Correlations Between Trace Elements in Selected Locations of the Human Brain in Individuals with Alcohol Use Disorder
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Population
4.2. Procedure of Tissue Collection
4.3. Analytical Procedure
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Griffith, G.C.; Butt, E.M.; Walker, J. The inorganic element content of certain human tissues. Ann. Intern. Med. 1954, 41, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Perry, H.M., Jr.; Tipton, I.H.; Schroeder, H.A.; Cook, M.J. Variability in the metal content of human organs. J. Lab. Clin Med. 1962, 60, 245–253. [Google Scholar]
- Tipton, I.H.; Schroeder, H.A.; Perry Jr, H.M.; Cook, M.J. Trace Elements in Human Tissue: III. Subjects from Africa, the near and Far East and Europe. Health Phys. 1965, 11, 403–451. [Google Scholar] [CrossRef] [PubMed]
- Grochowski, C.; Blicharska, E.; Baj, J.; Mierzwińska, A.; Brzozowska, K.; Forma, A.; Maciejewski, R. Serum iron, Magnesium, Copper, and Manganese Levels in Alcoholism: A Systematic Review. Molcules 2019, 24, 1361. [Google Scholar] [CrossRef]
- Verma, P.K.; Sharma, A.; Shankar, H.; Sharma, A.; Rao, D.N. Role of Trace Elements, Oxidative Stress and Immune System: A Triad in Premature Ovarian Failure. Biol. Trace Elem. Res. 2017, 184, 325–333. [Google Scholar] [CrossRef]
