Blood and Plasma Volumetric Absorptive Microsampling (VAMS) Coupled to LC-MS/MS for the Forensic Assessment of Cocaine Consumption
Abstract
1. Introduction
2. Results and Discussion
2.1. Sampling and Pretreatment Procedure Development
2.2. Method Validation
2.2.1. Linearity
2.2.2. Process Efficiency, Precision, Matrix Effect and Carry-Over
2.2.3. Stability
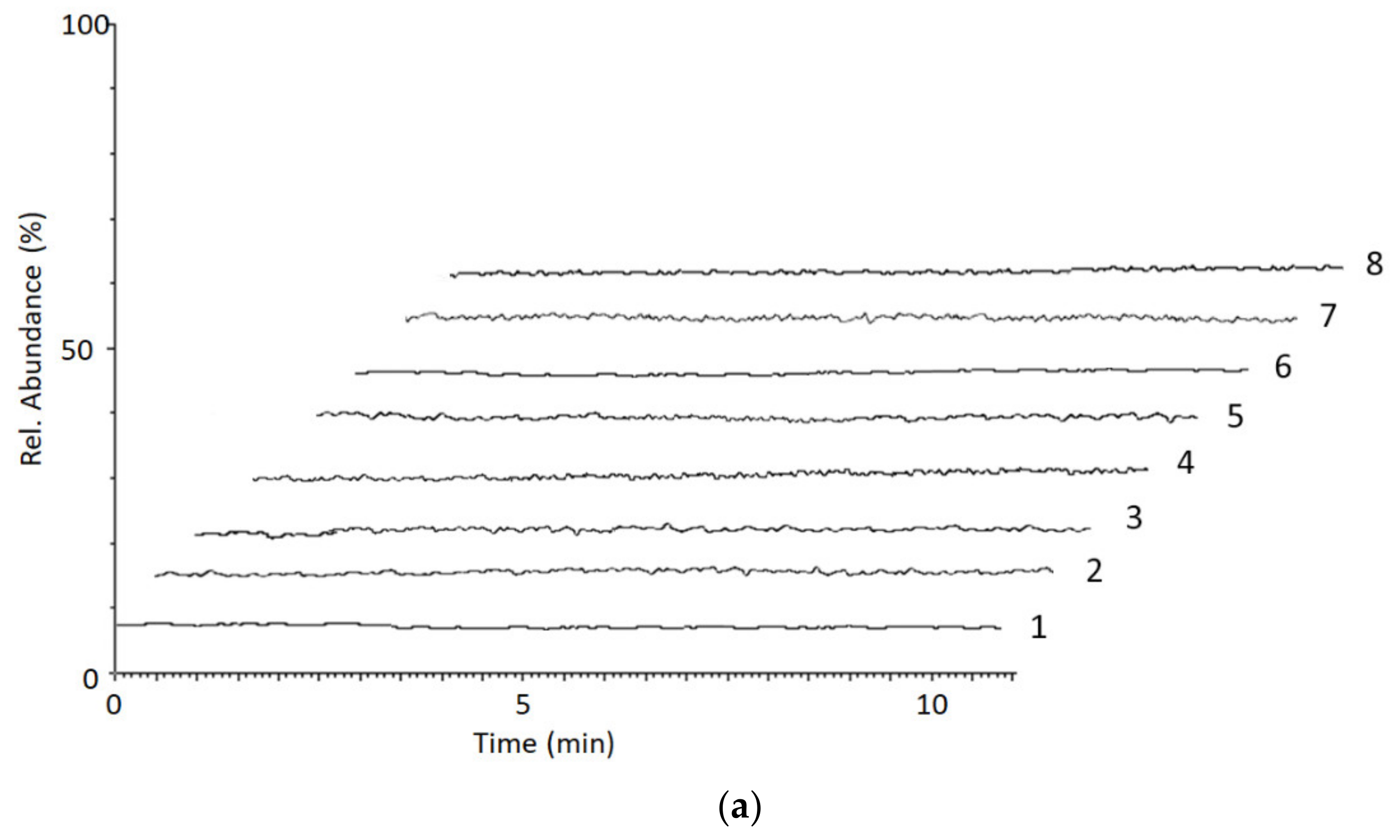
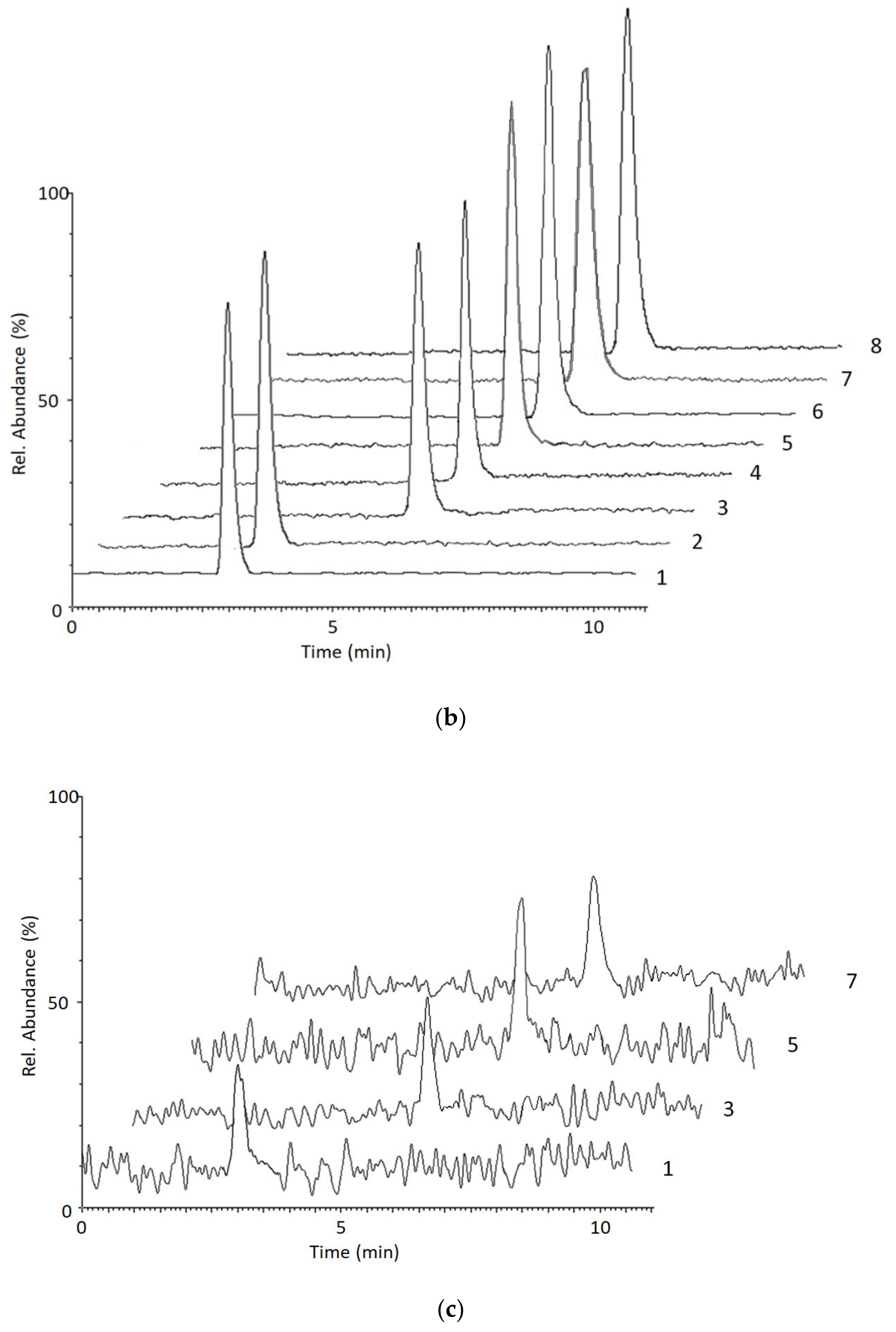
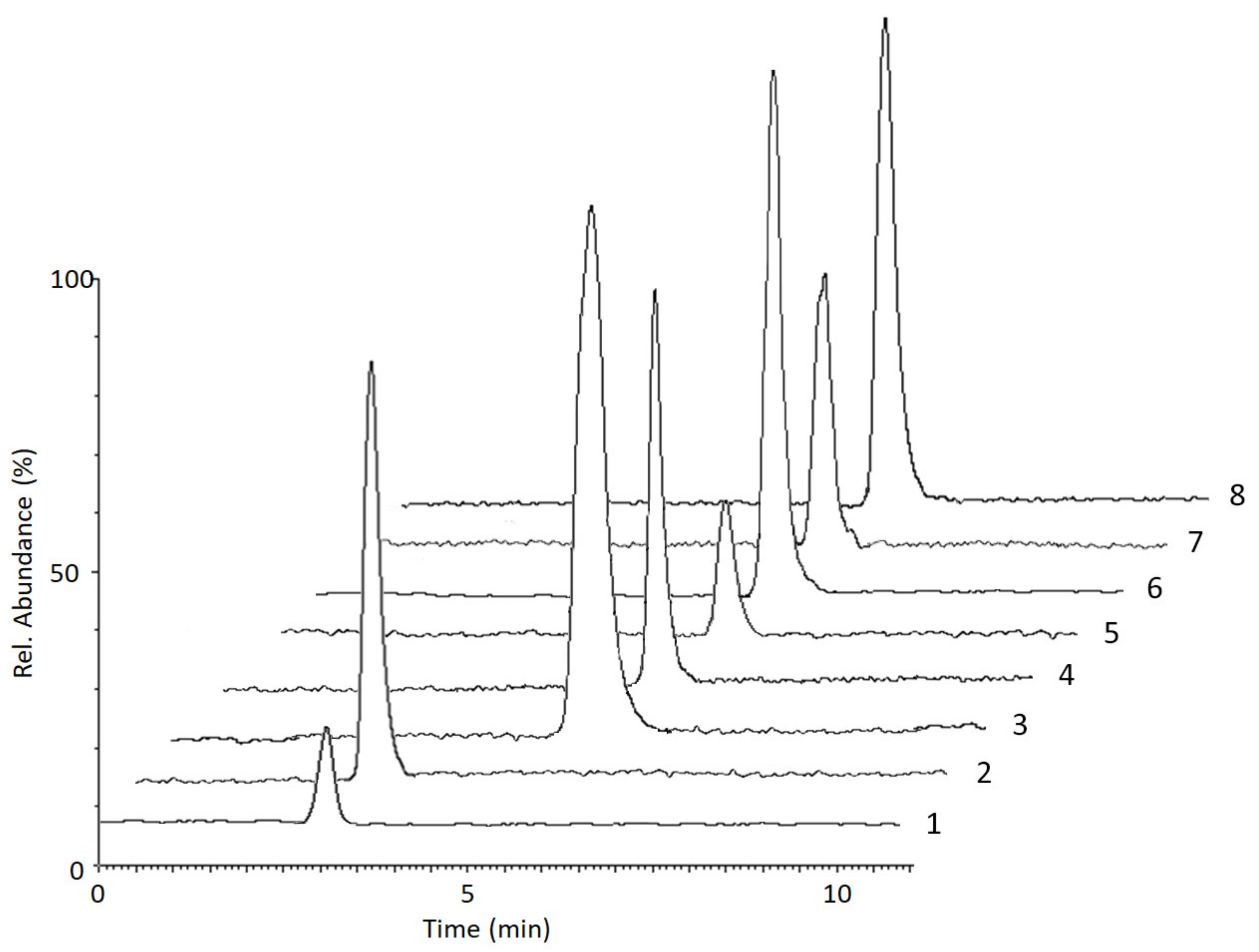
2.2.4. Selectivity
2.3. Analysis of Real Samples and Accuracy
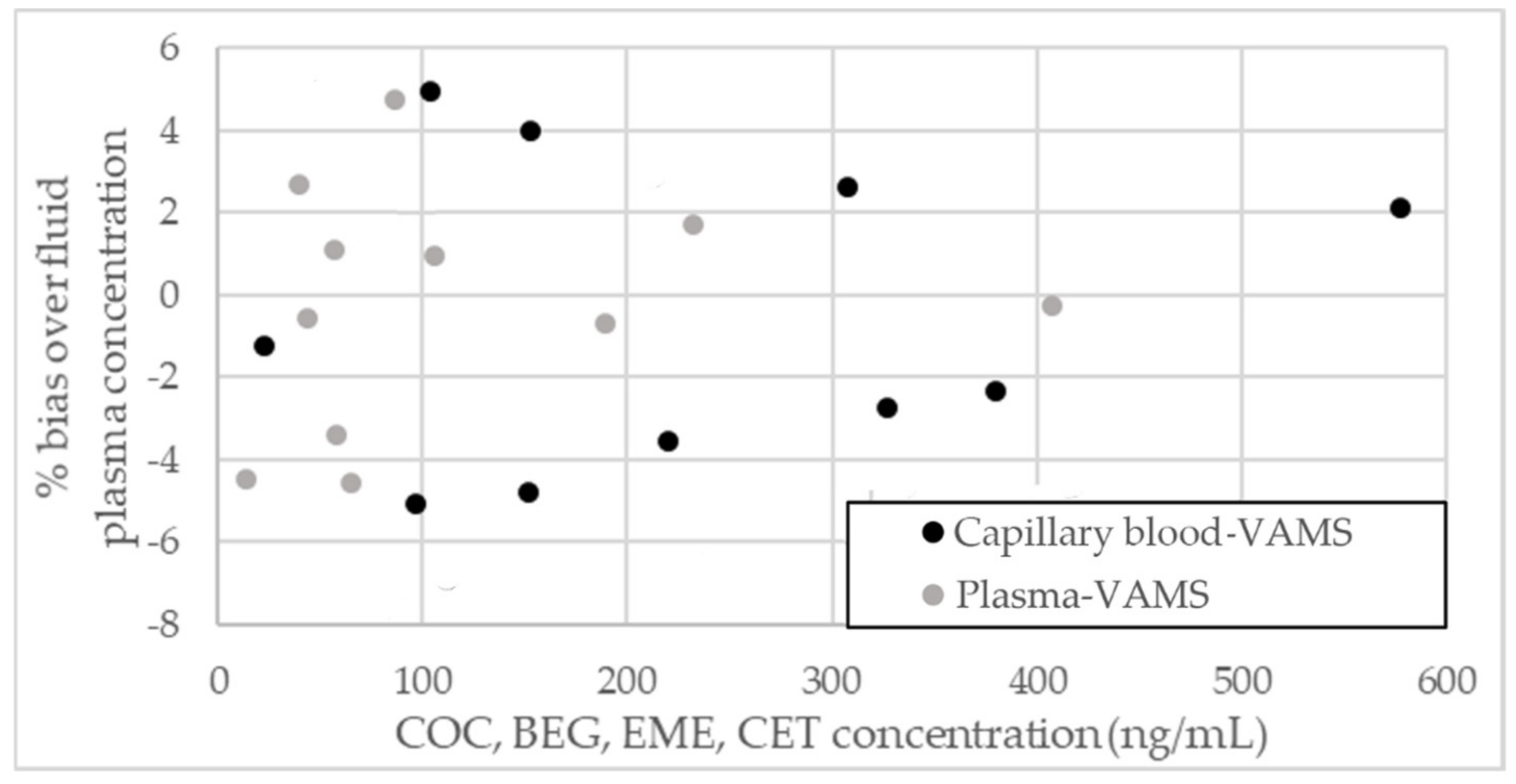
2.4. Comparison to Fluid Blood/Plasma Samples
3. Materials and Methods
3.1. Chemicals and Standard Solutions
3.2. LC-MS/MS Instrumentation and Conditions
3.3. Compliance with Ethical Standards
3.4. Microsampling: VAMS
3.5. Microsample Pretreatment: DPX
3.6. Method Validation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations Office on Drugs and Crime. World Drug Report 2019, Booklet 2; United Nations: Vienna, Austria, 2019; pp. 10–18. [Google Scholar]
- United Nations Office on Drugs and Crime, Statistics and Data—Annual Prevalence of the Use of Drugs by Region and Globally. Available online: https://dataunodc.un.org/drugs/prevalence_regional-2017 (accessed on 2 December 2019).
- Kim, S.T.; Park, T. Acute and chronic effects of cocaine on cardiovascular health. Int. J. Mol. Sci. 2019, 20, 584. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.J.; Rehm, J.; Fischer, B. Health outcomes associated with crack-cocaine use: Systematic review and meta-analyses. Drug Alcohol Depen. 2017, 180, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.; Uggen, C. Dealers, thieves, and the common determinants of drug and nondrug illegal earnings. Criminology 2012, 50, 1057–1087. [Google Scholar] [CrossRef]
- Cornish, J.W.; O’Brien, C.P. Crack cocaine abuse: An epidemic with many public health consequences. Ann. Rev. Pub. Health 1996, 17, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, S.; Mann, R.; Chipman, M.; Pakula, B.; Erickson, P.; Hathaway, A.; Macintyre, P. Driving behavior under the influence of cannabis or cocaine. Traffic Inj. Prev. 2008, 9, 190–194. [Google Scholar] [CrossRef] [PubMed]
