the issues regarding timely data reporting and lack of disclosure of the exact curcumin formulations used in these studies should not be neglected. This article is a snapshot of the current status of publicly available data on curcumin clinical trials and a detailed presentation of results obtained so far with some curcumin formulations. Phenomena related to the observed effects of curcumin shown in clinical trials are presented, and its modifying effect on gut microbiota and metabolic reprogramming is discussed. Based on available data, there is a strong indication that curcumin and its metabolites present molecules that do not necessarily need to be abundant in order to act locally and benefit systemically. Future clinical studies should be designed in a way that will take that fact into consideration.
1. Introduction
Therapeutic efforts in the mid 20th century—era preceding smart drugs—were commonly based on very rigid, almost technocratic, attitudes toward many diseases, including cancer. The introduction of highly cytotoxic and non-selectively acting compounds, thought to be potent enough to achieve a complete breakthrough in cancer treatment, did not fulfill early expectations [1]. While the effect of these early synthesized drugs was carefully monitored, they also needed to be continuously modified to improve selectivity and efficacy, while reducing toxic side effects and cross resistance [2]. Even improved versions of originally synthesized and applied compounds have been commonly used with limited benefit for patients and are associated with unavoidable side effects [3].
Numerous observational studies have recorded therapeutic responses varying among patients suffering from the same type of malignant tumor. This reflects heterogeneity that has yet to be fully understood. At minimum, it should be considered on two levels.
Between-tumor heterogeneity occurs as a consequence of patient-to-patient differences. It can be a major obstacle to predicting a therapeutic response, even in those patients whose tumors express a targetable marker [4]. Well-known examples are breast cancers with amplified receptor tyrosine-protein kinase erbB-2 (HER2/neu) [5]. Within-tumor heterogeneity, resulting from phenotypic and functional cellular diversity within the tumor, directs the outcome of antitumor therapy. This is commonly discussed in the context of therapeutic failure [6].
These obstacles in clinical oncology have been a beneficial moving force for encouraging multidisciplinary research efforts to discover compounds which would be not only highly therapeutically effective but also critically selective. New and powerful technologies have been the basis for advances in molecular biology and molecular medicine. In parallel, factual knowledge regarding the precise mechanisms by which certain molecules exert their mode of action became a clinical reality.
Many processes are very dynamic, including those relevant to tumor development and its response to therapy. They take place during specific time windows during which the host may be affected by varied and numerous external factors.
Currently, we are witnessing therapeutic decisions being guided by an understanding of the molecular features of tumor tissues which develop in the specific genetic background of its host [7]. Even in these situations, in which we appear to be able to precisely determine and characterize the molecular therapeutic target, the side effects of the therapy do not diminish to a satisfactory comfort level.
We are able to study already developed cancer from the point of its mutational status integration into the whole network of aberrant signaling pathways, which are tightly interconnected in the tumor. They are then still highly dependent on the specific parameters of the host itself. Consequently, various cancer features, with their well-known hallmarks, develop as a result of permanent interaction with the host [8]. Diet is of significant influence on these.
Indeed, the influence of diet and nutritional status on cancer occurrence has been investigated and discussed very intensively. While it is well recognized that certain diets definitely are beneficial when facing many diseases, including cancer, some recommendations and observations have yet to be confirmed in properly designed studies [9]. The Mediterranean diet is a good example of a healthy diet. It is based on consuming non-refined cereals, nuts, olive oil, legumes, fish and plenty of fresh fruits and vegetables. With a high content of antioxidative compounds and anti-inflammatory nutrients, the diet may be considered even as chemopreventive with respect to cancer. Still, one cannot mechanistically confirm protective effects of the diet itself just because the dietary pattern produces numerous positive effects during an extended period of time [10]. What all too often remains under the radar is the genetic constitution of the host.
2. Curcumin-Pleiotropic Molecule in a Setting of Personalized Medicine
In this complex setting, which tends to be and should be the basis for a precise and personalized therapeutic approach, it is very difficult to understand the exact molecular mechanism(s) of action of molecules that are pleiotropic. Curcumin, which is used in foods for its typical color, flavor and as a preservative, has been well known for its beneficial effects in traditional medicine for centuries. These days, numerous biological effects were shown to be the bona fide consequence of its three fundamental roles: an antioxidative role when present in low concentration [11], its protein binding properties [12] and its chelating activities [13]. Two of these properties make curcumin yet one more “double-edged sword molecule” because it can also act as a prooxidative molecule when applied in a high concentration [14], and has the potential to decrease systemically available iron [15]. However, the most recent data indicates that curcumin may enhance the effects of low-dose iron supplementation, especially in individuals with iron deficiency, who are otherwise healthy [16].
The natural source of curcumin (diferuloylmethane; IUPAC: (1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) is the rhizome of the medicinal plant, Curcuma longa, a perennial herb in the Zingiberaceae family [17]. The yellow color of the rhizome originates from a mixture of three curcuminoides, one of which is curcumin. The content of the commercial turmeric rhizomes can significantly vary as to curcuminoides content, within a range from 0.58% to 6.5% on a dry weight basis [18,19]. It is highly dependent on many factors, including the seed sources, plant age, harvesting and drying processes. The amount and composition of specific curcuminoides in the rhizome also vary. It is commonly reported to be a composition of curcumin (CUR—diferuloylmethane, 77%), demethoxycurcumin (DEM, 17%) and bis-demethoxycurcumin (bis-DEM, 3%) [20]. The stated percentage values are averaged and are considered “standard”.
3. Low Bioavailability: How to Understand the Mechanism of Action?
There are numerous studies showing curcumin’s low bioavailability, which is commonly estimated based on its very low level in plasma, when and if detectable. The most important factors, described in a recent review, which explain a low bioavailability, are its poor water solubility, chemical instability, rapid metabolism, short half-life and poor intestinal absorption [21].
Early experiments on the application of curcumin and its bioavailability in rats, showed that the feces contained 75% of the orally applied curcumin [22]. Similarly, oral administration of radiolabeled curcumin led to 89% radioactivity in feces and 6% in urine [23]. The poor bioavailability of curcumin has been one of the major challenges in understanding its mode of action, especially when interpreting the available data on its chemopreventive or beneficial effects in vivo, commonly in animals and rarely in humans. However, the fact that curcuminoides and their metabolites may be present in colon mucosa [24] has opened a quite broad horizon for exploring its effects on gut microbiota, which can manifest as various local and, especially, systemic effects. Its modulatory effect on gut microbiota may, at least in part, explain some interesting phenomena observed and published in the literature. Indeed, as clearly proposed in a recently published review [25], an existing discrepancy between curcumin’s poor bioavailability and chemical instability on the one hand, and convincingly manifested activities recorded in vitro and in vivo on the other hand, deserve to be considered. It is particularly important to understand the relevant aspects of the oral application of curcumin, considering the fact that it affects one complex superorganism, that the human host and its symbiotic microbiome represents [26].
The first record of curcumin application in modern medicine dates back to 1937, when it was used for treating 67 patients suffering from various forms of subacute, recurrent, or chronic cholecystitis [27]. The positive therapeutic response recorded then was the basis for the future interest in curcumin and its healing properties, especially its anti-inflammatory properties. They were among the first studied and, when considered from the perspective of the modern medical era, may be very interesting with respect to several cancer hallmarks, especially for preventing the tumor-promoting inflammation and metabolic reprogramming. Many of its biological roles, including these two, relate to its ability to act as a powerful electron donor and metal ion chelator, as already stated. A strong and well-known activity against reactive oxygen species (ROS) is due to the presence of conjugated double bonds in its chemical structure [28]. Already in 2009, it was shown that its α,β-unsaturated β-diketo moiety makes the chemical basis for metal ions chelation, as shown for iron [29].
Although commonly called a “drug” or “therapeutic”, and notwithstanding encouraging data that show its mostly beneficial effects in quite heterogeneous scenarios, as yet, curcumin cannot be and should not be considered any of these. There are many reasons for this, and some of them have been addressed and thoroughly discussed in several recent papers [30,31]. Without wanting to discourage further scientific research and medical therapeutic development involving curcumin, here, we plan to address several levels of inconsistency connected with the clinical trials currently listed, including those relating to the need for timely reporting the data/results [32].
4. Current Status of Curcumin-Related Oncological Clinical Studies Listed at ClinicalTrials.Gov
For the purpose of this review, we analyzed the current status of curcumin in cancer translational research during August and September of 2020, at several levels. First, we stratified clinical studies relating to cancer and curcumin currently listed at National Institutes of Health (NIH) clinicaltrails.gov, on the following parameters: the status of the study, the type of cancer and the formulation given. Second, we collected and selected data to be presented for showing the effect of precise curcumin formulations aimed at improving its bioavailability. For selected formulations currently used in clinical trials, the effects obtained in vitro and in animal models are also presented. This set of data is expanded by including previously published studies. A significant portion covers clinical trials and is published in the journals indexed in Web of Science that are also available at PubMed. Third, we discussed the effect of curcumin as presented in new data on the gut microbiota and some exciting aspects of curcumin’s systemic effects. Fourth, we presented curcumin’s possible effect in modifying cancer cell metabolism.
We summarized the status of currently listed clinical trials relating to curcumin in clinical oncology which are available at ClinicalTrials.gov, “a database of privately and publicly funded clinical studies conducted around the world”. The “condition or disease” was defined as “Cancer” and was combined with the word “Curcumin” under “Other Terms”. The search yielded 69 clinical studies. Out of these 69 clinical studies, 10 were not directly related to cancer treatment, but were mainly focused on testing curcumin’s effect in alleviating radiation/chemotherapy side effects.
Out of 59 studies that were or are, focused on cancer, 11 were excluded from the detailed analyses. Two studies were based on applying turmeric either alone (NCT03061591) or combined with Omega-3+ Vitamin D (NCT03290417). Two studies were based on applying curcumin with various polyphenols (NCT03482401) and anthocyanins (NCT01948661) in breast and colorectal cancer patients, respectively. Three studies were based on applying minimal curcumin and vitamin D in leukemia (NCT02100423), cervical/uterine (NCT03192059) and pancreatic cancer patients (NCT02336087). In the remaining two studies, curcumin was combined with Q10 for myelodysplastic syndrome (NCT00247026) and with fish oils for lung cancer patients (NCT03598309). Two chemopreventive studies (colon) compared the effect of curcumin vs. Sulindac (NCT00176618) and curcumin vs. Sulindac vs. rutin vs. quercetin (NCT00003365).
The Status of Clinical Studies with Curcumin Only (n = 48)
The remaining 48 studies may be stratified by different criteria, of which the status of the study may be a good indication of the current confusion regarding implementing curcumin into cancer clinical arena.
So far, 21 studies were indicated as completed (n = 21). The status of the remaining 27 studies varies: active, not recruiting (n = 5), recruiting (n = 4), not yet recruiting (n = 3), currently suspended due to COVID-19 (n = 1), terminated (n = 3), withdrawn (n = 4) and unknown status (n = 7).
We were particularly interested in the reason for the termination or the withdrawal of studies. For the two studies, the reason for termination was for “futility in view of the results of the interim analysis”, and “for futility in view of the results of the anticipated analysis”. The termination of the third study was attributed to “technology problems and cost constraints”. With respect to withdrawn studies, only one study (NCT00248053) claimed that: “Subsequent data generated by our collaborators have shown efficacy with curcumin and quercetin in 5 patients in a non-placebo-controlled trial”. There is no explanation for withdrawing the remaining three studies. With respect to common claims which tend to present curcumin and its derivatives as potent antitumor agents, this set of data is not optimistic.
Seven studies are presented with “unknown status”, because the status of the study has not been verified within the past two years. However, after performing an additional search, we were able to find out that one of these studies has published results in a peer-reviewed journal (NCT02138955) [33] and one study (NCT00689195) published results unrelated to curcumin [34].
For the rest of the five studies with “unknown status”, we were unable to find published data (NCT00973869, NCT02554344, NCT00295035, NCT00486460, NCT02321293).
We found that one study (NCT02724618) listed as “active, non-recruiting”, has results published with “nanocurcumin”, which was given to prostate cancer patients undergoing radiotherapy in a randomized, double-blind, placebo-controlled phase II trial. The precise formulation of the compound given: nanocurcumin-SinaCurcumin® used, was not described. No significant difference was shown between the SinaCurcumin® and placebo group with respect to radiation-induced cystitis, hematologic findings and apparent diffusion coefficient (ADC) [35].
Out of 21 studies with the status “completed”, only four have reported results obtained on the ClinicalTrials.gov web page (NCT01740323, NCT00641147, NCT00113841, NCT00365209). Out of these four studies, only one (NCT00641147) reported that results are published in a peer-reviewed journal [36]. The design of that study relied on a pure, 100% curcumin that was taken two times a day for 12 months in a cohort of patients (n = 22) who were family members of patients diagnosed with familial adenomatous polyposis (FAP) and having intestinal adenomas. While the treatment itself was well-tolerated, there was no significant difference between the mean number of polyps in the placebo group as compared with the curcumin group. The fact that the intake of 1.5 g curcumin twice daily is well-tolerated does not seem to be a surprise, since the earlier clinical research data showed good tolerance and safety at doses between 4 and 8 g/day, even up to 12 g/day [37]. However, it was very disappointing that there was no effect of curcumin on intestinal adenomas which was the primary target of the trial. Even more so, because previously, Carrol’s study showed a strong effect of curcumin with respect to a decrease in the number of aberrant crypt foci (ACF), which was achieved with oral intake of four grams of curcumin for a month [38]. We were able to find published results from seven other completed studies (NCT01160302, NCT01490996, NCT03211104, NCT03072992, NCT01917890, NCT01712542 and NCT02017353), which, unfortunately, have not reported results on the ClinicalTrials.gov website. The results from the first study (NCT01160302) will be explained in a subsection on microbiota and interleukin 17 (IL-17) [39]. The results from the second study (NCT01490996) were published in three papers, showing the effects of conventional cancer therapy combined with curcumin [40,41,42].
