Wine or Beer? Comparison, Changes and Improvement of Polyphenolic Compounds during Technological Phases
Abstract
1. Introduction
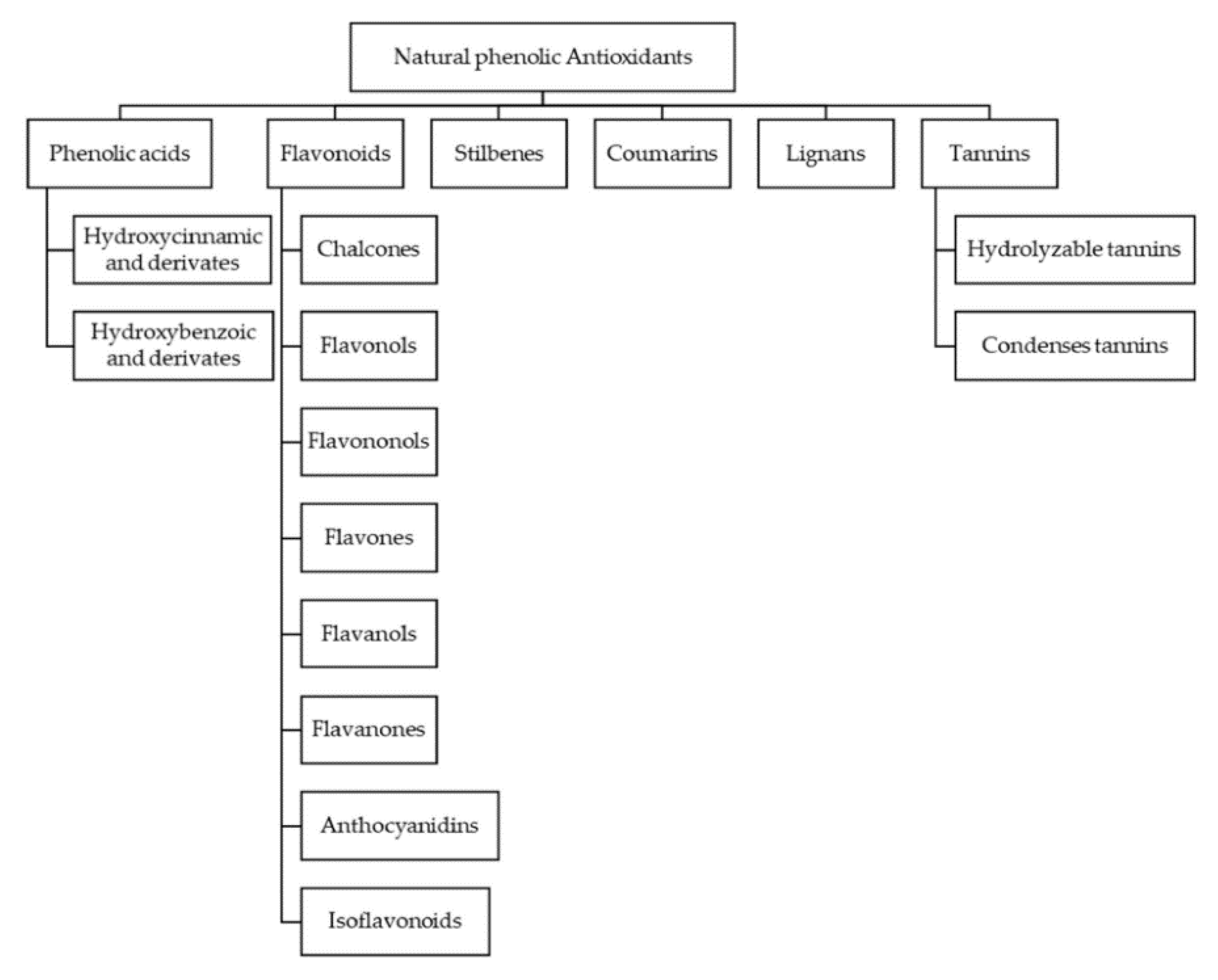
2. Bioactive Compounds in Beer and Wine
2.1. Analytical Methods for Determination of Antioxidant Activity in Beer and Wine
2.2. Non-Flavonoid Polyphenols in Wine and Beer
2.2.1. Hydroxycinnamic Acids
2.2.2. Hydroxybenzoic Acids
2.2.3. Stilbenes
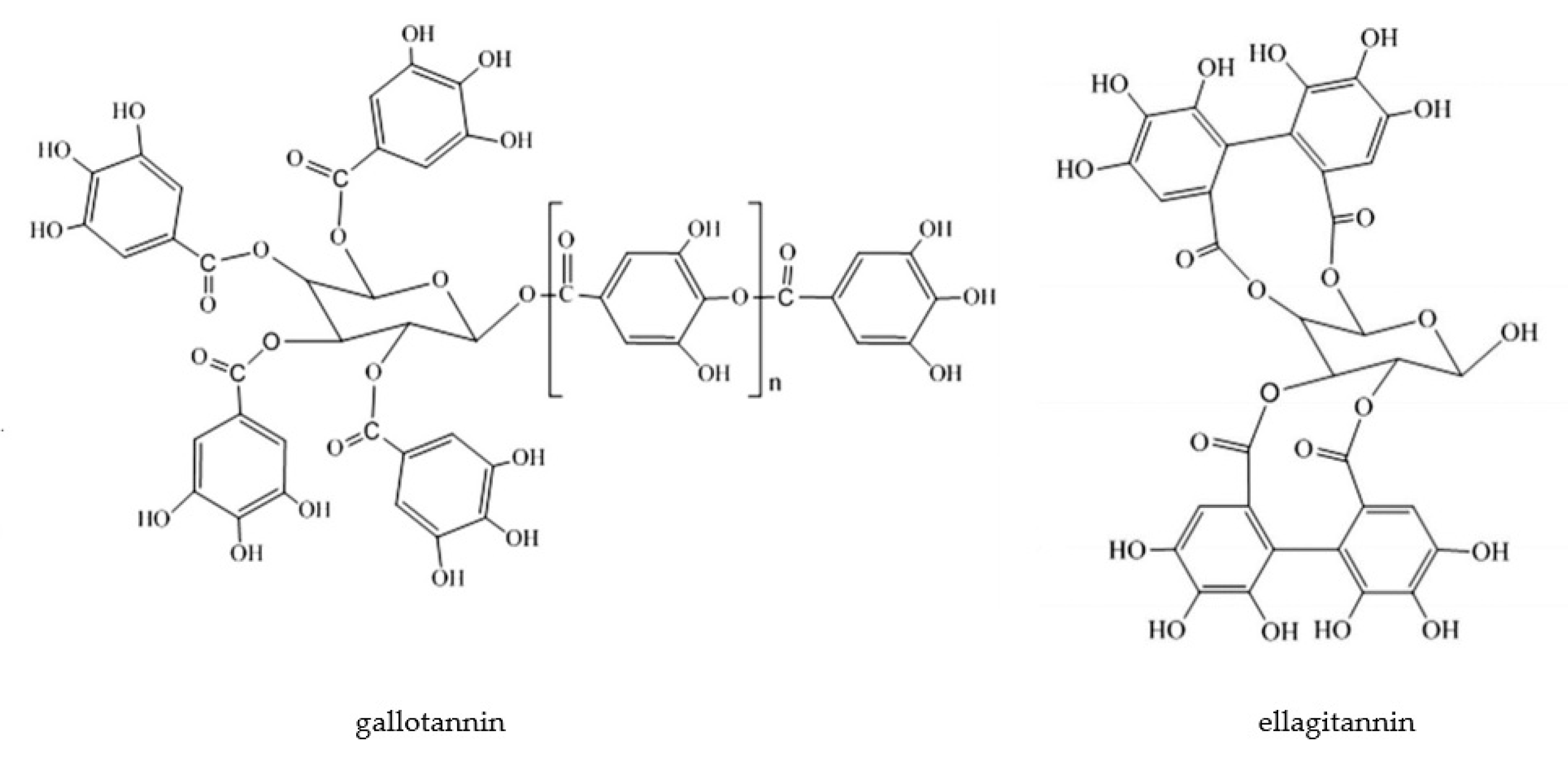
2.2.4. Hydrolysable Tannins
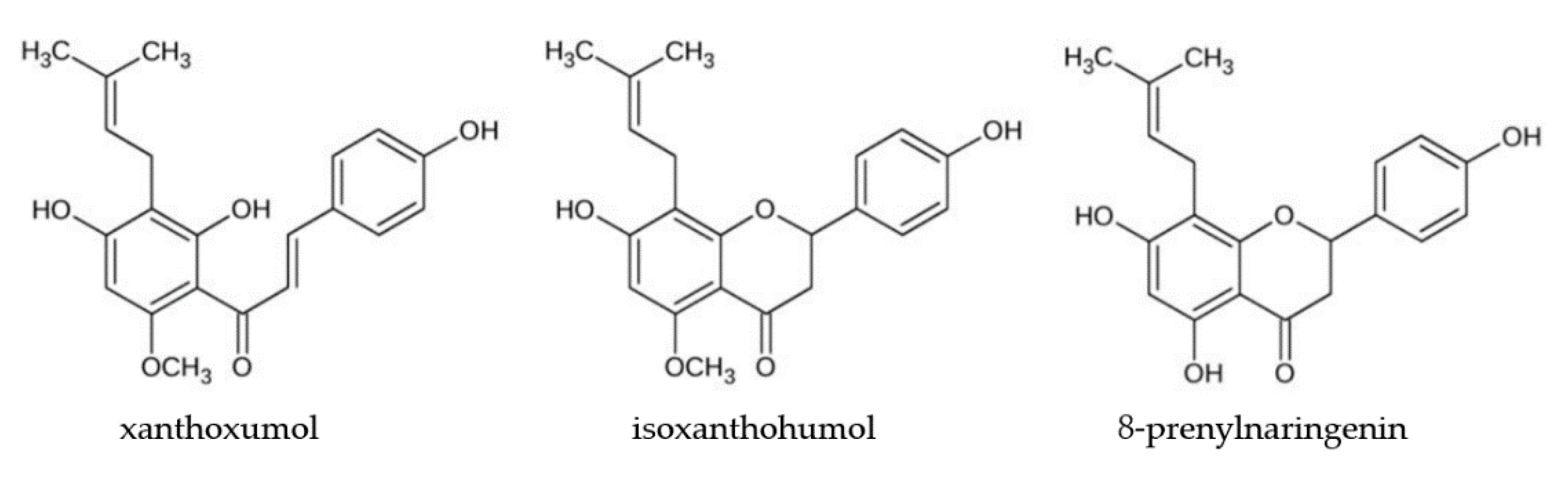
2.3. Flavonoid Compounds
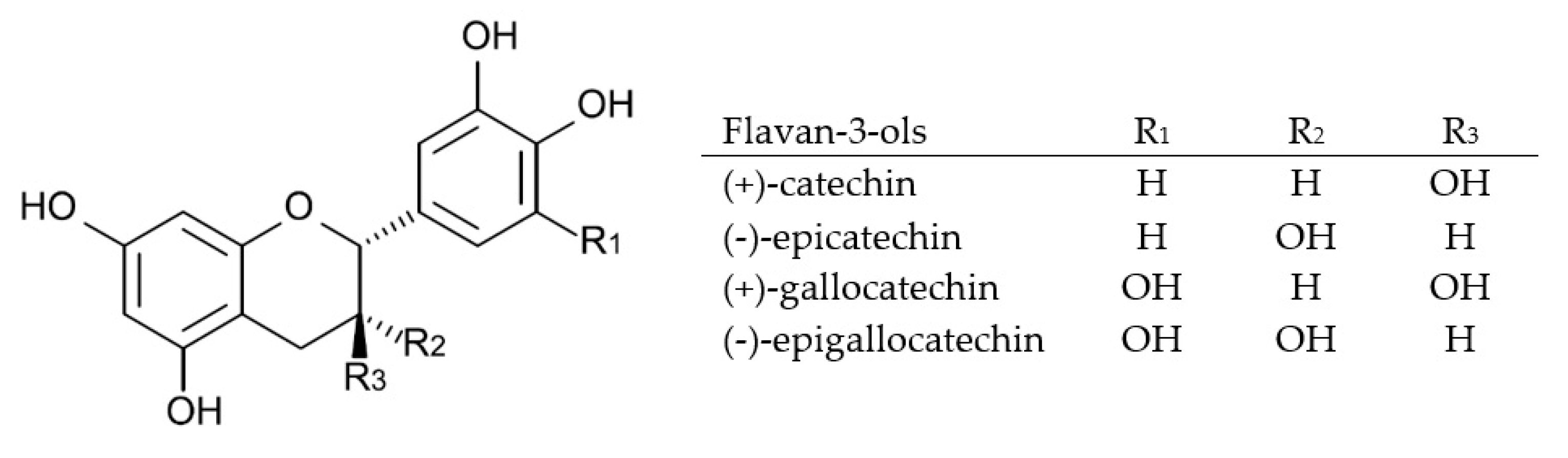
2.3.1. Flavan-3-ols and Condensed Tannins
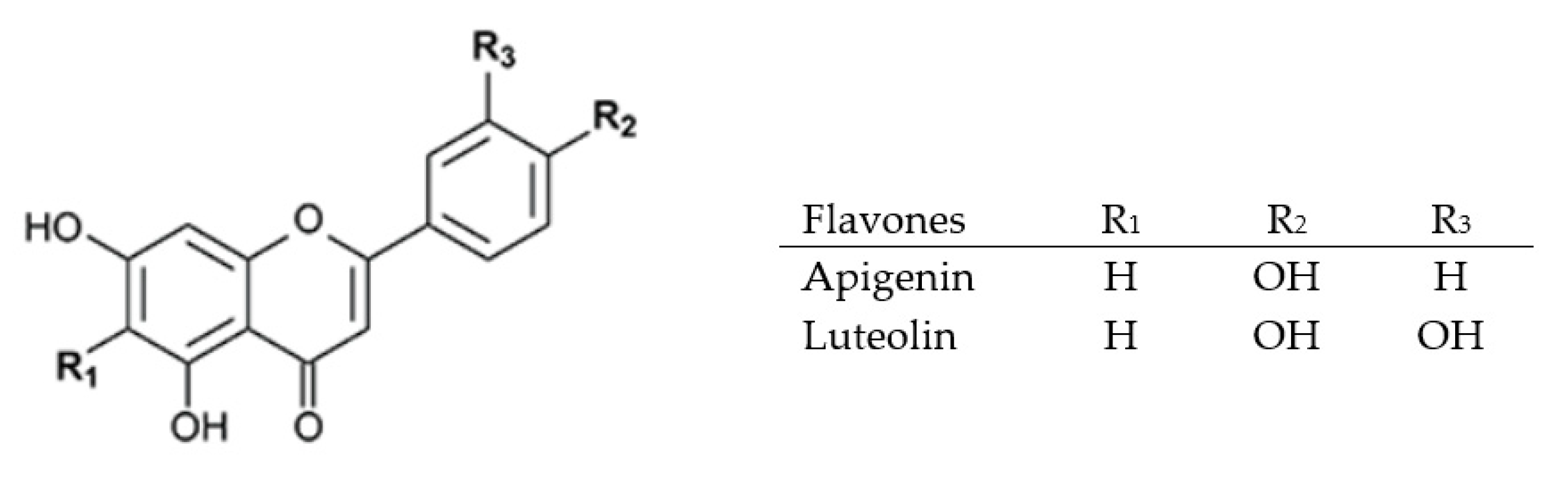
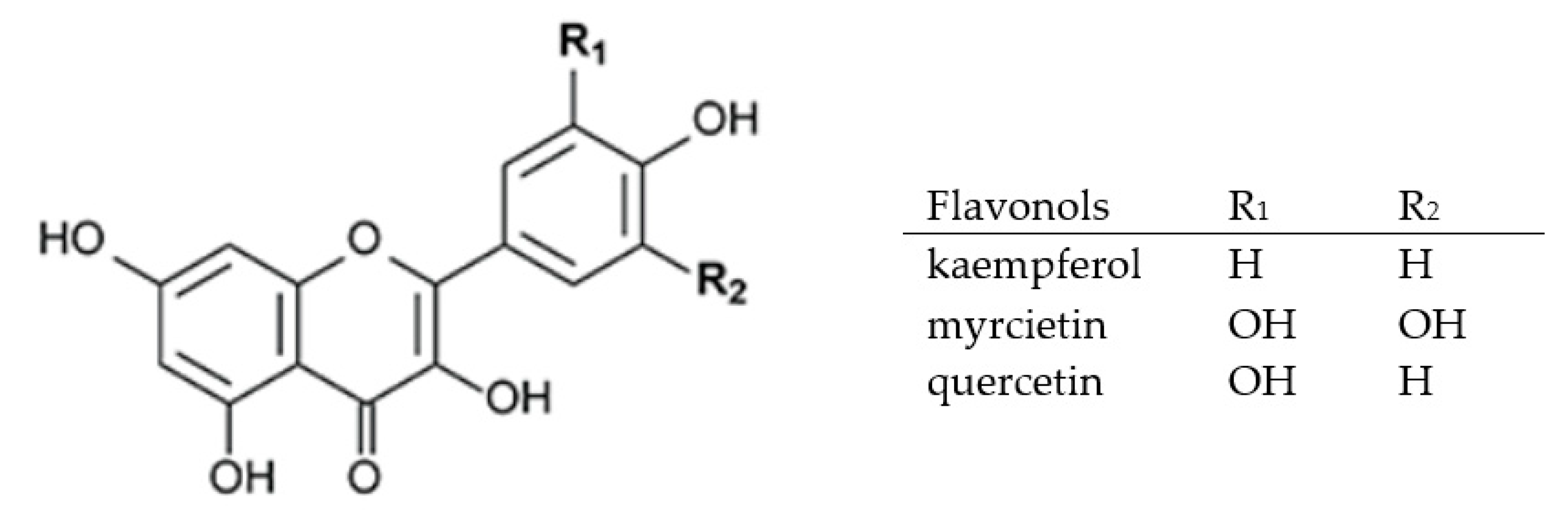
2.3.2. Flavones, Flavonols, and Flavanones
2.3.3. Anthocyanins
3. Impact of Technologies in Order to Increase Phenolics in Wine and Beer
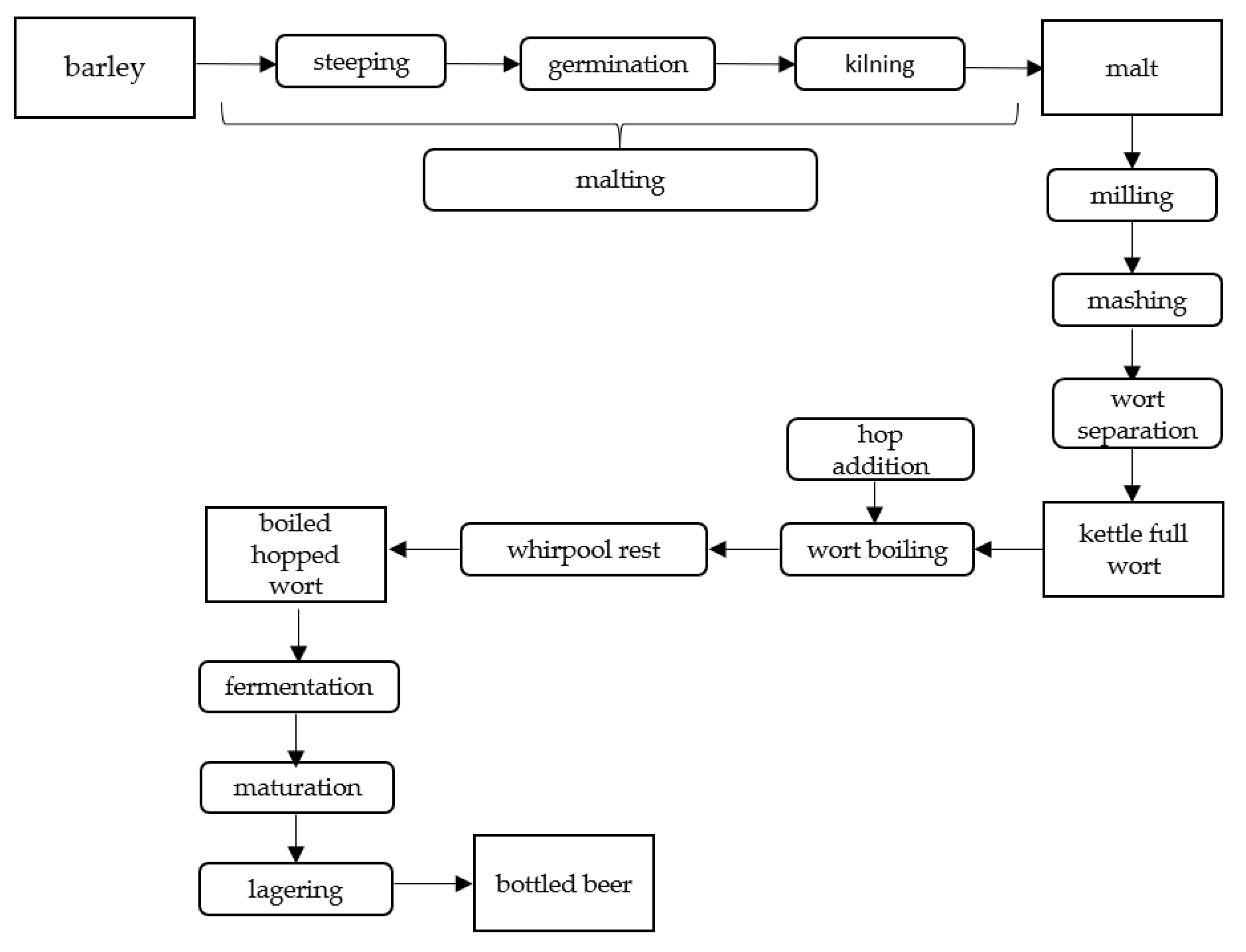
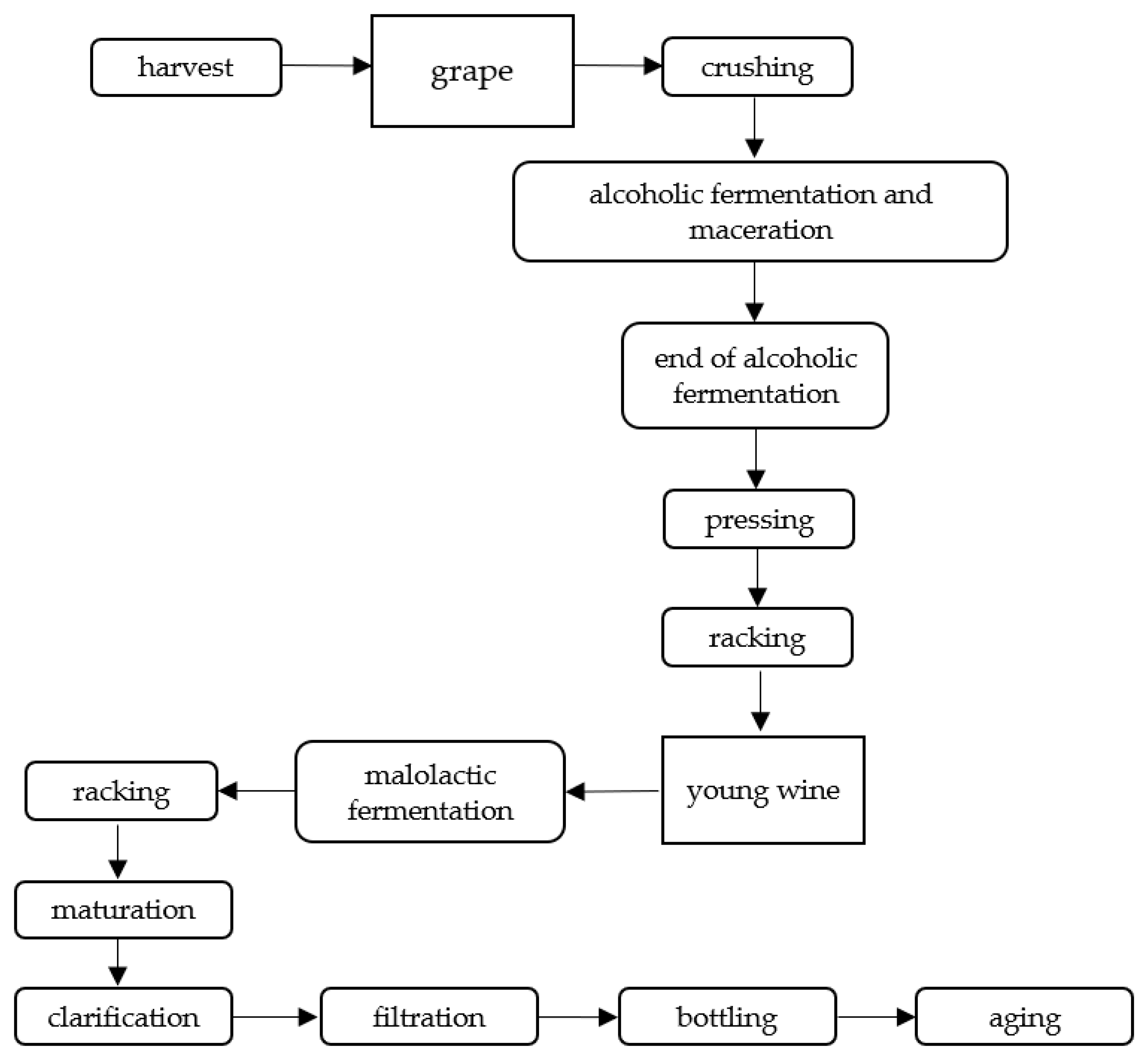
3.1. Raw Material
3.2. Changes during Alcoholic Fermentation
3.3. Maturation, Aging, and Storage
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, H.; Wang, H.; Li, H.; Goodman, S.; van der Leed, P.; Xu, Z.; Fortunato, A.; Yanga, P. The worlds of wine: Old, new and ancient. Wine Econ. Pol. 2018, 7, 178–182. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Ball, T.; Yu, L.; Li, Y.; Xing, F. Revealing a 5000-y-old beer recipe in china. Proc. Natl. Acad. Sci. USA 2016, 113, 6444–6448. [Google Scholar] [CrossRef] [PubMed]
- Roerecke, M.; Rehm, J. Alcohol consumption, drinking patterns, and ischemic heart disease: A narrative review of meta-analyses and a systematic review and meta-analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Med. 2014, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- Gromes, R.; Zeuch, M.; Piendl, A. Further investigations into the dietary fibre content of beers. Brau. Int. 2000, 18, 24–28. [Google Scholar]
- Powell, J.J.; McNaughton, S.A.; Jugdaohsingh, R.; Anderson, S.H.; Dear, J.; Khot, F.; Mowatt, L.; Gleason, K.L.; Sykes, M.; Thompson, R.P.; et al. A provisional database for the silicon content of foods in the United Kingdom. Br. J. Nutr. 2005, 94, 804–812. [Google Scholar] [CrossRef]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; Di Minno, G.; Ritieni, A. Red wine consumption and cardiovascular health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef]
- Di Pietro, M.B.; Bamforth, C.W. A comparison of the antioxidant potential of wine and beer. J. Inst. Brew. 2011, 117, 547–555. [Google Scholar] [CrossRef]
- Martinez-Gomez, A.; Caballero, I.; Blanco, C.A. Phenols and melanoidins as natural antioxidants in beer. structure, reactivity and antioxidant activity. Biomolecules 2020, 10, 400. [Google Scholar] [CrossRef]
- Habschied, K.; Lončarić, A.; Mastanjević, K. Screening of polyphenols and antioxidative activity in industrial beers. Foods 2020, 9, 238. [Google Scholar] [CrossRef]
- Horincar, G.; Enachi, E.; Bolea, C.; Râpeanu, G.; Aprodu, I. Value-added lager beer enriched with eggplant (Solanum melongena l.) peel extract. Molecules 2020, 25, 731. [Google Scholar] [CrossRef]
- Wannenmacher, J.; Gastl, M.; Becker, T. Phenolic substances in beer: Structural diversity, reactive potential and relevance for brewing process and beer quality. Compr. Rev. Food Sci. 2018, 17, 953–988. [Google Scholar] [CrossRef]
- Wannenmacher, J.; Cotterchio, C.; Schlumberger, M.; Reuber, V.; Gastl, M.; Becker, T. Technological influence on sensory stability and antioxidant activity of beers measured by ORAC and FRAP. J. Sci. Food Agric. 2019, 99, 6628–6637. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gil, A.; del Alamo-Sanza, M.; Sánchez-Gómez, R.; Nevares, I. Alternative woods in enology: Characterization of tannin and low molecular weight phenol compounds with respect to traditional oak woods. A Review. Molecules 2020, 25, 1474. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, J.; Vongluanngam, I.; Ricci, A.; Parpinello, G.P.; Versari, A. The oxygen consumption kinetics of commercial oenological tannins in model wine solution and chianti red wine. Molecules 2020, 25, 1215. [Google Scholar] [CrossRef]
- Afroz, R.; Tanvir, E.; Little, P. Honey-derived flavonoids: Natural products for the prevention of atherosclerosis and cardiovascular diseases. Clin. Exp. Pharmacol. 2016, 6, 1–4. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Rosenberg, I.; Uauy, R. History of modern nutrition science—Implications for current research, dietary guidelines, and food policy. BMJ 2018, 361, 2392. [Google Scholar] [CrossRef]
- Cheng, C.K.; Luo, J.; Lau, C.W.; Chen, Z.; Tian, X.Y.; Huang, Y. Pharmacological basis and new insights of resveratrol action in the cardiovascular system. Br. J. Pharmacol. 2019, 177, 1258–1277. [Google Scholar] [CrossRef]
- De Gaetano, G.; Cerletti, C.; Alkerwi, A.; Iacoviello, L.; Badimon, L.; Costanzo, S.; Pounis, G.; Trevisan, M.; Panico, S.; Stranges, S.; et al. Effects of moderate beer consumption on health and disease: A consensus document. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 443–467. [Google Scholar] [CrossRef]
- Redondo, N.; Nova, E.; Díaz-Prieto, L.E.; Marcos, A. Effects of moderate beer consumption on health. Nutr. Hosp. 2018, 35, 41–44. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Magraner, E.; Condines, X.; Valderas-Martínez, P.; Roth, I.; Arranz, S.; Casas, R.; Navarro, M.; Hervas, A.; Sisó, A.; et al. Effects of alcohol and polyphenols from beer on atherosclerotic biomarkers in high cardiovascular risk men: A randomized feeding trial. Nutr. Metab. Cardiovasc. Dis. 2014, 25, 36–45. [Google Scholar] [CrossRef]
- Lugasi, A. Polyphenol content and antioxidant properties of beer. Acta Aliment. 2003, 32, 181–192. [Google Scholar] [CrossRef]
- Arranz, S.; Valderas-Martínez, P.; Chiva-Blanch, G.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Estruch, R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients 2012, 4, 759–781. [Google Scholar] [CrossRef] [PubMed]
- Quifer, P.; Martínez, M.; Jáuregui, O.; Estruch, R.; Lamuela, R.; Chiva, G.; Vallverdú-Queralt, A. A comprehensive characterisation of beer polyphenols by high resolution mass spectrometry (LC–ESI-LTQ-Orbitrap-MS). Food Chem. 2014, 169, 336–343. [Google Scholar] [CrossRef]
- Čechovská, L.; Konečný, M.; Velíšek, J.; Cejpek, K. Effect of Maillard reaction on reducing power of malts and beers. Czech J. Food Sci. 2012, 30, 548–556. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Pietrafesa, A.; Siesto, G.; Pietrafesa, R.; Zambuto, M.; Romano, P. Use of Saccharomyces cerevisiae var. boulardii in co-fermentations with S. cerevisiae for the production of craft beers with potential healthy value-added. Int. J. Food Microbiol. 2018, 284, 22–30. [Google Scholar] [CrossRef]
- Lingua, M.S.; Neme Tauil, R.M.; Batthyány, C.; Wunderlin, D.A.; Baroni, M.V. Proteomic analysis of Saccharomyces cerevisiae to study the effects of red wine polyphenols on oxidative stress. J. Food Sci. Technol. 2019, 56, 4129–4138. [Google Scholar] [CrossRef]