- World Anti-Doping Agency. The World Anti-Doping Code International Standard, Prohibited List January 2019; World Anti-Doping Agency: Lausanne, Switzerland, 2018; p. 7. [Google Scholar]
- Meyer, M.R.; Schütz, A.; Maurer, H.H. Contribution of human esterases to the metabolism of selected drugs of abuse. Toxicol. Lett. 2015, 232, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Kagawa, M.; Fukui, Y. In vivo and in vitro studies on cocaine metabolism: Ecgonine methyl ester as a major metabolite of cocaine. Forensic Sci. Int. 1984, 26, 169–180. [Google Scholar] [CrossRef]
- Cone, E.J.; Tsadik, A.; Oyler, J.; Darwin, W.D. Cocaine metabolism and urinary excretion after different routes of administration. Ther. Drug Monit. 1998, 20, 556–560. [Google Scholar] [CrossRef]
- Fiorentin, T.R.; Scherer, J.N.; Marcelo, M.C.A.; Sousa, T.R.V.; Pechansky, F.; Ferrao, M.F.; Limberger, R.P. Comparison of cocaine/crack biomarkers concentrations in oral fluid, urine and plasma simultaneously collected from drug users. J. Anal. Toxicol. 2018, 42, 69–76. [Google Scholar] [CrossRef]
- Protti, M.; Rudge, J.; Sberna, A.E.; Gerra, G.; Mercolini, L. Dried haematic microsamples and LC–MS/MS for the analysis of natural and synthetic cannabinoids. J. Chromatogr. B 2017, 1044–1045, 77–86. [Google Scholar] [CrossRef]
- Protti, M.; Catapano, M.C.; Samolsky Dekel, B.G.; Rudge, J.; Gerra, G.; Somaini, L.; Mandrioli, R.; Mercolini, L. Determination of oxycodone and its major metabolites in haematic and urinary matrices: Comparison of traditional and miniaturised sampling approaches. J. Pharm. Biomed. Anal. 2018, 152, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; Freni, F.; Valentini, B.; Vignali, C.; Groppi, A.; Visonà, S.D.; Osculati, A.M.M.; Morini, L. Determination of antidepressants and antipsychotics in dried blood spots (DBSs) collected from post-mortem samples and evaluation of the stability over a three-month period. Molecules 2019, 24, art. no. 3636. [Google Scholar] [CrossRef]