- Cicero, C.E.; Mostile, G.; Vasta, R.; Rapisarda, V.; Signorelli, S.S.; Ferrante, M.; Zappia, M.; Nicoletti, A. Metals and neurodegenerative diseases. A systematic review. Environ. Res. 2017, 159, 82–94. [Google Scholar] [CrossRef]
- Grochowski, C.; Radzikowska, E.; Maciejewski, R. Neural stem cell therapy—Brief review. Clin. Neurol. Neurosurg. 2018, 173, 8–14. [Google Scholar] [CrossRef]
- Duce, J.A.; Bush, A.I. Biological metals and Alzheimer’s disease: Implications for therapeutics and diagnostics. Prog. Neurobiol. 2010, 92, 1–18. [Google Scholar] [CrossRef]
- Grochowski, C.; Litak, J.; Kulesza, B.; Szmygin, P.; Ziemianek, D.; Kamieniak, P.; Szczepanek, D.; Rola, R.; Trojanowski, T. Size and location correlations with higher rupture risk of intracranial aneurysms. J. Clin. Neurosci. 2018, 48, 181–184. [Google Scholar] [CrossRef]
- Grochowski, C.; Staśkiewicz, G. Ultra high field TOF-MRA: A method to visualize small cerebral vessels. 7 T TOF-MRA sequence parameters on different MRI scanners–Literature review. Neurol. Neurochir. Pol. 2017, 51, 411–418. [Google Scholar] [CrossRef]