- Grieco, F.; Carluccio, M.A.; Giovinazzo, G. Autochthonous Saccharomyces cerevisiae starter cultures enhance polyphenols content, antioxidant activity, and anti-inflammatory response of Apulian red wines. Foods 2019, 8, 453. [Google Scholar] [CrossRef] [PubMed]
- Generalić Mekinić, I.; Skračić, Ž.; Kokeza, A.; Soldo, B.; Ljubenkov, I.; Banović, M.; Skroza, D. Effect of winemaking on phenolic profile, colour components and antioxidants in Crljenak kaštelanski (sin. Zinfandel, Primitivo, Tribidrag) wine. J. Food Sci. Technol. 2019, 56, 1841–1853. [Google Scholar] [CrossRef] [PubMed]
- Mitić, M.; Kostić, D.; Pavlović, A. The phenolic composition and the antioxidant capacity of Serbian red wines. Adv. Technol. 2014, 3, 16–22. [Google Scholar] [CrossRef]
- Mitić, M.; Kostić, D.; Pavlović, A.; Micić, R.; Stojanović, B.; Paunović, D.; Dimitrijević, D. Antioxidant activity and polyphenol profile of Vranac red wines from Balkan region. Hem. Ind. 2016, 70, 265–275. [Google Scholar] [CrossRef]
- Kondrashov, A.; Ševčík, R.; Benákova, H.; Koštířová, M.; Štípek, S. The key role of grape variety for antioxidant capacity of red wines. e-SPEN Eur. e-J. Clin. Nutr. Metab. 2009, 4, 41–46. [Google Scholar] [CrossRef]
- Di Majo, D.; La Guardia, M.; Giammanco, S.; La Neve, L.; Giammanco, M. The antioxidant capacity of red wine in relationship with its polyphenolic constituents. Food Chem. 2008, 111, 45–49. [Google Scholar] [CrossRef]
- Raičević, D.; Božinović, Z.; Petkov, M.; Ivanova-Petropulos, V.; Kodžulović, V.; Mugoša, M.; Šućur, S.; Maraš, V. Polyphenolic content and sensory profile of Montenegrin Vranac wines produced with different oenological products and maceration. Maced. J. Chem. Chem. Eng. 2017, 36, 229–238. [Google Scholar] [CrossRef]
- Callemien, D.; Collin, S. Structure, organoleptic properties, quantification methods, and stability of phenolic compounds in beer-a review. Food Rev. Int. 2010, 26, 1–84. [Google Scholar] [CrossRef]
- Gerhäuser, C. Beer constituents as potential cancer chemopreventive agents. Eur. J. Cancer 2005, 41, 1941–1954. [Google Scholar] [CrossRef]
- Shahidi, F.; Nazk, M. Phenolics in Food and Nutraceuticals, 2nd ed.; CRC Press LLC: Boca Raton, FL, USA, 2004. [Google Scholar] [CrossRef]
- Andersen, M.L.; Gundermann, M.; Danielsen, B.P.; Lund, M.N. Kinetic models for the role of protein thiols during oxidation in beer. J. Agric. Food Chem. 2017, 65, 10820–10828. [Google Scholar] [CrossRef]
- Andersen, M.L.; Outtrup, H.; Skibsted, L.H. Potential antioxidants in beer assessed by ESR spin trapping. J. Agric. Food Chem. 2000, 48, 3106–3111. [Google Scholar] [CrossRef]
- Lindenmeier, M.; Faist, V.; Hofmann, T. Structural and functional characterization of pronyl-lysine, a novel protein modification in bread crust melanoidins showing in vitro antioxidative and phase I/II enzyme modulating activity. J. Agric. Food Chem. 2002, 50, 6997–7006. [Google Scholar] [CrossRef]
- Maillard, M.N.; Billaud, C.; Chow, Y.N.; Ordonaud, C.; Nicolas, J. Free radical scavenging, inhibition of polyphenoloxidase activity and copper chelating properties of model Maillard systems. LWT Food Sci. Technol. 2007, 40, 1434–1444. [Google Scholar] [CrossRef]
- Kunz, T.; Frenzel, J.; Wietstock, P.C.; Methner, F.J. Possibilities to improve the antioxidative capacity of beer by optimized hopping regimes. J. Inst. Brew. 2014, 120, 415–425. [Google Scholar] [CrossRef]
- Wietstock, P.; Kunz, T.; Shelhammer, T.; Schon, T.; Methner, F.J. Behaviour of antioxidants derived from hops during wort boiling. J. Inst. Brew. 2010, 116, 157–166. [Google Scholar] [CrossRef]
- Ting, P.L.; Lusk, L.; Refling, J.; Kay, S.; Ryder, D. Identification of antiradical hop compounds. J. Am. Soc. Brew. Chem. 2008, 66, 116–126. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Szwajgier, D. Content of individual phenolic acids in worts and beers and their possible contribution to the antiradical activity of beer. J. Inst. Brew. 2009, 115, 243–252. [Google Scholar] [CrossRef]
- Piazzon, A.; Forte, M.; Nardini, M. Characterization of phenolics content and antioxidant activity of different beer types. J. Agric. Food Chem. 2010, 58, 10677–10683. [Google Scholar] [CrossRef]
- Snopek, L.; Mlcek, J.; Sochorova, L.; Baron, M.; Hlavacova, I.; Jurikova, T.; Kizek, R.; Sedlackova, E.; Sochor, J. Contribution of red wine consumption to human health protection. Molecules 2018, 23, 1684. [Google Scholar] [CrossRef]
- Osorio-Paz, I.; Brunauer, R.; Alavez, S. Beer and its non-alcoholic compounds in health and disease. Crit. Rev. Food Sci. Nutr. 2019, 1, 1–14. [Google Scholar] [CrossRef]
- Di Renzo, L.; Marsella, L.T.; Carraro, A.; Valente, R.; Gualtieri, P.; Gratteri, S.; Tomasi, D.; Gaiotti, F.; De Lorenzo, A. Changes in LDL oxidative status and oxidative and inflammatory gene expression after red wine intake in healthy people: A randomized trial. Mediat. Inflamm. 2015, 2015, 317348:1–317348:13. [Google Scholar] [CrossRef]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Hassan, S.T.; Tenore, G.C.; Novellino, E. Effect of grape pomace polyphenols with or without pectin on TMAO serum levels assessed by LC/MS-based assay: A preliminary clinical study on overweight/obese subjects. Front. Pharmacol. 2019, 10, 575:1–575:11. [Google Scholar] [CrossRef]
- Nova, E.; San Mauro-Martín, I.; Díaz-Prieto, L.E.; Marcos, A. Wine and beer within a moderate alcohol intake is associated with higher levels of HDL-c and adiponectin. Nutr. Res. 2019, 63, 42–50. [Google Scholar] [CrossRef]
- Golan, R.; Gepner, Y.; Shai, I. Wine and health–new evidence. Eur. J. Clin. Clin. Nutr. 2018, 72, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Cachofeiro, V.; Millán, J.; Lahera, V.; Nieto, M.; Martin, R.; Bello, E.; Alvarez-Sala, L.; Nieto, M. Red wine intake but not other alcoholic beverages increases total antioxidant capacity and improves pro-inflammatory profile after an oral fat diet in healthy volunteers. Rev. Clín. Esp. 2015, 215, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Franchini, M.; Favaloro, E.J.; Targher, G. Moderate red wine consumption and cardiovascular disease risk: Beyond the “French paradox”. In Seminars in Thrombosis and Hemostasis; Favaloro, E.J., Levi, M., Lisman, T., Kwaan, H.C., Schulman, S., Eds.; Thieme Medical Publishers: Stuttgart, Germany, 2010; pp. 59–70. [Google Scholar]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; Amann, M.; Anderson, H.R.; Andrews, K.G.; Aryee, M.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- De Salvo, K.B.; Olson, R.; Casavale, K.O. Dietary guidelines for Americans. JAMA 2016, 315, 457–458. [Google Scholar] [CrossRef]
- Giovinazzo, G.; Ingrosso, I.; Paradiso, A.; De Gara, L.; Santino, A. Resveratrol biosynthesis: Plant metabolic engineering for nutritional improvement of food. Plant Foods Hum. Nutr. 2012, 67, 191–199. [Google Scholar] [CrossRef]
- Calabriso, N.; Scoditti, E.; Massaro, M.; Pellegrino, M.; Storelli, C.; Ingrosso, I.; Giovinazzo, G.; Carluccio, M.A. Multiple anti-inflammatory and anti-atherosclerotic properties of red wine polyphenolic extracts: Differential role of hydroxycinnamic acids, flavonols and stilbenes on endothelial inflammatory gene expression. Eur. J. Nutr. 2016, 55, 477–489. [Google Scholar] [CrossRef]