The third study, NCT03211104, did not precisely specify the formulation of curcumin (“curcuminoid powder in capsule form”) given to prostate cancer patients with intermittent androgen deprivation in a randomized, double-blind, placebo-controlled trial [43]. Increase in prostate-specific antigen (PSA) during the time of curcumin intake (6 months) was significantly less than in the placebo group (p =0.0259). The fourth study (NCT03072992) was based on CUC-1R applied intravenously together with paclitaxel to the patients with advanced, metastatic breast cancer.
The group of patients receiving CUC-1R + paclitaxel had statistically significant improved ORR (overall response rate) when compared with the group receiving paclitaxel + placebo (p < 0.01) [44].
The results from the fifth study (NCT01917890) were based on applying curcumin formulation BCM-95® to prostate cancer patients being treated with radiotherapy [45]. The sixth study (NCT01712542) was based on giving micellar NovaSol formulation to glioblastoma patients (57.4 mg curcumin, 11.2 mg demethoxycurcumin, 1.4 mg bisdemethoxycurcumin). Curcumin was present in tumorous tissue (average, 56 pg/mg) but its amount significantly varied among tumors (range 9–151 pg/mg) [46].
The seventh, open-label, non-randomized phase 2 study (NCT02017353), was based on the hypothesis that curcumin supplementation might influence inflammatory biomarker levels in patients (n = 7) suffering from endometrial carcinoma (EC). The patients consumed Curcumin Phytosome-Meriva® orally, 2 g/day, for 2 weeks. The results of the study were not definitely conclusive [47].
All together, among 48 analyzed studies based on application of curcumin, four studies reported results at ClinicalTrials.Gov., of which one published the data. Among 21 studies with the “completed” status, seven studies, resulting in nine papers, did not report the data at ClinicalTrials.Gov.
Based on these data, the problem becomes clear: the studies have been designed to explore the efficacy of curcumin being given to patients with various types of premalignant lesions/cancer, using heterogeneous curcumin formulations that are to be given (or were given) in daily doses which significantly differ. There are various application methods utilized during significantly different time periods. As well, the hosts (patients) differ in many respects, especially genetically (between-tumor heterogeneity). A host’s genetics is the only parameter that cannot be changed for improving curcumin clinical trials. Everything else, including the design of the studies and disclosing details on curcumin formulations given, can be and should be improved.
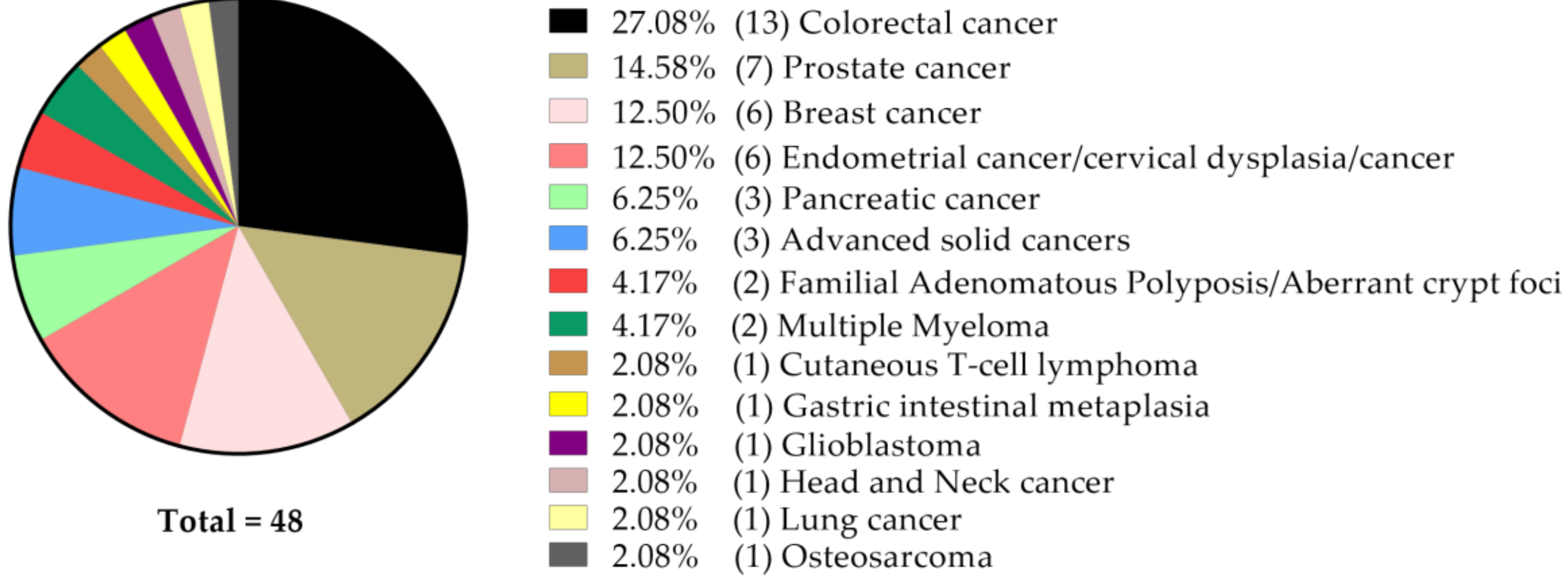
With respect to the type of cancer, a majority of the 48 studies focused on colorectal (n = 13) and prostate (n = 7) cancer, followed by breast (n = 6), uterine (endometrium and cervix, n = 6) and pancreatic cancer (n = 3) models (Figure 1).
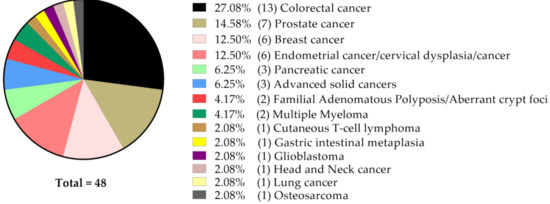
Figure 1.
Number and percentage of studies focused on a particular type of cancer, according to its anatomical origin.
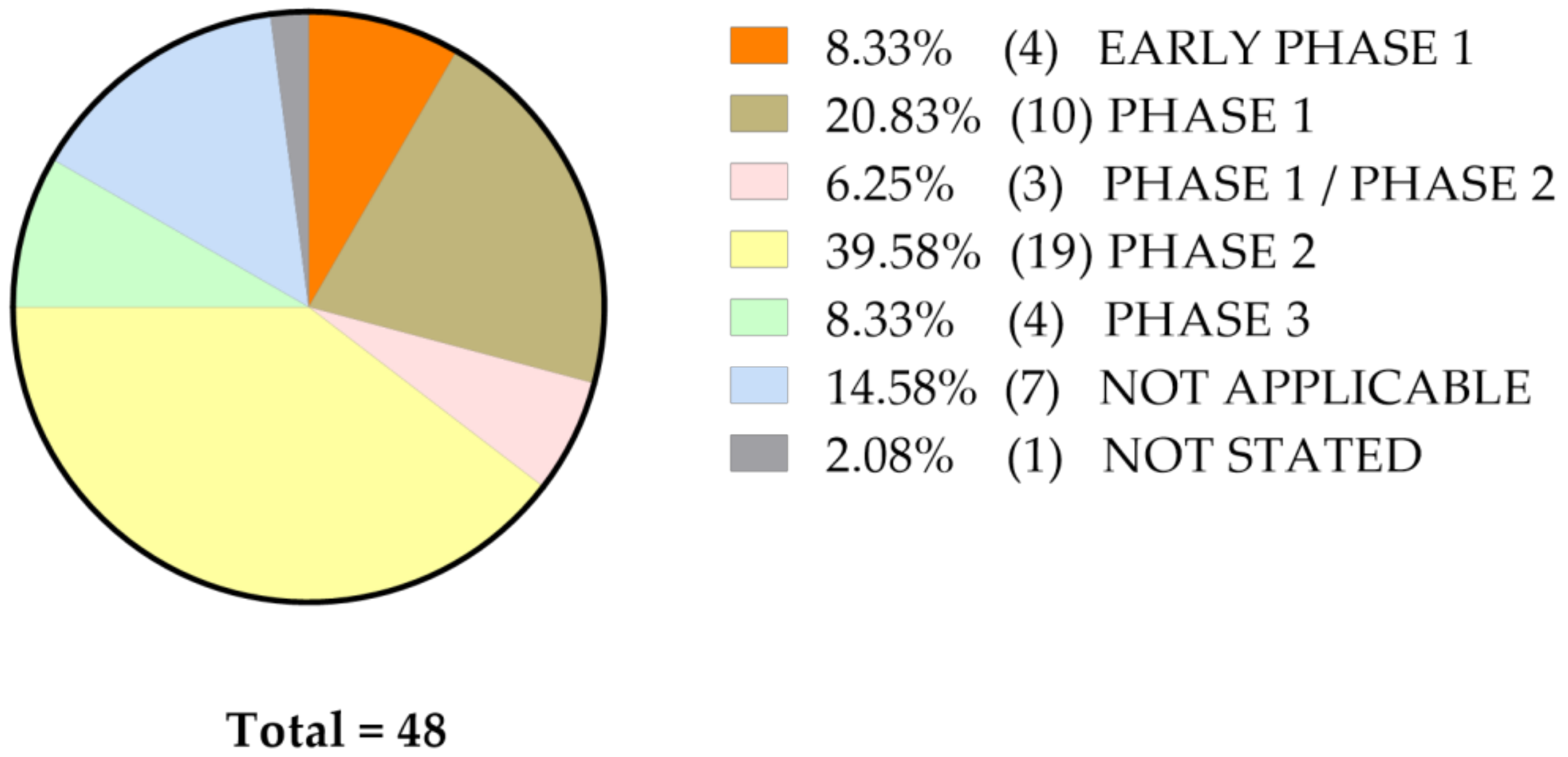
The inclusive criteria and final goals are highly variable, which leads to stratifying these studies in different stages of clinical trials, as shown on Figure 2.
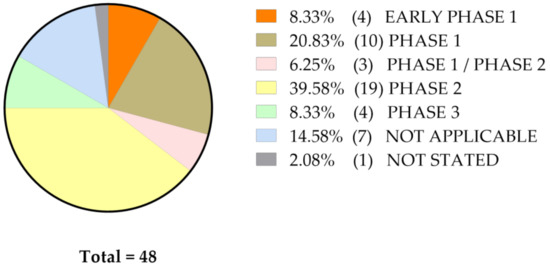
Figure 2.
Reported phases of clinical trials involving curcumin, regardless of its formulation and type of malignant disease.
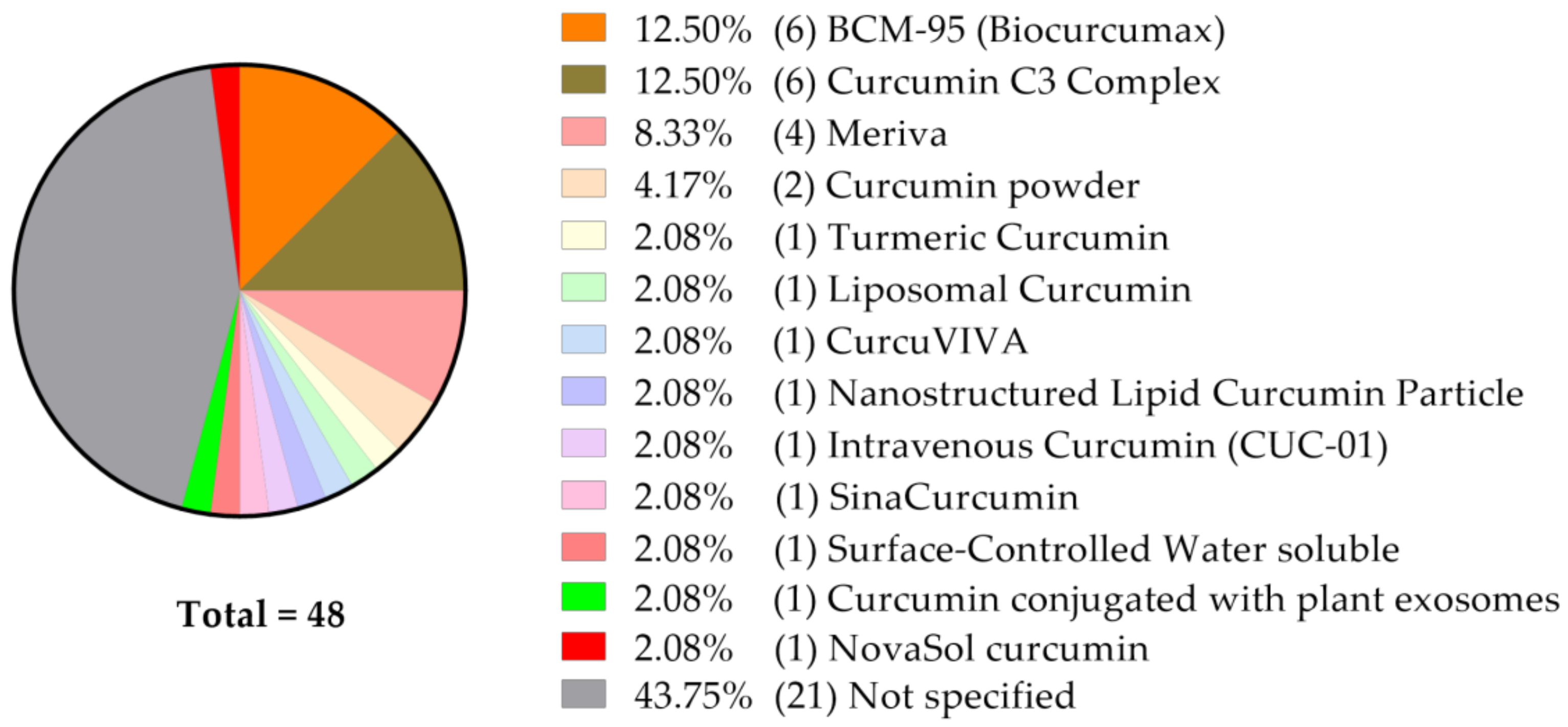
A very high portion of studies do not disclose the precise curcumin formulation given (Figure 3).