- Rehm, J.; Samokhvalov, A.V.; Neuman, M.G.; Room, R.; Parry, C.; Lönnroth, K.; Patra, J.; Poznyak, V.; Popova, S. The association between alcohol use, alcohol use disorders and tuberculosis (TB). A systematic review. BMC Public Health. 2009, 9, 450. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J. The risks associated with alcohol use and alcoholism. Alcohol Res Health. 2011, 34, 135–143. [Google Scholar]
- Dinsmore, W.W.; McMaster, D.; Callender, M.E.; Buchanan, K.D.; Love, A.H.G. Trace elements and alcohol. Sci. Total Environ. 1985, 42, 109–119. [Google Scholar] [CrossRef]
- Schott, J. Some Tests for the Equality of Covariance Matrices. J. Stat. Plan. Inference 2001, 94, 25–36. [Google Scholar] [CrossRef]
- O’Dell, B.L. Mineral interactions relevant to nutrient requirement. J. Nutr. 1989, 119, 1832–1838. [Google Scholar] [CrossRef]
- Elsenhans, B.; Schumann, K.; Forth, W. Toxic metals: Interactions with essential metals. In: Rowland IR, ed. Nutr. Toxic. Cancer. 1991, 8, 223–258. [Google Scholar]
- Telisman, S. Interactions of essential and/or toxic metals and metalloid regarding interindividual differences in susceptibility to various toxicants and chronic diseases in man. Arh. Hig. Rada. Toksikol. 1995, 46, 459–476. [Google Scholar]
