Unraveling the Antioxidant, Binding and Health-Protecting Properties of Phenolic Compounds of Beers with Main Human Serum Proteins: In Vitro and In Silico Approaches †
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Polyphenols, Flavonoids, Flavanols and Phenolic Acids Content of Beers
2.2. Beer Antioxidant Activities
2.3. Binding Properties of Beers and Some Phenolic Compounds with Main Human Proteins
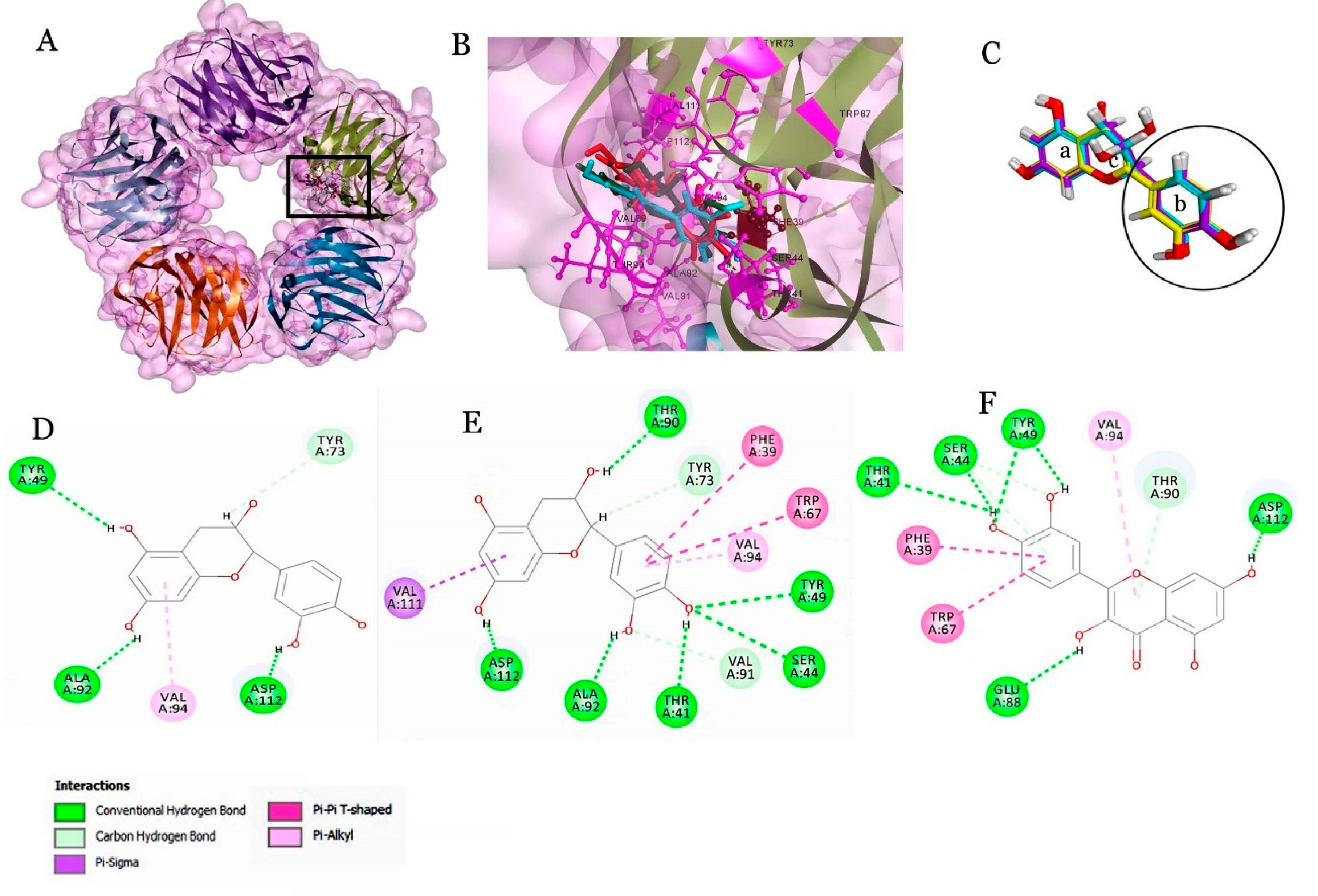
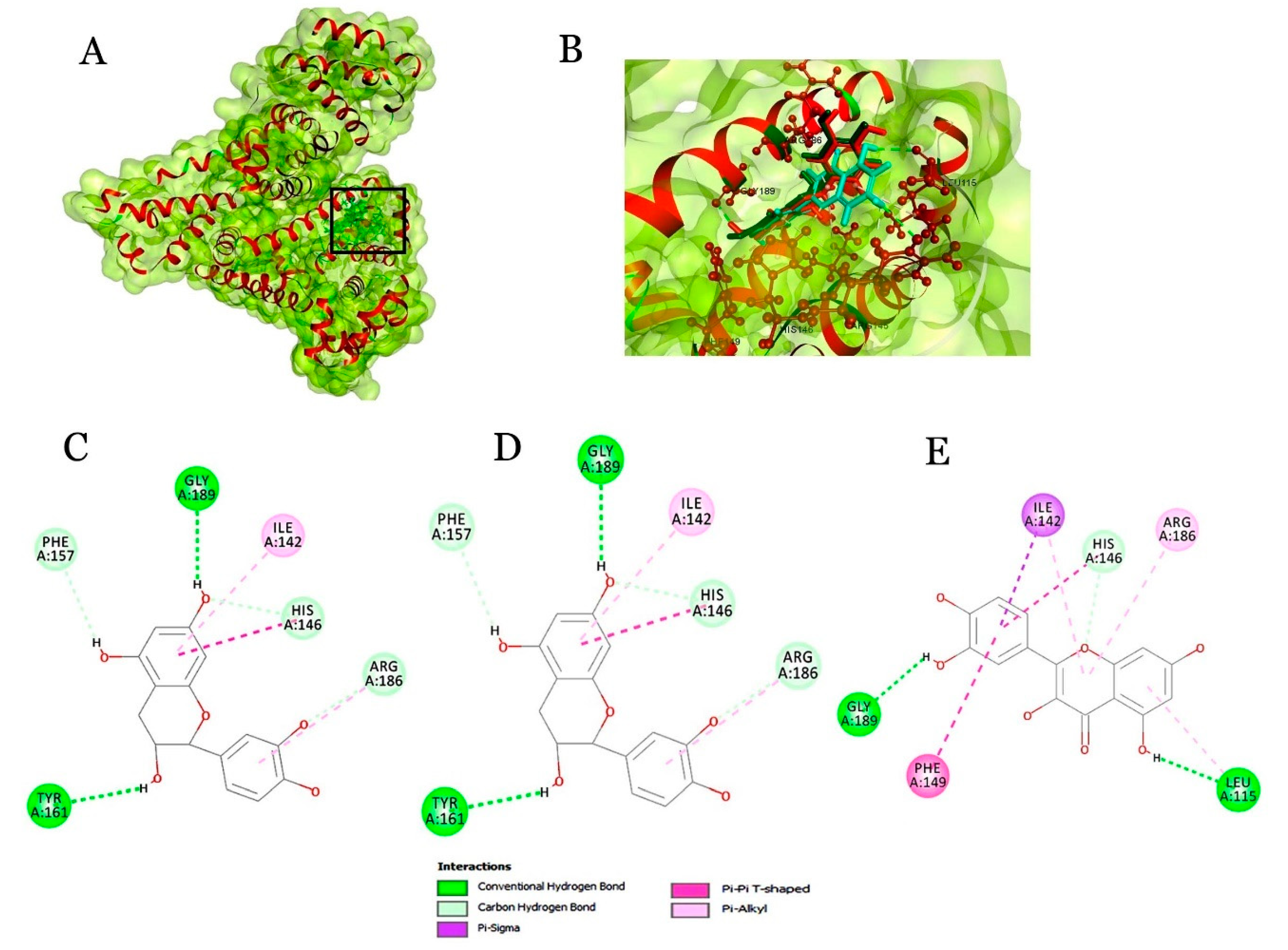
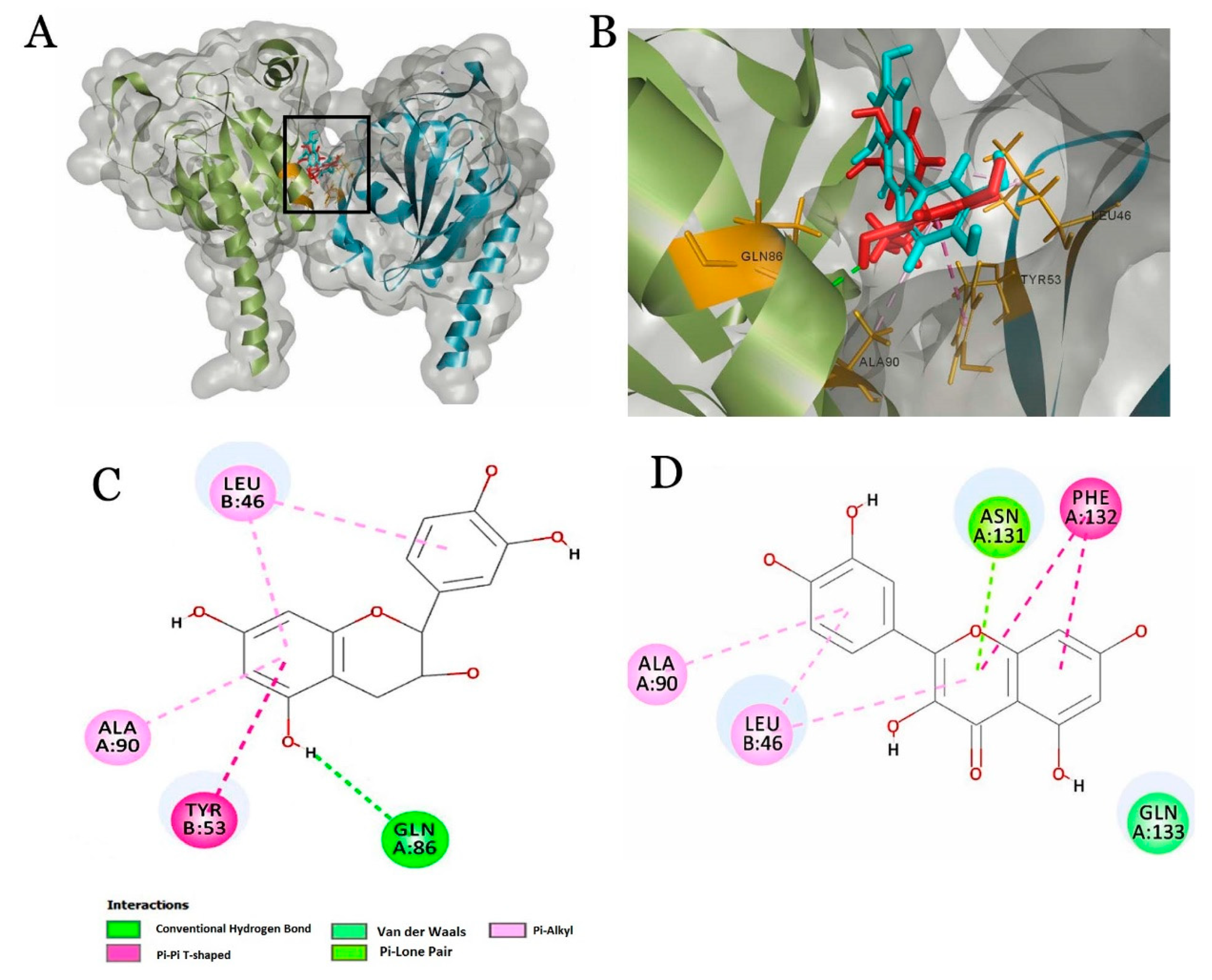
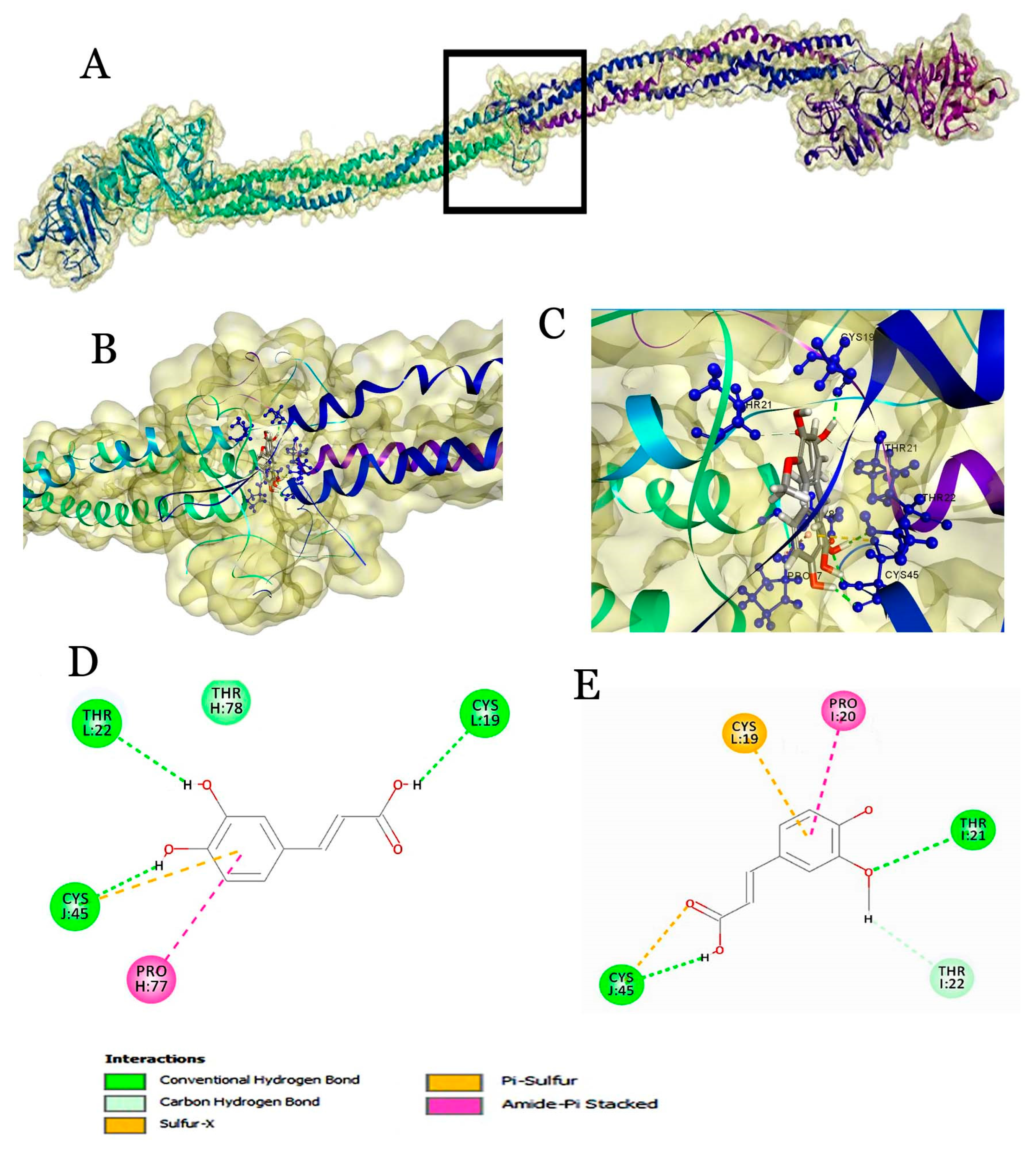
2.4. Molecular Docking of Beer Components with Serum Proteins
3. Materials and Methods
3.1. Materials
3.2. Samples
3.3. Analyses of Bioactive Compounds
3.4. Determination of Antioxidant Activities
3.5. Fluorimetric Measurements
3.6. Molecular Docking Studies Using Main Human Serum Proteins
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nardini, M.; Ghiselli, A. Determination of free and bound phenolic acids in beer. Food Chem. 2004, 84, 137–143. [Google Scholar] [CrossRef]
- Piazzon, A.; Forte, M.; Nardini, M. Characterization of Phenolics Content and Antioxidant Activity of Different Beer Types. J. Agric. Food Chem. 2010, 58, 10677–10683. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chen, W.; Lu, J.; Zhao, M. Phenolic profiles and antioxidant activities of commercial beers. Food Chem. 2010, 119, 1150–1158. [Google Scholar] [CrossRef]
- Wannenmacher, J.; Gastl, M.; Becker, T. Phenolic Substances in Beer: Structural Diversity, Reactive Potential and Relevance for Brewing Process and Beer Quality. Compr. Rev. Food Sci. Food Saf. 2018, 17, 953–988. [Google Scholar] [CrossRef]
- Cerrato-Alvarez, M.; Bernalte, E.; Bernalte-García, M.J.; Pinilla-Gil, E. Fast and direct amperometric analysis of polyphenols in beers using tyrosinase-modified screen-printed gold nanoparticles biosensors. Talanta 2019, 193, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M.; Foddai, M.S. Phenolics Profile and Antioxidant Activity of Special Beers. Molecules 2020, 25, 2466. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M.; Garaguso, I. Characterization of bioactive compounds and antioxidant activity of fruit beers. Food Chem. 2020, 305, 125437. [Google Scholar] [CrossRef]
- Nardini, M.; Natella, F.; Scaccini, C.; Ghiselli, A. Phenolic acids from beer are absorbed and extensively metabolized in humans. J. Nutr. Biochem. 2006, 17, 14–22. [Google Scholar] [CrossRef]
- Gorinstein, S.; Caspi, A.; Libman, I.; Leontowicz, H.; Leontowicz, M.; Tashma, Z.; Katrich, E.; Jastrzebski, Z.; Trakhtenberg, S. Bioactivity of beer and its influence on human metabolism. Int. J. Food Sci. Nutr. 2007, 58, 94–107. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Magraner, E.; Condines, X.; Valderas-Martínez, P.; Roth, I.; Arranz, S.; Casas, R.; Navarro, M.; Hervas, A.; Sisó, A.; et al. Effects of alcohol and polyphenols from beer on atherosclerotic biomarkers in high cardiovascular risk men: A randomized feeding trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 36–45. [Google Scholar] [CrossRef]