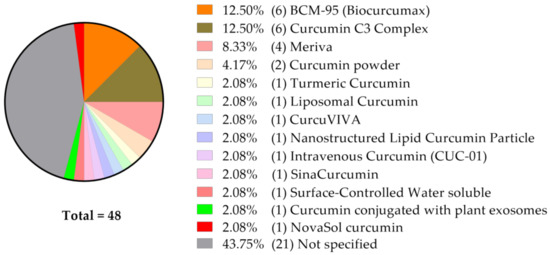
Figure 3.
Among 48 clinical studies currently listed at ClinicalTrials.gov and analyzed as described, 43.75% (n = 21) do not specify the curcumin formulation used/to be used.
5. The Effect of Curcumin Formulations Targeted at Improving its Bioavailability
Curcumin Phytosome-Meriva®, the formulation given in the last study described in the previous section, is only one of many curcumin-based formulations targeted at improving its bioavailability. Already in 1998, Shoba et al. described how piperine, the constituent of black pepper, then already known to be an inhibitor of hepatic and intestinal glucuronidation, increased curcumin’s bioavailability in humans by 2000%. This conclusion was based on curcumin’s value in serum [48]. Since then, many formulations were synthesized and tested [21].
As presented on Figure 3, BCM-95® (95% curcuminoid complex with preserved natural ratio of curcumin, demethoxycurcumin, and bisdemethoxycurcumin and essential oil of turmeric [49], Table 1), Curcumin C3 Complex® (reported formulations vary: curcumin: 77–90%, demethoxycurcumin: 6–17%, bis-demethoxycurcumin: 3–4% [50,51], Table 2) and Meriva® (curcumin, soy lecithin, microcrystalline cellulose, 18–20% curcuminoids [52], Table 3) appear to be the most commonly explored in the clinical trials we followed. For that reason, we have decided to present as much information as possible on these formulations when applied in vitro, in animal models and when tested in clinical trials.

Table 1.
Effects of BCM-95® (Biocurcumax™) obtained in vitro, in vivo and in clinical trials.

Table 2.
Effects of C3 Complex® obtained in vitro, in vivo and in clinical trials.

Table 3.
Effects of Meriva® obtained in vitro, in vivo and in clinical trials.
We extended our search to three currently promising formulations: liposomal curcumin (Lipocurc™, Table 4), the blood-brain barrier crossing Longvida® (solid lipid curcumin particle, phosphatidylcholine and 20% curcumin [53], Table 5) and a colloidal nanoparticle-based formulation of curcumin, Theracurmin™ (THC; 10% w/w of curcumin, 2% of other curcuminoids such as demethoxycurcumin and bisdemethoxycurcumin, 46% of glycerin, 4% of gum ghatti and 38% of water [54], Table 6), which was shown to exert a very strong antiproliferative effect, especially in combination with NAD(P)H quinone dehydrogenase 1 (NQO1) inhibitor [55]. For all formulations but Lipocurc™, the pharmacokinetics data obtained in humans were recently reviewed [56,57]. For Lipocurc™, the available data on humans indicates stable curcumin plasma concentrations during infusion, and soon thereafter, a rapid decline to an undetectable level [33].

Table 4.
Effects of Lipocurc™ obtained in vitro, in vivo and in clinical trials.

Table 5.
Effects of LongVida® obtained in vitro, in vivo and in clinical trials.

Table 6.
Effects of Theracurmin™ obtained in vitro, in vivo and in clinical trials.
It is well-known that curcumin may improve the therapeutic effect of many tested drugs. We have written about that earlier in the context of its inhibitory effect on nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) [58], in combined cancer therapy. Here, we present combined treatments for cancer with the earlier listed formulations in vitro, in animal models and in clinical studies. Case reports are not presented. Papers in which formulations were not specifically disclosed are not included in the tables presented here. Although the U.S. Food and Drug Administration (FDA) considers curcumin as a GRAS (Generally Recognized as Safe) molecule, some side effects were observed in clinical studies when higher doses were taken. These adverse effects are also shown in the tables.
6. Microbiota and Curcumin: Acting Locally Benefiting Systemically?
It is now well-established that curcumin can influence intestinal microflora directly [118] and indirectly through the effect of active curcumin’s metabolites on metabolic activity of gut microbiota. The major processes included are demethoxylation, reduction, hydroxylation, methylation and acetylation of the parent compound. This was shown for the first time in 2015, when 23 metabolites were characterized after incubating human stool with 100 μM curcumin [119]. There are many research results showing curcumin’s effect on gut microbiota in rats, mice and humans. However, it has to be clearly stated that one must avoid making any kind of parallel between curcumin’s effect on gut microbiota based on results obtained in different species, because there is a significant difference in the relative abundance of major gut bacterial families and genera in mice, rats, non-human primates and in humans [120].
A prospective, single-center, evaluator-blinded randomized pilot study including a total of 32 adults, showed that turmeric/curcumin influences the content of gut microbiota in healthy humans in a highly personalized way. However, changes in the pattern of microbial species abundance were highly similar in a turmeric group (1 g turmeric root—6 g daily (Curcuma longa) plus 1.25 mg black pepper-derived extract of piperine alkaloid (BioPerine)) and a curcumin group (combination of C3—6 g daily, and BioPerine). This suggests that curcumin indeed has a major influence on microbiota composition alterations. An increase of Clostridium spp. and Bacteroides spp. was particularly strong [121]. An increase of Bacteroides spp. was also shown in mice fed a curcumin diet [122].
In another mouse model, in which animals were fed a diet containing 0.2% (w/w) nanoparticle curcumin—Meriva®, the clinical presentation of experimentally induced inflammatory bowel disease (IBD) was significantly improved and was associated with an increase of Clostridium cluster IV and Clostridium subcluster XIVa. The stool contained a high level of one anti-inflammatory short-chain fatty acid (SCFA)—butyrate. The content level of CD4+ Foxp3+ regulatory T cells and CD103+ CD8α− CD11c+ dendritic cells in intestinal mucosa was high [123]. These findings agree with previously published research papers, unrelated to curcumin, showing butyrate (a) as an inducer of Treg cell differentiation in colon, which is crucial for suppressing inflammatory and allergic responses [124], and (b) as a suppressor of colonic epithelial stem/progenitor cell proliferation [125]. Thus, it seems that some beneficial effects of curcumin obtained locally and systemically, with respect to various pathological conditions, may indeed be related to its effect on butyrate-producing gut bacteria.
This may be of a particular relevance for appreciating and understanding the beneficial effects of curcumin observed in colon cancer patients, known since 2005 [91]. We can now consider those data from a very interesting perspective, relating to curcumin’s effect on microbiota. Earlier this year, Sánchez-Alcoholado et al. have shown that colon cancer patients have significantly decreased abundance of Bacteroidetes [126]. If curcumin can induce increased abundance of Bacteroides spp. (which should be in accord with Reference [121]), then maybe the butyrate potentially benefited the participants in the study published back in 2005 [91].
In our recent paper, we considered the importance of curcumin with respect to interleukin-17 (IL-17)-related effects in humans, especially in the field of oncology [127]. We discussed the fact that curcumin strongly decreases the serum level of IL-17 at one hour post-ingestion of C3 Complex® (p = 0.0342), as shown in 15 head and neck squamous cell carcinoma patients and eight healthy volunteers [39]. The possible reason for IL-17 decrease may well be the curcumin’s effect on gut microbiota.
A mouse model of experimental colitis was explored by Zhao and collaborators, showing that activation of intestinal dendritic cells by curcumin leads to enhanced suppressive functions of Treg cells joined with a decrease in several mediators of inflammation in colonic mucosa, including IL-17. Of note, the decrease was measurable, but did not reach statistical significance [128]. In humans, using an organ culture chamber, Larussa et al. have shown, based on 35 human gastric biopsies, that 200 μM curcumin significantly decreases IL-17 in both gastric biopsies (p = 0.0003) and culture supernatants (p = 0.0001) [129]. The mechanism described may facilitate the persistence of the bacterium locally due to a decreased inflammatory reaction [129]. However, one may ask whether decreased IL-17 production in the colon, as a consequence of the presence of curcumin/curcumin’s metabolites, may also be beneficial for the host, due to the possible influence even on such harmful systemic effects as hypertension [130], mediated by IL-17.
It is well-known and was recently reviewed in depth that the role of IL-17 in cancer may be suppressive or promotive [131]. For that reason, the precise role of this cytokine, in a well-defined cellular setting, should be entirely ascertained before coming to a firm conclusion on curcumin’s role through modulation of IL-17 secretion.
There are data showing that gut microbiota depletion by antibiotics leads to a significant decrease in subcutaneous tumor burden in pancreatic cancer and melanoma models in mice. This is only in animals having mature T and B lymphocytes [132]. In those animals, there was a significant increase in anti-tumor interferon gamma(IFNγ)-secreting T cells (IFNγ + CD3+), associated with a decrease in IL17a (IL17a + CD3+) and IL10 (IL10 + CD4 + CD3+) secreting immune populations. If the animals were treated with IL-17a neutralizing monoclonal antibody, the tumor-attenuating effect of antibiotics was abrogated. Thus, at least in this mouse model, the essential role of IL-17a in tumorigenesis seems to be clearly confirmed.
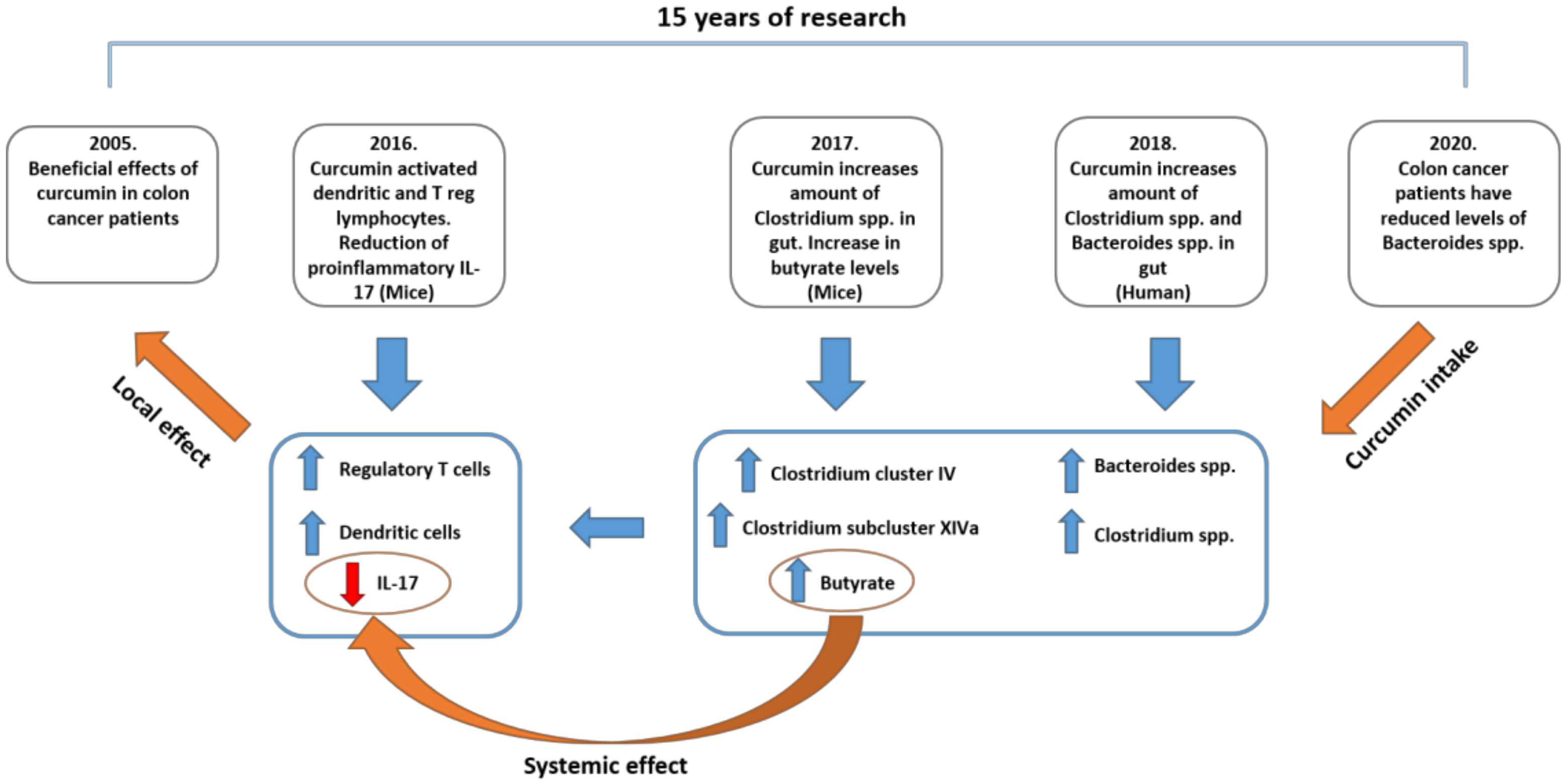
Based on all these data, especially on the very recent data, it appears that curcumin does not need to be measurable in plasma in order to exert an effect. It has the ability to modulate the gut microbiota and reprogram differentiation of crucial T-cell population(s) in the gut. Thus, indeed, it may have the potential to act locally while also reaching numerous targets systemically (Figure 4).