- Giovinazzo, G.; Grieco, F. Functional properties of grape and wine polyphenols. Plant Foods Hum. Nutr. 2015, 70, 454–462. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Duk, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. BioFactors 2018, 44, 16–25. [Google Scholar] [CrossRef]
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An important scaffold for medicinal chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D. Tannins from foods to combat diseases. Int. J. Pharma Res. Rev. 2015, 4, 40–44. [Google Scholar]
- Rufián Henares, J.; Morales, F. Functional properties of melanoidins: In vitro antioxidant, antimicrobial and antihypertensive activities. Food Res. Int. 2013, 40, 995–1002. [Google Scholar] [CrossRef]
- Samuels, J.; Shashidharamurthy, R.; Rayalam, S. Novel anti-obesity effects of beer hops compound xanthohumol: Role of AMPK signalling pathway. Nutr. Metab. 2018, 15, 42:1–42:11. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hansen, P.E.; Wang, G.; Qiu, L.; Dong, J.; Yin, H.; Qian, Z.; Yang, M.; Miao, J. Pharmacological profile of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus). Molecules 2015, 20, 754–779. [Google Scholar] [CrossRef] [PubMed]
- Rivero, D.; Pérez, S.; González, M.L.; Valls, V.; Codoñer, P.; Muñiz, P. Inhibition of induced DNA oxidative damage by beers: Correlation with the content of polyphenols and melanoidins. J. Agric. Food Chem. 2005, 53, 3637–3642. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, H.; Sun, G.; Yang, B.; Zhao, M. Assessment of endogenous antioxidative compounds and antioxidant activities of lager beers. J. Sci. Food Agric. 2013, 93, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Liu, Y.M.; Wang, J.; Wang, X.N.; Li, C.Y. Anti-inflammatory effect of the blueberry anthocyanins malvidin-3-glucoside and malvidin-3-galactoside in endothelial cells. Molecules 2014, 19, 12827–12841. [Google Scholar] [CrossRef]
- Radonjić, S.; Košmerl, T.; Ota, A.; Prosen, H.; Maraš, V.; Demšar, L.; Polak, T. Technological and microbiological factors affecting polyphenolic profile of Montenegrin red wines. Chem. Ind. Chem. Eng. Q. 2019, 25, 309–319. [Google Scholar] [CrossRef]
- Pajović-Šćepanović, R.; Wendelin, S.; Eder, R. Phenolic composition and varietal discrimination of Montenegrin red wines (Vitis vinifera var. Vranac, Kratošija, and Cabernet Sauvignon). Eur. Food Res. Technol. 2018, 244, 2243–2254. [Google Scholar] [CrossRef]
- Lima, A.; Oliveira, C.; Santos, C.; Campos, F.M.; Couto, J.A. Phenolic composition of monovarietal red wines regarding volatile phenols and its precursors. Eur. Food Res. Technol. 2018, 244, 1985–1994. [Google Scholar] [CrossRef]
- Bartolomé, B.; Peña-Neira, A.; Gómez-Cordovés, C. Phenolics and related substances in alcohol-free beers. Eur. Food Res. Technol. 2000, 210, 419–423. [Google Scholar] [CrossRef]
- Dvořáková, M.; Hulín, P.; Karabín, M.; Dostálek, P. Determination of polyphenols in beer by an effective method based on solid-phase extraction and high performance liquid chromatography with diode-array detection. Czech J. Food Sci. 2007, 25, 182–188. [Google Scholar] [CrossRef]
- Jandera, P.; Škeříková, V.; Řehová, L.; Hájek, T.; Baldriánová, L.; Škopová, G.; Kellner, V.; Horna, A. RP-HPLC analysis of phenolic compounds and flavonoids in beverages and plant extracts using a CoulArray detector. J. Sep. Sci. 2005, 28, 1005–1022. [Google Scholar] [CrossRef]
- McMurrough, I.; Roche, G.P.; Cleary, K.G. Phenolic acids in beers and worts. J. Inst. Brew. 1984, 90, 181–187. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, W.; Lu, J.; Zhao, M. Phenolic profiles and antioxidant activities of commercial beers. Food Chem. 2010, 119, 1150–1158. [Google Scholar] [CrossRef]
- Floridi, S.; Montanari, L.; Marconi, O.; Fantozzi, P. Determination of free phenolic acids in wort and beer by coulometric array detection. J. Agric. Food Chem. 2003, 51, 1548–1554. [Google Scholar] [CrossRef]
- Marques, D.; Cassis, M.; Quelhas, J.; Bertozzi, J.; Visentainer, J.; Oliveira, C.; Monteiro, A. Characterization of craft beers and their bioactive compounds. Chem. Eng. Trans. 2017, 57, 1747–1752. [Google Scholar]
- Marova, I.; Parilova, K.; Friedl, Z.; Obruca, S.; Duronova, K. Analysis of phenolic compounds in lager beers of different origin: A contribution to potential determination of the authenticity of Czech beer. Chromatographia 2011, 73, 83–95. [Google Scholar] [CrossRef]
- Alonso Garcıa, A.; Cancho Grande, B.; Simal Gandara, J. Development of a rapid method based on solid-phase extraction and liquid chromatography with ultraviolet absorbance detection for the determination of polyphenols in alcohol-free beers. J. Chromatogr. A 2004, 1054, 175–180. [Google Scholar] [CrossRef]
- Kellner, V.; Jurková, M.; Čulík, J.; Horák, T.; Čejka, P. Some phenolic compounds in Czech hops and beer of pilsner type. Brew. Sci. 2007, 60, 31–37. [Google Scholar]
- Socha, R.; Pająk, P.; Fortuna, T.; Buksa, K. Antioxidant activity and the most abundant phenolics in commercial dark beers. Int. J. Food Prop. 2017, 20, 1–15. [Google Scholar] [CrossRef]
- Nardini, M.; Ghiselli, A. Determination of free and bound phenolic acids in beer. Food Chem. 2004, 84, 137–143. [Google Scholar] [CrossRef]
- Lamuela-Raventos, R.M.; Romero-Perez, A.I.; Waterhouse, A.L.; Carmen de la Torre-Borona, M. Direct HPLC Analysis of cis- and trans-resveratrol and piceid isomers in Spanish red Vitis vinifera wines. J. Agric. Food Chem. 1995, 43, 281–283. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Rotchés-Ribalta, M.; Zamora-Ros, R.; Llorach, R.; Lamuela-Raventós, R.M.; Andrés-Lacueva, C. Determination of resveratrol and piceid in beer matrices by solid-phase extraction and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 698–705. [Google Scholar] [CrossRef]
- Paulo, L.; Domingues, F.; Queiroz, J.A.; Gallardo, E. Development and validation of an analytical method for the determination of trans- and cis-resveratrol in wine: Analysis of its contents in 186 Portuguese red wines. J. Agric. Food Chem. 2011, 59, 2157–2168. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Muntean, C. Ultraviolet irradiation of trans-resveratrol and HPLC determination of trans-resveratrol and cis-resveratrol in Romanian red wines. J. Chromatogr. Sci. 2012, 50, 920–927. [Google Scholar] [CrossRef]
- Zoechling, A.; Reiter, E.; Eder, R.; Wendelin, S.; Leiber, F.; Jungbauer, A. The flavonoid kaempferol is responsible for majority of estrogenic activity in red wine. Am. J. Enol. Vitic. 2009, 60, 223–232. [Google Scholar]
- Basalekou, M.; Kyraleou, M.; Pappas, C.; Tarantilis, P.; Kotseridis, Y.; Kallithraka, S. Proanthocyanidin content as an astringency estimation tool and maturation index in red and white winemaking technology. Food Chem. 2019, 299, 125–135. [Google Scholar] [CrossRef]
- Stark, T.; Wollmann, N.; Wenker, K.; Lösch, S.; Glabasnia, A.; Hofmann, T. Matrix-calibrated LC-MS/MS quantitation and sensory evaluation of oak ellagitannins and their transformation products in red wines. J. Agric. Food Chem. 2010, 58, 6360–6369. [Google Scholar] [CrossRef]