- Spaggiari, G.; Cignarelli, A.; Sansone, A.; Baldi, M.; Santi, D. To beer or not to beer: A meta-analysis of the effects of beer consumption on cardiovascular health. PLoS ONE 2020, 15, e0233619. [Google Scholar] [CrossRef]
- Osorio-Paz, I.; Brunauer, R.; Alavez, S. Beer and its non-alcoholic compounds in health and disease. Crit. Rev. Food Sci. Nutr. 2019, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, B.; Peña-Neira, A.; Gómez-Cordovés, C. Phenolics and related substances in alcohol-free beers. Eur. Food Res. Technol. 2000, 210, 419–423. [Google Scholar] [CrossRef]
- Humia, B.V.; Santos, K.S.; Barbosa, A.M.; Sawata, M.; Mendonça, M.C.; Padilha, F.F. Beer Molecules and Its Sensory and Biological Properties: A Review. Molecules 2019, 24, 1568. [Google Scholar] [CrossRef] [PubMed]
- Boronat, A.; Soldevila-Domenech, N.; Rodríguez-Morató, J.; Martínez-Huélamo, M.; Lamuela-Raventós, R.; De La Torre, R. Beer Phenolic Composition of Simple Phenols, Prenylated Flavonoids and Alkylresorcinols. Molecules 2020, 25, 2582. [Google Scholar] [CrossRef] [PubMed]
- Mitić, S.S.; Paunović, D.Đ.; Pavlović, A.N.; Tošić, S.B.; Stojković, M.B.; Mitić, M. Phenolic Profiles and Total Antioxidant Capacity of Marketed Beers in Serbia. Int. J. Food Prop. 2013, 17, 908–922. [Google Scholar] [CrossRef]
- Szwajgier, D. Content of Individual Phenolic Acids in Worts and Beers and their Possible Contribution to the Antiradical Activity of Beer. J. Inst. Brew. 2009, 115, 243–252. [Google Scholar] [CrossRef]
- Gasowski, B.; Leontowicz, M.; Leontowicz, H.; Katrich, E.; Lojek, A.; Číž, M.; Trakhtenberg, S.; Gorinstein, S. The influence of beer with different antioxidant potential on plasma lipids, plasma antioxidant capacity, and bile excretion of rats fed cholesterol-containing and cholesterol-free diets. J. Nutr. Biochem. 2004, 15, 527–533. [Google Scholar] [CrossRef]
- Gorinstein, S.; Zemser, M.; Berliner, M.; Goldstein, R.; Libman, I.; Trakhtenberg, S.; Caspi, A. Moderate beer consumption and positive biochemical changes in patients with coronary atherosclerosis. J. Intern. Med. 1997, 242, 219–224. [Google Scholar] [CrossRef]
- Poloni, D.M.; Dangles, O.; Vinson, J.A. Binding of Plant Polyphenols to Serum Albumin and LDL: Healthy Implications for Heart Disease. J. Agric. Food Chem. 2019, 67, 9139–9147. [Google Scholar] [CrossRef]
- Dufour, C.; Dangles, O. Flavonoid–serum albumin complexation: Determination of binding constants and binding sites by fluorescence spectroscopy. Biochim. Biophys. Acta 2005, 1721, 164–173. [Google Scholar] [CrossRef]