- Abu-Rabie, P.; Neupane, B.; Spooner, N.; Rudge, J.; Denniff, P.; Mulla, H.; Pandya, H. Validation of methods for determining pediatric midazolam using wet whole blood and volumetric absorptive microsampling. Bioanalysis 2019, 11, 1737–1754. [Google Scholar] [CrossRef] [PubMed]
- Denniff, P.; Parry, S.; Dopson, W.; Spooner, N. Quantitative bioanalysis of paracetamol in rats using volumetric absorptive microsampling (VAMS). J. Pharm. Biomed. Anal. 2015, 108, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Kip, A.E.; Kiers, K.C.; Rosing, H.; Schellens, J.H.M.; Beijnen, J.H.; Dorlo, T.P.C. Volumetric absorptive microsampling (VAMS) as an alternative to conventional dried blood spots in the quantification of miltefosine in dried blood samples. J. Pharm. Biomed. Anal. 2017, 135, 160–166. [Google Scholar] [CrossRef]
- Qu, Y.; Brady, K.; Apilado, R.; O’Malley, T.; Reddy, S.; Chitkara, P.; Ibarra, C.; Alexander, R.V.; Dervieux, T. Capillary blood collected on volumetric absorptive microsampling (VAMS) device for monitoring hydroxychloroquine in rheumatoid arthritis patients. J. Pharm. Biomed. Anal. 2017, 140, 334–341. [Google Scholar] [CrossRef]
- Protti, M.; Mandrioli, R.; Mercolini, L. Perspectives and strategies for anti-doping analysis. Bioanalysis 2019, 11, 149–152. [Google Scholar] [CrossRef]
- Mercolini, L.; Protti, M. Biosampling strategies for emerging drugs of abuse: Towards the future of toxicological and forensic analysis. J. Pharm. Biomed. Anal. 2016, 130, 202–219. [Google Scholar] [CrossRef]
- Xie, I.; Xu, Y.; Anderson, M.; Wang, M.; Xue, L.; Breidinger, S.; Goykhman, D.; Woolf, E.J.; Bateman, K.P. Extractability-mediated stability bias and hematocrit impact: High extraction recovery is critical to feasibility of volumetric adsorptive microsampling (VAMS) in regulated bioanalysis. J. Pharm. Biomed. Anal. 2018, 156, 58–66. [Google Scholar] [CrossRef]
- Protti, M.; Mandrioli, R.; Mercolini, L. Tutorial: Volumetric absorptive microsampling (VAMS). Anal. Chim. Acta 2019, 1046, 32–47. [Google Scholar] [CrossRef]