- García-Estévez, I.; Escribano-Bailón, M.T.; Rivas-Gonzalo, J.C.; Alcalde-Eon, C. Validation of a mass spectrometry method to quantify oak ellagitannins in wine samples. J. Agric. Food Chem. 2012, 60, 1373–1379. [Google Scholar] [CrossRef]
- Jourdes, M.; Michel, J.; Saucier, C.; Quideau, S.; Teissedre, P.L. Identification, amounts, and kinetics of extraction of C-glucosidic ellagitannins during wine aging in oak barrels or in stainless steel tanks with oak chips. Anal. Bioanal. Chem. 2011, 401, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Guo, A.; Zhang, Y.; Wang, H.; Liu, Y.; Li, H.J. A review on astringency and bitterness perception of tannins in wine. Trends Food Sci. Technol. 2014, 40, 6–19. [Google Scholar] [CrossRef]
- Niculescu, V.C.; Paun, N.; Ionete, R.E. The evolution of polyphenols from grapes to wines. In Grapes and Wines—Advances in Production, Processing, Analysis and Valorization; Jordão, A.M., Cosme, F., Eds.; Intech Open: London, UK, 2017. [Google Scholar] [CrossRef]
- Barnaba, C.; Dellacassa, E.; Nicolini, G.; Nardin, T.; Malacarne, M.; Larcher, R. Identification and quantification of 56 targeted phenols in wines, spirits, and vinegars by online solid-phase extraction—Ultrahigh-performance liquid chromatography—Quadrupole-orbitrap mass spectrometry. J. Chromatogr. A 2015, 1423, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Banegil, M.; Hurtado-sánchez, M.C.; Galeano-Díaz, T.; Durán-Merás, I. Front-face fluorescence spectroscopy combined with second-order multivariate algorithms for the quantification of polyphenols in red wine samples. Food Chem. 2016, 220, 168–176. [Google Scholar] [CrossRef]
- Kustrin, S.; Hettiarachchi, C.; Morton, D.; Razic, S. Analysis of phenolics in wine by high performance thin-layer chromatography with gradient elution and high resolution plate imaging. J. Pharm. Biomed. Anal. 2014, 102, 93–99. [Google Scholar] [CrossRef]
- Justesen, U.; Knuthsen, P.; Leth, T. Quantitative analysis of flavonols, flavones, and flavanones in fruits, vegetables and beverages by high-performance liquid chromatography with photo-diode array and mass spectrometric detection. J. Chromatogr. A 1998, 799, 101–110. [Google Scholar] [CrossRef]
- Achilli, G.; Cellerino, G.P.; Gamache, P.H. Identification and determination of phenolic constituents in natural beverages and plant extracts by means of a coulometric electrode array system. J. Chromatogr. A 1993, 632, 111–117. [Google Scholar] [CrossRef]
- Martinez-Periñan, E.; Hernández-Artiga, M.P.; Palacios-Santander, J.M.; ElKaoutit, M.; Naranjo-Rodriguez, I.; Bellido-Milla, D. Estimation of beer stability by sulphur dioxide and polyphenol determination. Evaluation of a laccase-sonogel-carbon biosensor. Food Chem. 2011, 127, 234–239. [Google Scholar] [CrossRef]
- Sýs, M.; Metelka, R.; Vytřas, K. Comparison of tyrosinase biosensor based on carbon nanotubes with DPPH spectrophotometric assay in determination of TEAC in selected Moravian wines. Monatsh. Chem. Chem. Mon. 2015, 146, 813–817. [Google Scholar] [CrossRef]
- Martysiak-Żurowska, D.; Wenta, W. A Comparison of ABTS and DPPH methods for assessing the total antioxidant capacity of human milk. Acta Sci. Pol. Technol. Aliment. 2012, 1, 83–89. [Google Scholar]
- Özyürek, M.; Güçlü, K.; Tütem, E.; Sözgen-Başkan, K.; Erçaǧ, E.; Çelik, S.E.; Baki, S.; Yildiz, L.; Karaman, Ş.; Apak, R. A comprehensive review of CUPRAC methodology. Anal. Methods 2011, 3, 2439–2453. [Google Scholar] [CrossRef]
- Šeruga, M.; Novak, I.; Jakobek, L. Determination of polyphenols content and antioxidant activity of some red wines by differential pulse voltammetry, HPLC and spectrophotometric methods. Food Chem. 2011, 124, 1208–1216. [Google Scholar] [CrossRef]
- Đorđević, N.O.; Pejin, B.; Novaković, M.M.; Stanković, D.M.; Mutić, J.J.; Pajović, S.B.; Tešević, V.V. Some chemical characteristics and antioxidant capacity of novel Merlot wine clones developed in Montenegro. Sci. Hortic. 2017, 225, 505–511. [Google Scholar] [CrossRef]
- Ricci, A.; Teslic, N.; Petropolus, V.; Parpinello, G.P.; Versari, A. Fast analysis of total polyphenol content and antioxidant activity in wines and oenological tannins using a flow injection system with tandem diode array and electrochemical detections. Food Anal. Methods 2019, 12, 347–354. [Google Scholar] [CrossRef]
- Minkova, S.; Hristova-Avakumova, N.; Traykov, T.; Nikolova, K.; Hadjimitova, V. Antioxidant scavenging capacity of Bulgarian red wines by chemiluminescent and stable free radical methods. Bulg. Chem. Commun. 2019, 51, 299–303. [Google Scholar]
- García-Guzmán, J.J.; López-Iglesias, D.; Cubillana-Aguilera, L.; Lete, C.; Lupu, S.; Palacios-Santander, J.M.; Bellido-Milla, D. Assessment of the polyphenol indices and antioxidant capacity for beers and wines using a tyrosinase-based biosensor prepared by sinusoidal current method. Sensors 2018, 19, 66. [Google Scholar] [CrossRef] [PubMed]
- Mikyška, A.; Dušek, M. How wort boiling process affects flavonoid polyphenols in beer. Kvas. Prum. 2019, 65, 192–200. [Google Scholar] [CrossRef]
- Šućur, S.; Maraš, V.; Kodžulović, V.; Raičević, J.; Mugoša, M.; Jug, T.; Košmerl, T. The impact of different commercial yeasts on quality parameters of Montenegrin red wine—Vranac and Kratošija. Biol. Eng. Med. 2016, 1. [Google Scholar] [CrossRef]
- Mattivi, F.; Nicolini, G. Analysis of polyphenols and resveratrol in Italian wines. BioFactors 1997, 6, 445–448. [Google Scholar] [CrossRef]
- Martínez, A.; Vegara, S.; Herranz-López, M.; Martí, N.; Valero, M.; Micol, V.; Saura, D. Kinetic changes of polyphenols, anthocyanins and antioxidant capacity in forced aged hibiscus ale beer. J. Inst. Brew. 2017, 123, 58–65. [Google Scholar] [CrossRef]
- Chen, O.; Blumberg, J. Flavonoids in Beer and Their Potential Benefit on the Risk of Cardiovascular Disease. In Beer in Health and Disease Prevention, 1st ed.; Preedy, V.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 831–841. [Google Scholar]
- Galarce, O.; Pavon, J.; Aranda, M.; Novoa, L.; Henriquez, K. Chemometric Optimization of QuEChERS extraction method for polyphenol determination in beers by liquid chromatography with ultraviolet detection. Food Anal. Methods 2018, 12, 448–457. [Google Scholar] [CrossRef]
- Lentz, M. The impact of simple phenolic compounds on beer aroma and flavor. Fermentation 2018, 4, 20. [Google Scholar] [CrossRef]
- Pihlava, J.M.; Kurtelius, T.; Hurme, T. Total hordatine content in different types of beers. J. Inst. Brew. 2016, 122, 212–217. [Google Scholar] [CrossRef]
- Ferreira-Lima, N.; Vallverdú-Queralt, A.; Meudec, E.; Pinasseau, L.; Verbaere, A.; Bordignon-Luiz, M.T.; Le Guernevé, C.; Cheynier, V.; Sommerer, N. Quantification of hydroxycinnamic derivatives in wines by UHPLC-MRM-MS. Anal. Bioanal. Chem. 2018, 410, 3483–3490. [Google Scholar] [CrossRef] [PubMed]
- Kallitraka, S.; Tsoutsouras, E.; Tzourou, E.; Lanaridis, P. Principal phenolic compounds in Greek red wines. Food Chem. 2006, 99, 784–793. [Google Scholar] [CrossRef]