- Gorinstein, S.; Caspi, A.; Zemser, M.; Libman, I.; Goshev, I.; Trakhtenberg, S. Plasma circulating fibrinogen stability and moderate beer consumption. J. Nutr. Biochem. 2003, 14, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Gorinstein, S.; Caspi, A.; Rosen, A.; Goshev, I.; Zemser, M.; Weisz, M.; Añon, M.C.; Libman, I.; Lerner, H.T.; Trakhtenberg, S. Structure characterization of human serum proteins in solution and dry state. J. Peptide Res. 2002, 59, 71–78. [Google Scholar] [CrossRef]
- Tung, W.-C.; Rizzo, B.; Dabbagh, Y.; Saraswat, S.; Romanczyk, M.; Codorniu-Hernández, E.; Rebollido-Rios, R.; Needs, P.W.; Kroon, P.A.; Rakotomanomana, N.; et al. Polyphenols bind to low density lipoprotein at biologically relevant concentrations that are protective for heart disease. Arch. Biochem. Biophys. 2020, 694, 108589. [Google Scholar] [CrossRef] [PubMed]
- Habschied, K.; Lončarić, A.; Mastanjević, K. Screening of Polyphenols and Antioxidative Activity in Industrial Beers. Foods 2020, 9, 238. [Google Scholar] [CrossRef] [PubMed]
- Gorinstein, S.; Caspi, A.; Zemser, M.; Trakhtenberg, S. Comparative contents of some phenolics in beer, red and white wines. Nutr. Res. 2000, 20, 131–139. [Google Scholar] [CrossRef]
- Dvorakova, M.; Hulin, P.; Karabin, M.; Dostálek, P. Determination of polyphenols in beer by an effective method based on solid-phase extraction and high performance liquid chromatography with diode-array detection. Czech J. Food Sci. 2008, 25, 182–188. [Google Scholar] [CrossRef]
- Wang, C.; Xie, Y.; Wang, H.; Bai, Y.; Dai, C.; Li, C.; Xu, X.; Zhou, G. Phenolic compounds in beer inhibit formation of polycyclic aromatic hydrocarbons from charcoal-grilled chicken wings. Food Chem. 2019, 294, 578–586. [Google Scholar] [CrossRef]
- Zhao, H.; Li, H.; Sun, G.; Yang, B.; Zhao, M. Assessment of endogenous antioxidative compounds and antioxidant activities of lager beers. J. Sci. Food Agric. 2012, 93, 910–917. [Google Scholar] [CrossRef]
- Pattanayak, R.; Basak, P.; Sen, S.; Bhattacharyya, M. An insight to the binding of ellagic acid with human serum albumin using spectroscopic and isothermal calorimetry studies. Biochem. Biophys. Rep. 2017, 10, 88–93. [Google Scholar] [CrossRef]
- Latruffe, N.; Menzel, M.; Delmas, D.; Buchet, R.; Lançon, A. Compared Binding Properties between Resveratrol and Other Polyphenols to Plasmatic Albumin: Consequences for the Health Protecting Effect of Dietary Plant Microcomponents. Molecules 2014, 19, 17066–17077. [Google Scholar] [CrossRef]
- Leontowicz, H.; Leontowicz, M.; Latocha, P.; Jesion, I.; Park, Y.S.; Katrich, E.; Barasch, D.; Nemirovski, A.; Gorinstein, S. Bioactivity and nutritional properties of hardy kiwifruit Actinidia arguta in comparison with Actinidia deliciosa ‘Hayward’ and Actinidia eriantha ‘Bidan’. Food Chem. 2016, 196, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Park, Y.S.; Park, Y.-K.; Ham, K.-S.; Kang, S.-G.; Shafreen, R.M.B.; Lakshmi, S.A.; Gorinstein, S. Characterization of Bioactive Ligands with Antioxidant Properties of Kiwifruit and Persimmon Cultivars Using In Vitro and in Silico Studies. Appl. Sci. 2020, 10, 4218. [Google Scholar] [CrossRef]
