Enhanced Fire Safety of Rigid Polyurethane Foam via Synergistic Effect of Phosphorus/Nitrogen Compounds and Expandable Graphite
Abstract
1. Introduction
2. Results and Discussion
2.1. Thermal Degradation Behavior Analysis
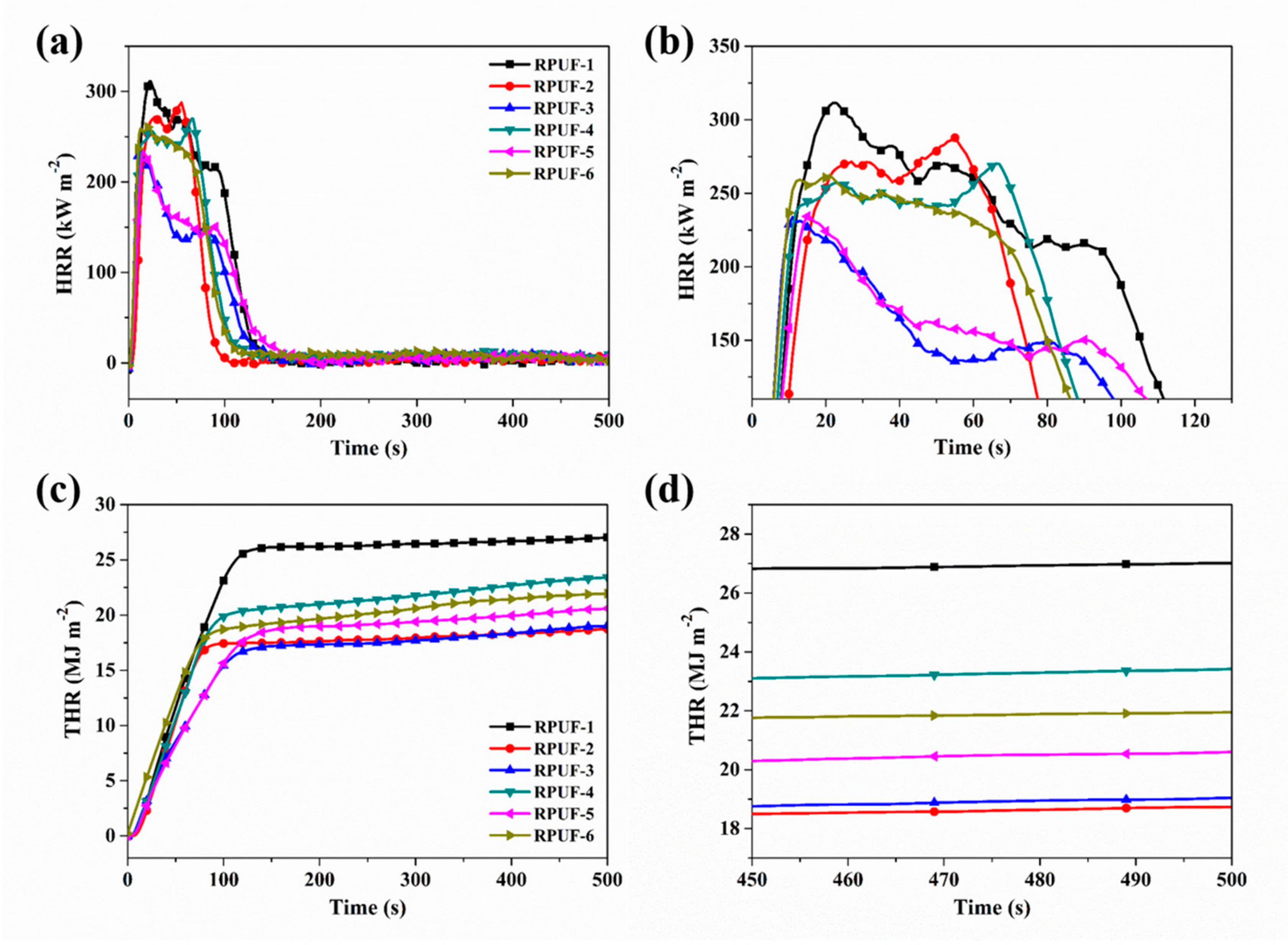
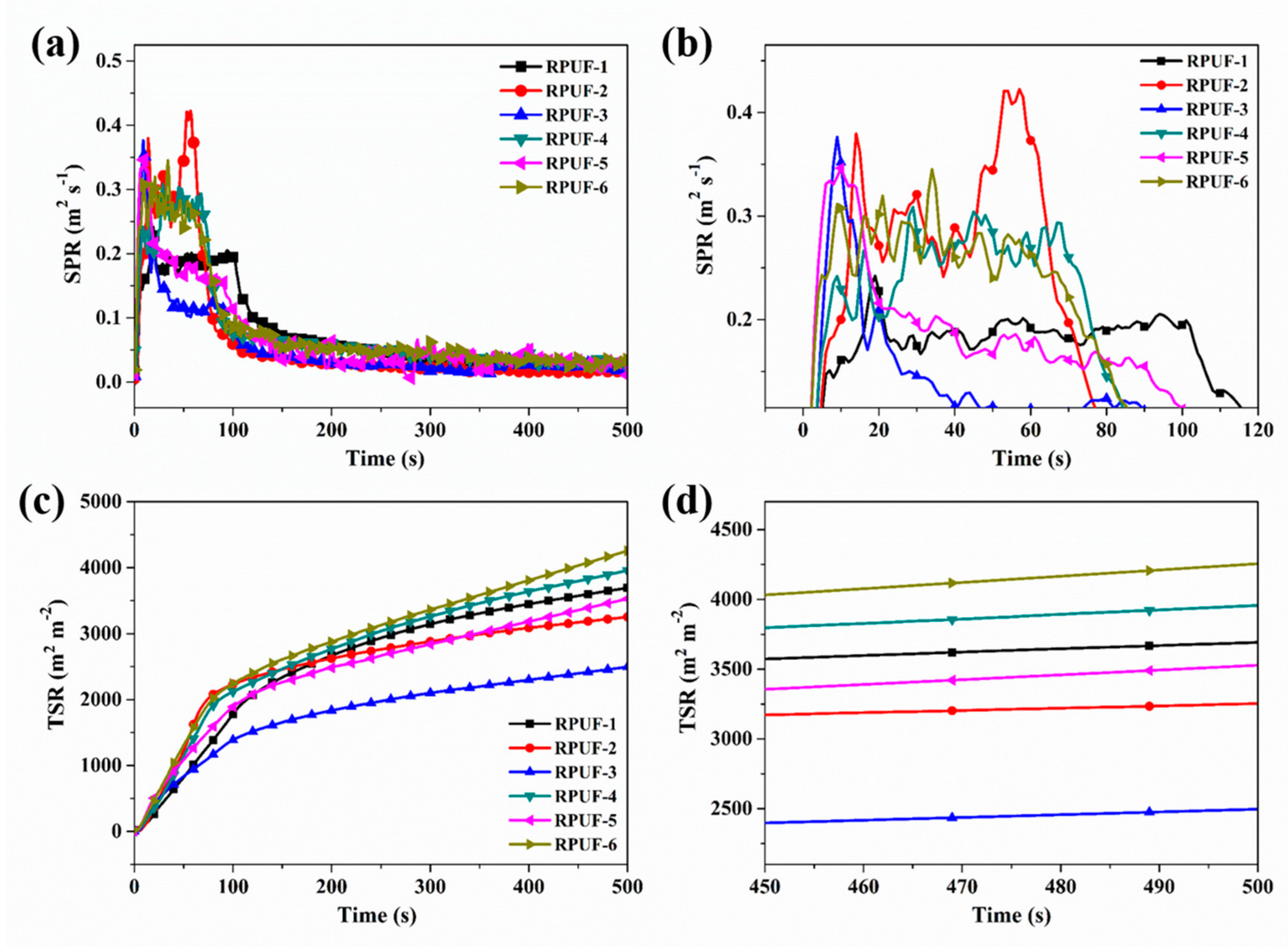
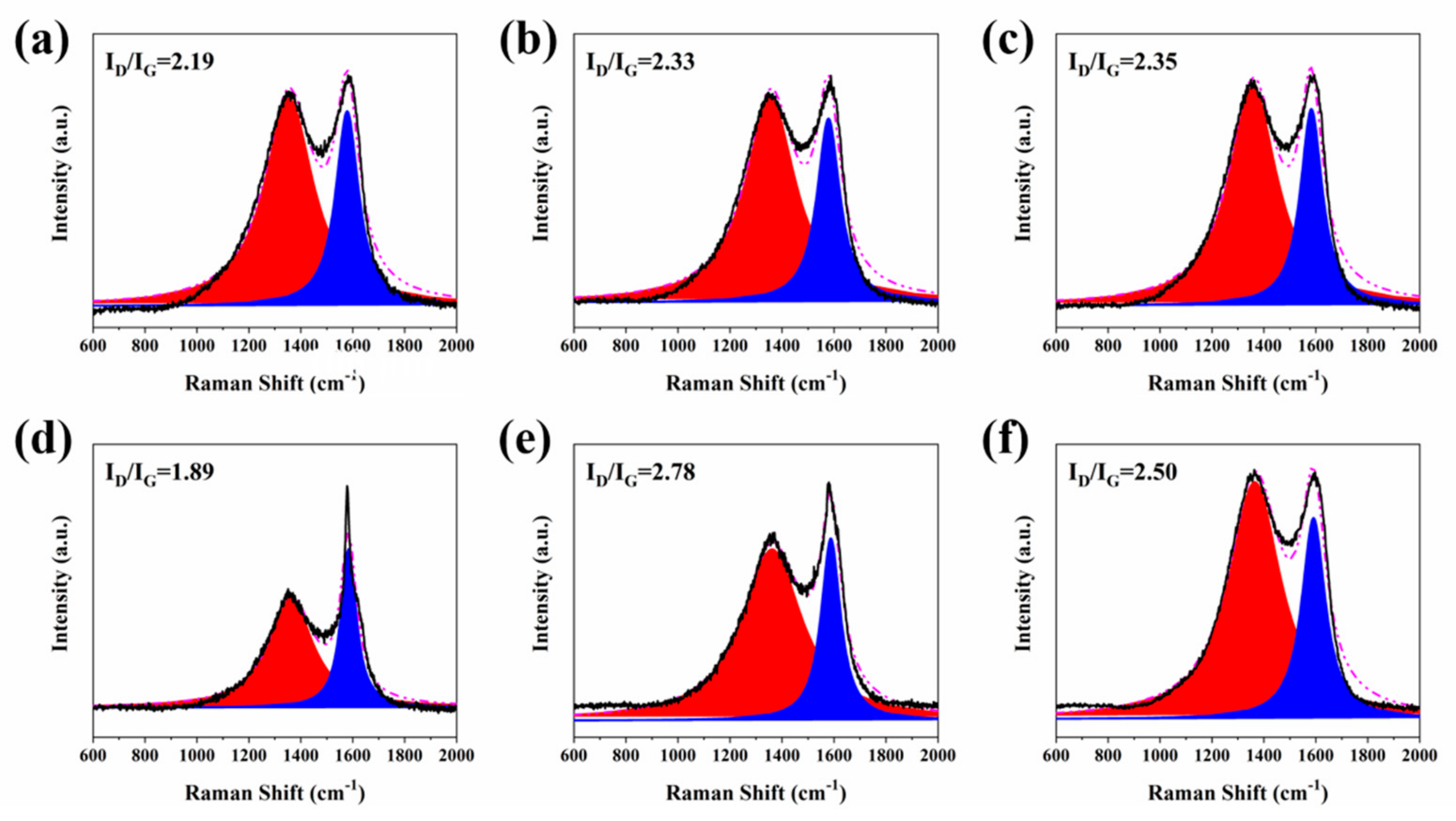
2.2. Combustion Behavior Evaluation of RPUF and Its Composites
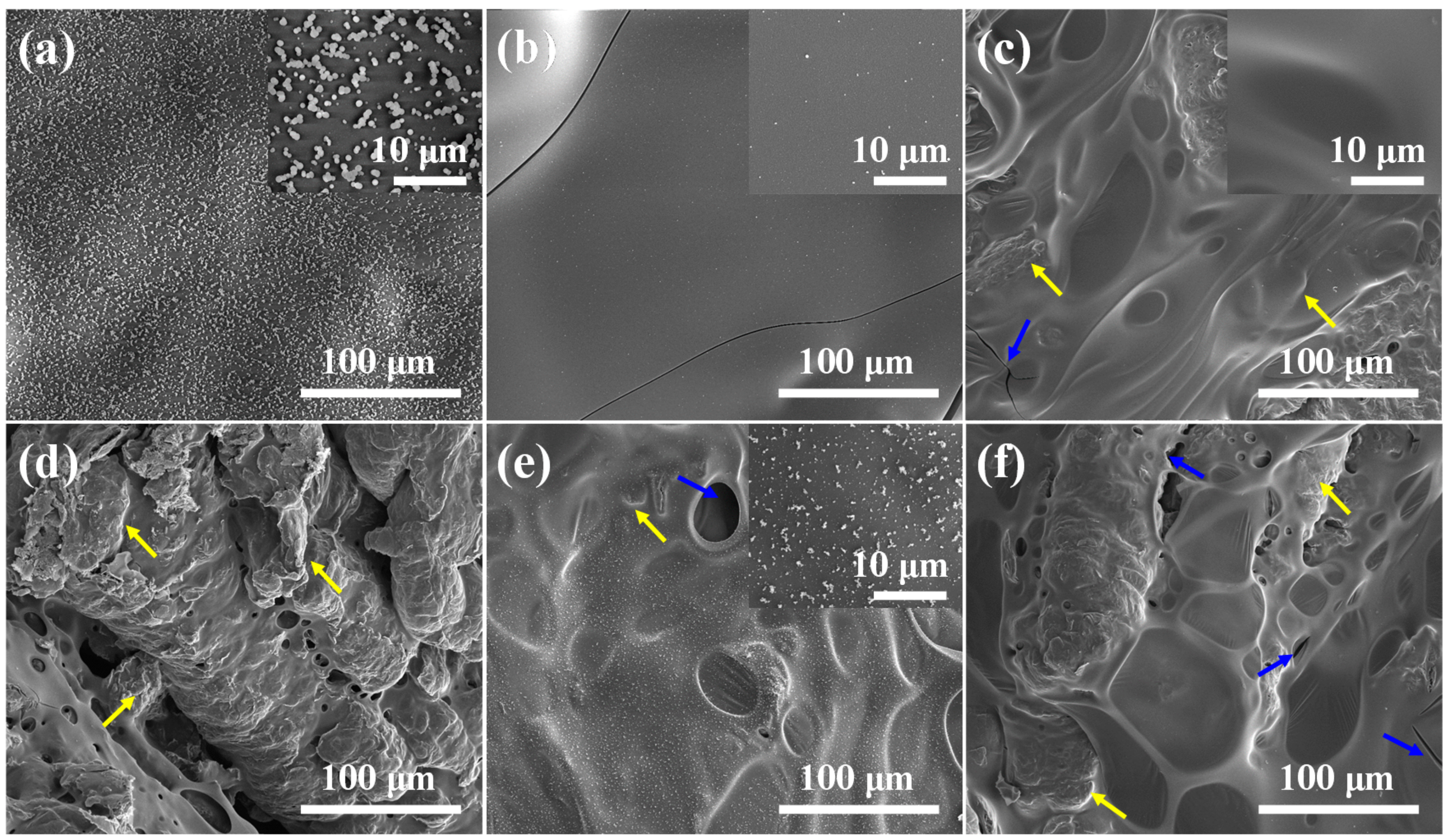
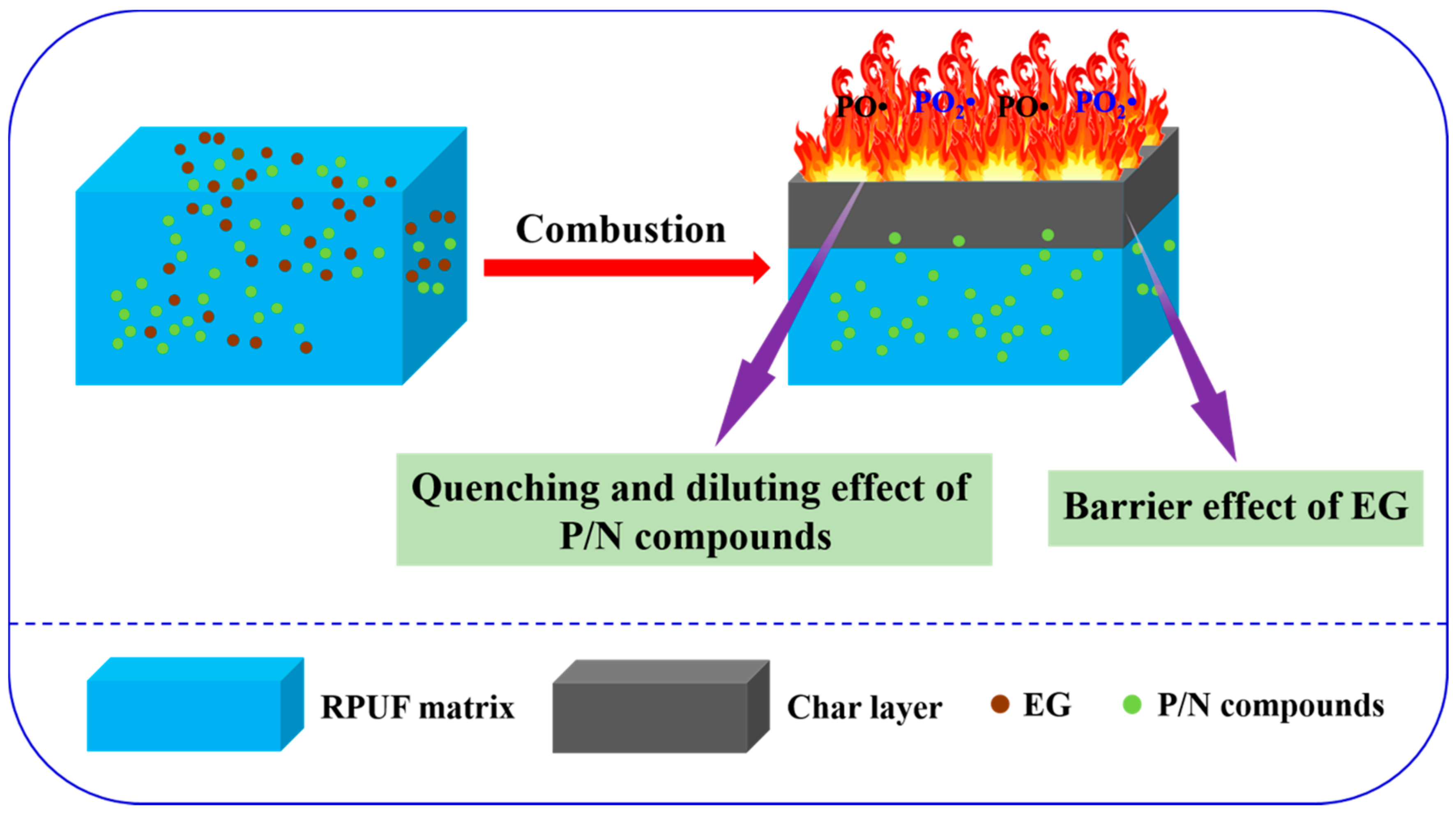
2.3. Flame-Retardant Mechanism
3. Experimental
3.1. Raw Materials
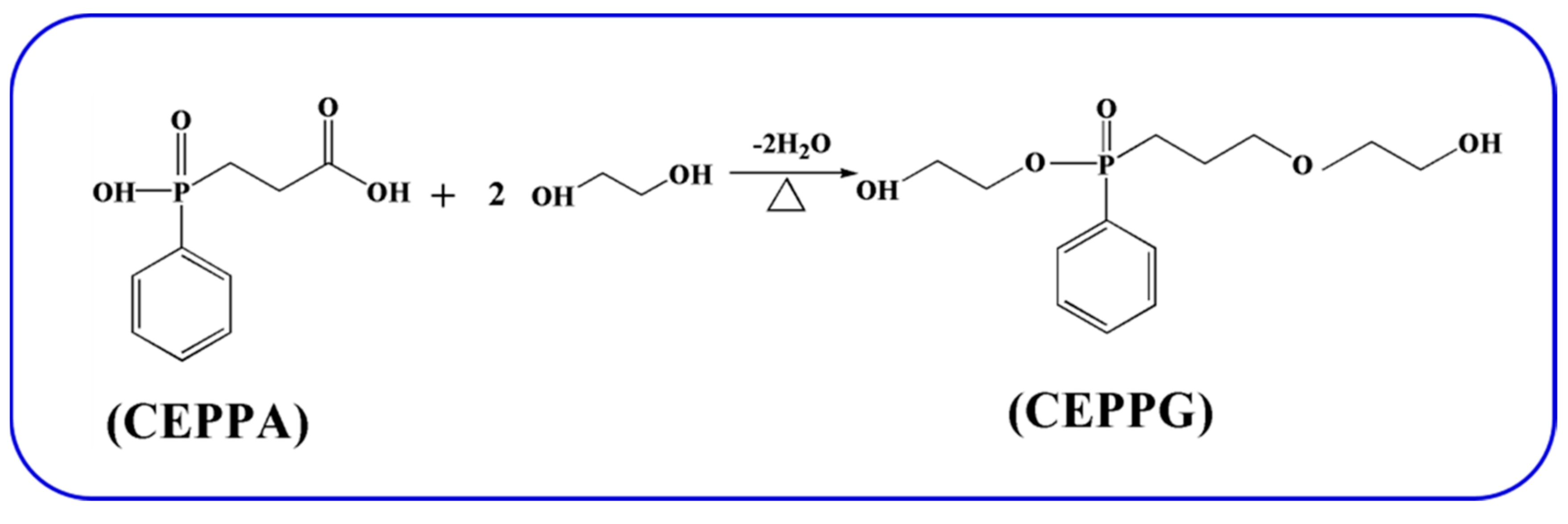
3.2. Preparation of 2-Carboxyethyl Phenylphosphinic Acid Glycol Ester (CEPPG)
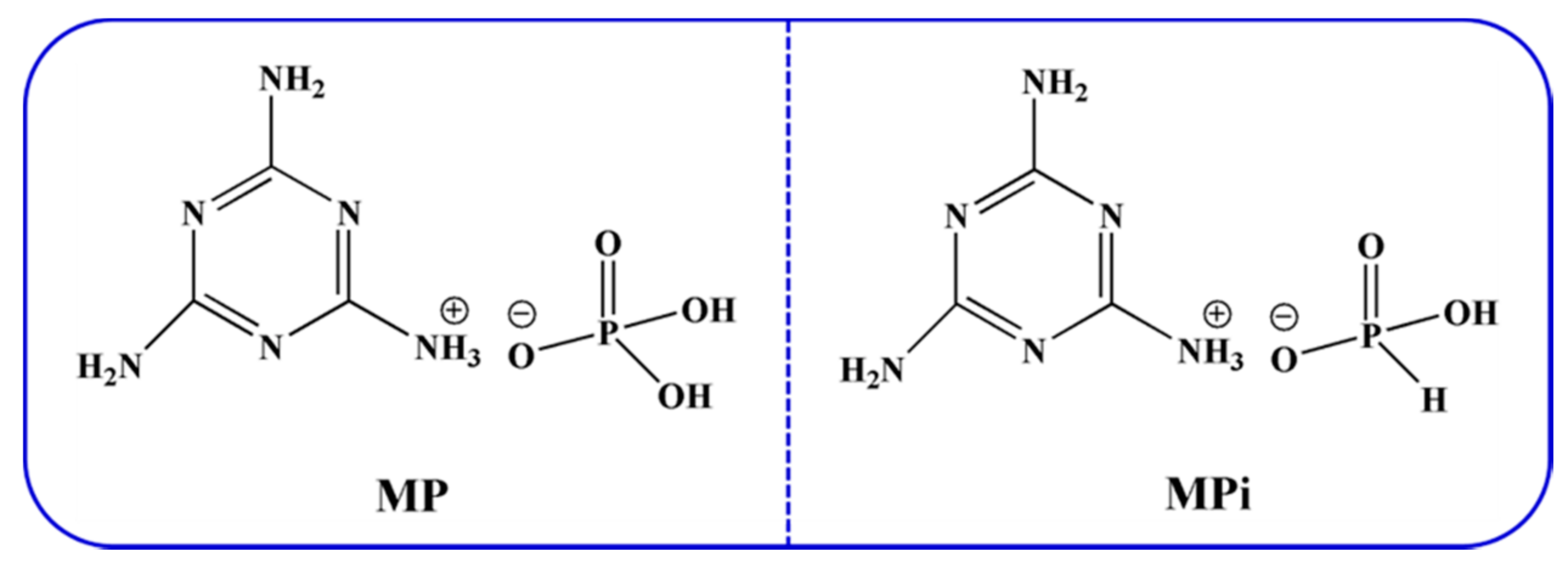
3.3. Preparation of Melamine Phosphate (MP) and Melamine Phosphite (MPi)
3.4. Fabrication of Flame Retardant RPPU Composites
3.5. Instruments and Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Borreguero, A.M.; Velencoso, M.M.; Rodriguez, J.F.; Serrano, A.; Carrero, M.J.; Ramos, M.J. Synthesis of aminophosphonate polyols and polyurethane foams with improved fire retardant properties. J. Appl. Polym. Sci. 2019, 136, 10. [Google Scholar] [CrossRef]
- Luo, F.; Wu, K.; Li, D.; Zheng, J.; Guo, H.; Zhao, Q.; Lu, M. A novel intumescent flame retardant with nanocellulose as charring agent and its flame retardancy in polyurethane foam. Polym. Compos. 2017, 38, 2762–2770. [Google Scholar] [CrossRef]
- Chiu, S.H.; Wu, C.L.; Lee, H.T.; Gu, J.H.; Suen, M.C. Synthesis and characterisation of novel flame retardant polyurethanes containing designed phosphorus units. J. Polym. Res. 2016, 23, 10. [Google Scholar] [CrossRef]
- Liu, C.; Fang, Y.F.; Miao, X.M.; Pei, Y.B.; Yan, Y.; Xiao, W.J.; Wu, L.B. Facile fabrication of superhydrophobic polyurethane sponge towards oil water separation with exceptional flame-retardant performance. Sep. Purif. Technol. 2019, 229, 10. [Google Scholar] [CrossRef]
- Yang, H.Y.; Liu, H.Y.; Jiang, Y.P.; Chen, M.F.; Wan, C.J. Density effect on flame retardancy, thermal degradation, and combustibility of rigid polyurethane foam modified by expandable graphite or ammonium polyphosphate. Polymers 2019, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Li, J.F.; Zhou, X. A flame retardant rigid polyurethane foam system including functionalized graphene oxide. Polym. Compos. 2019, 40, 1274–1282. [Google Scholar] [CrossRef]