- Mitić, S.; Paunović, D.; Pavlović, A.; Tošić, S.; Stojković, M.; Mitić, M. Phenolic profiles and total antioxidant capacity of marketed beers in Serbia. Int. J. Food Prop. 2014, 17, 908–922. [Google Scholar] [CrossRef]
- Moura, N.; Cazaroti, T.; Dias, N.; Fernandes, P.; Monteiro, M.; Perrone, D.; Guedes, A. Phenolic compounds of Brazilian beers from different types and styles and application of chemometrics for modeling antioxidant capacity. Food Chem. 2016, 199, 105–113. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Wine phenolics. Ann. N. Y. Acad. Sci. 2002, 957, 21–36. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Kalantari, H.; Das, D.K. Physiological effects of resveratrol. BioFactors 2010, 36, 401–406. [Google Scholar] [CrossRef]
- Wu, J.M.; Hsieh, T.C. Resveratrol: A cardioprotective substance. Ann. N. Y. Acad. Sci. 2011, 1215, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A. Effects of resveratrol supplementation on plasma lipids: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2013, 71, 822–835. [Google Scholar] [CrossRef]
- Penumathsa, S.V.; Maulik, N. Resveratrol: A promising agent in promoting cardioprotection against coronary heart disease. Can. J. Physiol. Pharmacol. 2009, 87, 275–286. [Google Scholar] [CrossRef]
- Sakata, Y.; Zhuang, H.; Kwansa, H.; Koehler, R.C.; Doré, S. Resveratrol protects against experimental stroke: Putative neuroprotective role of heme oxygenase 1. Exp. Neurol. 2010, 224, 325–329. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Anti-oxidative action of resveratrol: Implications for human health. Arab. J. Chem. 2011, 4, 293–298. [Google Scholar] [CrossRef]
- Hussein, M.A. A convenient mechanism for the free radical scavenging activity of resveratrol. Int. J. Phytomed. 2011, 3, 459–469. [Google Scholar]
- Godelmann, R.; Fang, F.; Humpfer, E.; Schütz, B.; Bansbach, M.; Schäfer, H.; Spraul, M. Targeted and Nontargeted Wine Analysis by 1H NMR spectroscopy combined with multivariate statistical analysis. diferentiation of important parameters: Grape variety, geographical origin, year of vintage. J. Agric. Food Chem. 2013, 61, 5610–5619. [Google Scholar] [CrossRef] [PubMed]
- Cheiran, K.P.; Raimundo, V.P.; Manfroi, V.; Cheiran, K.P.; Rodrigues, E.; Anzanello, M.J.; Raimundo, V.P.; Frazzon, J.; Kahmann, A. Simultaneous identification of low-molecular weight phenolic and nitrogen compounds in craft beers by HPLC-ESI-MS/MS. Food Chem. 2019, 286, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Molina, L.; Ruiz, A.; Luisa, F.M. A novel multicommuted fluorimetric optosensor for determination of resveratrol in beer. Talanta 2010, 83, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Jerkovic, V.; Collin, S. Fate of resveratrol and piceid through different hop processings and storage times. J. Agric. Food Chem. 2008, 56, 584–590. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of resveratrol: In vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042:1–837042:13. [Google Scholar] [CrossRef]
- Chira, K.; Pacella, N.; Jourdes, M.; Teissedre, P.-L. Chemical and sensory evaluation of Bordeaux wines (Cabernet-Sauvignon and Merlot) and correlation with wine age. Food Chem. 2011, 126, 1971–1977. [Google Scholar] [CrossRef] [PubMed]
- Markoski, M.M.; Garavaglia, J.; Oliveira, A.; Olivaes, J.; Marcadenti, A. Molecular properties of red wine compounds and cardiometabolic benefits. Nutr. Metab. Insights 2016, 9, 51–57. [Google Scholar] [CrossRef]
- Feng, W.; Hao, Z.; Li, M. Isolation and Structure Identification of Flavonoids. In Flavonoids—From Biosynthesis to Human Health; Justino, G.C., Ed.; Intech Open: London, UK, 2017. [Google Scholar] [CrossRef]
- Escobar-Cévoli, R.; Castro-Espín, C.; Béraud, V.; Buckland, G.; Zamora-Ros, R.; Béraud, G.B.V. An overview of global flavonoid intake and its food sources. In Flavonoids—From Biosynthesis to Human Health; Justino, G.C., Ed.; Intech Open: London, UK, 2017. [Google Scholar] [CrossRef]
- Elrod, S.M. Xanthohumol and the medicinal benefits of beer. In Polyphenols: Mechanisms of Action in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2018; pp. 19–32. [Google Scholar]
- Andrés-Iglesias, C.; Blanco, C.A.; Blanco, J.; Montero, O. Mass spectrometry-based metabolomics approach to determine differential metabolites between regular and non-alcohol beers. Food Chem. 2014, 157, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Page, J.E. Xanthohumol and related prenylflavonoids from hops and beer: To your good health! Phytochemistry 2004, 65, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, S.; Burkard, M.; Biendl, M.; Lauer, U.M.; Frank, J.; Busch, C. Prenylated chalcones and flavonoids for the prevention and treatment of cancer. Nutrition 2016, 32, 1171–1178. [Google Scholar] [CrossRef]
- Back, W.; Zurcher, A.; Wunderlich, S. Verfahren zur Herstellung eines xanthohumolhaltigen Getränkes aus Malz- und/oder Rohfruchtwürze sowie derart hergestelltes Getränk. DE10256166A1 2004, A1, 1–5. [Google Scholar]
- Collin, S.; Jerkovic, V.; Bröhan, M.; Callemien, D. Polyphenols and beer quality. In Natural Products, 1st ed.; Ramawat, K.G., Mérillon, J.M., Eds.; Springer: Heidelberg, Germany, 2013; pp. 2334–2353. [Google Scholar]
- Garrido, J.; Borges, F. Wine and grape polyphenols—A chemical perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef]
- Dadic, M.; Belleau, G. Polyphenols and beer flavor. Proc. Am. Soc. Brew. Chem. 1973, 31, 107–114. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Gerhäuser, C.; Becker, H. Phenolic Compounds in Beer. In Beer in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2008; Chapter 12; ISBN 9780080453521. [Google Scholar]
- de Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J. Quantitative analysis of flavan-3-ols in Spanish foodstuffs and beverages. J. Agric. Food Chem. 2000, 48, 5331–5337. [Google Scholar] [CrossRef] [PubMed]
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Törrönen, A.R. Contents of anthocyanins and ellagitannins in selected foods consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619. [Google Scholar] [CrossRef]
- Dixon, R.A.; Xie, D.Y.; Sharma, S.B. Proanthocyanidins—A final frontier in flavonoid research? New Phytol. 2005, 165, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin--like compounds—Nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- McCullough, M.L.; Peterson, J.J.; Patel, R.; Jacques, P.F.; Shah, R.; Dwyer, J.T. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am. J. Clin. Nutr. 2012, 95, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef]
- Cheynier, V.; Dueñas-Paton, M.; Salas, E.; Maury, C.; Souquet, J.M.; Sarni-Manchado, P.; Fulcrand, H. Structure and properties of wine pigments and tannins. Am. J. Enol. Vitic. 2006, 57, 298–305. [Google Scholar]
- Gerhäuser, C.; Alt, A.P.; Klimo, K.; Knauft, J.; Frank, N.; Becker, H. Isolation and potential cancer chemopreventive activities of phenolic compounds of beer. Phytochem. Rev. 2002, 1, 369–377. [Google Scholar] [CrossRef]
- Oregon State University Home Page. Available online: https://lpi.oregonstate.edu/mic/dietary-factors/phytochemicals/flavonoids (accessed on 13 October 2020).