- Mercolini, L.; Protti, M.; Catapano, M.C.; Rudge, J.; Sberna, A.E. LC-MS/MS and volumetric absorptive microsampling for quantitative bioanalysis of cathinone analogues in dried urine, plasma and oral fluid samples. J. Pharm. Biomed. Anal. 2016, 123, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Barroso, M.; Gallardo, E.; Queiroz, J.A. Bioanalytical methods for the determination of cocaine and metabolites in human biological samples. Bioanalysis 2009, 1, 977–1000. [Google Scholar] [CrossRef] [PubMed]
- Barroso, M.; Gallardo, E. Assessing cocaine abuse using LC-MS/MS measurements in biological specimens. Bioanalysis 2015, 7, 1497–1525. [Google Scholar] [CrossRef] [PubMed]
- Menzies, E.L.; Archer, J.R.H.; Dargan, P.I.; Parkin, M.C.; Yamamoto, T.; Wood, D.M.; Braithwaite, R.A.; Elliott, S.P.; Kicman, A.T. Detection of cocaine and its metabolites in whole blood and plasma following a single dose, controlled administration of intranasal cocaine. Drug Test. Anal. 2019, 11, 1419–1430. [Google Scholar] [CrossRef]
- Dempsey, S.K.; Moeller, F.G.; Poklis, J.L. Rapid Separation and Quantitation of Cocaine and its Metabolites in Human Serum by Differential Mobility Spectrometry-tandem Mass Spectrometry (DMS-MS-MS). J. Anal. Toxicol. 2018, 42, 518–524. [Google Scholar] [CrossRef]
- Fiorentin, T.R.; D’Avila, F.B.; Comiran, E.; Zamboni, A.; Scherer, J.N.; Pechansky, F.; Borges, P.E.M.; Fröehlich, P.E.; Limberger, R.P. Simultaneous determination of cocaine/crack and its metabolites in oral fluid, urine and plasma by liquid chromatography-mass spectrometry and its application in drug users. J. Pharmacol. Toxicol. Methods 2017, 86, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Thibert, V.; Legeay, P.; Chapuis-Hugon, F.; Pichon, V. Molecularly imprinted polymer for the selective extraction of cocaine and its metabolites, benzoylecgonine and ecgonine methyl ester, from biological fluids before LC-MS analysis. J. Chromatogr. B 2014, 949–950, 16–23. [Google Scholar] [CrossRef]
- Sánchez-González, J.; García-Carballal, S.; Cabarcos, P.; Tabernero, M.J.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Determination of cocaine and its metabolites in plasma by porous membrane-protected molecularly imprinted polymer micro-solid-phase extraction and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2016, 1451, 15–22. [Google Scholar] [CrossRef]