- Xu, D.F.; Yu, K.J.; Qian, K. Effect of tris(1-chloro-2-propyl)phosphate and modified aramid fiber on cellular structure, thermal stability and flammability of rigid polyurethane foams. Polym. Degrad. Stabil. 2017, 144, 207–220. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, H.Y.; Yu, B.; Shi, Y.Q.; Wang, W.; Song, L.; Hu, Y.; Zhang, Y.M. Phosphorus and nitrogen-containing polyols: Synergistic effect on the thermal property and flame retardancy of rigid polyurethane foam composites. Ind. Eng. Chem. Res. 2016, 55, 10813–10822. [Google Scholar] [CrossRef]
- Zhang, M.; Luo, Z.Y.; Zhang, J.W.; Chen, S.G.; Zhou, Y.H. Effects of a novel phosphorus-nitrogen flame retardant on rosin-based rigid polyurethane foams. Polym. Degrad. Stabil. 2015, 120, 427–434. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Zhou, Y.; Hu, L. The study of mechanical behavior and flame retardancy of castor oil phosphate-based rigid polyurethane foam composites containing expanded graphite and triethyl phosphate. Polym. Degrad. Stabil. 2013, 98, 2784–2794. [Google Scholar] [CrossRef]
- Xu, W.; Wang, G.; Zheng, X. Research on highly flame-retardant rigid PU foams by combination of nanostructured additives and phosphorus flame retardants. Polym. Degrad. Stabil. 2015, 111, 142–150. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Qi, X.; Qian, L. The pyrolysis behaviors of phosphorus-containing organosilicon compound modified APP with different polyether segments and their flame retardant mechanism in polyurethane foam. Compos. Part. B Eng. 2019, 173, 106784. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Qian, L. The pyrolysis behaviors of phosphorus-containing organosilicon compound modified ammonium polyphosphate with different phosphorus-containing groups, and their different flame-retardant mechanisms in polyurethane foam. RSC Adv. 2018, 8, 27470–27480. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, G.; Xu, W. Roles of organically-modified montmorillonite and phosphorous flame retardant during the combustion of rigid polyurethane foam. Polym. Degrad. Stabil. 2014, 101, 32–39. [Google Scholar] [CrossRef]