- Qian, J. The efficiency of flavonoids in polar extracts of Lycium chinense Mill fruits as free radical scavenger. Food Chem. 2004, 87, 283–288. [Google Scholar] [CrossRef]
- Gonçalves, S.; Santos, N.C.; Martins-Silva, J.; Saldanha, C. Fluorescence spectroscopy evaluation of fibrinogen–β-estradiol binding. J. Photochem. Photobiol. B Biol. 2007, 86, 170–176. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, X.-F.; Huang, J.-Y. Resveratrol Binding to Fibrinogen and its Biological Implication. Food Biophys. 2011, 7, 35–42. [Google Scholar] [CrossRef]
- Gligorijević, N.; Radomirović, M.; Rajkovic, A.; Nedić, O.; Cirkovic-Velickovic, T. Fibrinogen Increases Resveratrol Solubility and Prevents it from Oxidation. Foods 2020, 9, 780. [Google Scholar] [CrossRef]
- Sierksma, A.; Van Der Gaag, M.S.; Kluft, C.; Hendriks, H.F.J. Moderate alcohol consumption reduces plasma C-reactive protein and fibrinogen levels; a randomized, diet-controlled intervention study. Eur. J. Clin. Nutr. 2002, 56, 1130–1136. [Google Scholar] [CrossRef]
- Saxena, M.; Saxena, J.; Pradhan, A. Flavonoids and phenolic acids as antioxidants in plants and human health. Int. J. Pharm. Sci. Rev. Res. 2012, 16, 130–134. [Google Scholar]
- Ghiselli, A.; Natella, F.; Guidi, A.; Montanari, L.; Fantozzi, P.; Scaccini, C. Beer increases plasma antioxidant capacity in humans. J. Nutr. Biochem. 2000, 11, 76–80. [Google Scholar] [CrossRef]
- Arranz, S.; Chiva-Blanch, G.; Valderas-Martínez, P.; Remon, A.M.; Raventós, R.M.L.; Estruch, R. Wine, Beer, Alcohol and Polyphenols on Cardiovascular Disease and Cancer. Nutrients 2012, 4, 759–781. [Google Scholar] [CrossRef] [PubMed]
- Padro, T.; Muñoz-García, N.; Vilahur, G.; Chagas, P.; Deyà, A.; Antonijoan, R.M.; Badimon, L. Moderate Beer Intake and Cardiovascular Health in Overweight Individuals. Nutrients 2018, 10, 1237. [Google Scholar] [CrossRef] [PubMed]
- Kumaresan, P.R.; Devaraj, S.; Huang, W.; Lau, E.Y.; Liu, R.; Lam, K.S.; Jialal, I. Synthesis and Characterization of a Novel Inhibitor of C-Reactive Protein–Mediated Proinflammatory Effects. Metab. Syndr. Relat. Disord. 2013, 11, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.; Van Der Heijden, R.; Heeringa, P.; Kaijzel, E.; Verschuren, L.; Blomhoff, R.; Kooistra, T.; Kleemann, R. Epicatechin attenuates atherosclerosis and exerts anti-inflammatory effects on diet-induced human-CRP and NFκB in vivo. Atherosclerosis 2014, 233, 149–156. [Google Scholar] [CrossRef]
- Nagai, T.; Anzai, T.; Kaneko, H.; Mano, Y.; Anzai, A.; Maekawa, Y.; Takahashi, T.; Meguro, T.; Yoshikawa, T.; Fukuda, K. C-reactive protein overexpression exacerbates pressure overload-induced cardiac remodeling through enhanced inflammatory response. Hypertension 2011, 57, 208–215. [Google Scholar] [CrossRef]
- Moua, E.D.; Hu, C.; Day, N.; Hord, N.G.; Takata, Y. Coffee Consumption and C-Reactive Protein Levels: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1349. [Google Scholar] [CrossRef]