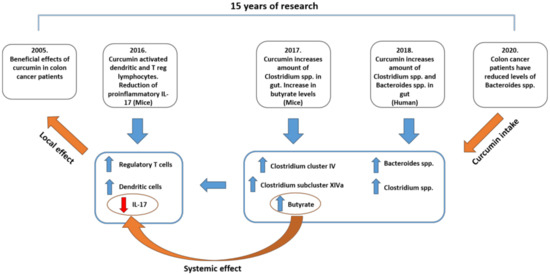
Figure 4.
A proposed model for explaining systemic effects consequential to curcumin’s local activity. The local effect obtained in the clinic back in 2005 can be explained through recent findings on gut microbiota.
7. Curcumin in Targeting Metabolic Liabilities in Cancer
Metabolic heterogeneity needs to be considered in the context of the developmental program of epithelial-to-mesenchymal transition (EMT), which allows cancer cells to acquire features needed for their survival. These include increased migratory potential, an ability to invade tissues around the primary tumor, extravasation into lymphatics or blood vessels and migration to distant sites in the body [133]. This factor significantly contributes to microenvironment-mediated metabolic heterogeneity of cancer tissue. Proliferation of disseminated cancer cells at distant sites is possible because the migrated cells pass through a mesenchymal-to-epithelial transition (MET) once they reach their destination [134]. Metabolic reprogramming in cancer, involving an increase in glucose uptake, glycolytic flux and lactate production, contributes to the acquisition of cancer stem cell properties and tumor aggressiveness [135]. This takes place by an orchestrated activity of expressing EMT-inducing transcription factors (TF) and their transcriptional targets, some of which are metabolic enzymes.
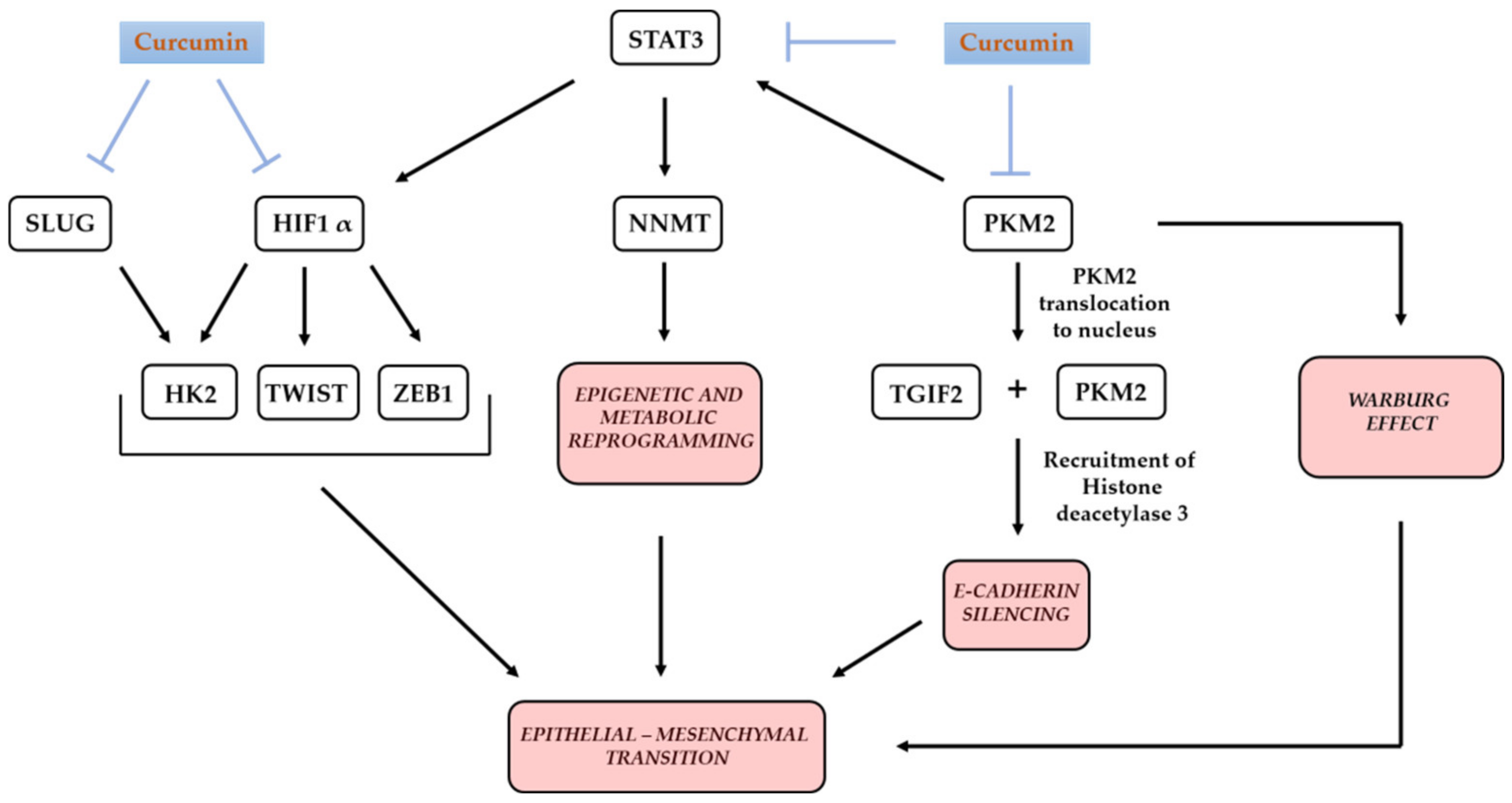
In 2006, the suppressive effect of curcumin on transcriptional activity of HIF1A gene under hypoxia was shown [136]. The protein product coded by this gene, Hypoxia-Inducible Factor 1-Alpha-HIF1α, is a transcription factor, central to metabolic reprogramming in cancer and EMT-induction [137].
HIF1α contributes to EMT at many levels, which can be significantly affected with pleiotropic curcumin’s action. For example, through silencing of HIF1A, curcumin can silence expression of its transcriptional target hexokinase 2 (HK2), the enzyme that catalyzes the first step of glycolysis (phosphorylation of glucose to glucose-6-phosphate). Increased expression of HK2 under hypoxia directed by HIF1α has been reported to promote EMT in tongue squamous carcinoma [138]. In addition to through HIF1α, curcumin can influence the multiple, cell-type-specific transcriptional control of HK2 by decreasing the activity of STAT3 [139] and snail family transcriptional repressor 2-SLUG. In 2016, the existence of this second scenario was shown in breast cancer cell lines [140]. Dissociation of HK2 from the colon cancer cells mitochondria through an unknown mechanism was also shown [141].
Pyruvate kinase isoform PKM2, which catalyzes the final step of glycolysis and promotes the Warburg effect, and is a positive regulator of STAT3 activity [142], has been shown to promote EMT. Nuclear translocation of PKM2 and its direct interaction with a transcriptional cofactor repressor TGF-β-induced factor homeobox 2 (TGIF2), causes recruitment of histone deacetylase 3 to E-cadherin promoter, leading to EMT through suppression of E-cadherin transcription [143]. Curcumin has been shown to downregulate the expression of PKM2 [144,145] and to influence its nuclear localization, albeit in a cell-type-specific manner [145]. In line with curcumin’s negative effect on PKM2 expression and nuclear localization, upregulation of E-cadherin upon curcumin treatment has been reported in various types of cancer cells [146,147,148,149]. Concomitant with reducing HIF1α expression, curcumin reduces the expression of EMT-inducing factors Twist Family bHLH Transcription Factor (TWIST) and Zinc Finger E-Box Binding Homeobox 1 (ZEB1) [150], since HIF1α-binds to hypoxia-response elements (HRE) in their promoters [151,152].
In addition to hypoxia, nutrients availability has a profound influence on the interdependent processes of metabolic and epigenetic reprogramming in cancer [153]. Prolonged glucose deprivation has been shown to contribute to the phenotypic plasticity of ovarian serous carcinoma cells (OSCS), characterized by acquiring stem cell properties and resistance to glucose restriction, dependent on the expression of nicotinamide N-methyltransferase (NNMT) [154]. This enzyme methylates nicotinamide (NAM) and impairs methylation of histones and other proteins in cancer cells [155]. The increased expression of NNMT was shown in numerous, various malignant tumors [156]. Its active presence induces epigenetic and metabolic reprogramming for promoting the mesenchymal phenotype of cancer cells through increasing migration, invasion, proliferation and survival. On the other hand, knockdown of NNMT suppresses cancer cell migration and EMT. NNMT was shown to be a critical regulator of EMT in esophageal cancer cell lines and was suggested to be a potential therapeutic target for metastatic squamous cell carcinomas [157].
NNMT is under the transcriptional control of STAT3, which is often overexpressed in various types of cancer. Curcumin, by direct binding to cysteine residue 259, prevents STAT3 phosphorylation, dimerization and translocation into the nucleus, as shown in H-Ras-transformed breast epithelial cells, H-Ras MCF10A [158].
In the cancer cells which rely on STAT3-mediated increase of NNMT expression, this can be of the utmost importance. NNMT is thought to be “a poor prognostic biomarker for patients with solid tumors”, based on a meta-analysis performed on 3340 patients [159]. If the cells lose NNMT, as a result of curcumin’s action, then they lose a powerful oncogenic player—a master metabolic regulator of cancer-associated fibroblasts (CAF)—as shown in high-grade OSCS [160]. In line with the negative effect of curcumin on STAT3 activity, downregulated expression of NNMT was shown in vitro, in MDA-MB-468 breast cancer cells and HT29 colon cancer cells [161].
The activity of NNMT promoter is regulated in a complex, tissue-specific fashion [162]. Keeping in mind the importance of the cell-type-specific modes of transcriptional control, it deserves to be explored in depth in which types of tumors curcumin exerts its negative effect on EMT through STAT3-mediated silencing of NNMT, as well as HK2.
A simplified presentation of some aspects of curcumin’s modifying effects on metabolic reprogramming and EMT is shown in Figure 5.
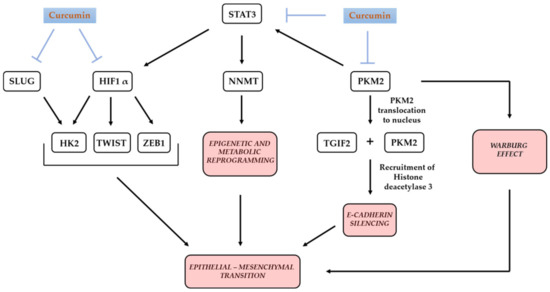
Figure 5.
Simplified presentation of an inhibitory effect of curcumin on key molecular players at the cross-roads of epithelial-to-mesenchymal transition and metabolic reprogramming in cancer cell.
Curcumin-Mediated Suppression of NNMT and Other Metabolic Enzymes in Circumventing Therapy Resistance
The connection between nutrient status, NNMT expression and its role in EMT should be considered with respect to cancer therapy. It has been recently reported that the expression of NNMT in human esophageal squamous cell carcinoma cell lines decreases sensitivity to 5-Fluorouracil (5-FU) by promoting the Warburg effect. In a cell line with high NNMT expression (TE1 cells), the sensitivity was low, while the highly 5-FU-sensitive EC1 and Eca109 cells had a low NNMT expression. Downregulation of NNMT in TE1 cells increased their sensitivity to 5-FU, accompanied by lower glucose consumption, lactate production, and the expression of hexokinase 2, lactate dehydrogenase A and phosphoglycerate mutase 1 [163]. Curcumin was shown to increase the sensitivity of cancer cells to 5-FU through the silencing of NF-κB [58]. Considering its pleiotropism and ability to modify activity of numerous cellular molecules, it is conceivable that enhanced sensitivity to 5-FU by curcumin (Table 1 and Table 2) may be a direct consequence of its silencing effect on the axis STAT3/NNMT, and not only NF-κB and its targets. Decrease in the strength of the axis STAT3/HK2, which was recently unquestionably shown as a mechanism responsible for 5-FU resistance in HNSCC [164], may also be the mechanism with which curcumin increases sensitivity to conventional chemotherapy in vitro [61,66,67,68,72,84,96].
Finally, some metabolic enzymes were shown to be not only crucial for sustaining cancer cells’ metabolic needs, but also for development of therapy resistance. For example, phosphoglycerate dehydrogenase (PHGDH), the rate-limiting enzyme of de novo serine biosynthesis, is commonly overexpressed in malignant tumors and was recently shown to promote bortezomib resistance of multiple myeloma cells [165]. Serine hydroxymethyltransferase (SHMT2) catalyzes the conversion of serine to glycine, and is essential to cell survival under hypoxic condition, when it is under transcriptional control of HIF-1α [166]. Silencing of SHMT2 increases sensitivity of hepatocellular cancer cells to doxorubicin [167]. To which extent curcumin’s direct binding to these two enzymes [12,168] contributes to previously shown reversion of the chemoresistant phenotypes related to these two drugs [169] remains to be explored.