- Xu, Z.B.; Kong, W.W.; Zhou, M.X.; Peng, M. Eeffect of surface modification of montmorillonite on the properties of rigid polyurethane foam composites. Chin. J. Polym. Sci. 2010, 28, 615–624. [Google Scholar] [CrossRef]
- Xu, W.Z.; Wang, G.S.; Xu, J.Y.; Liu, Y.C.; Chen, R.; Yan, H.Y. Modification of diatomite with melamine coated zeolitic imidazolate framework-8 as an effective flame retardant to enhance flame retardancy and smoke suppression of rigid polyurethane foam. J. Hazard. Mater. 2019, 379, 10. [Google Scholar] [CrossRef]
- Wang, J.; Ma, C.; Mu, X.; Cai, W.; Liu, L.; Zhou, X.; Hu, W.; Hu, Y. Construction of multifunctional MoSe2 hybrid towards the simultaneous improvements in fire safety and mechanical property of polymer. J. Hazard. Mater. 2018, 352, 36–46. [Google Scholar] [CrossRef]
- Xu, Q.W.; Zhai, H.M.; Wang, G.J. Mechanism of smoke suppression by melamine in rigid polyurethane foam. Fire Mater. 2015, 39, 271–282. [Google Scholar] [CrossRef]
- Akdogan, E.; Erdem, M.; Ureyen, M.E.; Kaya, M. Rigid polyurethane foams with halogen-free flame retardants: Thermal insulation, mechanical, and flame retardant properties. J. Appl. Polym. Sci. 2020, 137, 47611. [Google Scholar] [CrossRef]
- Chai, H.; Duan, Q.L.; Jiang, L.; Sun, J.H. Effect of inorganic additive flame retardant on fire hazard of polyurethane exterior insulation material. J. Therm. Anal. Calorim. 2019, 135, 2857–2868. [Google Scholar] [CrossRef]
- Wang, Y.T.; Wang, F.; Dong, Q.X.; Xie, M.C.; Liu, P.; Ding, Y.F.; Zhang, S.M.; Yang, M.S.; Zheng, G.Q. Core-shell expandable graphite @ aluminum hydroxide as a flame-retardant for rigid polyurethane foams. Polym. Degrad. Stabil. 2017, 146, 267–276. [Google Scholar] [CrossRef]
- Wu, S.; Deng, D.; Zhou, L.; Zhang, P.; Tang, G. Flame retardancy and thermal degradation of rigid polyurethane foams composites based on aluminum hypophosphite. Mater. Res. Express 2019, 6, 105365. [Google Scholar] [CrossRef]
- Xu, W.Z.; Liu, L.; Wang, S.Q.; Hu, Y. Synergistic effect of expandable graphite and aluminum hypophosphite on flame-retardant properties of rigid polyurethane foam. J. Appl. Polym. Sci. 2015, 132, 42842. [Google Scholar] [CrossRef]
- Tang, Q.; Song, Y.; He, J.; Yang, R. Synthesis and characterization of inherently flame-retardant and anti-dripping thermoplastic poly(imides-urethane)s. J. Appl. Polym. Sci. 2014, 131, 40801. [Google Scholar] [CrossRef]