- Darret, G.; Couzy, F.; Antoine, J.M.; Magliola, C.; Mareschi, J.P. Estimation of Minerals and Trace Elements Provided by Beverages for the Adult in France. Ann. Nutr. Metab. 1986, 30, 335–344. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, P.; Wan, H.; Wang, Y.; Hao, P.; Liu, Y.; Liu, J. Selenium-Chromium (VI) Interaction Regulates the Contents and Correlations of Trace Elements in Chicken Brain and Serum. Biol. Trace Elem. Res. 2018, 181, 154–163. [Google Scholar] [CrossRef]
- Rahil-Khazen, R.; Bolann, B.J.; Ulvik, R.J. Correlations of trace element levels within and between different normal autopsy tissues analyzed by inductively coupled plasma atomic emission spectrometry (ICP-AES). BioMetals 2002, 15, 87–98. [Google Scholar] [CrossRef]
- Tanaka, M.; Matsugi, E.; Miyasaki, K. PIXE measurement applied to trace elemental analysis of human tissues. Nucl. Instrum. Meth. B. 1987, 22, 152–155. [Google Scholar] [CrossRef]
- Rahil-Khazen, R.; Bolann, B.J.; Myking, A.; Ulvik, R.J. Multi-element analysis of trace element levels in human autopsy tissues by using inductively coupled atomic emission spectrometry technique (ICP-AES). J. Trace Elem. Med. Biol. 1987, 16, 15–25. [Google Scholar] [CrossRef]
- Gulati, A.; Chandishwar, N.; Shanker, K.; Srimal, R.C.; Dhawan, K.N.; Bhargava, K.P. Effect of alcohols on the permeability of blood-brain barrier. Pharm. Res. Commun. 1985, 17, 85–93. [Google Scholar] [CrossRef]