- Mangnus, L.; Van Steenbergen, H.W.; Nieuwenhuis, W.P.; Reijnierse, M.; Mil, A.H.M.V.D.H.-V. Moderate use of alcohol is associated with lower levels of C reactive protein but not with less severe joint inflammation: A cross-sectional study in early RA and healthy volunteers. RMD Open 2018, 4, e000577. [Google Scholar] [CrossRef]
- Li, Q.-Y.; Li, H.-Y.; Fu, G.; Yu, F.; Wu, Y.; Zhao, M.-H. Autoantibodies against C-Reactive Protein Influence Complement Activation and Clinical Course in Lupus Nephritis. J. Am. Soc. Nephrol. 2017, 28, 3044–3054. [Google Scholar] [CrossRef]
- Arques, S. Human serum albumin in cardiovascular diseases. Eur. J. Intern. Med. 2018, 52, 8–12. [Google Scholar] [CrossRef]
- Chung, S.S.; Kim, M.; Youn, B.S.; Lee, N.S.; Park, J.W.; Lee, I.K.; Lee, Y.S.; Kim, J.B.; Cho, Y.M.; Lee, H.K.; et al. Glutathione peroxidase 3 mediates the antioxidant effect of peroxisome proliferator-activated receptor gamma in human skeletal muscle cells. Mol. Cell Biol. 2009, 29, 20–30. [Google Scholar] [CrossRef]
- Nagata, H.; Takekoshi, S.; Takagi, T.; Honma, T.; Watanabe, K. Antioxidative action of flavonoids, quercetin and catechin, mediated by the activation of glutathione peroxidase. Tokai J. Exp. Clin. Med. 1999, 24, 1–11. [Google Scholar] [PubMed]
- Pang, P.; Abbott, M.; Abdi, M.; Fucci, Q.-A.; Chauhan, N.; Mistri, M.; Proctor, B.; Chin, M.; Wang, B.; Yin, W.; et al. Pre-clinical model of severe glutathione peroxidase-3 deficiency and chronic kidney disease results in coronary artery thrombosis and depressed left ventricular function. Nephrol. Dial. Transplant. 2017, 33, 923–934. [Google Scholar] [CrossRef]
- Pastori, D.; Pignatelli, P.; Farcomeni, A.; Menichelli, D.; Nocella, C.; Carnevale, R.; Violi, F. Aging-Related Decline of Glutathione Peroxidase 3 and Risk of Cardiovascular Events in Patients with Atrial Fibrillation. J. Am. Hear. Assoc. 2016, 5, e003682. [Google Scholar] [CrossRef] [PubMed]
- Domingues, M.M.; Macrae, F.L.; Mcpherson, H.R.; Bridge, K.I.; Ajjan, R.A.; Ridger, V.C.; Connell, S.D.; Philippou, H.; Ari, R.A.S. Thrombin and fibrinogen, impact clot structure by marked effects on intra fibrillar structure and proto fibril packing. Blood J. Am. Soc. Hematol. 2019, 127, 487–496. [Google Scholar]
- Luo, X.; Du, C.; Cheng, H.; Chen, J.; Lin, C. Study on the Anticoagulant or Procoagulant Activities of Type II Phenolic Acid Derivatives. Molecules 2017, 22, 2047. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Ziewiecki, R.; Saluk, J.; Ponczek, M.; Pawlaczyk, I.; Krotkiewski, H.; Wachowicz, B.; Nowak, P. Thrombin inhibitory activity of some polyphenolic compounds. Med. Chem. Res. 2013, 23, 2324–2337. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Feucht, W.; Polster, J. Nuclei of Plants as a Sink for Flavanols. Zeitschrift für Naturforschung C 2001, 56, 479–482. [Google Scholar] [CrossRef]