8. Conclusions
Curcumin’s influence on energy metabolism and stress-response-relevant proteins is tightly linked to redox status in cancer [170]. Curcumin’s activity is mediated by its oxidative metabolites. The varying extent of its redox-dependent bioactivation in diverse in vitro and in vivo models contributes to the current inconclusive results regarding its influence on a particular target [171]. Still, its beneficial effects shown in studies performed in vitro, in vivo and in clinical trials should be considered from as many perspectives as possible. The varying effects of curcumin, which could be due to its metabolites, are a potential advantage, applied in the proper context [172]. The major obstacles to obtaining consistent and measurable results when studying curcumin’s effect in the context of a specific cancer cell line in vitro or cancer in vivo are accentuated by the numerous variables in a vivid biological system. They are inherent in the tumor cell tissue of origin, are driven by oncogenotype (mutations in different cancer-relevant genes result in different phenotypes of tumors arising in the same tissue) and arise by the interaction with tumor microenvironment (vascularization-dependent nutrient and oxygen availability, local nutrient milieu, dependent on non-malignant cells and host microbiome) [173]. It is conceivable that this metabolic heterogeneity and flexibility makes cancer treatment, including targeting its metabolic liabilities, difficult to treat [174]. Still, there are numerous excellent research data which should accompany double-blinded, placebo-controlled clinical trials, and become a moving force for ever more fruitful research and eventual therapy. For meaningful basic medical science, curcumin’s effects need to be critically measured, presented and put in the context of findings that do not necessarily relate to curcumin research. The scope for understanding the way of acting of a pleiotropic molecule must be very broad. At the same time, several aspects of clinical trials nesed to be improved so that these efforts can be applied toward the particular cancer and other targets needing reliable attention.
Author Contributions
Conceptualization, K.G.T.; methodology, K.G.T; validation K.G.T., M.T. and A.M.; formal analysis, K.G.T., I.S., M.T. and N.Đ.; investigation, K.G.T., R.N.K., I.S., M.T. and N.Đ.; resources, K.G.T. and N.Đ.; data curation, K.G.T., R.N.K., I.S. and N.Đ.; draft writing—K.G.T.; writing—review and editing, K.G.T., R.N.K., I.S., N.Đ., M.T. and A.M.; visualization, K.G.T., M.T. and I.S.; supervision, K.G.T.; project administration, K.G.T.; funding acquisition, K.G.T. and N.Đ. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the Croatian Science Foundation under its grant: IP-2016-06-4404, NRF2 at the crossroads of epigenetic remodeling, metabolism and proliferation of cancer cells, to K.G.T.—PI, and the financial support of the University of Zagreb for the research: Polymorphism of the NQO1 and presence of CD45+ and CD8+ in the tumor stroma—predictors of the therapy response?
Acknowledgments
The authors are grateful to Aaron Etra for his work on English editing. Even with our best efforts, we are aware that some articles may have been missed and apologize to any authors whose work was inadvertently not included.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Farber, S.; Diamond, L.K. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N. Engl. J.Med. 1948, 238, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Rixe, O.; Ortuzar, W.; Alvarez, M.; Parker, R.; Reed, E.; Paull, K.; Fojo, T. Oxaliplatin, tetraplatin, cisplatin, and carboplatin: Spectrum of activity in drug-resistant cell lines and in the cell lines of the National Cancer Institute’s Anticancer Drug Screen panel. Biochem. Pharmacol. 1996, 52, 1855–1865. [Google Scholar] [CrossRef]
- Weiss, R.B.; Christian, M.C. New cisplatin analogues in development. A review. Drugs 1993, 46, 360–377. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.C.; Sorger, P.K. Combination Cancer Therapy Can Confer Benefit via Patient-to-Patient Variability without Drug Additivity or Synergy. Cell 2017, 171, 1678–1691. [Google Scholar] [CrossRef] [PubMed]
- Gajria, D.; Chandarlapaty, S. HER2-amplified breast cancer: Mechanisms of trastuzumab resistance and novel targeted therapies. Expert Rev. Anticanc. 2011, 11, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.; Pusztai, L.; Swanton, C. Cancer heterogeneity: Implications for targeted therapeutics. Br. J. Cancer 2013, 108, 479–485. [Google Scholar] [CrossRef]
- Yuan, M.; Huang, L.L.; Chen, J.H.; Wu, J.; Xu, Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal. Transduct Target. Ther. 2019, 4, 61. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Moazzen, S.; van der Sloot, K.J.W.; Bock, G.H.; Alizadeh, B.Z. Systematic review and meta-analysis of diet quality and colorectal cancer risk: Is the evidence of sufficient quality to develop recommendations? Crit Rev. Food Sci. Nutr. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean Diet: A Review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.L.; Huang, T.S.; Lin, J.K. Curcumin, an antioxidant and anti-tumor promoter, induces apoptosis in human leukemia cells. Biochim. Biophys Acta 1996, 1317, 95–100. [Google Scholar] [CrossRef]
- Abegg, D.; Frei, R.; Cerato, L.; Prasad Hari, D.; Wang, C.; Waser, J.; Adibekian, A. Proteome-Wide Profiling of Targets of Cysteine reactive Small Molecules by Using Ethynyl Benziodoxolone Reagents. Angew Chem. Int. Ed. Engl. 2015, 54, 10852–10857. [Google Scholar] [CrossRef]
- Mary, C.P.V.; Vijayakumar, S.; Shankar, R. Metal chelating ability and antioxidant properties of Curcumin-metal complexes-A DFT approach. J. Mol. Graph. Model. 2018, 79, 1–14. [Google Scholar] [CrossRef]
- Paciello, F.; Fetoni, A.R.; Mezzogori, D.; Rolesi, R.; Di Pino, A.; Paludetti, G.; Grassi, C.; Troiani, D. The dual role of curcumin and ferulic acid in counteracting chemoresistance and cisplatin-induced ototoxicity. Sci. Rep. 2020, 10, 1063. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Morón, E.; Calderón-Montaño, J.M.; Salvador, J.; Robles, A.; López-Lázaro, M. The dark side of curcumin. Int. J. Cancer 2010, 126, 1771–1775. [Google Scholar] [CrossRef]
- Tiekou Lorinczova, H.; Fitzsimons, O.; Mursaleen, L.; Renshaw, D.; Begum, G.; Zariwala, M.G. Co-Administration of Iron and a Bioavailable Curcumin Supplement Increases Serum BDNF Levels in Healthy Adults. Antioxidants 2020, 9, 645. [Google Scholar] [CrossRef]
- Ammon, H.P.; Wahl, M.A. Pharmacology of Curcuma longa. Planta Med. 1991, 57, 1–7. [Google Scholar] [CrossRef]
- Tayyem, R.F.; Heath, D.D.; Al-Delaimy, W.K.; Rock, C.L. Curcumin content of turmeric and curry powders. Nutr. Cancer 2006, 55, 126–131. [Google Scholar] [CrossRef]
- Thangavel, K.D.K. Determination of curcumin, starch and moisture content in turmeric by Fourier transform near infrared spectroscopy (FT-NIR). Eng. Agr. Environ. Food 2019, 12, 264–269. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 5, 787–809. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef]
- Wahlstrom, B.; Blennow, G. A study on the fate of curcumin in the rat. Acta Pharmacol. Toxicol. (Copenh) 1978, 43, 86–92. [Google Scholar] [CrossRef]
- Holder, G.M.; Plummer, J.L.; Ryan, A.J. The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica 1978, 8, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Irving, G.R.; Howells, L.M.; Sale, S.; Kralj-Hans, I.; Atkin, W.S.; Clark, S.K.; Britton, R.G.; Jones, D.J.; Scott, E.N.; Berry, D.P.; et al. Prolonged biologically active colonic tissue levels of curcumin achieved after oral administration--a clinical pilot study including assessment of patient acceptability. Cancer Prev. Res. 2013, 6, 119–128. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between Gut Microbiota and Curcumin: A New Key of Understanding for the Health Effects of Curcumin. Nutrients 2020, 12, 2499. [Google Scholar] [CrossRef]
- Salvucci, E. The human-microbiome superorganism and its modulation to restore health. Int. J. Food Sci. Nutr. 2019, 70, 781–795. [Google Scholar] [CrossRef]
- Oppenheimer, A. Turmeric (curcumin) in biliary diseases. Lancet 1937, 229, 619–621. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- Jiao, Y.; Wilkinson, J.t.; Di, X.; Wang, W.; Hatcher, H.; Kock, N.D.; D’Agostino, R., Jr.; Knovich, M.A.; Torti, F.M.; Torti, S.V. Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood 2009, 113, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Devassy, J.G.; Nwachukwu, I.D.; Jones, P.J. Curcumin and cancer: Barriers to obtaining a health claim. Nutr. Rev. 2015, 73, 155–165. [Google Scholar] [CrossRef]
- DeVito, N.J.; Bacon, S.; Goldacre, B. Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: A cohort study. Lancet 2020, 395, 361–369. [Google Scholar] [CrossRef]
- Greil, R.; Greil-Ressler, S.; Weiss, L.; Schonlieb, C.; Magnes, T.; Radl, B.; Bolger, G.T.; Vcelar, B.; Sordillo, P.P. A phase 1 dose-escalation study on the safety, tolerability and activity of liposomal curcumin (Lipocurc™) in patients with locally advanced or metastatic cancer. Cancer Chemother. Pharmacol. 2018, 82, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Pires, N.; Gota, V.; Gulia, A.; Hingorani, L.; Agarwal, M.; Puri, A. Safety and pharmacokinetics of Withaferin-A in advanced stage high grade osteosarcoma: A phase I trial. J. Ayurveda Integr. Med. 2020, 11, 68–72. [Google Scholar] [CrossRef]
- Saadipoor, A.; Razzaghdoust, A.; Simforoosh, N.; Mahdavi, A.; Bakhshandeh, M.; Moghadam, M.; Abdollahi, H.; Mofid, B. Randomized, double-blind, placebo-controlled phase II trial of nanocurcumin in prostate cancer patients undergoing radiotherapy. Phytother. Res. 2019, 33, 370–378. [Google Scholar] [CrossRef]
- Cruz-Correa, M.; Hylind, L.M.; Marrero, J.H.; Zahurak, M.L.; Murray-Stewart, T.; Casero, R.A., Jr.; Montgomery, E.A.; Iacobuzio-Donahue, C.; Brosens, L.A.; Offerhaus, G.J.; et al. Efficacy and Safety of Curcumin in Treatment of Intestinal Adenomas in Patients With Familial Adenomatous Polyposis. Gastroenterology 2018, 155, 668–673. [Google Scholar] [CrossRef]
- Lao, C.D.; Ruffin, M.T.t.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.E.; Benya, R.V.; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens, F.L., Jr.; et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev. Res. (Phila) 2011, 4, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Latimer, B.; Ekshyyan, O.; Nathan, N.; Moore-Medlin, T.; Rong, X.; Ma, X.; Khandelwal, A.; Christy, H.T.; Abreo, F.; McClure, G.; et al. Enhanced Systemic Bioavailability of Curcumin Through Transmucosal Administration of a Novel Microgranular Formulation. Anticancer Res. 2015, 35, 6411–6418. [Google Scholar]
- Howells, L.M.; Iwuji, C.O.O.; Irving, G.R.B.; Barber, S.; Walter, H.; Sidat, Z.; Griffin-Teall, N.; Singh, R.; Foreman, N.; Patel, S.R.; et al. Curcumin Combined with FOLFOX Chemotherapy Is Safe and Tolerable in Patients with Metastatic Colorectal Cancer in a Randomized Phase IIa Trial. J. Nutr. 2019, 149, 1133–1139. [Google Scholar] [CrossRef]
- James, M.I.; Iwuji, C.; Irving, G.; Karmokar, A.; Higgins, J.A.; Griffin-Teal, N.; Thomas, A.; Greaves, P.; Cai, H.; Patel, S.R.; et al. Curcumin inhibits cancer stem cell phenotypes in ex vivo models of colorectal liver metastases, and is clinically safe and tolerable in combination with FOLFOX chemotherapy. Cancer Lett. 2015, 364, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Irving, G.R.; Iwuji, C.O.; Morgan, B.; Berry, D.P.; Steward, W.P.; Thomas, A.; Brown, K.; Howells, L.M. Combining curcumin (C3-complex, Sabinsa) with standard care FOLFOX chemotherapy in patients with inoperable colorectal cancer (CUFOX): Study protocol for a randomised control trial. Trials 2015, 16, 110. [Google Scholar] [CrossRef]