- Haorah, J. Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. J. Leukoc. Biol. 2005, 78, 1223–1232. [Google Scholar] [CrossRef]
- Kornhuber, J.; Kaiserauer, C.H.; Kornhuber, A.W.; Kornhuber, M.E. Alcohol Consumption and Blood-Cerebrospinal Fluid Barrier Dysfunction in Man. Neuroscience 1987, 79, 218–222. [Google Scholar] [CrossRef]
- Kumar, V.; Gill, K.D. Oxidative Stress and Mitochondrial Dysfunction in Aluminium Neurotoxicity and Its Amelioration: A Review. NeuroToxicology 2014, 41, 154–166. [Google Scholar] [CrossRef]
- Puntarulo, S. Iron, Oxidative Stress and Human Health. Mol. Aspects Med. 2005, 26, 299–312. [Google Scholar] [CrossRef]
- Deibel, M.; Ehmann, W.; Markesbery, W. Copper, Iron, and Zinc Imbalances in Severely Degenerated Brain Regions in Alzheimer’s Disease: Possible Relation to Oxidative Stress. J. Neurol. Sci. 1996, 143, 137–142. [Google Scholar] [CrossRef]
- Lochhead, J.J.; McCaffrey, G.; Quigley, C.E.; Finch, J.; DeMarco, K.M.; Nametz, N.; Davis, T.P. Oxidative Stress Increases Blood–Brain Barrier Permeability and Induces Alterations in Occludin during Hypoxia–Reoxygenation. J. Cereb. Blood Flow Metab. 2010, 30, 1625–1636. [Google Scholar] [CrossRef]
- Bolognin, S.; Messori, L.; Zatta, P. Metal Ion Physiopathology in Neurodegenerative Disorders. Neuro. Mol. Med. 2009, 11, 223–238. [Google Scholar] [CrossRef]