- Pozo-Bayón, M.Á.; Hernández, M.T.; Martín-Álvarez, A.P.J.; Polo, M.C. Study of Low Molecular Weight Phenolic Compounds during the Aging of Sparkling Wines Manufactured with Red and White Grape Varieties. J. Agric. Food Chem. 2003, 51, 2089–2095. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Singh, R.P.; Murthy, K.N.C.; Jayaprakasha, G.K. Studies on the Antioxidant Activity of Pomegranate (Punicagranatum) Peel and Seed Extracts Using In Vitro Models. J. Agric. Food Chem. 2002, 50, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Dassault Systemes. BIOVIA, Discovery Studio Modeling Environment; Release 4.5; Dassault Systemes: San Diego, CA, USA, 2015. [Google Scholar]
| Beer Code | Style | Country of Production | Alcohol Strength % Vol | Total Pol., mg GAE/L | Total Flavonoids, mg CE/L | Total Flavanols, mg CE/L | β-Carot, % AA | ABTS, mM TE |
|---|---|---|---|---|---|---|---|---|
| MACC | Pale lager | Israel | 5.0 | 510.1 ± 10.1 b | 45.1 ± 0.5 b | 40.3 ± 1.8 a | 28.1 ± 0.8 b | 2.06 ± 0.01 b |
| GOLD | Dark lager | Israel | 4.9 | 552.6 ± 9.6 a,b | 48.9 ± 0.8 a,b | 23.3 ± 1.4 c | 30.7 ± 1.2 a,b | 2.21 ± 0.02 a,b |
| HEIN | Pale lager | Israel | 5.0 | 466.3 ± 6.2 c | 41.3 ± 0.7 c | 21.9 ± 1.5 d | 25.2 ± 0.7 c | 1.88 ± 0.02 c |
| CARL | Pale lager | Israel | 5.0 | 450.5 ± 5.5 c,d | 40.1 ± 0.6 c,d | 21.2 ± 0.9 d | 24.6 ± 0.8 d | 1.82 ± 0.01 c,d |
| MGD | Pale lager | USA | 4.6 | 456.7 ± 7.2 c,d | 40.8 ± 0.9 c,d | 16.3 ± 0.4 e | 25.1 ± 1.1 c | 1.85 ± 0.01 c,d |
| COR | Pale lager | Mexico | 4.5 | 442.1 ± 4.3 d | 35.8 ± 0.5 d | 19.2 ± 0.8 d,e | 24.2 ± 0.7 d | 1.79 ± 0.03 d |
| ORJB | Pale lager | Netherlands | 5.0 | 482.3 ± 6.8 b,c | 42.9 ± 0.9 b,c | 29.6 ± 1.5 b | 26.7 ± 1.0 b,c | 1.95 ± 0.01 b,c |
| AMST | Pale lager | Netherlands | 4.1 | 501.3 ± 7.5 b,c | 44.3 ± 1.3 b,c | 21.6 ± 0.9 d | 27.6 ± 1.1 b,c | 2.02 ± 0.01 b |
| KAM | Pale lager | Bulgaria | 4.4 | 647.4 ± 11.3 a | 51.1 ± 1.4 a | 25.4 ± 1.1 b,c | 33.6 ± 1.3 a | 2.61 ± 0.05 a |
| ROST | Golden lager | Germany | 4.9 | 668.3 ± 13.3 a | 52.5 ± 1.5 a | 26.4 ± 1.2 b,c | 34.5 ± 1.2 a | 2.68 ± 0.03 a |
| ŻYW | Blond lager | Poland | 5.6 | 471.3 ± 7.2 c | 41.5 ± 0.9 c | 22.2 ± 1.0 c,d | 26.6 ± 1.0 b,c | 1.90 ± 0.02 b,c |
| Beer Code | Caffeic Acid | Ferulic Acid | Catechin | Epicatechin | Quercetin |
|---|---|---|---|---|---|
| MACC | 2.17 ± 0.08 b,c | 14.10 ± 0.39 b | 3.03 ± 0.06 b | 1.09 ± 0.08 a,b | 1.40 ± 0.08 b |
| GOLD | 2.34 ± 0.07 b | 15.22 ± 0.54 a,b | 3.27 ± 0.09 a,b | 1.17 ± 0.07 a,b | 1.52 ± 012 a,b |
| HEIN | 1.97 ± 0.07 c,d | 12.92 ± 0.45 c,d | 2.78 ± 0.07 b,c | 0.99 ± 0.05 b,c | 1.24 ± 0.07 c |
| CARL | 1.91 ± 0.04 c,d | 12.48 ± 0.32 d | 2.69 ± 0.09 c | 0.96 ± 0.09 b,c | 1.28 ± 0.08 b,c |
| MGD | 1.94 ± 0.05 c,d | 12.62 ± 0.35 c,d | 2.69 ± 0.08 c | 0.97 ± 0.06 b,c | 1.25 ± 0.11 b,c |
| COR | 1.87 ± 0.06 d | 12.23 ± 0.44 d | 2.64 ± 0.08 c | 0.94 ± 0.07 c | 1.21 ± 0.07 c |