- Perez-Vizcaino, F.; Duarte, J. Flavonols and cardiovascular disease. Mol. Asp. Med. 2010, 31, 478–494. [Google Scholar] [CrossRef]
- Forster, A.; Beck, B.; Schmidt, R.; Jansen, C.; Mellenthin, A. On the composition of low molecular polyphenols in different varieties of hops and from two growing areas. Mon. Brauwiss. 2002, 55, 98–108. [Google Scholar]
- Gangopadhyay, N.; Rai, D.K.; Brunton, N.P.; Gallagher, E.; Hossain, M.B. Antioxidant-guided isolation and mass spectrometric identification of the major polyphenols in barley (Hordeum vulgare) grain. Food Chem. 2016, 210, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Taylor, A.W.; Deinzer, M.L. Quantitative analysis of xanthohumol and related prenylfiavonoids in hops and beer by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 1999, 832, 97–107. [Google Scholar] [CrossRef]
- Moritz Seliger, J.; Misuri, L.; Maser, E.; Hintzpeter, J. The hop-derived compounds xanthohumol, isoxanthohumol and 8-prenylnaringenin are tight-binding inhibitors of human aldo-keto reductases 1B1 and 1B10. J. Enzyme Inhib. Med. Chem. 2018, 33, 607–614. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Yoon, Y.; Yoon, H.; Park, H.-M.; Song, S.; Yeum, K.-J.J.N. Dietary anthocyanins against obesity and inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef]
- Cassidy, A.; Rimm, E.B.; O’Reilly, É.J.; Logroscino, G.; Kay, C.; Chiuve, S.E.; Rexrode, K.M. Dietary flavonoids and risk of stroke in women. Stroke 2012, 43, 946–951. [Google Scholar] [CrossRef]
- Cassidy, A.; Mukamal, K.J.; Liu, L.; Franz, M.; Eliassen, A.H.; Rimm, E.B. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 2013, 127, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Bertoia, M.; Chiuve, S.; Flint, A.; Forman, J.; Rimm, E.B. Habitual intake of anthocyanins and flavanones and risk of cardiovascular disease in men. Am. J. Clin. Nutr. 2016, 104, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Munoz, N.; Winterhalter, P.; Weber, F.; Gomez, M.V.; Gomez-Alonso, S.; Garcia- Romero, E.; Hermosin-Gutierrez, I. Structure elucidation of peonidin 3,7-O-β-diglucoside isolated from Garnacha Tintorera (Vitis vinifera L.) grapes. J. Agric. Food Chem. 2010, 58, 11105–11111. [Google Scholar] [CrossRef]
- Hannah, L.; Roehrdanz, P.R.; Ikegami, M.; Shepard, A.V.; Shaw, M.R.; Tabor, G.; Zhi, L.; Marquet, P.A.; Hijmans, R.J. Climate change, wine, and conservation. Proc. Natl. Acad. Sci. USA 2013, 110, 6907–6912. [Google Scholar] [CrossRef]
- Jones, G.V.; White, M.A.; Cooper, O.R.; Storchmann, K. Climate change and global wine quality. Clim. Chang. 2005, 73, 319–343. [Google Scholar] [CrossRef]
- Bogićević, M.; Maraš, V.; Mugoša, M.; Kodžulović, V.; Raičević, J.; Šućur, S.; Failla, O. The effect of early leaf removal and cluster thinning treatments on berry growth and grape composition in cultivars Vranac and Cabernet Sauvignon. Chem. Biol. Technol. Agric. 2015, 2, 13. [Google Scholar] [CrossRef]
- Kemp, B.S.; Harrison, R.; Creasy, G.L. Effect of mechanical leaf removal and its timing on flavan-3-ol composition and concentrations in Vitis vinifera L. cv. Pinot noir wine. Aust. J. Grape Wine Res. 2011, 17, 270–279. [Google Scholar] [CrossRef]
- Gatti, M.; Bernizzoni, F.; Civardi, S.; Poni, S. Effects of cluster thinning and pre-flowering leaf removal on growth and grape composition in cv. Sangiovese. Am. J. Enol. Vitic. 2012, 63, 325–332. [Google Scholar] [CrossRef]
- Lee, J.; Skinkis, P.A. Oregon ‘Pinot noir’ grape anthocyanin enhancement by early leaf removal. Food Chem. 2013, 139, 893–901. [Google Scholar] [CrossRef]
- Košmerl, T.; Bertalanič, L.; Maraš, V.; Kodžulović, V.; Šućur, S.; Abramovič, H. Impact of Yield on Total polyphenols, Anthocyanins, Reducing Sugars and Antioxidant potential in White and Red Wines Produced from Montenegrin Autochthonous Grape Varieties. Food Sci. Technol. 2013, 1, 7–15. [Google Scholar] [CrossRef]
- Vezzulli, S.; Civardi, S.; Ferrari, F.; Bavaresco, L. Methyl jasmonate treatment as a trigger of resveratrol synthesis in cultivated grapevine. Am. J. Enol. Vitic. 2007, 58, 530–533. [Google Scholar]
- Santamaria, A.R.; Mulinacci, N.; Valletta, A.; Innocenti, M.; Pasqua, G. Effects of elicitors on the production of resveratrol and viniferins in cell cultures of Vitis vinifera L. cv Italia. J. Agric. Food Chem. 2011, 59, 9094–9101. [Google Scholar] [CrossRef]
- Giacosa, S.; Ossola, C.; Botto, R.; Río Segade, S.; Paissoni, M.A.; Pollon, M.; Gerbi, V.; Rolle, L. Impact of specific inactive dry yeast application on grape skin mechanical properties, phenolic compounds extractability, and wine composition. Food Res. Int. 2019, 116, 1084–1093. [Google Scholar] [CrossRef]
- Narziss, L. Polyphenolgehalt und Polymerisationsindex von Gersten und Kleinmalzen. Mon. Brauwiss. 1976, 29, 9–19. [Google Scholar]
- Almaguer, C.; Schonberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus—A story that begs to be told. A review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
- Holtekjølen, A.K.; Kinitz, C.; Knutsen, S.H. Flavanol and bound phenolic acid contents in different barley varieties. J. Agric. Food Chem. 2006, 54, 2253–2260. [Google Scholar] [CrossRef] [PubMed]
- Penalvo, J.L.; Haajanen, K.M.; Botting, N.; Adlercreutz, H. Quantification of lignans in food using isotope dilution gas chromatography/mass spectrometry. J. Agric. Food Chem. 2005, 53, 9342–9347. [Google Scholar] [CrossRef]
- Kohyama, N.; Ono, H. Hordatine a beta-d-glucopyranoside from ungerminated barley grains. J. Agric. Food Chem. 2013, 61, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.O.; Curto, A.F.; Guido, L.F. Determination of phenolic content in different barley varieties and corresponding malts by liquid chromatography-diode array detection-electrospray ionization tandem mass spectrometry. Antioxidants 2015, 4, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Dvořáková, M.; Douanier, M.; Jurková, M.; Kellner, V.; Dostálek, P. Comparison of antioxidant activity of barley (hordeum vulgare l.) and malt extracts with the content of free phenolic compounds measured by high performance liquid chromatography coupled with coularray detector. J. Inst. Brew. 2008, 114, 150–159. [Google Scholar] [CrossRef]
- Dvořáková, M.; Moreira, M.M.; Dostálek, P.; Skulilova, Z.; Guido, L.F.; Barros, A.A. Characterization of monomeric and oligomeric flavan-3-ols from barley and malt by liquid chromatography-ultraviolet detection-electrospray ionization mass spectrometry. J. Chromatogr. A 2008, 1189, 398–405. [Google Scholar] [CrossRef]
- Madhujith, T.; Shahidi, F. Antioxidative and antiproliferative properties of selected barley (Hordeum vulgare L.) cultivars and their potential for inhibition of low-density lipoprotein (LDL) cholesterol oxidation. J. Agric. Food Chem. 2007, 55, 5018–5024. [Google Scholar] [CrossRef]
- Madhujith, T.; Shahidi, F. Antioxidant potential of barley as affected by alkaline hydrolysis and release of insoluble-bound phenolics. Food Chem. 2009, 117, 615–620. [Google Scholar] [CrossRef]
- Leitao, C.; Marchioni, E.; Bergaentzlé, M.; Zhao, M.; Didierjean, L.; Miesch, L.; Holder, E.; Miesch, M.; Ennahar, S. Fate of polyphenols and antioxidant activity of barley throughout malting and brewing. J. Cereal Sci. 2012, 55, 318–322. [Google Scholar] [CrossRef]
- Inns, E.L.; Buggey, L.A.; Booer, C.; Nursten, H.E.; Ames, J.M. Effect of heat treatment on the antioxidant activity, color, and free phenolic acid profile of malt. J. Agric. Food Chem. 2007, 55, 6539–6546. [Google Scholar] [CrossRef]
- Muñoz-Insa, A.; Gastl, M.; Becker, T. Variation of sunstruck flavor-related substances in malted barley, triticale and spelt. Eur. Food Res. Technol. 2016, 242, 11–23. [Google Scholar] [CrossRef]
- Stavri, M.; Schneider, R.; O’ Donnell, G.; Lechner, D.; Bucar, F.; Gibbons, S. The antimycobacterial components of hops (Humulus lupulus) and their dereplication. Phytother. Res. 2004, 18, 774–776. [Google Scholar] [CrossRef] [PubMed]
- Prencipe, F.B.; Brighenti, V.; Rodolfi, M.; Mongelli, A.; dall’Asta, C.; Ganino, T.; Bruni, R.; Pellati, F. Development of a new high-performance liquid chromatography method with diode array and electrospray ionization-mass spectrometry detection for the metabolite fingerprinting of bioactive compounds in Humulus lupulus L. J. Chromatogr. A 2014, 1349, 50–59. [Google Scholar] [CrossRef]
- Forster, A.; Beck, B.; Schmidt, R.; Jansen, C.; Mellenthin, A. U ber die Zusammensetzung von niedermolekularen Polyphenolen in verschiedenen Hopfensorten und zwei Anbaugebieten. Mon. Brauwiss. 2002, 55, 98–108. [Google Scholar]
- McMurrough, I.; Hennigan, G.P.; Loughrey, M.J. Quantitative analysis of hop flavonols using high-performance liquid chromatography. J. Agric. Food Chem. 1982, 30, 1102–1106. [Google Scholar] [CrossRef]
- Jerkovic, V.; Collin, S. Occurrence of resveratrol and piceid in American and European hop cones. J. Agric. Food Chem. 2007, 55, 8754–8758. [Google Scholar] [CrossRef]
- Jerkovic, V.; Callemien, D.; Collin, S. Determination of stilbenes in hop pellets from different cultivars. J. Agric. Food Chem. 2005, 53, 4202–4206. [Google Scholar] [CrossRef]
- Inui, T.; Okumura, K.; Matsui, H.; Hosoya, T.; Kumazawa, S. Effect of harvest time on some in vitro functional properties of hop polyphenols. Food Chem. 2007, 225, 69–76. [Google Scholar] [CrossRef]