- Bowman, A.B.; Kwakye, G.F.; Herrero Hernández, E.; Aschner, M. Role of Manganese in Neurodegenerative Diseases. J. Trace Elem. Med. Biol. 2011, 25, 191–203. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
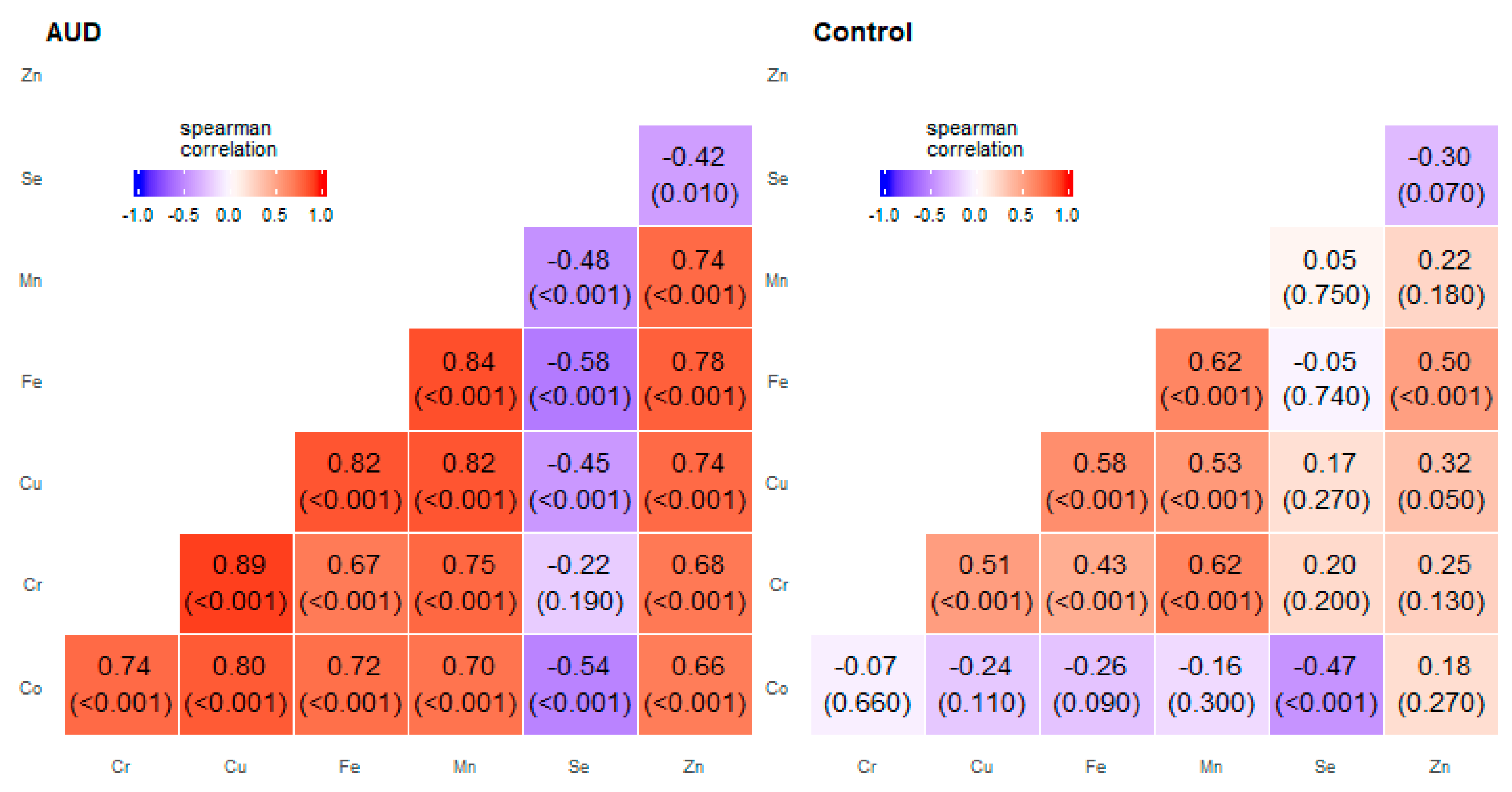
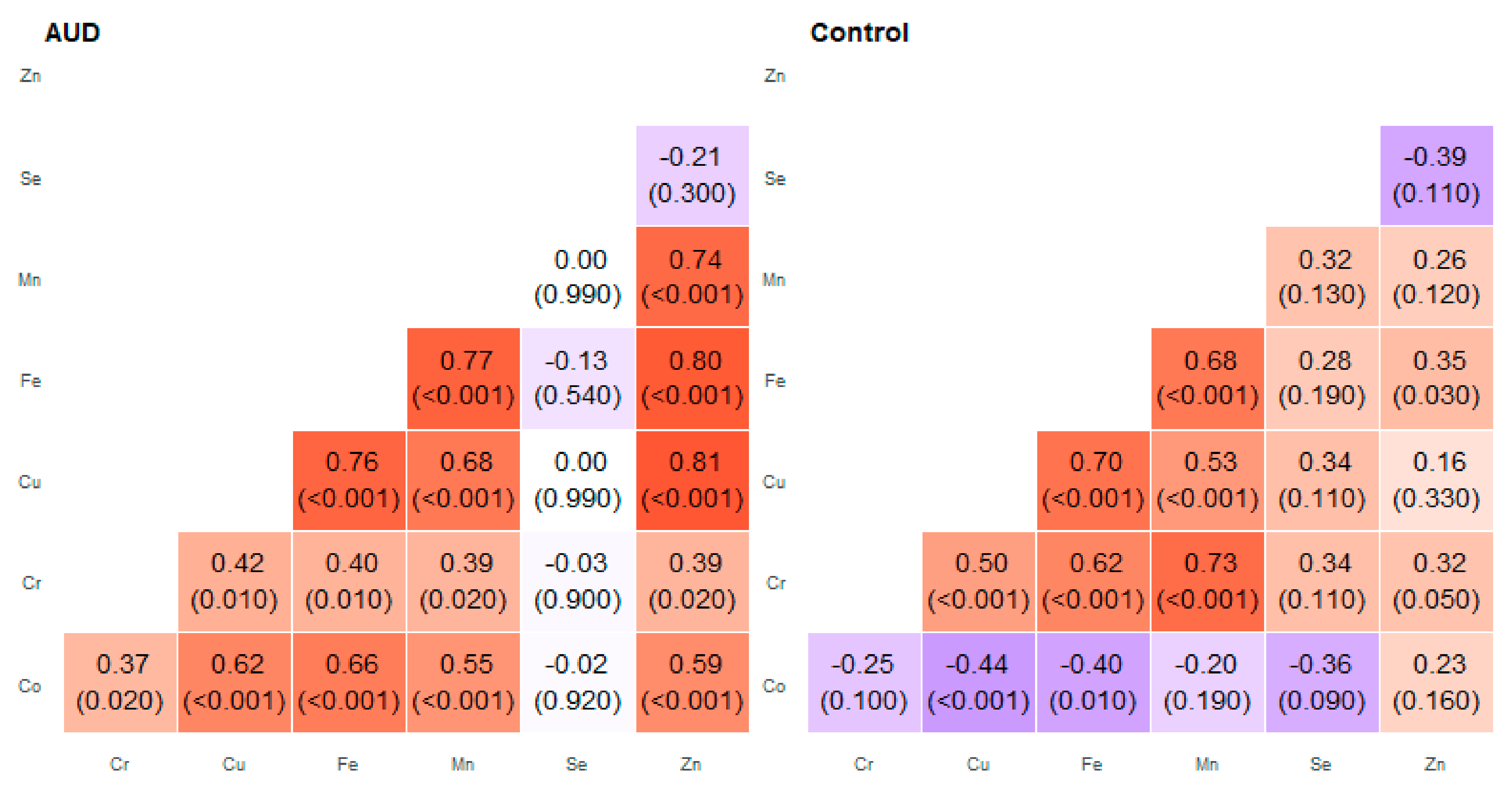
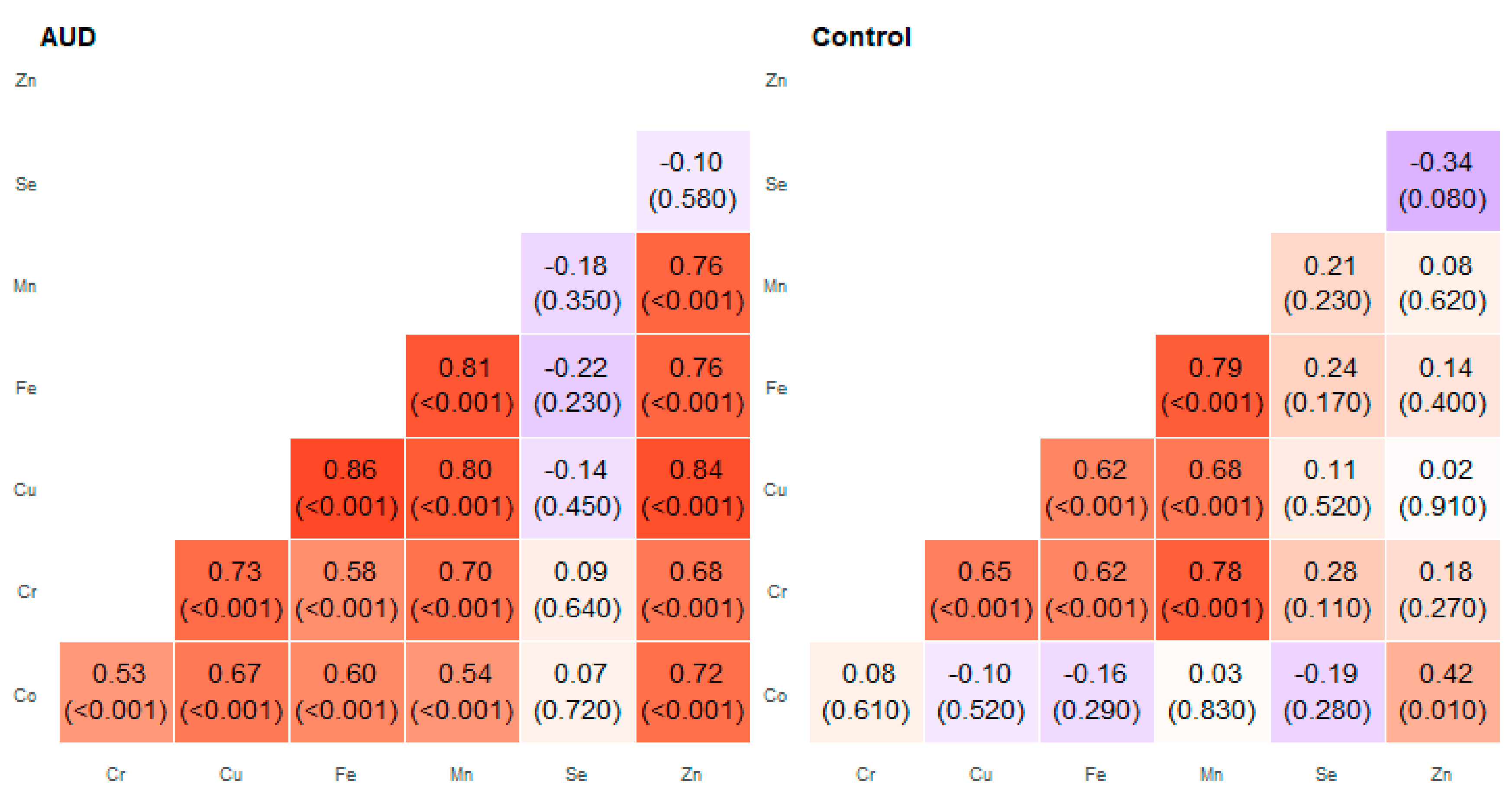
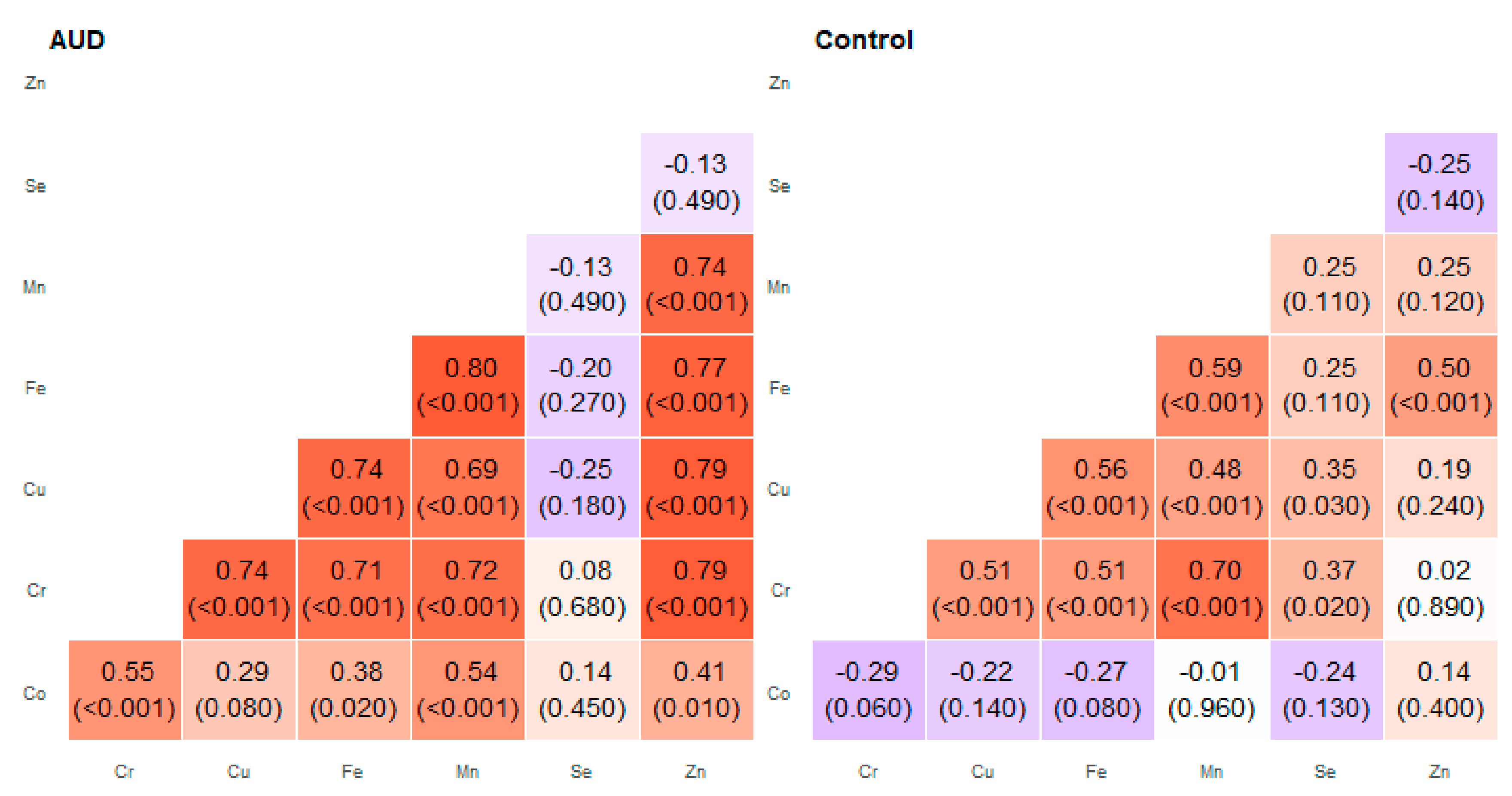
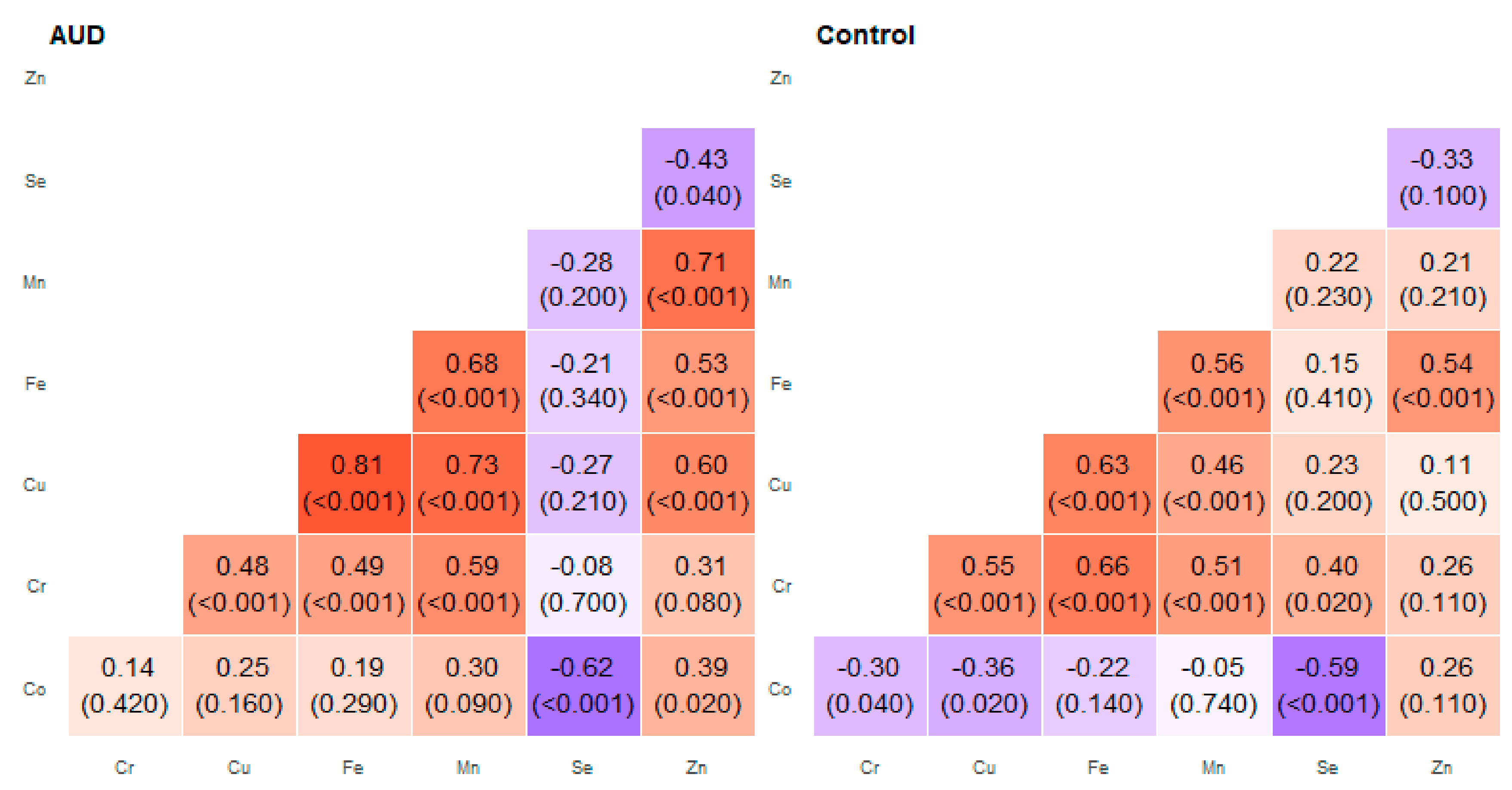
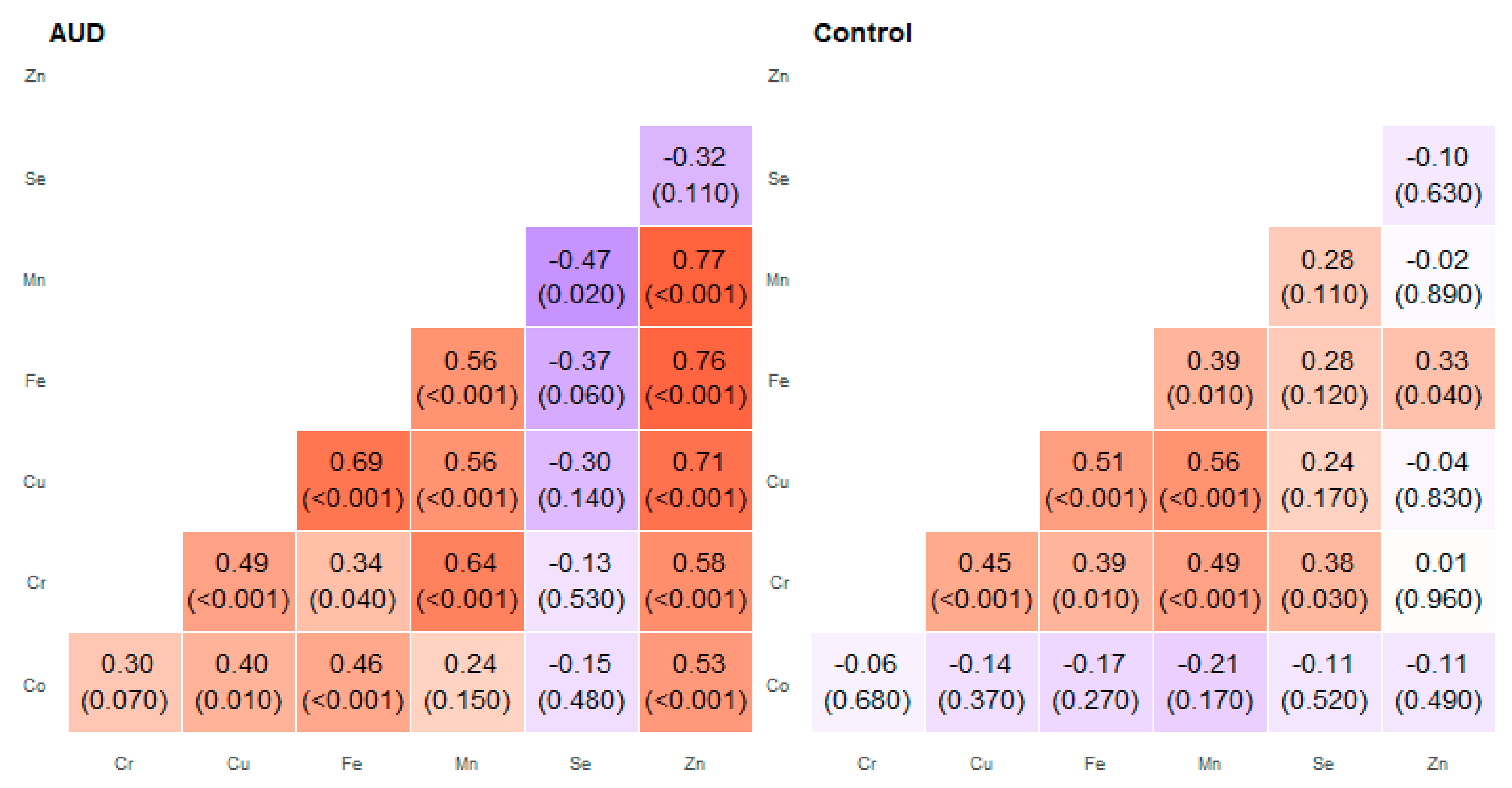
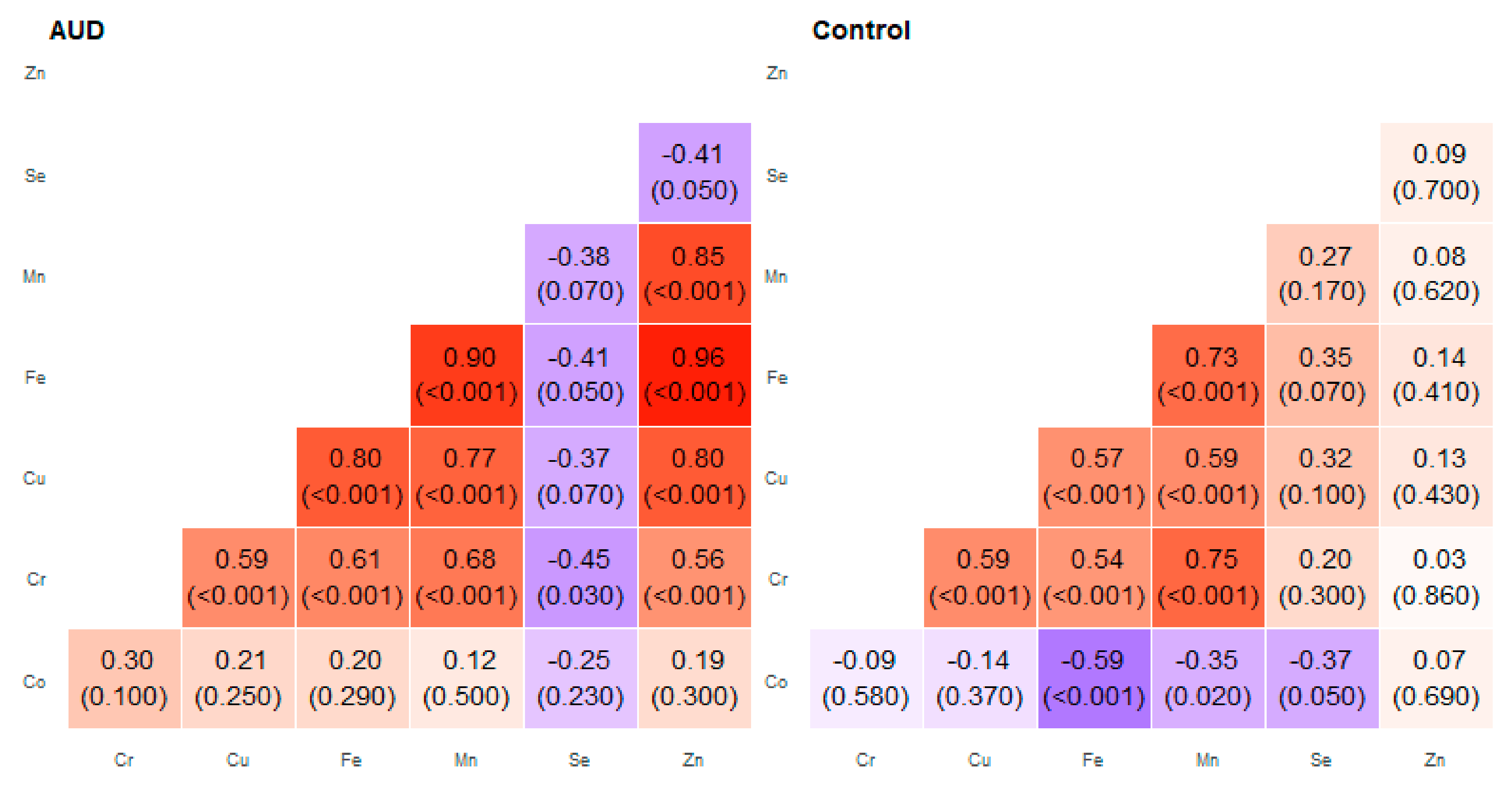
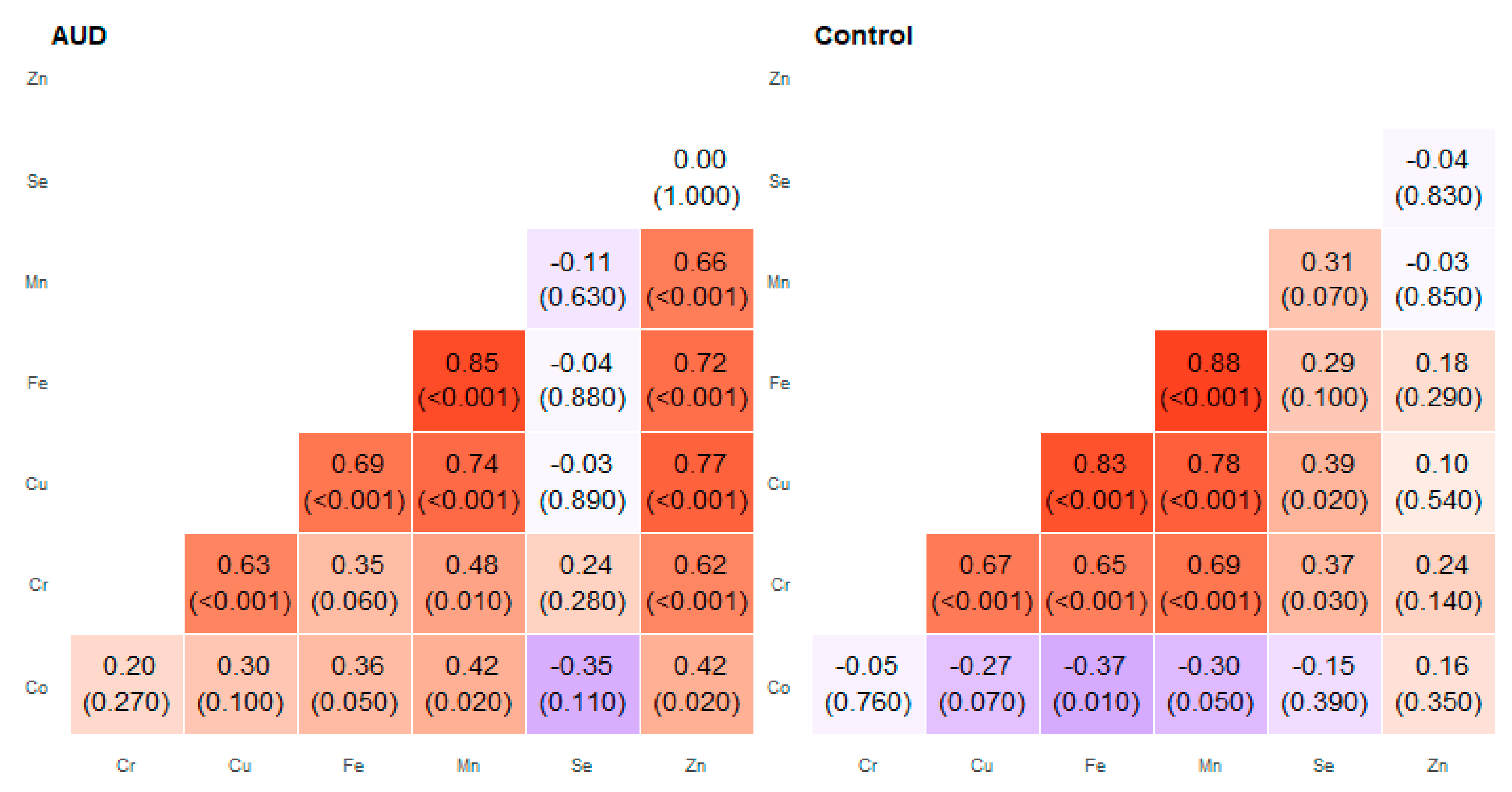
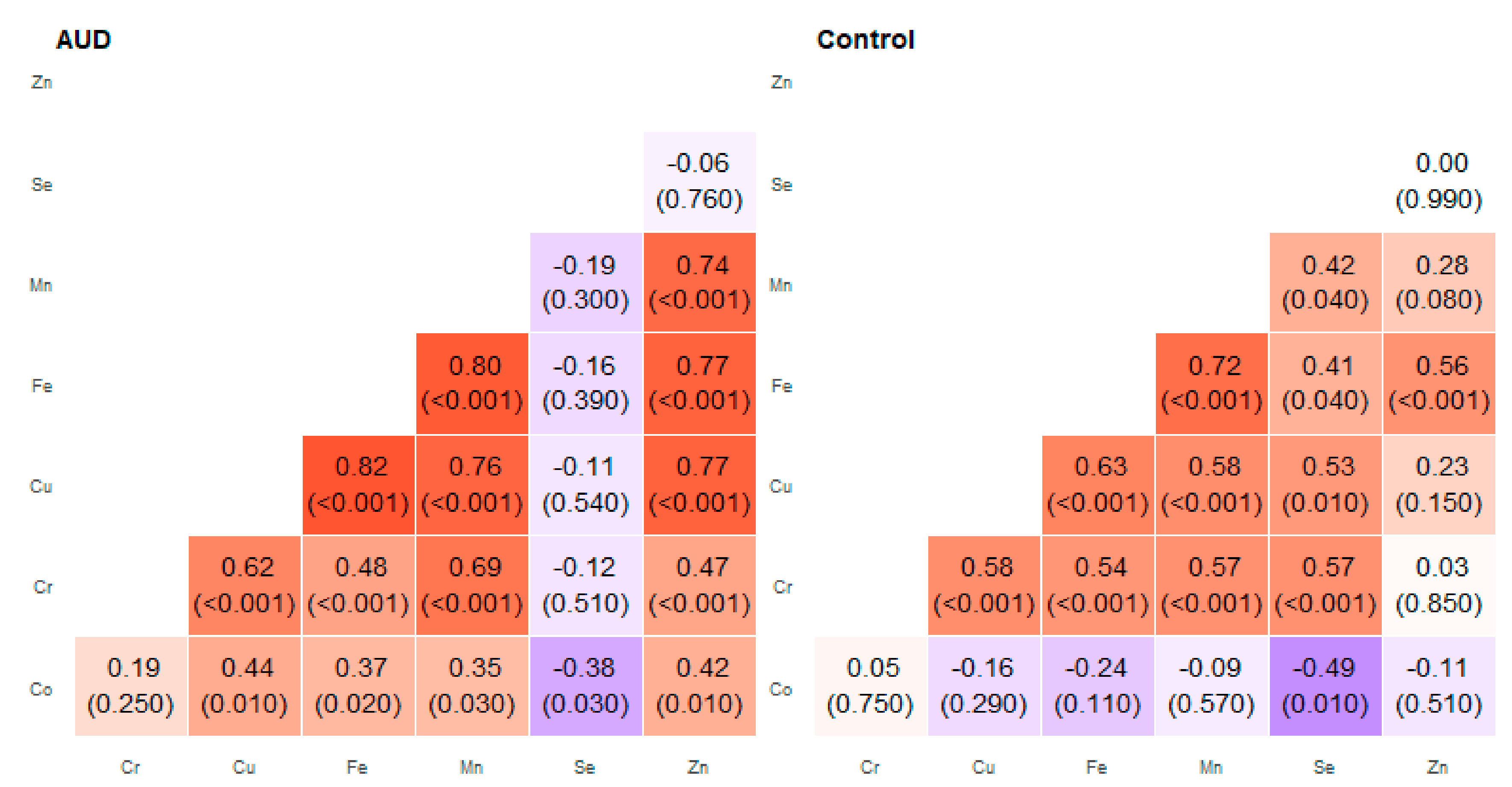
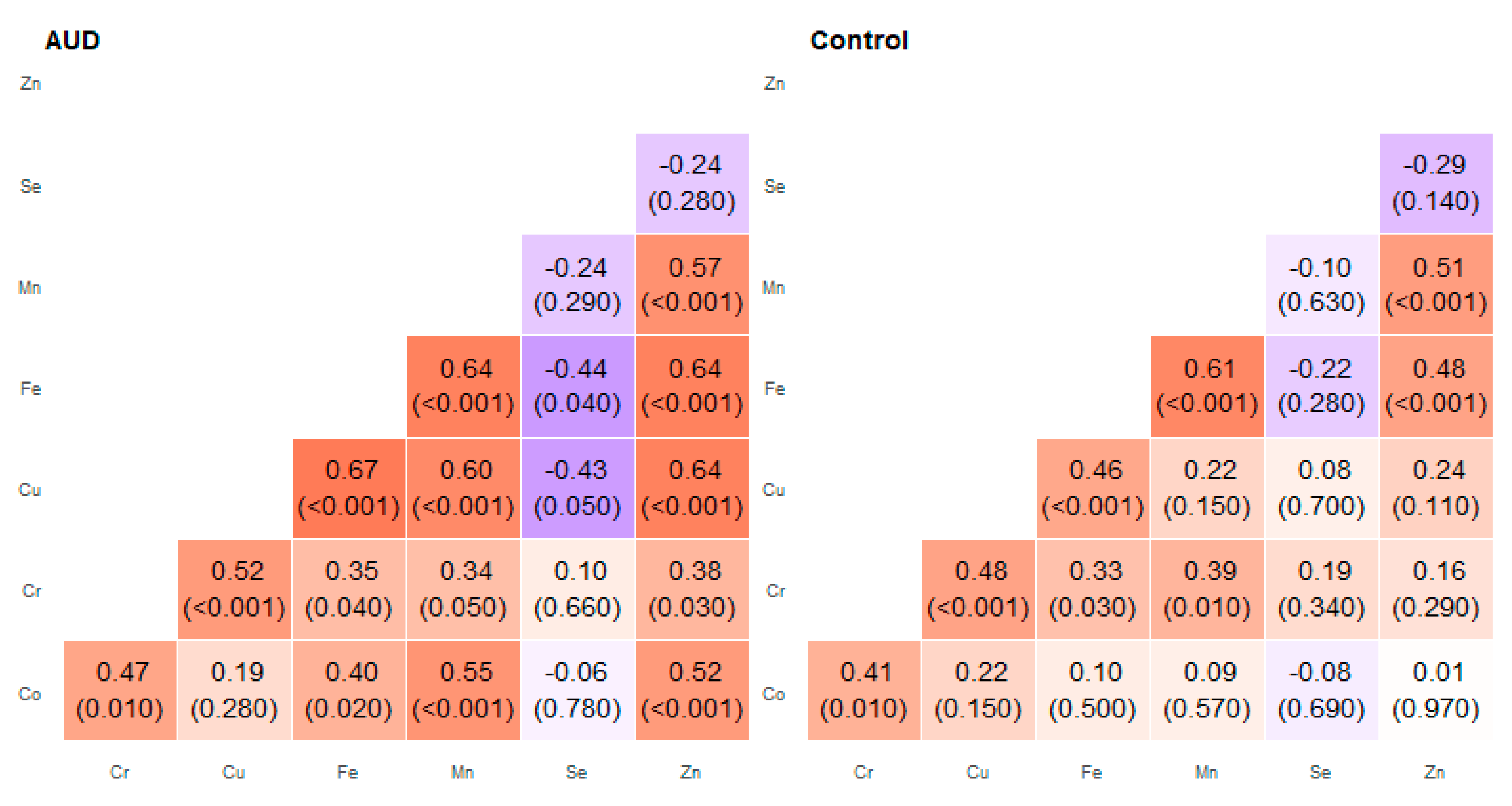

| Pair of Trace Elements | Alcohol Use Disorder Group | Control Group | ||
|---|---|---|---|---|
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | |
| Co–Cr | 0.03 | 0.74 | −0.30 | 0.41 |
| Co–Cu | 0.21 | 0.62 | −0.44 | 0.22 |
| Co–Fe | 0.19 | 0.72 | −0.59 | 0.62 |
| Co–Mn | 0.12 | 0.69 | −0.35 | 0.09 |
| Cr–Cu | 0.42 | 0.74 | 0.45 | 0.67 |
| Cr–Mn | 0.34 | 0.72 | 0.39 | 0.78 |
| Cu–Fe | 0.67 | 0.86 | 0.46 | 0.83 |
| Cu–Mn | 0.56 | 0.8 | 0.22 | 0.78 |
| Cu–Zn | 0.52 | 0.84 | −0.10 | 0.32 |
| Fe–Mn | 0.45 | 0.90 | 0.39 | 0.88 |
| Fe–Zn | 0.26 | 0.97 | 0.10 | 0.56 |
| Mn–Zn | 0.57 | 0.85 | −0.03 | 0.51 |