| ORJB | 2.07 ± 0.08 c | 13.31 ± 0.54 c | 2.83 ± 0.05 b,c | 1.02 ± 0.07 b | 1.32 ± 0.13 b |
| AMST | 2.12 ± 0.06 b,c | 13.89 ± 0.48 b,c | 2.99 ± 0.09 b,c | 1.07 ± 0.07 b | 1.37 ± 0.01 b |
| KAM | 2.73 ± 0.06 a | 17.87 ± 0.61 a | 3.82 ± 0.12 a | 1.37 ± 0.08 a | 1.77 ± 0.12 a |
| ROST | 2.83 ± 0.08 a | 18.47 ± 0.51 a | 3.98 ± 0.15 a | 1.42 ± 0.09 a | 1.83 ± 0.07 a |
| ŻYW | 2.08 ± 0.07 c | 13.01 ± 0.36 c | 2.26 ± 0.08 d | 1.01 ± 0.07 b | 1.29 ± 0.09 b,c |
| Beer Code | λem (nm) | FI (A.U.) | Binding to HSA (%) | λem (nm) | FI (A.U.) | Binding to PCF (%) |
|---|---|---|---|---|---|---|
| MACC | 349 | 731.8 ± 2.1 c,d | 24.1 ± 2.5 b | 347 | 674.9 ± 2.8 d | 14.1 ± 0.9 c |
| GOLD | 350 | 713.6 ± 2.6 d | 26.0 ± 2.8 a,b | 348 | 666.3 ± 1.2 e | 15.1 ± 1.2 b |
| HEIN | 347 | 750.9 ± 3.8 b,c | 22.2 ± 2.7 c | 346 | 684.3 ± 2.7 c | 12.8 ± 0.8 d |
| CARL | 346 | 759.4 ± 3.5 b,c | 21.3 ± 2.6 c,d | 346 | 683.6 ± 2.8 c | 12.9 ± 1.1 d |
| MGD | 346 | 755.3 ± 4.2 b,c | 21.7 ± 1.9 c,d | 346 | 685.9 ± 2.1 c | 12.6 ± 0.9 d |
| COR | 345 | 763.9 ± 4.3 b,c | 20.8 ± 1.5 d | 346 | 686.7 ± 2.7 c | 12.5 ± 1.1 d |
| ORJB | 348 | 745.1 ± 5.8 c | 22.8 ± 1.9c | 346 | 680.0 ± 3.0 c | 13.3 ± 1.2 c,d |
| AMST | 349 | 735.9 ± 5.5 c,d | 23.7 ± 1.3 b,c | 346 | 676.5 ± 4.1 d | 13.8 ± 1.1 c,d |
| KAM | 350 | 671.3 ± 6.3 e | 30.4 ± 1.4a | 350 | 646.7 ± 4.3 f | 17.6 ± 1.5 a |
| ROST | 351 | 663.2 ± 7.3 e | 31.3 ± 1.5 a | 350 | 642.4 ± 4.2 f | 18.1 ± 1.3 a |
| ŻYW | 347 | 748.8 ± 4.2 c | 22.4 ± 0.9 c | 346 | 682.8 ± 4.0 c | 13.0 ± 0.9 c,d |
| EtOH | 344 | 934.8 ± 2.9 a | 3.1 ± 0.2 f | 343 | 764.3 ± 5.9 a,b | 2.6 ± 0.1 f |
| Catechin | 348 | 743.8 ± 4.4 c | 22.9 ± 1.9 c | 344 | 736.9 ± 5.7 b | 6.1 ± 0.7 e |
| Epicatechin | 348 | 745.7 ± 4.8 c | 22.7 ± 2.0 c | 344 | 738.7 ± 5.4 b | 6.9 ± 0.9 e |
| Quercetin | 347 | 754.4 ± 4.7 b,c | 21.8 ± 2.1 c,d | 344 | 743.2 ± 6.1 b | 5.3 ± 0.5 e,f |
| Caffeic acid | 345 | 820.1 ± 3.2 a,b | 14.9 ± 1.5 e | 360 | 667.9 ± 4.9 e | 14.9 ± 1.1 c |
| Ferulic acid | 345 | 803.6 ± 5.2 b | 16.7 ± 1.5 d,e | 360 | 663.9 ± 4.9 e | 15.4 ± 0.9 b |
| HSA/buffer | 343 | 964.7 ± 3.8 a | - | - | - | - |
| PCF/buffer | - | - | - | 344 | 784.8 ± 4.9 a | - |
| Ligand Name | Dock Score | Bond Formation | Chain | Interacting Amino Acids |
|---|---|---|---|---|
| Human C-Reactive Protein | ||||
| Catechin | 62.693 | 2(H-bond),1(Pi-sigma) | Chain A | ALA92, VAL94, ASP112 |
| Epicatechin | 60.268 | 3(H),1(Pi-alkyl), 1(carbon-H) | Chain A | PHE39, THR41, SER44, TYR49, TRP67, TYR73, THR90, VAL91, ALA92, VAL94, ASP112, VAL111 |
| Ferulic acid | 55.343 | 6(H),1(Pi-sigma),1(Pi-alkyl), 2(carbon-H) | Chain A | TYR49, TYR73, ALA92, VAL94, ASP112 |
| Caffeic acid | 53.062 | 2(H),1(Pi-alkyl),2(carbon-H) | Chain A | TYR73, VAL89, ALA92, VAL94, ASP112 |
| Quercetin | 58.609 | 6(H),2(Pi-Pi),1(Pi-alkyl),1(carbon-H) | Chain A | PHE39, THR41, SER44, TYR49, TRP67, THR90, GLU88, VAL94, ASP112 |
| Human Serum Albumin | ||||