- Kavalier, A.R.; Litt, A.; Ma, C.; Pitra, N.J.; Coles, M.C.; Kennelly, E.J.; Matthews, P.D. Phytochemical and morphological characterization of hop (Humulus lupulus L.) cones over five developmental stages using high performance liquid chromatography coupled to time-of-flight mass spectrometry, ultrahigh performance liquid chromatography photodiode array detection, and light microscopy techniques. J. Agric. Food Chem. 2011, 59, 4783–4793. [Google Scholar] [CrossRef]
- Olšovska, J.; Kamenık, Z.; Čejka, P.; Jurkova, M.; Mikyška, A. Ultra-high-performance liquid chromatography profiling method for chemical screening of proanthocyanidins in Czech hops. Talanta 2013, 116, 919–926. [Google Scholar] [CrossRef]
- Mikyška, A.; Krofta, K.; Haškova, D.; Culık, J.; Čejka, P. The influence of hopping on formation of carbonyl compounds during storage of beer. J. Inst. Brew. 2012, 117, 47–54. [Google Scholar] [CrossRef]
- Krofta, K.; Mikyška, A.; Haškova, D. Antioxidant characteristics of hops and hop products. J. Inst. Brew. 2008, 114, 160–166. [Google Scholar] [CrossRef]
- Mikyška, A.; Krofta, K. Assessment of changes in hop resins and polyphenols during long-term storage. J. Inst. Brew. 2012, 118, 269–279. [Google Scholar] [CrossRef]
- Steenackers, B.; De Cooman, L.; De Vos, D. Chemical transformations of characteristic hop secondary metabolites in relation to beer properties and the brewing process: A review. Food Chem. 2015, 172, 742–756. [Google Scholar] [CrossRef] [PubMed]
- Jaskula-Goiris, B.; Goiris, K.; Syryn, E.; Van Opstaele, F.; De Rouck, G.; Aerts, G.; De Cooman, L. The use of hop polyphenols during brewing to improve flavor quality and stability of pilsner beer. J. Am. Soc. Brew. Chem. 2014, 72, 175–183. [Google Scholar] [CrossRef]
- Muñoz-Insa, A.; Gastl, M.; Becker, T. Use of polyphenol-rich hop products to reduce sunstruck flavor in beer. J. Am. Soc. Brew. Chem. 2015, 73, 228–235. [Google Scholar] [CrossRef]
- Szwajgier, D. Dry and wet milling of malt. A preliminary study comparing fermentable sugar, total protein, total phenolics and the ferulic acid content in non-hopped worts. J. Inst. Brew. 2012, 117, 569–577. [Google Scholar] [CrossRef]
- Vanbeneden, N.; Van Roey, T.; Willems, F.; Delvaux, F.; Delvaux, F.R. Release of phenolic flavour precursors during wort production: Influence of process parameters and grist composition on ferulic acid release during brewing. Food Chem. 2008, 111, 83–91. [Google Scholar] [CrossRef]
- Coghe, S.; Benoot, K.; Delvaux, F.; Vanderhaegen, B.; Delvaux, F.R. Ferulic acid release and 4-vinylguaiacol formation during brewing and fermentation: Indications for feruloyl esterase activity in Saccharomyces cerevisiae. J. Agric. Food Chem. 2004, 52, 602–608. [Google Scholar] [CrossRef]
- Schwarz, K.J.; Boitz, L.I.; Methner, F.J. Release of phenolic acids and amino acids during mashing dependent on temperature, pH, time and raw materials. J. Am. Soc. Brew. Chem. 2012, 70, 290–295. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, M. Effects of mashing on total phenolic contents and antioxidant activities of malts and worts. Int. J. Food Sci. Technol. 2012, 47, 240–247. [Google Scholar] [CrossRef]
- Fumi, M.D.; Galli, R.; Lambri, M.; Donadini, G.; De Faveri, D.M. Effect of full-scale brewing process on polyphenols in italian all-malt and maize adjunct lager beers. J. Food Compos. Anal. 2011, 24, 568–573. [Google Scholar] [CrossRef]
- Pascoe, H.M.; Ames, J.M.; Sachin, C. Critical stages of the brewing process for changes in antioxidant activity and levels of phenolic compounds in ale. J. Am. Soc. Brew. Chem. 2003, 61, 203–209. [Google Scholar] [CrossRef]
- Forster, A.; Gahr, A. On the fate of certain hop substances during dry hopping. Brew. Sci. 2013, 66, 93–103. [Google Scholar]
- Wietstock, P.C.; Kunz, T.; Methner, F.J. Influence of hopping technology on oxidative stability and staling-related carbonyls in pale lager beer. Brew. Sci. 2016, 69, 73–84. [Google Scholar]
- Brandolini, V.; Fiore, C.; Maietti, A.; Tedeschi, P.; Romano, P. Influence of Saccharomyces cerevisiae strains on wine total antioxidant capacity evaluated by photochemiluminescence. World J. Microbiol. Biotechnol. 2007, 23, 581–586. [Google Scholar] [CrossRef]
- Kostadinović, S.; Wilkens, A.; Stefova, M.; Ivanova, V.; Vojnovski, B.; Mirhosseini, H.; Winterhalter, P. Stilbene levels and antioxidant activity of Vranec and Merlot wines from Macedonia: Effect of variety and enological practices. Food Chem. 2012, 135, 3003–3009. [Google Scholar] [CrossRef]
- Carrascosa, A.V.; Bartolome, B.; Robredo, S.; Leon, A.; Cebollero, E.; Juega, M.; Nunez, Y.P.; Martinez, M.C.; Martinez-Rodriguez, A.J. Influence of locally-selected yeast on the chemical and sensorial properties of Albariño white wines. LWT Food Sci. Technol. 2012, 46, 319–325. [Google Scholar] [CrossRef]
- Carew, A.L.; Dugald, P.S.; Close, C.; Curtin, C.; Dambergs, R.G. Yeast effects on Pinot noir wine phenolics, color, and tannin composition. J. Agric. Food Chem. 2013, 61, 9892–9898. [Google Scholar] [CrossRef]
- Caridi, A.; Sidari, R.; Solieri, L.; Cufari, A.; Giudici, P. Wine colour adsorption phenotype: An inheritable quantitative trait loci of yeasts. J. Appl. Microbiol. 2007, 103, 735–742. [Google Scholar] [CrossRef]
- Bautista-Ortın, A.; Fernandez-Fernandez, J.I.; Lopez-Roca, J.M.; Gomez-Plaza, E. The effects of enological practices in anthocyanins, phenolic compounds and wine colour and their dependence on grape characteristics. J. Food Comp. Anal. 2007, 20, 546–552. [Google Scholar] [CrossRef]
- Mojsov, K.; Ziberoski, J.; Bozinovic, Z.; Petreska, M. A comparison of effects of three commercial pectolytic enzyme preparations in red winemaking. Int. J. Pure Appl. Sci. Technol. 2010, 1, 127–136. [Google Scholar] [CrossRef]
- Rıo Segade, S.; Pace, C.; Torchio, F.; Giacosa, S.; Gerbi, V.; Rolle, L. Impact of maceration enzymes on skin softening and relationship with anthocyanin extraction in wine grapes with different anthocyanin profiles. Food Res. Int. 2015, 71, 50–57. [Google Scholar] [CrossRef]
- González-Neves, G.; Favre, G.; Piccardo, D.; Gil, G. Anthocyanin profile of young red wines of Tannat, Syrah and Merlot made using maceration enzymes and cold soak. Int. J. Food Sci. Technol. 2016, 51, 260–267. [Google Scholar] [CrossRef]
- Aguilar, T.; Loyola, C.; de Bruijn, J.; Bustamante, L.; Vergara, C.; von Baer, D.; Mardones, C.; Serra, I. Effect of thermomaceration and enzymatic maceration on phenolic compounds of grape must enriched by grape pomace, vine leaves and canes. Eur. Food Res. Technol. 2016, 242, 1149–1158. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Topics related to aging. In Understanding Wine Chemistry; Waterhouse, A.L., Sacks, G.L., Jeffery, D.W., Eds.; John Wiley & Sons, Ltd.: West Sussex, UK, 2016; pp. 294–317. ISBN 978-1-118-73070-6. [Google Scholar]
- Sarni-Manchado, P.; Fulcrand, H.; Souquet, J.M.; Cheynier, V.; Moutounet, M. Stability and color of unreported wine anthocyanin-derived pigments. J. Food Sci. 1996, 61, 938–941. [Google Scholar] [CrossRef]
- Asenstorfer, R.E.; Hayasaka, Y.; Jones, G.P. Isolation and structures of oligomeric wine pigments by bisulfite-mediated ion-exchange chromatography. J. Agric. Food Chem. 2001, 49, 5957–5963. [Google Scholar] [CrossRef]
- Mateus, N.; Silva, A.M.; Rivas-Gonzalo, J.C.; Santos-Buelga, C.; De Freitas, V. A new class of blue anthocyanin-derived pigments isolated from red wines. J. Agric. Food Chem. 2003, 51, 1919–1923. [Google Scholar] [CrossRef]
- Remy, S.; Fulcrand, H.; Labarbe, B.; Cheynier, V.; Moutounet, M. First confirmation in red wine of products resulting from direct anthocyanin-tannin reactions. J. Sci. Food Agric. 2000, 80, 745–751. [Google Scholar] [CrossRef]
- He, F.; Mu, L.; Yan, G.L.; Liang, N.N.; Pan, Q.H.; Wang, L.; Reeves, M.J.; Duan, C.Q. Biosynthesis of anthocyanins and their regulation in coloured grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef]
- Grainger, K.; Tattersall, H. Wine Production: Vine to Bottle, 2nd ed.; John Wiley & Sons, Ltd.: West Sussex, UK, 2016; pp. 138–143. ISBN 978-1-405-17354-4. [Google Scholar]
- Glabasnia, A.; Hofmann, T. Sensory-directed identification of taste-active ellagitannins in American (Quercus alba L.) and European oak wood (Quercus robur L.) and quantitative analysis in bourbon whiskey and oak-matured red wines. J. Agric. Food Chem. 2006, 54, 3380–3390. [Google Scholar] [CrossRef]
- Rasines-Perea, Z.; Jacquet, R.; Jourdes, M.; Quideau, S.; Teissedre, P.L. Ellagitannins and flavano-ellagitannins: Red wines tendency in different areas, barrel origin and ageing time in barrel and bottle. Biomolecules 2019, 9, 316. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.; Albertin, W.; Jourdes, M.; Le Floch, A.; Giordanengo, T.; Mourey, N.; Teissedre, P.L. Variations in oxygen and ellagitannins, and organoleptic properties of red wine aged in French oak barrels classified by a near infrared system. Food Chem. 2016, 204, 381–390. [Google Scholar] [CrossRef]
- Navarro, M.; Kontoudakis, N.; Giordanengo, T.; Gómez-Alonso, S.; García-Romero, E.; Fort, F.; Canals, J.M.; Hermosín-Gutíerrez, I.; Zamora, F. Oxygen consumption by oak chips in a model wine solution; Influence of the botanical origin, toast level and ellagitannin content. Food Chem. 2016, 199, 822–827. [Google Scholar] [CrossRef] [PubMed]