- Jia, D.; Guo, X.; He, J.; Yang, R. An anti-melt dripping, high char yield and flame-retardant polyether rigid polyurethane foam. Polym. Degrad. Stabil. 2019, 167, 189–200. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, S.; Liang, R.; Liao, Z.; You, G. A green highly-effective surface flame-retardant strategy for rigid polyurethane foam: Transforming UV-cured coating into intumescent self-extinguishing layer. Compos. Part. A Appl. Sci. Manuf. 2019, 125, 105534. [Google Scholar] [CrossRef]
- Xue, B.; Qin, R.; Shao, M.; Li, S.; Niu, M. Improving the flame retardancy of PET fiber by constructing the carbon microspheres based melamine polyphosphate powder. J. Text. Inst. 2019, 111, 597–603. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Nando, G.B.; Naik, Y.P.; Singha, N.K. Halogen-free flame retardant PUF: Effect of melamine compounds on mechanical, thermal and flame retardant properties. Polym. Degrad. Stabil. 2010, 95, 1138–1145. [Google Scholar] [CrossRef]
- Yang, H.; Song, L.; Tai, Q.; Wang, X.; Yu, B.; Yuan, Y.; Hu, Y.; Yuen, R.K.K. Comparative study on the flame retarded efficiency of melamine phosphate, melamine phosphite and melamine hypophosphite on poly(butylene succinate) composites. Polym. Degrad. Stabil. 2014, 105, 248–256. [Google Scholar] [CrossRef]
- Cheng, J.J.; Qu, W.J.; Sun, S.H. Mechanical properties improvement and fire hazard reduction of expandable graphite microencapsulated in rigid polyurethane foams. Polym. Compos. 2019, 40, E1006–E1014. [Google Scholar] [CrossRef]
- Wang, G.; Bai, S. Synergistic effect of expandable graphite and melamine phosphate on flame-retardant polystyrene. Appl. Polym. Sci. 2017, 134, 45474. [Google Scholar] [CrossRef]
- Pang, X.Y.; Xin, Y.P.; Shi, X.Z.; Xu, J.Z. Effect of different size-modified expandable graphite and ammonium polyphosphate on the flame retardancy, thermal stability, physical, and mechanical properties of rigid polyurethane foam. Polym. Eng. Sci. 2019, 59, 1381–1394. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, Y.; Guo, X.; Chen, L.; Xu, T.; Jia, D. Structure and flame-retardant actions of rigid polyurethane foams with expandable graphite. Polymers 2019, 11, 686. [Google Scholar] [CrossRef]