- Feliu, C.; Millart, H.; Guillemin, H.; Vautier, D.; Binet, L.; Fouley, A.; Djerada, Z. Validation of a fast UPLC-MS/MS method for quantitative analysis of opioids, cocaine, amphetamines (and their derivatives) in human whole blood. Bioanalysis 2015, 7, 2685–2700. [Google Scholar] [CrossRef]
- De Lima Feltraco Lizot, L.; da Silva, A.C.C.; Bastiani, M.F.; Hahn, R.Z.; Bulcão, R.; Perassolo, M.S.; Antunes, M.V.; Linden, R. Simultaneous determination of cocaine, ecgonine methyl ester, benzoylecgonine, cocaethylene and norcocaine in dried blood spots by ultra-performance liquid chromatography coupled to tandem mass spectrometry. Forensic Sci. Int. 2019, 298, 408–416. [Google Scholar] [CrossRef]
- Moretti, M.; Visonà, S.D.; Freni, F.; Tomaciello, I.; Vignali, C.; Groppi, A.; Tajana, L.; Osculati, A.M.M.; Morini, L. A liquid chromatography–tandem mass spectrometry method for the determination of cocaine and metabolites in blood and in dried blood spots collected from postmortem samples and evaluation of the stability over a 3-month period. Drug Test. Anal. 2018, 10, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Sadler Simões, S.; Castañera Ajenjo, A.; João Dias, M. Dried blood spots combined to an UPLC–MS/MS method for the simultaneous determination of drugs of abuse in forensic toxicology. J. Pharm. Biomed. Anal. 2018, 147, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Alfazil, A.A.; Anderson, R.A. Stability of Benzodiazepines and Cocaine in Blood Spots Stored on Filter Paper. J. Anal. Toxicol. 2008, 32, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Ambach, L.; Menzies, E.; Parkin, M.C.; Kicman, A.; Archer, J.R.H.; Wood, D.M.; Dargan, P.I.; Stove, C. Quantification of cocaine and cocaine metabolites in dried blood spots from a controlled administration study using liquid chromatography-tandem mass spectrometry. Drug Test. Anal. 2019, 11, 709–720. [Google Scholar] [CrossRef]