| Region | Statistic | p-value |
|---|---|---|
| Dorsal anterior cingular cortex | 56.91 | <0.001 |
| The head of the caudate nucleus | 26.58 | <0.001 |
| Frontal part of the thalamus | 41.46 | <0.001 |
| The foot of the hippocampus | 27.39 | <0.001 |
| Inferior longitudinal fasciculus | 40.44 | <0.001 |
| Frontal part of the insula | 37.78 | <0.001 |
| Nucleus accumbens | 38.32 | <0.001 |
| Post central gyrus | 38.04 | <0.001 |
| Frontal cortex | 42.20 | <0.001 |
| Superior longitudinal fasciculus | 29.71 | <0.001 |
| Total (average value) | 65.09 | <0.001 |
| Parameter | Control Group | AUD Group | p |
|---|---|---|---|
| Age (mean ± SD) | 50.3 ± 19.1 | 47.9 ± 13.3 | 0.41 |
| Sex (n, %) | Women: 8 (26%) Men: 23 (74%) | Women: 10 (32%) Men: 21 (68%) | 0.58 |
| Weight (mean ± SD) | 82.0 ± 20.9 | 81.4 ± 25.4 | 0.64 |
| BMI (kg/m2, mean ± SD) | 28.5 ± 7.3 | 26.5 ± 7.4 | 0.65 |
| Wavelength | DL | Range | Uncertainty | |
|---|---|---|---|---|
| nm | mg L−1 | mg L−1 | % | |
| Co | 238,892 | 0.00029 | DL-20 | 1.6 |
| Cr | 267,716 | 0.00033 | DL-20 | 4.0 |
| Cu | 327,395 | 0.00027 | DL-20 | 9.6 |
| Fe | 238,204 | 0.00084 | DL-100 | 1.6 |
| Mn | 257,610 | 0.00021 | DL-20 | 1.8 |
| Se | 196,026 | 0.0011 | DL-10 | 16.4 |