- Choi, Y.H.; Han, D.H.; Kim, S.W.; Kim, M.J.; Sung, H.H.; Jeon, H.G.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Lee, H.M.; et al. A randomized, double-blind, placebo-controlled trial to evaluate the role of curcumin in prostate cancer patients with intermittent androgen deprivation. Prostate 2019, 79, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Saghatelyan, T.; Tananyan, A.; Janoyan, N.; Tadevosyan, A.; Petrosyan, H.; Hovhannisyan, A.; Hayrapetyan, L.; Arustamyan, M.; Arnhold, J.; Rotmann, A.R.; et al. Efficacy and safety of curcumin in combination with paclitaxel in patients with advanced, metastatic breast cancer: A comparative, randomized, double-blind, placebo-controlled clinical trial. Phytomedicine 2020, 70, 153218. [Google Scholar] [CrossRef]
- Hejazi, J.; Rastmanesh, R.; Taleban, F.A.; Molana, S.H.; Hejazi, E.; Ehtejab, G.; Hara, N. Effect of Curcumin Supplementation During Radiotherapy on Oxidative Status of Patients with Prostate Cancer: A Double Blinded, Randomized, Placebo-Controlled Study. Nutr. Cancer 2016, 68, 77–85. [Google Scholar] [CrossRef]
- Dützmann, S.; Schiborr, C.; Kocher, A.; Pilatus, U.; Hattingen, E.; Weissenberger, J.; Geßler, F.; Quick-Weller, J.; Franz, K.; Seifert, V.; et al. Intratumoral Concentrations and Effects of Orally Administered Micellar Curcuminoids in Glioblastoma Patients. Nutr. Cancer 2016, 68, 943–948. [Google Scholar] [CrossRef]
- Tuyaerts, S.; Rombauts, K.; Everaert, T.; Van Nuffel, A.M.T.; Amant, F. A Phase 2 Study to Assess the Immunomodulatory Capacity of a Lecithin-based Delivery System of Curcumin in Endometrial Cancer. Front. Nutr. 2018, 5, 138. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Antony, B.; Merina, B.; Iyer, V.S.; Judy, N.; Lennertz, K.; Joyal, S. A Pilot Cross-Over Study to Evaluate Human Oral Bioavailability of BCM-95CG (Biocurcumax), A Novel Bioenhanced Preparation of Curcumin. Indian J. Pharm Sci. 2008, 70, 445–449. [Google Scholar] [CrossRef]
- Garcea, G.; Jones, D.J.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J.; Berry, D.P. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br. J. Cancer 2004, 90, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Dahmke, I.N.; Backes, C.; Rudzitis-Auth, J.; Laschke, M.W.; Leidinger, P.; Menger, M.D.; Meese, E.; Mahlknecht, U. Curcumin intake affects miRNA signature in murine melanoma with mmu-miR-205-5p most significantly altered. PLoS ONE 2013, 8, e81122. [Google Scholar] [CrossRef]
- Cuomo, J.; Appendino, G.; Dern, A.S.; Schneider, E.; McKinnon, T.P.; Brown, M.J.; Togni, S.; Dixon, B.M. Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. J. Nat. Prod. 2011, 74, 664–669. [Google Scholar] [CrossRef]
- Gota, V.S.; Maru, G.B.; Soni, T.G.; Gandhi, T.R.; Kochar, N.; Agarwal, M.G. Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J. Agric. Food Chem. 2010, 58, 2095–2099. [Google Scholar] [CrossRef]
- Sasaki, H.; Sunagawa, Y.; Takahashi, K.; Imaizumi, A.; Fukuda, H.; Hashimoto, T.; Wada, H.; Katanasaka, Y.; Kakeya, H.; Fujita, M.; et al. Innovative preparation of curcumin for improved oral bioavailability. Biol. Pharm. Bull. 2011, 34, 660–665. [Google Scholar] [CrossRef]
- Mizumoto, A.; Ohashi, S.; Kamada, M.; Saito, T.; Nakai, Y.; Baba, K.; Hirohashi, K.; Mitani, Y.; Kikuchi, O.; Matsubara, J.; et al. Combination treatment with highly bioavailable curcumin and NQO1 inhibitor exhibits potent antitumor effects on esophageal squamous cell carcinoma. J. Gastroenterol. 2019, 54, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, R. Bioavailable curcumin formulations: A review of pharmacokinetic studies in healthy volunteers. J. Integr. Med. 2018, 16, 367–374. [Google Scholar] [CrossRef]
- Jamwal, R. Corrigendum to “Bioavailable curcumin formulations: A review of pharmacokinetic studies in healthy volunteers” [J. Integr. Med. 16 (2018) 367–374]. J. Integr. Med. 2019, 17, 310. [Google Scholar] [CrossRef]
- Troselj, K.G.; Kujundzic, R.N. Curcumin in combined cancer therapy. Curr. Pharm.Des. 2014, 20, 6682–6696. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Kraehe, P.; Lueders, C.; Shayan, P.; Goel, A.; Shakibaei, M. Curcumin suppresses crosstalk between colon cancer stem cells and stromal fibroblasts in the tumor microenvironment: Potential role of EMT. PLoS ONE 2014, 9, e107514. [Google Scholar] [CrossRef]
- Toden, S.; Okugawa, Y.; Buhrmann, C.; Nattamai, D.; Anguiano, E.; Baldwin, N.; Shakibaei, M.; Boland, C.R.; Goel, A. Novel Evidence for Curcumin and Boswellic Acid-Induced Chemoprevention through Regulation of miR-34a and miR-27a in Colorectal Cancer. Cancer Prev. Res. (Phila) 2015, 8, 431–443. [Google Scholar] [CrossRef]
- Yoshida, K.; Toden, S.; Ravindranathan, P.; Han, H.; Goel, A. Curcumin sensitizes pancreatic cancer cells to gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1 expression. Carcinogenesis 2017, 38, 1036–1046. [Google Scholar] [CrossRef]
- Toden, S.; Okugawa, Y.; Jascur, T.; Wodarz, D.; Komarova, N.L.; Buhrmann, C.; Shakibaei, M.; Boland, C.R.; Goel, A. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis 2015, 36, 355–367. [Google Scholar] [CrossRef]
- Kuriakose, M.A.; Ramdas, K.; Dey, B.; Iyer, S.; Rajan, G.; Elango, K.K.; Suresh, A.; Ravindran, D.; Kumar, R.R.; Prathiba, R.; et al. A Randomized Double-Blind Placebo-Controlled Phase IIB Trial of Curcumin in Oral Leukoplakia. Cancer Prev. Res. (Phila) 2016, 9, 683–691. [Google Scholar] [CrossRef]
- Purbadi, S.; Rustamadji, P.; Prijanti, A.R.; Sekarutami, S.M.; Sutrisna, B.; Suyatna, F.D.; Andrijono. Biocurcumin as Radiosensitiser for Cervical Cancer Study (BRACES): A Double-Blind Randomised Placebo-Controlled Trial. Evid. Based Complement. Alternat. Med. 2020, 2020, 1986793. [Google Scholar] [CrossRef] [PubMed]
- Arun, P.; Sagayaraj, A.; Azeem Mohiyuddin, S.M.; Santosh, D. Role of turmeric extract in minimising mucositis in patients receiving radiotherapy for head and neck squamous cell cancer: A randomised, placebo-controlled trial. J. Laryngol. Otol. 2020, 7, 1–6. [Google Scholar] [CrossRef]
- Shakibaei, M.; Buhrmann, C.; Kraehe, P.; Shayan, P.; Lueders, C.; Goel, A. Curcumin chemosensitizes 5-fluorouracil resistant MMR-deficient human colon cancer cells in high density cultures. PLoS ONE 2014, 9, e85397. [Google Scholar] [CrossRef]
- Du, B.; Shim, J.S. Targeting Epithelial-Mesenchymal Transition (EMT) to Overcome Drug Resistance in Cancer. Molecules 2016, 21, 965. [Google Scholar] [CrossRef]
- Shakibaei, M.; Kraehe, P.; Popper, B.; Shayan, P.; Goel, A.; Buhrmann, C. Curcumin potentiates antitumor activity of 5-fluorouracil in a 3D alginate tumor microenvironment of colorectal cancer. BMC Cancer 2015, 15, 250. [Google Scholar] [CrossRef]
- Siddappa, G.; Kulsum, S.; Ravindra, D.R.; Kumar, V.V.; Raju, N.; Raghavan, N.; Sudheendra, H.V.; Sharma, A.; Sunny, S.P.; Jacob, T.; et al. Curcumin and metformin-mediated chemoprevention of oral cancer is associated with inhibition of cancer stem cells. Mol. Carcinog. 2017, 56, 2446–2460. [Google Scholar] [CrossRef]
- Ravindranathan, P.; Pasham, D.; Balaji, U.; Cardenas, J.; Gu, J.; Toden, S.; Goel, A. A combination of curcumin and oligomeric proanthocyanidins offer superior anti-tumorigenic properties in colorectal cancer. Sci. Rep. 2018, 8, 13869. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Takada, Y.; Kondo, S.; Sawaya, R.; Aggarwal, B.B.; Kondo, Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: Role of Akt and extracellular signal-regulated kinase signaling pathways. Mol. Pharmacol. 2007, 72, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Sung, B.; Kunnumakkara, A.B.; Sethi, G.; Anand, P.; Guha, S.; Aggarwal, B.B. Curcumin circumvents chemoresistance in vitro and potentiates the effect of thalidomide and bortezomib against human multiple myeloma in nude mice model. Mol. Cancer Ther. 2009, 8, 959–970. [Google Scholar] [CrossRef]
- Kim, T.; Davis, J.; Zhang, A.J.; He, X.; Mathews, S.T. Curcumin activates AMPK and suppresses gluconeogenic gene expression in hepatoma cells. Biochem. Biophys. Res. Commun. 2009, 388, 377–382. [Google Scholar] [CrossRef]
- Clark, C.A.; McEachern, M.D.; Shah, S.H.; Rong, Y.; Rong, X.; Smelley, C.L.; Caldito, G.C.; Abreo, F.W.; Nathan, C.O. Curcumin inhibits carcinogen and nicotine-induced Mammalian target of rapamycin pathway activation in head and neck squamous cell carcinoma. Cancer Prev. Res. (Phila) 2010, 3, 1586–1595. [Google Scholar] [CrossRef]
- Kakarala, M.; Brenner, D.E.; Korkaya, H.; Cheng, C.; Tazi, K.; Ginestier, C.; Liu, S.; Dontu, G.; Wicha, M.S. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res. Treat. 2010, 122, 777–785. [Google Scholar] [CrossRef]
- Tu, S.P.; Jin, H.; Shi, J.D.; Zhu, L.M.; Suo, Y.; Lu, G.; Liu, A.; Wang, T.C.; Yang, C.S. Curcumin induces the differentiation of myeloid-derived suppressor cells and inhibits their interaction with cancer cells and related tumor growth. Cancer Prev. Res. (Phila) 2012, 5, 205–215. [Google Scholar] [CrossRef]
- Alexandrow, M.G.; Song, L.J.; Altiok, S.; Gray, J.; Haura, E.B.; Kumar, N.B. Curcumin: A novel Stat3 pathway inhibitor for chemoprevention of lung cancer. Eur. J. Cancer Prev. 2012, 21, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Sonavane, K.; Phillips, J.; Ekshyyan, O.; Moore-Medlin, T.; Roberts Gill, J.; Rong, X.; Lakshmaiah, R.R.; Abreo, F.; Boudreaux, D.; Clifford, J.L.; et al. Topical curcumin-based cream is equivalent to dietary curcumin in a skin cancer model. J. Skin Cancer 2012, 2012, 147863. [Google Scholar] [CrossRef]
- Khandelwal, A.R.; Rong, X.; Moore-Medlin, T.; Ekshyyan, O.; Abreo, F.; Gu, X.; Nathan, C.A. Photopreventive Effect and Mechanism of AZD4547 and Curcumin C3 Complex on UVB-Induced Epidermal Hyperplasia. Cancer Prev. Res. (Phila) 2016, 9, 296–304. [Google Scholar] [CrossRef]
- Colacino, J.A.; McDermott, S.P.; Sartor, M.A.; Wicha, M.S.; Rozek, L.S. Transcriptomic profiling of curcumin-treated human breast stem cells identifies a role for stearoyl-coa desaturase in breast cancer prevention. Breast Cancer Res. Treat. 2016, 158, 29–41. [Google Scholar] [CrossRef]
- Messner, D.J.; Robinson, T.; Kowdley, K.V. Curcumin and Turmeric Modulate the Tumor-Promoting Effects of Iron In Vitro. Nutr. Cancer 2017, 69, 481–489. [Google Scholar] [CrossRef]
- Khandelwal, A.R.; Moore-Medlin, T.; Ekshyyan, O.; Gu, X.; Abreo, F.; Nathan, C.O. Local and systemic Curcumin C3 complex inhibits 4NQO-induced oral tumorigenesis via modulating FGF-2/FGFR-2 activation. Am. J. Cancer Res. 2018, 8, 2538–2547. [Google Scholar]
- Di Meo, F.; Filosa, S.; Madonna, M.; Giello, G.; Di Pardo, A.; Maglione, V.; Baldi, A.; Crispi, S. Curcumin C3 complex(R)/Bioperine(R) has antineoplastic activity in mesothelioma: An in vitro and in vivo analysis. J. Exp. Clin. Cancer Res. 2019, 38, 360. [Google Scholar] [CrossRef]
- Lin, Y.G.; Kunnumakkara, A.B.; Nair, A.; Merritt, W.M.; Han, L.Y.; Armaiz-Pena, G.N.; Kamat, A.A.; Spannuth, W.A.; Gershenson, D.M.; Lutgendorf, S.K.; et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin. Cancer Res. 2007, 13, 3423–3430. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Diagaradjane, P.; Guha, S.; Deorukhkar, A.; Shentu, S.; Aggarwal, B.B.; Krishnan, S. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin. Cancer Res. 2008, 14, 2128–2136. [Google Scholar] [CrossRef]