| Catechin | 53.679 | 2(H),1(Pi-Pi),2(Pi-alkyl),3(carbon-H) | Chain A | ILE142,HIS146,PHE157,TYR161,ARG186, GLY189 |
| Epicatechin | 53.033 | 2(H),2(Pi-Pi),1(Pi-alkyl),1(Pi-sigma) | Chain A | ILE142, HIS146, PHE149, TYR161, ARG186, GLY189, LEU115 |
| Ferulic acid | 39.165 | 1(Pi-Pi),1(Pi-alkyl) | Chain A | ILE142, PHE157 |
| Caffeic acid | 36.825 | 3(Pi-Pi),1(Pi-alkyl),1(Van der Waals) | Chain A | ILE142, PHE157, HIS146, GLY189, LYS190 |
| Quercetin | 51.170 | 2(H),1(Pi-Pi),1(Pi-alkyl),1(carbon-H), 1(Pi-sigma) | Chain A | ILE142, HIS146, PHE149, TYR161, ARG186, GLY189 |
| Human glutathione peroxidase 3 (GPX3) | ||||
| Epicatechin | 103.364 | 2(H),1(Pi-Pi),3(Pi-alkyl) | Chain A | LEU46, TYR53, GLN86, ALA90 |
| Ferulic acid | 82.449 | 1(Pi-Pi),1(Pi-alkyl), 1(carbon-H) | Chain A | TYR53, ALA90, ASN131 |
| Caffeic acid | 83.956 | 1(Pi-Pi),1(Pi-alkyl), 1(carbon-H) | Chain A | TYR53, ALA90, ASN131 |
| Quercetin | 102.459 | 1(amide-Pi),2(Pi-alkyl), 1(Pi-lone), 1(Van der Waals) | Chain A | ALA90, ASN131, LEU46, PHE132, GLN133 |
| Human Fibrinogen | ||||
| Ferulic acid | 95.517 | 2(H),1(Pi-amide),1(Pi-S), 1(carbon-H) | Chain J (α), Chain I (β), chain L (γ) | CYS19, PRO20, THR21, THR22, CYS45 |
| Caffeic acid | 95.095 | 3(H),1(Pi-amide),1(Pi-S), 1(carbon-H),1(Van der Waals) | Chain J (α), Chain H (β), Chain L (γ) | CYS19, THR22, CYS45, THR78, PRO77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafreen, R.M.B.; Lakshmi, S.A.; Pandian, S.K.; Park, Y.S.; Kim, Y.M.; Paśko, P.; Deutsch, J.; Katrich, E.; Gorinstein, S. Unraveling the Antioxidant, Binding and Health-Protecting Properties of Phenolic Compounds of Beers with Main Human Serum Proteins: In Vitro and In Silico Approaches. Molecules 2020, 25, 4962. https://doi.org/10.3390/molecules25214962
Shafreen RMB, Lakshmi SA, Pandian SK, Park YS, Kim YM, Paśko P, Deutsch J, Katrich E, Gorinstein S. Unraveling the Antioxidant, Binding and Health-Protecting Properties of Phenolic Compounds of Beers with Main Human Serum Proteins: In Vitro and In Silico Approaches. Molecules. 2020; 25(21):4962. https://doi.org/10.3390/molecules25214962
Chicago/Turabian StyleShafreen, Raja Mohamed Beema, Selvaraj Alagu Lakshmi, Shunmugiah Karutha Pandian, Yong Seo Park, Young Mo Kim, Paweł Paśko, Joseph Deutsch, Elena Katrich, and Shela Gorinstein. 2020. "Unraveling the Antioxidant, Binding and Health-Protecting Properties of Phenolic Compounds of Beers with Main Human Serum Proteins: In Vitro and In Silico Approaches" Molecules 25, no. 21: 4962. https://doi.org/10.3390/molecules25214962
APA StyleShafreen, R. M. B., Lakshmi, S. A., Pandian, S. K., Park, Y. S., Kim, Y. M., Paśko, P., Deutsch, J., Katrich, E., & Gorinstein, S. (2020). Unraveling the Antioxidant, Binding and Health-Protecting Properties of Phenolic Compounds of Beers with Main Human Serum Proteins: In Vitro and In Silico Approaches. Molecules, 25(21), 4962. https://doi.org/10.3390/molecules25214962