- Xi, W.; Qian, L.; Chen, Y.; Wang, J.; Liu, X. Addition flame-retardant behaviors of expandable graphite and bis (2-hydroxyethyl) aminol-methyl-phosphonic acid dimethyl ester in rigid polyurethane foams. Polym. Degrad. Stabil. 2015, 122, 36–43. [Google Scholar] [CrossRef]
- Wang, S.; Qian, L.; Xin, F. The synergistic flame-retardant behaviors of pentaerythritol phosphate and expandable graphite in rigid polyurethane foams. Polym. Compos. 2018, 39, 329–336. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Zhang, S.; Guo, J.; Tang, W.; Li, H.; Gu, X. Effects of carboxymethyl chitosan microencapsulated melamine polyphosphate on the flame retardancy and water resistance of thermoplastic polyurethane. Polym. Degrad. Stabil. 2019, 160, 168–176. [Google Scholar] [CrossRef]
- Feng, F.; Qian, L. The flame retardant behaviors and synergistic effect of expandable graphite and dimethyl methylphosphonate in rigid polyurethane foams. Polym. Compos. 2014, 35, 301–309. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, C.; Liu, L.; Fu, L.; Yu, B.; Lv, Y.; Yang, F.; Song, P. Strengthening, toughing and thermally stable ultra-thin MXene nanosheets/polypropylene nanocomposites via nanoconfinement. Chem. Eng. J. 2019, 378, 1222267. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Gui, Z.; Yuan, B.H.; Hu, Y.; Zheng, Y.Y. Flammability of polystyrene/aluminim phosphinate composites containing modified ammonium polyphosphate. J. Therm. Anal. Calorim. 2018, 131, 1067–1077. [Google Scholar] [CrossRef]
- Han, S.; Zhu, X.; Chen, F.; Chen, S.; Liu, H. Flame-retardant system for rigid polyurethane foams based on diethyl bis (2-hydroxyethyl) aminomethylphosphonate and in-situ exfoliated clay. Polym. Degrad. Stabil. 2020, 177, 109178. [Google Scholar] [CrossRef]
- Singh, H.; Jain, A.K. Ignition, combustion, toxicity, and fire retardancy of polyurethane foams: A comprehensive review. J. Appl. Polym. Sci. 2009, 111, 1115–1143. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. Thermal decomposition, combustion and fire-retardancy of polyurethanes—A review of the recent literature. Polym. Int. 2004, 53, 1585–1610. [Google Scholar] [CrossRef]