- Siddiqui, R.A.; Hassan, S.; Harvey, K.A.; Rasool, T.; Das, T.; Mukerji, P.; DeMichele, S. Attenuation of proteolysis and muscle wasting by curcumin c3 complex in MAC16 colon tumour-bearing mice. Br. J. Nutr. 2009, 102, 967–975. [Google Scholar] [CrossRef]
- Phillips, J.M.; Clark, C.; Herman-Ferdinandez, L.; Moore-Medlin, T.; Rong, X.; Gill, J.R.; Clifford, J.L.; Abreo, F.; Nathan, C.O. Curcumin inhibits skin squamous cell carcinoma tumor growth in vivo. Otolaryngol. Head Neck Surg. 2011, 145, 58–63. [Google Scholar] [CrossRef]
- Phillips, J.; Moore-Medlin, T.; Sonavane, K.; Ekshyyan, O.; McLarty, J.; Nathan, C.A. Curcumin inhibits UV radiation-induced skin cancer in SKH-1 mice. Otolaryngol. Head Neck Surg. 2013, 148, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wu, R.; Gaspar, J.M.; Sargsyan, D.; Su, Z.Y.; Zhang, C.; Gao, L.; Cheng, D.; Li, W.; Wang, C.; et al. DNA methylome and transcriptome alterations and cancer prevention by curcumin in colitis-accelerated colon cancer in mice. Carcinogenesis 2018, 39, 669–680. [Google Scholar] [CrossRef]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef]
- Garcea, G.; Berry, D.P.; Jones, D.J.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J. Consumption of the putative chemopreventive agent curcumin by cancer patients: Assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 120–125. [Google Scholar]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef]
- Golombick, T.; Diamond, T.H.; Badmaev, V.; Manoharan, A.; Ramakrishna, R. The potential role of curcumin in patients with monoclonal gammopathy of undefined significance--its effect on paraproteinemia and the urinary N-telopeptide of type I collagen bone turnover marker. Clin. Cancer Res. 2009, 15, 5917–5922. [Google Scholar] [CrossRef] [PubMed]
- Rai, B.; Kaur, J.; Jacobs, R.; Singh, J. Possible action mechanism for curcumin in pre-cancerous lesions based on serum and salivary markers of oxidative stress. J. Oral. Sci. 2010, 52, 251–256. [Google Scholar] [CrossRef]
- Golombick, T.; Diamond, T.H.; Manoharan, A.; Ramakrishna, R. Monoclonal gammopathy of undetermined significance, smoldering multiple myeloma, and curcumin: A randomized, double-blind placebo-controlled cross-over 4g study and an open-label 8g extension study. Am. J. Hematol. 2012, 87, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Guha, S.; Krishnan, S.; Diagaradjane, P.; Gelovani, J.; Aggarwal, B.B. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007, 67, 3853–3861. [Google Scholar] [CrossRef] [PubMed]
- Epelbaum, R.; Schaffer, M.; Vizel, B.; Badmaev, V.; Bar-Sela, G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr. Cancer 2010, 62, 1137–1141. [Google Scholar] [CrossRef]
- Kanai, M.; Yoshimura, K.; Asada, M.; Imaizumi, A.; Suzuki, C.; Matsumoto, S.; Nishimura, T.; Mori, Y.; Masui, T.; Kawaguchi, Y.; et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother. Pharmacol. 2011, 68, 157–164. [Google Scholar] [CrossRef]
- Ibrahim, A.; El-Meligy, A.; Fetaih, H.; Dessouki, A.; Stoica, G.; Barhoumi, R. Effect of curcumin and Meriva on the lung metastasis of murine mammary gland adenocarcinoma. In Vivo 2010, 24, 401–408. [Google Scholar]
- Chandra, D.; Jahangir, A.; Cornelis, F.; Rombauts, K.; Meheus, L.; Jorcyk, C.L.; Gravekamp, C. Cryoablation and Meriva have strong therapeutic effect on triple-negative breast cancer. Oncoimmunology 2016, 5, e1049802. [Google Scholar] [CrossRef]
- Belcaro, G.; Hosoi, M.; Pellegrini, L.; Appendino, G.; Ippolito, E.; Ricci, A.; Ledda, A.; Dugall, M.; Cesarone, M.R.; Maione, C.; et al. A controlled study of a lecithinized delivery system of curcumin (Meriva®) to alleviate the adverse effects of cancer treatment. Phytother. Res. 2014, 28, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Howells, L.M.; Sale, S.; Sriramareddy, S.N.; Irving, G.R.; Jones, D.J.; Ottley, C.J.; Pearson, D.G.; Mann, C.D.; Manson, M.M.; Berry, D.P.; et al. Curcumin ameliorates oxaliplatin-induced chemoresistance in HCT116 colorectal cancer cells in vitro and in vivo. Int. J. Cancer 2011, 129, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Ledda, A.; Belcaro, G.; Dugall, M.; Luzzi, R.; Scoccianti, M.; Togni, S.; Appendino, G.; Ciammaichella, G. Meriva®, a lecithinized curcumin delivery system, in the control of benign prostatic hyperplasia: A pilot, product evaluation registry study. Panminerva Med. 2012, 54, 17–22. [Google Scholar]
- Panahi, Y.; Saadat, A.; Beiraghdar, F.; Sahebkar, A. Adjuvant therapy with bioavailability-boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: A randomized double-blind placebo-controlled trial. Phytother. Res. 2014, 28, 1461–1467. [Google Scholar] [CrossRef]
- Pastorelli, D.; Fabricio, A.S.C.; Giovanis, P.; D’Ippolito, S.; Fiduccia, P.; Soldà, C.; Buda, A.; Sperti, C.; Bardini, R.; Da Dalt, G.; et al. Phytosome complex of curcumin as complementary therapy of advanced pancreatic cancer improves safety and efficacy of gemcitabine: Results of a prospective phase II trial. Pharmacol. Res. 2018, 132, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.P.; Mukerjee, A.; Gdowski, A.; Helson, L.; Bouchard, A.; Majeed, M.; Vishwanatha, J.K. Curcumin-ER Prolonged Subcutaneous Delivery for the Treatment of Non- Small Cell Lung Cancer. J. Biomed. Nanotechnol. 2016, 12, 679–688. [Google Scholar] [CrossRef]
- Withers, S.S.; York, D.; Johnson, E.; Al-Nadaf, S.; Skorupski, K.A.; Rodriguez, C.O., Jr.; Burton, J.H.; Guerrero, T.; Sein, K.; Wittenburg, L.; et al. In vitro and in vivo activity of liposome-encapsulated curcumin for naturally occurring canine cancers. Vet. Comp. Oncol. 2018, 16, 571–579. [Google Scholar] [CrossRef]
- Bolger, G.T.; Licollari, A.; Tan, A.; Greil, R.; Vcelar, B.; Greil-Ressler, S.; Weiss, L.; Schönlieb, C.; Magnes, T.; Radl, B.; et al. Pharmacokinetics of liposomal curcumin (Lipocurc™) infusion: Effect of co-medication in cancer patients and comparison with healthy individuals. Cancer Chemother. Pharmacol. 2019, 83, 265–275. [Google Scholar] [CrossRef]
- Maiti, P.; Al-Gharaibeh, A.; Kolli, N.; Dunbar, G.L. Solid Lipid Curcumin Particles Induce More DNA Fragmentation and Cell Death in Cultured Human Glioblastoma Cells than Does Natural Curcumin. Oxid. Med. Cell Longev. 2017, 2017, 9656719. [Google Scholar] [CrossRef]
- Hazarey, V.K.; Sakrikar, A.R.; Ganvir, S.M. Efficacy of curcumin in the treatment for oral submucous fibrosis-A randomized clinical trial. J. Oral. Maxillofac. Pathol. 2015, 19, 145–152. [Google Scholar] [CrossRef]
- Milano, F.; Mari, L.; van de Luijtgaarden, W.; Parikh, K.; Calpe, S.; Krishnadath, K.K. Nano-curcumin inhibits proliferation of esophageal adenocarcinoma cells and enhances the T cell mediated immune response. Front. Oncol. 2013, 3, 137. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Ho, J.N.; Kook, H.R.; Lee, S.; Oh, J.J.; Hong, S.K.; Lee, S.E.; Byun, S.S. Theracurmin® efficiently inhibits the growth of human prostate and bladder cancer cells via induction of apoptotic cell death and cell cycle arrest. Oncol. Rep. 2016, 35, 1463–1472. [Google Scholar] [CrossRef]
- Ide, H.; Lu, Y.; Noguchi, T.; Muto, S.; Okada, H.; Kawato, S.; Horie, S. Modulation of AKR1C2 by curcumin decreases testosterone production in prostate cancer. Cancer Sci. 2018, 109, 1230–1238. [Google Scholar] [CrossRef]
- Adachi, S.; Hamoya, T.; Fujii, G.; Narita, T.; Komiya, M.; Miyamoto, S.; Kurokawa, Y.; Takahashi, M.; Takayama, T.; Ishikawa, H.; et al. Theracurmin inhibits intestinal polyp development in Apc-mutant mice by inhibiting inflammation-related factors. Cancer Sci. 2020, 111, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- De Velasco, M.A.; Lu, Y.; Kura, Y.; China, T.; Inoue, Y.; Nakayama, A.; Okada, H.; Horie, S.; Uemura, H.; Ide, H. Chemopreventive effects of nanoparticle curcumin in a mouse model of Pten-deficient prostate cancer. Hum. Cell 2020, 33, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Izumi, Y.; Yamamoto, J.; Nomori, H. Coadministration of erlotinib and curcumin augmentatively reduces cell viability in lung cancer cells. Phytother. Res. 2014, 28, 728–735. [Google Scholar] [CrossRef]
- Kanai, M.; Otsuka, Y.; Otsuka, K.; Sato, M.; Nishimura, T.; Mori, Y.; Kawaguchi, M.; Hatano, E.; Kodama, Y.; Matsumoto, S.; et al. A phase I study investigating the safety and pharmacokinetics of highly bioavailable curcumin (Theracurmin) in cancer patients. Cancer Chemother. Pharmacol. 2013, 71, 1521–1530. [Google Scholar] [CrossRef]
- Feng, W.; Wang, H.; Zhang, P.; Gao, C.; Tao, J.; Ge, Z.; Zhu, D.; Bi, Y. Modulation of gut microbiota contributes to curcumin-mediated attenuation of hepatic steatosis in rats. Biochim. Biophys Acta. Gen. Subj. 2017, 1861, 1801–1812. [Google Scholar] [CrossRef]
- Lou, Y.; Zheng, J.; Hu, H.; Lee, J.; Zeng, S. Application of ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry to identify curcumin metabolites produced by human intestinal bacteria. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 985, 38–47. [Google Scholar] [CrossRef]
- Nagpal, R.; Wang, S.; Solberg Woods, L.C.; Seshie, O.; Chung, S.T.; Shively, C.A.; Register, T.C.; Craft, S.; McClain, D.A.; Yadav, H. Comparative Microbiome Signatures and Short-Chain Fatty Acids in Mouse, Rat, Non-human Primate, and Human Feces. Front. Microbiol. 2018, 9, 2897. [Google Scholar] [CrossRef]
- Peterson, C.T.; Vaughn, A.R.; Sharma, V.; Chopra, D.; Mills, P.J.; Peterson, S.N.; Sivamani, R.K. Effects of Turmeric and Curcumin Dietary Supplementation on Human Gut Microbiota: A Double-Blind, Randomized, Placebo-Controlled Pilot Study. J. Evid. Based Integr. Med. 2018, 23, 2515690X18790725. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liu, L.; Ji, H.F. Regulative effects of curcumin spice administration on gut microbiota and its pharmacological implications. Food Nutr. Res. 2017, 61, 1361780. [Google Scholar] [CrossRef]
- Ohno, M.; Nishida, A.; Sugitani, Y.; Nishino, K.; Inatomi, O.; Sugimoto, M.; Kawahara, M.; Andoh, A. Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells. PLoS ONE 2017, 12, e0185999. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Kaiko, G.E.; Ryu, S.H.; Koues, O.I.; Collins, P.L.; Solnica-Krezel, L.; Pearce, E.J.; Pearce, E.L.; Oltz, E.M.; Stappenbeck, T.S. The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell 2016, 165, 1708–1720. [Google Scholar] [CrossRef]
- Sánchez-Alcoholado, L.; Ordóñez, R.; Otero, A.; Plaza-Andrade, I.; Laborda-Illanes, A.; Medina, J.A.; Ramos-Molina, B.; Gómez-Millán, J.; Queipo-Ortuño, M.I. Gut Microbiota-Mediated Inflammation and Gut Permeability in Patients with Obesity and Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 6782. [Google Scholar] [CrossRef]
- Kujundžić, R.N.; Stepanić, V.; Milković, L.; Gašparović, A.; Tomljanović, M.; Trošelj, K.G. Curcumin and its Potential for Systemic Targeting of Inflamm-Aging and Metabolic Reprogramming in Cancer. Int. J. Mol. Sci. 2019, 20, 1180. [Google Scholar] [CrossRef]
- Zhao, H.M.; Xu, R.; Huang, X.Y.; Cheng, S.M.; Huang, M.F.; Yue, H.Y.; Wang, X.; Zou, Y.; Lu, A.P.; Liu, D.Y. Curcumin improves regulatory T cells in gut-associated lymphoid tissue of colitis mice. World J. Gastroenterol. 2016, 22, 5374–5383. [Google Scholar] [CrossRef]
- Larussa, T.; Gervasi, S.; Liparoti, R.; Suraci, E.; Marasco, R.; Imeneo, M.; Luzza, F. Downregulation of Interleukin- (IL-) 17 through Enhanced Indoleamine 2,3-Dioxygenase (IDO) Induction by Curcumin: A Potential Mechanism of Tolerance towards Helicobacter pylori. J. Immunol. Res. 2018, 2018, 3739593. [Google Scholar] [CrossRef] [PubMed]