| Zn | 213,857 | 0.00022 | DL-20 | 3.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grochowski, C.; Szukała, M.; Litak, J.; Budny, A.; Proch, J.; Majerek, D.; Blicharska, E.; Niedzielski, P. Correlations Between Trace Elements in Selected Locations of the Human Brain in Individuals with Alcohol Use Disorder. Molecules 2020, 25, 359. https://doi.org/10.3390/molecules25020359
Grochowski C, Szukała M, Litak J, Budny A, Proch J, Majerek D, Blicharska E, Niedzielski P. Correlations Between Trace Elements in Selected Locations of the Human Brain in Individuals with Alcohol Use Disorder. Molecules. 2020; 25(2):359. https://doi.org/10.3390/molecules25020359
Chicago/Turabian StyleGrochowski, Cezary, Magdalena Szukała, Jakub Litak, Agnieszka Budny, Jędrzej Proch, Dariusz Majerek, Eliza Blicharska, and Przemysław Niedzielski. 2020. "Correlations Between Trace Elements in Selected Locations of the Human Brain in Individuals with Alcohol Use Disorder" Molecules 25, no. 2: 359. https://doi.org/10.3390/molecules25020359
APA StyleGrochowski, C., Szukała, M., Litak, J., Budny, A., Proch, J., Majerek, D., Blicharska, E., & Niedzielski, P. (2020). Correlations Between Trace Elements in Selected Locations of the Human Brain in Individuals with Alcohol Use Disorder. Molecules, 25(2), 359. https://doi.org/10.3390/molecules25020359