- Du, J.Z.; Alain, G.; Zeng, H.Y.; Feng, B.; Xu, S.; Zhou, E.G.; Shi, X.K.; Jin, L. Electron beam irradiation crosslinking and flame retardant of ethylene vinyl acetate using melamine-formaldehyde microencapsulated layered double hydroxides. J. Nanosci. Nanotechnol. 2020, 20, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Wu, K.; Luo, F.B.; Guo, Y.Y.; Zhang, S.H.; Du, X.X.; Zhu, Q.Q.; Lu, M.G. An efficient phosphonate-based ionic liquid on flame retardancy and mechanical property of epoxy resin. J. Mater. Sci. 2017, 52, 13992–14003. [Google Scholar] [CrossRef]
- Latha, G.; Natarajan, M.; Murugavel, S.C. Synthesis and characterization of cardo-based phosphorous-containing flame-retardant aromatic polyesters. High. Perform. Polym. 2016, 28, 1218–1227. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, L.; Fu, L.; Liu, C.; Yu, B.; Yang, F.; Hu, Y. Sodium alginate-templated synthesis of g-C3N4/carbon spheres/Cu ternary nanohybrids for fire safety application. J. Colloid Interface Sci. 2019, 539, 1–10. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, C.; Duan, Z.; Yu, B.; Liu, M.; Song, P. Interface engineering of MXene towards super-tough and strong polymer nanocomposites with high ductility and excellent fire safety. Chem. Eng. J. 2020, 399, 125829. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, B.; Duan, L.; Gui, Z.; Wang, B.; Hu, Y.; Yuen, R.K.K. Graphitic carbon nitride/phosphorus-rich aluminum phosphinates hybrids as smoke suppressants and flame retardants for polystyrene. J. Hazard. Mater. 2017, 332, 87–96. [Google Scholar] [CrossRef]
- Akech, S.R.O.; Harrison, O.; Saha, A. Removal of a potentially hazardous chemical, tetrakis (hydroxymethyl) phosphonium chloride from water using biochar as a medium of adsorption. Environ. Technol. Innov. 2018, 12, 196–210. [Google Scholar] [CrossRef]
- Yuan, Y.; Yu, B.; Shi, Y.Q.; Ma, C.; Song, L.; Hu, W.Z.; Hu, Y. Highly efficient catalysts for reducing toxic gases generation change with temperature of rigid polyurethane foam nanocomposites: A comparative investigation. Compos. Part. A Appl. Sci. Manuf. 2018, 112, 142–154. [Google Scholar] [CrossRef]
- Wang, L.; Tawiah, B.; Shi, Y.; Cai, S.; Rao, X.; Liu, C.; Yang, Y.; Yang, F.; Yu, B.; Liang, Y.; et al. Highly effective flame-retardant rigid polyurethane foams: Fabrication and applications in inhibition of coal combustion. Polymers 2019, 11, 1776. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Ma, C.; Shi, Y.Q.; Song, L.; Hu, Y.; Hu, W.Z. Highly-efficient reinforcement and flame retardancy of rigid polyurethane foam with phosphorus-containing additive and nitrogen-containing compound. Mater. Chem. Phys. 2018, 211, 42–53. [Google Scholar] [CrossRef]