- Madhur, M.S.; Lob, H.E.; McCann, L.A.; Iwakura, Y.; Blinder, Y.; Guzik, T.J.; Harrison, D.G. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 2010, 55, 500–507. [Google Scholar] [CrossRef]
- Vitiello, G.A.; Miller, G. Targeting the interleukin-17 immune axis for cancer immunotherapy. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef]
- Sethi, V.; Kurtom, S.; Tarique, M.; Lavania, S.; Malchiodi, Z.; Hellmund, L.; Zhang, L.; Sharma, U.; Giri, B.; Garg, B.; et al. Gut Microbiota Promotes Tumor Growth in Mice by Modulating Immune Response. Gastroenterology 2018, 155, 33–37. [Google Scholar] [CrossRef]
- Serrano-Gomez, S.J.; Maziveyi, M.; Alahari, S.K. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol. Cancer 2016, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.H.; Donaher, J.L.; Murphy, D.A.; Chau, S.; Yang, J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 2012, 22, 725–736. [Google Scholar] [CrossRef]
- Wei, Q.; Qian, Y.; Yu, J.; Wong, C.C. Metabolic rewiring in the promotion of cancer metastasis: Mechanisms and therapeutic implications. Oncogene 2020, 39, 6139–6156. [Google Scholar] [CrossRef]
- Bae, M.K.; Kim, S.H.; Jeong, J.W.; Lee, Y.M.; Kim, H.S.; Kim, S.R.; Yun, I.; Bae, S.K.; Kim, K.W. Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1. Oncol. Rep. 2006, 15, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Majeed, M.; Sahebkar, A. Curcumin: A potent agent to reverse epithelial-to-mesenchymal transition. Cell Oncol. (Dordr) 2019, 42, 405–421. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Liang, J.; Li, W.; Zhu, Y.; Zhang, M.; Wang, C.; Hou, J. Deregulation of Hexokinase II Is Associated with Glycolysis, Autophagy, and the Epithelial-Mesenchymal Transition in Tongue Squamous Cell Carcinoma under Hypoxia. Biomed. Res. Int. 2018, 2018, 8480762. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhang, L.F.; Zhang, H.W.; Hu, S.; Lu, M.H.; Liang, S.; Li, B.; Li, Y.; Li, D.; Wang, E.D.; et al. A novel miR-155/miR-143 cascade controls glycolysis by regulating hexokinase 2 in breast cancer cells. EMBO J. 2012, 31, 1985–1998. [Google Scholar] [CrossRef]
- Geng, C.; Li, J.; Ding, F.; Wu, G.; Yang, Q.; Sun, Y.; Zhang, Z.; Dong, T.; Tian, X. Curcumin suppresses 4-hydroxytamoxifen resistance in breast cancer cells by targeting SLUG/Hexokinase 2 pathway. Biochem. Biophys Res. Commun. 2016, 473, 147–153. [Google Scholar] [CrossRef]
- Wang, K.; Fan, H.; Chen, Q.; Ma, G.; Zhu, M.; Zhang, X.; Zhang, Y.; Yu, J. Curcumin inhibits aerobic glycolysis and induces mitochondrial-mediated apoptosis through hexokinase II in human colorectal cancer cells in vitro. Anticancer Drugs 2015, 26, 15–24. [Google Scholar] [CrossRef]
- Yang, P.; Li, Z.; Fu, R.; Wu, H.; Li, Z. Pyruvate kinase M2 facilitates colon cancer cell migration via the modulation of STAT3 signalling. Cell Signal. 2014, 26, 1853–1862. [Google Scholar] [CrossRef]
- Hamabe, A.; Konno, M.; Tanuma, N.; Shima, H.; Tsunekuni, K.; Kawamoto, K.; Nishida, N.; Koseki, J.; Mimori, K.; Gotoh, N.; et al. Role of pyruvate kinase M2 in transcriptional regulation leading to epithelial-mesenchymal transition. Proc. Natl. Acad. Sci. USA 2014, 111, 15526–15531. [Google Scholar] [CrossRef]
- Siddiqui, F.A.; Prakasam, G.; Chattopadhyay, S.; Rehman, A.U.; Padder, R.A.; Ansari, M.A.; Irshad, R.; Mangalhara, K.; Bamezai, R.N.K.; Husain, M.; et al. Curcumin decreases Warburg effect in cancer cells by down-regulating pyruvate kinase M2 via mTOR-HIF1alpha inhibition. Sci. Rep. 2018, 8, 8323. [Google Scholar] [CrossRef]
- Mojzeš, A.; Tomljanović, M.; Milković, L.; Kujundžić, R.N.; Gašparović, A.; Trošelj, K.G. Cell-Type Specific Metabolic Response of Cancer Cells to Curcumin. Int. J. Mol. Sci. 2020, 21, 1661. [Google Scholar] [CrossRef]
- Lee, A.Y.; Fan, C.C.; Chen, Y.A.; Cheng, C.W.; Sung, Y.J.; Hsu, C.P.; Kao, T.Y. Curcumin Inhibits Invasiveness and Epithelial-Mesenchymal Transition in Oral Squamous Cell Carcinoma Through Reducing Matrix Metalloproteinase 2, 9 and Modulating p53-E-Cadherin Pathway. Integr. Cancer Ther. 2015, 14, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, H.; Xu, C.; Song, L.; Huang, L.; Lai, Y.; Wang, Y.; Chen, H.; Gu, D.; Ren, L.; et al. Curcumin inhibits tumor epithelialmesenchymal transition by downregulating the Wnt signaling pathway and upregulating NKD2 expression in colon cancer cells. Oncol. Rep. 2016, 35, 2615–2623. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mazumdar, M.; Chakraborty, S.; Manna, A.; Saha, S.; Khan, P.; Bhattacharjee, P.; Guha, D.; Adhikary, A.; Mukhjerjee, S.; et al. Curcumin inhibits breast cancer stem cell migration by amplifying the E-cadherin/beta-catenin negative feedback loop. Stem. Cell Res. Ther. 2014, 5, 116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cheng, X.; Gao, Y.; Zhang, C.; Bao, J.; Guan, H.; Yu, H.; Lu, R.; Xu, Q.; Sun, Y. Curcumin inhibits metastasis in human papillary thyroid carcinoma BCPAP cells via down-regulation of the TGF-beta/Smad2/3 signaling pathway. Exp. Cell Res. 2016, 341, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Ali, S.; Ahmad, A.; Azmi, A.S.; Li, Y.; Banerjee, S.; Kong, D.; Sethi, S.; Aboukameel, A.; Padhye, S.B.; et al. Hypoxia-induced aggressiveness of pancreatic cancer cells is due to increased expression of VEGF, IL-6 and miR-21, which can be attenuated by CDF treatment. PLoS ONE 2012, 7, e50165. [Google Scholar] [CrossRef]
- Yang, M.H.; Wu, M.Z.; Chiou, S.H.; Chen, P.M.; Chang, S.Y.; Liu, C.J.; Teng, S.C.; Wu, K.J. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat. Cell Biol. 2008, 10, 295–305. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, X.; Peng, Y.; Wu, M.; Zhang, P.; Xie, R.; Wu, Y.; Yan, Q.; Liu, S.; Wang, J. HIF-1alpha Promotes Epithelial-Mesenchymal Transition and Metastasis through Direct Regulation of ZEB1 in Colorectal Cancer. PLoS ONE 2015, 10, e0129603. [Google Scholar] [CrossRef]
- Yu, X.; Ma, R.; Wu, Y.; Zhai, Y.; Li, S. Reciprocal Regulation of Metabolic Reprogramming and Epigenetic Modifications in Cancer. Front. Genet. 2018, 9, 394. [Google Scholar] [CrossRef]
- Kanska, J.; Aspuria, P.P.; Taylor-Harding, B.; Spurka, L.; Funari, V.; Orsulic, S.; Karlan, B.Y.; Wiedemeyer, W.R. Glucose deprivation elicits phenotypic plasticity via ZEB1-mediated expression of NNMT. Oncotarget 2017, 8, 26200–26220. [Google Scholar] [CrossRef]
- Ulanovskaya, O.A.; Zuhl, A.M.; Cravatt, B.F. NNMT promotes epigenetic remodeling in cancer by creating a metabolic methylation sink. Nat. Chem. Biol. 2013, 9, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, D.B.; Waring, R.H.; Parsons, R.B.; Barlow, D.J.; Williams, A.C. Nicotinamide N-Methyltransferase: Genomic Connection to Disease. Int. J. Tryptophan Res. 2020, 13, 1178646920919770. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, L.; Wang, W.; Ma, S.; Liu, H.; Zang, X.; Zhang, Y.; Guan, F. Downregulation of nicotinamide N-methyltransferase inhibits migration and epithelial-mesenchymal transition of esophageal squamous cell carcinoma via Wnt/β-catenin pathway. Mol. Cell Biochem. 2019, 460, 93–103. [Google Scholar] [CrossRef]
- Hahn, Y.I.; Kim, S.J.; Choi, B.Y.; Cho, K.C.; Bandu, R.; Kim, K.P.; Kim, D.H.; Kim, W.; Park, J.S.; Han, B.W.; et al. Curcumin interacts directly with the Cysteine 259 residue of STAT3 and induces apoptosis in H-Ras transformed human mammary epithelial cells. Sci. Rep. 2018, 8, 6409. [Google Scholar] [CrossRef]
- Li, S.; Qiao, L.; Yang, Z.; He, C. Prognostic Value of Nicotinamide N-Methyltransferase Expression in Patients With Solid Tumors: A Systematic Review and Meta-Analysis. Front. Physiol. 2018, 9, 1407. [Google Scholar] [CrossRef]
- Eckert, M.A.; Coscia, F.; Chryplewicz, A.; Chang, J.W.; Hernandez, K.M.; Pan, S.; Tienda, S.M.; Nahotko, D.A.; Li, G.; Blaženović, I.; et al. Proteomics reveals NNMT as a master metabolic regulator of cancer-associated fibroblasts. Nature 2019, 569, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Tomida, M.; Ohtake, H.; Yokota, T.; Kobayashi, Y.; Kurosumi, M. Stat3 up-regulates expression of nicotinamide N-methyltransferase in human cancer cells. J. Cancer Res. Clin. Oncol. 2008, 134, 551–559. [Google Scholar] [CrossRef]
- Pissios, P. Nicotinamide N-Methyltransferase: More Than a Vitamin B3 Clearance Enzyme. Trends Endocrinol. Metab. 2017, 28, 340–353. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, D.; Wang, W.; Zhang, L.; Liu, H.; Ma, S.; Guo, W.; Yao, M.; Zhang, K.; Li, W.; et al. Nicotinamide N-methyltransferase decreases 5-fluorouracil sensitivity in human esophageal squamous cell carcinoma through metabolic reprogramming and promoting the Warburg effect. Mol. Carcinog 2020, 59, 940–954. [Google Scholar] [CrossRef]
- Li, W.C.; Huang, C.H.; Hsieh, Y.T.; Chen, T.Y.; Cheng, L.H.; Chen, C.Y.; Liu, C.J.; Chen, H.M.; Huang, C.L.; Lo, J.F.; et al. Regulatory Role of Hexokinase 2 in Modulating Head and Neck Tumorigenesis. Front. Oncol. 2020, 10, 176. [Google Scholar] [CrossRef]
- Wu, X.; Xia, J.; Zhang, J.; Zhu, Y.; Wu, Y.; Guo, J.; Chen, S.; Lei, Q.; Meng, B.; Kuang, C.; et al. Phosphoglycerate dehydrogenase promotes proliferation and bortezomib resistance through increasing reduced glutathione synthesis in multiple myeloma. Br. J. Haematol. 2020, 190, 52–66. [Google Scholar] [CrossRef]
- Ye, J.; Fan, J.; Venneti, S.; Wan, Y.W.; Pawel, B.R.; Zhang, J.; Finley, L.W.; Lu, C.; Lindsten, T.; Cross, J.R.; et al. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov. 2014, 4, 1406–1417. [Google Scholar] [CrossRef]
- Woo, C.C.; Chen, W.C.; Teo, X.Q.; Radda, G.K.; Lee, P.T. Downregulating serine hydroxymethyltransferase 2 (SHMT2) suppresses tumorigenesis in human hepatocellular carcinoma. Oncotarget 2016, 7, 53005–53017. [Google Scholar] [CrossRef]
- Angelo, L.S.; Maxwell, D.S.; Wu, J.Y.; Sun, D.; Hawke, D.H.; McCutcheon, I.E.; Slopis, J.M.; Peng, Z.; Bornmann, W.G.; Kurzrock, R. Binding partners for curcumin in human schwannoma cells: Biologic implications. Bioorg. Med. Chem. 2013, 21, 932–939. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, Q.; Li, Y.; Tang, H.; Liu, W.; Yang, X. Doxorubicin and curcumin co-delivery by lipid nanoparticles for enhanced treatment of diethylnitrosamine-induced hepatocellular carcinoma in mice. Eur. J. Pharm. Biopharm. 2015, 93, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Pignanelli, C.; Ma, D.; Noel, M.; Ropat, J.; Mansour, F.; Curran, C.; Pupulin, S.; Larocque, K.; Wu, J.; Liang, G.; et al. Selective Targeting of Cancer Cells by Oxidative Vulnerabilities with Novel Curcumin Analogs. Sci. Rep. 2017, 7, 1105. [Google Scholar] [CrossRef]
- Edwards, R.L.; Luis, P.B.; Varuzza, P.V.; Joseph, A.I.; Presley, S.H.; Chaturvedi, R.; Schneider, C. The anti-inflammatory activity of curcumin is mediated by its oxidative metabolites. J. Biol. Chem. 2017, 292, 21243–21252. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, F.; Demiray, M. A Realistic View on “The Essential Medicinal Chemistry of Curcumin”. ACS Med. Chem. Lett. 2017, 8, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; DeBerardinis, R.J. Mechanisms and Implications of Metabolic Heterogeneity in Cancer. Cell Metab. 2019, 30, 434–446. [Google Scholar] [CrossRef]
- Ward, P.S.; Thompson, C.B. Metabolic reprogramming: A cancer hallmark even warburg did not anticipate. Cancer Cell 2012, 21, 297–308. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).