- D’Urso, A.; Rudge, J.; Patsalos, P.N.; de Grazia, U. Volumetric Absorptive Microsampling: A New Sampling Tool for Therapeutic Drug Monitoring of Antiepileptic Drugs. Ther. Drug Monit. 2019, 41, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Verheijen, R.B.; Thijssen, B.; Atrafi, F.; Schellens, J.H.M.; Rosing, H.; de Vries, N.; Beijnen, J.H.; Mathijssen, R.H.J.; Steeghs, N.; Huitema, A.D.R. Validation and clinical application of an LC-MS/MS method for the quantification of everolimus using volumetric absorptive microsampling. J. Chromatogr. B 2019, 1104, 234–239. [Google Scholar] [CrossRef]
- Moorthy, G.S.; Vedar, C.; DiLiberto, M.A.; Zuppa, A.F. A patient-centric liquid chromatography-tandem mass spectrometry microsampling assay for analysis of cannabinoids in human whole blood: Application to pediatric pharmacokinetic study. J. Chromatogr. B 2019, 1130–1131, 121828. [Google Scholar] [CrossRef]
- Mandrioli, R.; Protti, M.; Mercolini, L. New-generation, non-SSRI antidepressants: Therapeutic Drug Monitoring and pharmacological interactions. Part 1: SNRIs, SMSs, SARIs. Curr. Med. Chem. 2018, 25, 772–792. [Google Scholar] [CrossRef]
- Mandrioli, R.; Protti, M.; Mercolini, L. Evaluation of the pharmacokinetics, safety and clinical efficacy of ziprasidone for the treatment of schizophrenia and bipolar disorder. Exp. Opin. Drug Metab. Toxicol. 2015, 11, 149–174. [Google Scholar] [CrossRef]
- Mandrioli, R.; Mercolini, L. Discontinued anxiolytic drugs (2009–2014). Exp. Opin. Invest. Drugs 2015, 24, 557–573. [Google Scholar] [CrossRef]
- Mandrioli, R.; Protti, M.; Mercolini, L. Novel atypical antipsychotics: Metabolism and Therapeutic Drug Monitoring (TDM). Curr. Drug Metab. 2015, 16, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Duthaler, U.; Suenderhauf, C.; Gaugler, S.; Vetter, B.; Krähenbühl, S.; Hammann, F. Development and validation of an LC-MS/MS method for the analysis of ivermectin in plasma, whole blood, and dried blood spots using a fully automatic extraction system. J. Pharm. Biomed. Anal. 2019, 172, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Mercolini, L.; Mandrioli, R.; Protti, M.; Conca, A.; Albers, L.J.; Raggi, M.A. Dried blood spot testing: A novel approach for the therapeutic drug monitoring of ziprasidone-treated patients. Bioanalysis 2014, 6, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Mercolini, L.; Mandrioli, R.; Gerra, G.; Raggi, M.A. Analysis of cocaine and two metabolites in dried blood spots by liquid chromatography with fluorescence detection: A novel test for cocaine and alcohol intake. J. Chromatogr. B 2010, 1217, 7242–7248. [Google Scholar] [CrossRef]