- Hejna, A.; Kirpluks, M.; Kosmela, P.; Cabulis, U.; Haponiuk, J.; Piszczyk, L. The influence of crude glycerol and castor oil-based polyol on the structure and performance of rigid polyurethane-polyisocyanurate foams. Ind. Crop. Prod. 2017, 95, 113–125. [Google Scholar] [CrossRef]
- Jiao, L.; Xiao, H.; Wang, Q.; Sun, J. Thermal degradation characteristics of rigid polyurethane foam and the volatile products analysis with TG-FTIR-MS. Polym. Degrad. Stabil. 2013, 98, 2687–2696. [Google Scholar] [CrossRef]
- Li, L.J.; Duan, R.T.; Zhang, J.B.; Wang, X.L.; Chen, L.; Wang, Y.Z. Phosphorus-containing poly (ethylene terephthalate): Solid-state polymerization and its sequential distribution. Ind. Eng. Chem. Res. 2013, 52, 5326–5333. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds for preparation of flame retardant RPPU composites are available from the authors. |
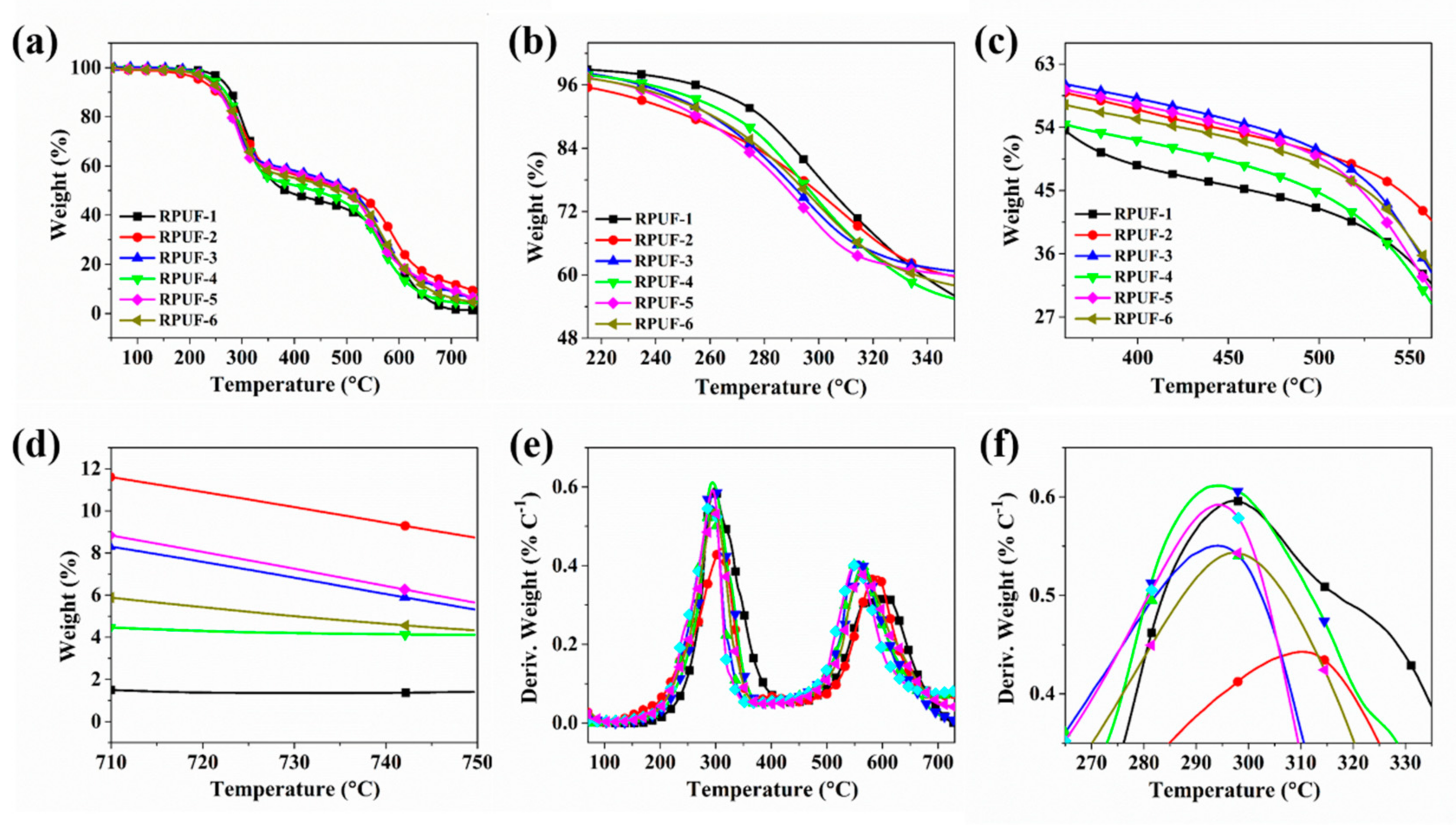




| Sample No. | T−5 (°C) | T−50 (°C) | Tmax (°C) | Residues at 750 °C (wt.%) | |
|---|---|---|---|---|---|
| Step 1 | Step 2 | ||||
| RPUF-1 | 260.8 | 383.4 | 297.4 | 604.6 | 1.41 |
| RPUF-2 | 220.4 | 504.6 | 310.2 | 590.3 | 8.74 |
| RPUF-3 | 240.6 | 505.4 | 294.1 | 556.5 | 5.32 |
| RPUF-4 | 245.1 | 437.9 | 294.4 | 564.4 | 4.18 |
| RPUF-5 | 236.6 | 497.7 | 294.3 | 550.6 | 5.65 |
| RPUF-6 | 236.2 | 486.4 | 297.2 | 565.4 | 4.33 |
| Sample No. | t1/t2 (s) | Ignite the Cotton | Rating |
|---|---|---|---|
| RPUF-1 | ― | No | NR |
| RPUF-2 | 19.7/0.0 | No | V-1 |
| RPUF-3 | 6.9/0.0 | No | V-0 |
| RPUF-4 | 15.7/0.0 | No | V-1 |
| RPUF-5 | 2.4/0.0 | No | V-0 |
| RPUF-6 | 17.8/0.0 | No | V-1 |
| Sample No. | TTI (s) | PHRR (kW m−2) | THR (MJ m−2) | PSRR (m2 s−1) | TSR (m2 m−2) |
|---|---|---|---|---|---|
| Error | ±1 | ±21 | ±2 | ±0.02 | ±696 |
| RPUF-1 | 5 | 312 | 27.0 | 0.24 | 3698 |
| RPUF-2 | 6 | 287 | 18.8 | 0.42 | 3255 |
| RPUF-3 | 4 | 233 | 19.0 | 0.38 | 2489 |
| RPUF-4 | 4 | 270 | 23.6 | 0.31 | 3957 |
| RPUF-5 | 3 | 235 | 20.6 | 0.35 | 3525 |
| RPUF-6 | 3 | 262 | 27.0 | 0.34 | 4248 |
| Sample No. | C (At.%) | O (At.%) | N (At.%) | P (At.%) | N/P | O/P | |