- García-Estévez, I.; Alcalde-Eon, C.; Martínez-Gil, A.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T.; Nevares, I.; Del Alamo-Sanza, M. An approach to the study of the interactions between ellagitannins and oxygen during oak wood aging. J. Agric. Food Chem. 2017, 65, 6369–6378. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, S.; Lefeuvre, D.; Jacquet, R.; Jourdes, M.; Ducasse, L.; Galland, S.; Grelard, A.; Saucier, C.; Teissedre, P.L.; Dangles, O.; et al. Physicochemical studies of new anthocyano-ellagitannin hybrid pigments: About the origin of the influence of oak C-glycosidic ellagitannins on wine color. Eur. J. Org. Chem. 2010, 2010, 55–63. [Google Scholar] [CrossRef]
- Zhang, B.; Cai, J.; Duan, C.Q.; Reeves, M.J.; He, F. A review of polyphenolics in oak woods. Int. J. Mol. Sci. 2015, 16, 6978–7014. [Google Scholar] [CrossRef]
- Quideau, S.; Jourdes, M.; Lefeuvre, D.; Montaudon, D.; Saucier, C.; Glories, Y.; Pardon, P.; Pourquier, P. The chemistry of wine polyphenolic C-glycosidic ellagitannins targeting human topoisomerase II. Chem. A Eur. J. 2005, 11, 6503–6513. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Rodríguez-Pulido, F.J.; Hernanz, D.; Escudero-Gilete, M.L.; Heredia, F.J. Determination of phenolic substances of seeds skins and stems from white grape marc by near-infrared hyperspectral imaging. Aust. J. Grape Wine Res. 2016, 22, 11–15. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Hernanz, D.; Escudero-Gilete, M.L.; Heredia, F.J. The use of grape seed by-products rich in flavonoids to improve the antioxidant potential of red wines. Molecules 2016, 21, 1526. [Google Scholar] [CrossRef]
- Rivero, F.J.; Gordillo, B.; Jara-Palacios, M.J.; González-Miret, M.L.; Heredia, F.J. Effect of addition of overripe seeds from white grape by-products during red wine fermentation on wine colour and phenolic composition. LWT Food Sci. Technol. 2017, 84, 544–550. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Gordillo, B.; González-Miret, M.L.; Hernanz, D.; Escudero-Gilete, M.L.; Heredia, F.J. Comparative study of the enological potential of different winemaking Byproducts: Implications in the antioxidant activity and color expression of red wine anthocyanins in a model solution. J. Agric. Food Chem. 2014, 62, 6975–6983. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Gilete, M.L.; Hernanz, D.; Galán-Lorente, C.; Heredia, F.J.; Jara-Palacios, M.J. Potential of cooperage byproducts rich in ellagitannins to improve the antioxidant activity and color expression of red wine anthocyanins. Foods 2019, 8, 336. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Verachtert, H.; Derdelinckx, G. The chemistry of beer aging—A critical review. Food Chem. 2006, 95, 357–381. [Google Scholar] [CrossRef]
- Karabín, M.; Rýparová, A.; Jelínek, L.; Kunz, T.; Wietstock, P.; Methner, F.J.; Dostálek, P. Relationship of iso-α-acid content and endogenous antioxidative potential during storage of lager beer. J. Inst. Brew. 2014, 120, 212–219. [Google Scholar] [CrossRef]
- Li, H.; Zhao, M.; Cui, C.; Sun, W.; Zhao, H. Antioxidant activity and typical ageing compounds: Their evolutions and relationships during the storage of lager beers. Int. J. Food Sci. Technol. 2016, 51, 2026–2033. [Google Scholar] [CrossRef]
- Moll, M.; Fonknechten, G.; Carnielo, M.; Flayeux, R. Changes in polyphenols from raw materials to finished beer. MBAA Tech. Q. 1984, 21, 79–87. [Google Scholar]
- Callemien, D.; Collin, S. Involvement of flavanoids in beer color instability during storage. J. Agric. Food Chem. 2007, 55, 9066–9073. [Google Scholar] [CrossRef]
- Intelmann, D.; Haseleu, G.; Dunkel, A.; Lagemann, A.; Stephan, A.; Hofmann, T. Comprehensive sensomics analysis of hop-derived bitter compounds during storage of beer. J. Agric. Food Chem. 2011, 59, 1939–1953. [Google Scholar] [CrossRef]
- Heuberger, A.L.; Broeckling, C.D.; Lewis, M.R.; Salazar, L.; Bouckaert, P.; Prenni, J.E. Metabolomic profiling of beer reveals effect of temperature on non-volatile small molecules during short-term storage. Food Chem. 2012, 135, 1284–1289. [Google Scholar] [CrossRef]
- Jerkovic, V.; Nguyen, F.; Timmermanns, A.; Collin, S. Comparison of procedures for resveratrol analysis in beer: Assessment of stilbenoids stability through wort fermentation and beer aging. J. Inst. Brew. 2008, 114, 143–149. [Google Scholar] [CrossRef]
- Đorđević, S.; Popović, D.; Despotović, S.; Veljović, M.; Atanacković, M.; Cvejić, J.; Nedović, V.; Leskosek-Cukalovik, I. Extracts of medicinal plants as functional beer additives. Chem. Ind. Chem. Eng. Q. 2016, 22, 301–308. [Google Scholar] [CrossRef]
- Ulloa, P.A.; Vidal, J.; Ávila, M.I.; Labbe, M.; Cohen, S.; Salazar, F.N. Effect of the addition of propolis extract on bioactive compounds and antioxidant activity of craft beer. J. Chem. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Veljović, M.; Despotović, S.; Pecić, S.; Davidović, S.; Djordjević, R.; Vukosavljević, P.; Leskosek-Cukalovik, I. The influence of raw materials and fermentation conditions on the polyphenol content of grape beer. In Proceedings of the 6th Central European Congress on Food, CEFood 2012, Novi Sad, Serbia, 23–26 May 2012; Lević, J., Nedović, V., Ilić, N., Tumbas, V., Kalušević, A., Eds.; University of Novi Sad, Institute of Food Technology: Novi Sad, Serbia, 2012; pp. 1137–1141. [Google Scholar]
- Lasanta, C.; Durán-Guerrero, E.; Díaz, A.B.; Castro, R. Influence of fermentation temperature and yeast type on the chemical and sensory profile of handcrafted beers. J. Sci. Food Agric. 2020, 1–8. [Google Scholar] [CrossRef]
- Nardini, M.; Garaguso, I. Characterization of bioactive compounds and antioxidant activity of fruit beers. Food Chem. 2020, 305. [Google Scholar] [CrossRef]
- Gasiński, A.; Kawa-Rygielska, J.; Szumny, A.; Czubaszek, A.; Gąsior, J.; Pietrzak, W. Volatile compounds content, physicochemical parameters, and antioxidant activity of beers with addition of mango fruit (Mangifera Indica). Molecules 2020, 25, 3033. [Google Scholar] [CrossRef]




| Red Wine | References | Beer | References | |
|---|---|---|---|---|
| Cinnamic Acids (mg/L) | ||||
| ferulic acid | 0.05–10.43 | [30,70,71,72] | 0.01–5.04 | [12,45,46,73,74,75,76,77,78,79,80,81,82] |
| p-coumaric acid | 0.02–8.00 | [27,30,70,71,72] | 0.003–55.80 | [9,12,45,73,78,79] |
| caffeic acid | 0.02–644.50 | [27,30,70,71,83] | 0.00–23.50 | [9,45,46,73,74,75,76,77,78,79,81,82,84] |
| Hydroxybenzoic Acids (mg/L) | ||||
| gallic acid | 27.10–66.10 | [28,71] | 0.00–142.20 | [9,46,73,74,75,76,77,78,80,81,82] |
| protocatechuic acid | 0.91–1.78 | [28,30] | 0.01–5.10 | [12,75,76,77,78,80,81,82,84] |
| p-hydroxybenzoic acid | 2.75–6.20 | [28] | 0.00–16.84 | [12,45,73,75,76,78] |
| Stilbenes (mg/L) | ||||
| resveratrol | 0,51–11.70 | [28,85] | 0.002–0.081 | [86] |
| trans-resveratrol | 0.21–23.00 | [27,70,71,87,88,89] | - | - |
| cis-resveratrol | 0.01–7.00 | [71,87,88] | - | - |
| total stilbenes | 1.00–5.50 | [71] | - | - |
| Tannins (mg/L) | ||||
| hydrolysable tannins | 0.4–50.0 | [90,91,92,93,94] | 1.5 | [81] |
| Flavan-3-ols (mg/L) | ||||
| catechin | 6.98–91.99 | [27,30,36] | 0.03–6.54 | [12,73,74,75,77,80,81,82] |
| epicatechin | 8.07–52.85 | [27,30,36] | 0–4.55 | [9,74,75,80,81,82] |
| Flavones (mg/L) | ||||
| luteolin | 0.20–1.00 | [95,96,97,98] | 0.10–0.19 | [82] |
| apigenin | 0.00–4.70 | [99] | 0.80–0.81 | [82] |
| Flavonols (mg/L) | ||||
| myricetin | 0.70–30.40 | [27,30] | 0.15–0.16 | [82] |
| quercetin | 1.27–65.90 | [27,30,36] | 0.06–1.79 | [74,80,82] |
| kaempferol | 0.61–26.80 | [27] | 0.10–1.64 | [80,100] |
| Flavanones (mg/L) | ||||
| naringenin | 0.90 to 4.20 | [89] | 0.06–2.34 | [80] |
| Beer | Wine | ||||
|---|---|---|---|---|---|
| Parameter | Range | Reference | Parameter | Range | Reference |
| Total polyphenols (FC method); mgGAE/L | 127–855 | [7,9,12,77] | Total polyphenols (FC method); mgGAE/L | 860.2–2912.0 | [27,30,31,71] |
| Total anthocyanogens; mg/L | 19.0–84.5 | [12,110] | Total anthocyanins; mg/L | 21–1011 | [71,111,112] |
| Antioxidant activity; DPPH; mmol TE/L | 0.55–6.67 | [9,78,113] | Antioxidant activity; ABTS; mmol TE/L | 7.5–96.4 | [27,30,71] |
| Antioxidant activity; FRAP; mmol TE/L | 0.862–1.271 | [12] | Antioxidant activity; FRAP; mmol TE/L | 6.9–15.2 | [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radonjić, S.; Maraš, V.; Raičević, J.; Košmerl, T. Wine or Beer? Comparison, Changes and Improvement of Polyphenolic Compounds during Technological Phases. Molecules 2020, 25, 4960. https://doi.org/10.3390/molecules25214960
Radonjić S, Maraš V, Raičević J, Košmerl T. Wine or Beer? Comparison, Changes and Improvement of Polyphenolic Compounds during Technological Phases. Molecules. 2020; 25(21):4960. https://doi.org/10.3390/molecules25214960
Chicago/Turabian StyleRadonjić, Sanja, Vesna Maraš, Jovana Raičević, and Tatjana Košmerl. 2020. "Wine or Beer? Comparison, Changes and Improvement of Polyphenolic Compounds during Technological Phases" Molecules 25, no. 21: 4960. https://doi.org/10.3390/molecules25214960
APA StyleRadonjić, S., Maraš, V., Raičević, J., & Košmerl, T. (2020). Wine or Beer? Comparison, Changes and Improvement of Polyphenolic Compounds during Technological Phases. Molecules, 25(21), 4960. https://doi.org/10.3390/molecules25214960