- Mercolini, L.; Mandrioli, R.; Sorella, V.; Somaini, L.; Giocondi, D.; Serpelloni, G.; Raggi, M.A. Dried blood spots: Liquid chromatography-mass spectrometry analysis of Δ9-tetrahydrocannabinol and its main metabolites. J. Chromatogr. A 2013, 1271, 33–40. [Google Scholar] [CrossRef]
- Protti, M.; Vignali, A.; Sanchez Blanco, T.; Rudge, J.; Bugamelli, F.; Ferranti, A.; Mandrioli, R.; Mercolini, L. Enantioseparation and determination of asenapine in biological fluid micromatrices by HPLC with diode array detection. J. Sep. Sci. 2018, 41, 1257–1265. [Google Scholar] [CrossRef]
- Uchimura, T.; Kato, M.; Saito, T.; Kinoshita, H. Prediction of human blood-to-plasma drug concentration ratio. Biopharm. Drug Dispos. 2010, 31, 286–297. [Google Scholar] [CrossRef]
- Ellefsen, K.N.; da Costa, J.L.; Concheiro, M.; Anizan, S.; Barnes, A.J.; Pirard, S.; Gorelick, D.A.; Huestis, M.A. Cocaine and metabolite concentrations in DBS and venous blood after controlled intravenous cocaine administration. Bioanalysis 2015, 7, 2041–2056. [Google Scholar] [CrossRef]
- European Medicines Agency, Guideline on Bioanalytical Method Validation. Available online: https://www.ema.europa.eu/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 2 December 2019).
- Food and Drug Administration, Bioanalytical Method Validation—Guidance for Industry. Available online: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf (accessed on 2 December 2019).
- World Anti-Doping Agency, International Standard for Laboratories. Available online: https://www.wada-ama.org/sites/default/files/resources/files/isl_nov2019.pdf (accessed on 2 December 2019).
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Validation of Analytical Procedures: Text and Methodology Q2(R1). Available online: https://database.ich.org/sites/default/files/Q2_R1__Guideline.pdf (accessed on 2 December 2019).