|---|---|---|---|---|---|---|---|
| RPUF-3 | External char | 79.22 | 8.77 | 11.19 | 0.82 | 13.65 | 10.70 |
| Internal char | 82.59 | 7.56 | 8.99 | 0.85 | 10.58 | 8.89 | |
| RPUF-5 | External char | 75.43 | 10.1 | 13.28 | 1.2 | 11.07 | 8.42 |
| Internal char | 84.45 | 6.17 | 8.42 | 0.96 | 8.77 | 6.43 | |
| Sample No. | RPUF-1 | RPUF-2 | RPUF-3 | RPUF-4 | RPUF-5 | RPUF-6 |
|---|---|---|---|---|---|---|
| LY4110 (g) | 100 | 54.94 | 54.94 | 54.94 | 54.94 | 54.94 |
| CEPPG (g) | 0 | 45.06 | 45.06 | 45.06 | 45.06 | 45.06 |
| A33 (g) | 1 | 1 | 1 | 1 | 1 | 1 |
| LC (g) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Water (g) | 2 | 2 | 2 | 2 | 2 | 2 |
| Si-Oil (g) | 2 | 2 | 2 | 2 | 2 | 2 |
| TEOA (g) | 3 | 3 | 3 | 3 | 3 | 3 |
| PM-200 (g) | 150 | 126.3 | 126.3 | 126.3 | 126.3 | 126.3 |
| EG (g) | 0 | 0 | 7.5 | 7.5 | 8.82 | 8.82 |
| MP (g) | 0 | 0 | 7.5 | 0 | 8.82 | 0 |
| Mpi (g) | 0 | 0 | 0 | 7.5 | 0 | 8.82 |
| P/N compounds wt.% | 0 | 19.2 | 22.4 | 22.4 | 23.0 | 23.0 |
| EG wt.% | 0 | 0 | 3.2 | 3.2 | 3.8 | 3.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Zhang, P.; Shi, Y.; Rao, X.; Cai, S.; Fu, L.; Feng, Y.; Wang, L.; Zheng, X.; Yang, W. Enhanced Fire Safety of Rigid Polyurethane Foam via Synergistic Effect of Phosphorus/Nitrogen Compounds and Expandable Graphite. Molecules 2020, 25, 4741. https://doi.org/10.3390/molecules25204741
Liu C, Zhang P, Shi Y, Rao X, Cai S, Fu L, Feng Y, Wang L, Zheng X, Yang W. Enhanced Fire Safety of Rigid Polyurethane Foam via Synergistic Effect of Phosphorus/Nitrogen Compounds and Expandable Graphite. Molecules. 2020; 25(20):4741. https://doi.org/10.3390/molecules25204741
Chicago/Turabian StyleLiu, Chuan, Ping Zhang, Yongqian Shi, Xiaohui Rao, Suncheng Cai, Libi Fu, Yuezhan Feng, Liancong Wang, Xueqin Zheng, and Wei Yang. 2020. "Enhanced Fire Safety of Rigid Polyurethane Foam via Synergistic Effect of Phosphorus/Nitrogen Compounds and Expandable Graphite" Molecules 25, no. 20: 4741. https://doi.org/10.3390/molecules25204741
APA StyleLiu, C., Zhang, P., Shi, Y., Rao, X., Cai, S., Fu, L., Feng, Y., Wang, L., Zheng, X., & Yang, W. (2020). Enhanced Fire Safety of Rigid Polyurethane Foam via Synergistic Effect of Phosphorus/Nitrogen Compounds and Expandable Graphite. Molecules, 25(20), 4741. https://doi.org/10.3390/molecules25204741