Sample Availability: No sample is available from the authors. |
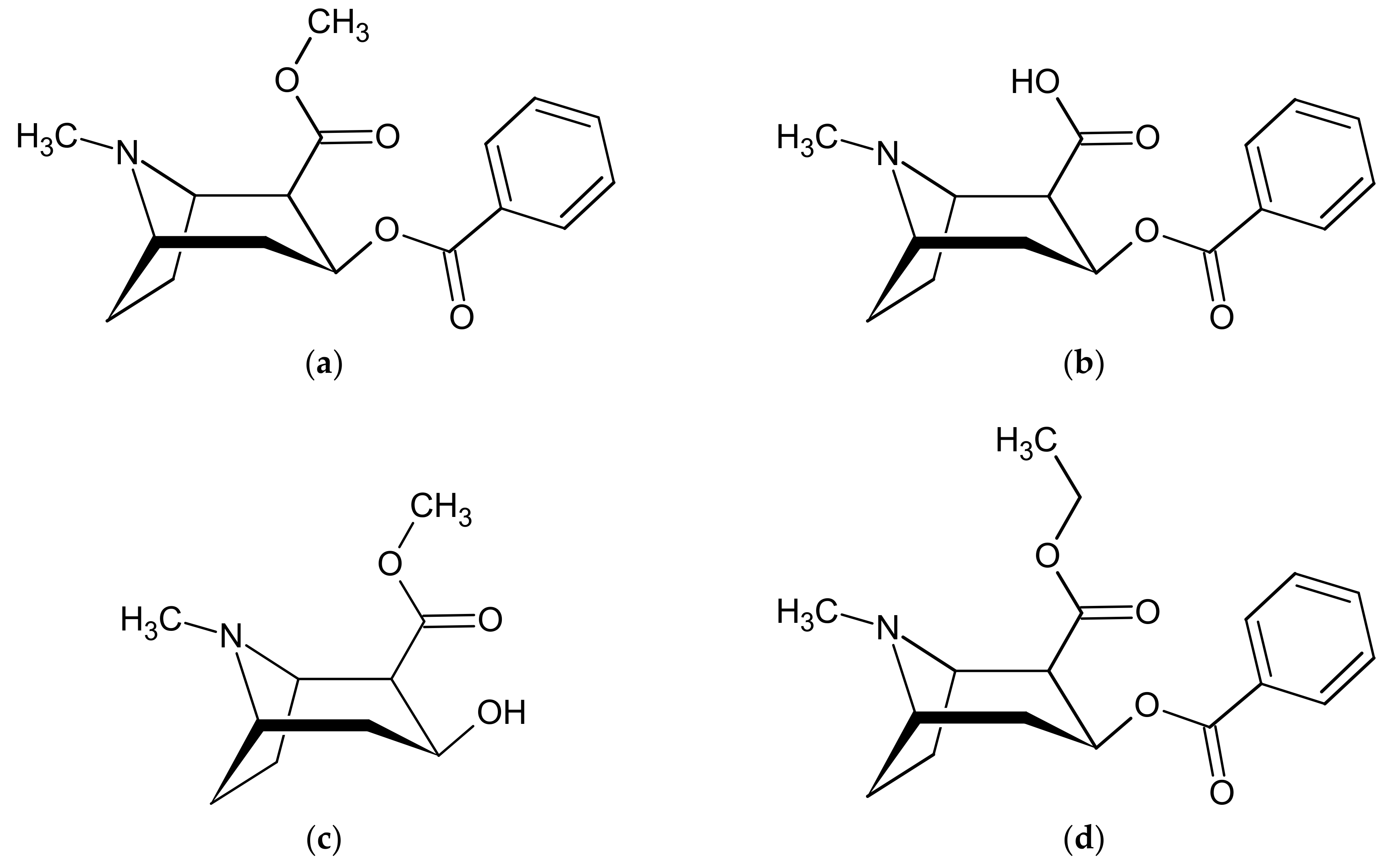
| Tip Contact Time (s) | Sampling Volume (µL) | Volume Accuracy (% of Theoretical Value) | Volume Precision (Relative Standard Deviation, RSD%) |
|---|---|---|---|
| 1 | 17.06 | 85.3 | 3.0 |
| 2 | 18.46 | 92.3 | 2.2 |
| 3 | 19.14 | 95.7 | 1.1 |
| 5 | 20.02 | 100.1 | 1.3 |
| 10 | 19.90 | 99.5 | 0.9 |
| 15 | 19.98 | 99.9 | 0.9 |
| 20 | 20.20 | 101.0 | 1.0 |
| Analyte | Matrix | Linearity Range (ng/mL) | Linearity Equation 1 | r2 | LOQ (ng/mL) | LOD (ng/mL) |
|---|---|---|---|---|---|---|
| COC | Blood VAMS | 2.0–500 | y = 0.446x + 0.003 | 0.9995 | 2.0 | 0.6 |
| Plasma VAMS | 2.0–500 | y = 0.121x + 0.005 | 0.9997 | 2.0 | 0.6 | |
| BEG | Blood VAMS | 1.0–500 | y = 0.861x + 0.003 | 0.9992 | 1.0 | 0.3 |
| Plasma VAMS | 1.0–500 | y = 0.224x + 0.006 | 0.9996 | 1.0 | 0.3 | |
| EME | Blood VAMS | 2.5–500 | y = 0.030x + 0.004 | 0.9993 | 2.5 | 0.8 |
| Plasma VAMS | 2.5–500 | y = 0.083x + 0.008 | 0.9995 | 2.5 | 0.8 | |
| CET | Blood VAMS | 2.0–500 | y = 0.428x + 0.004 | 0.9991 | 2.0 | 0.6 |
| Plasma VAMS | 2.0–500 | y = 0.141x + 0.008 | 0.9994 | 2.0 | 0.6 |
| Analyte | Concentration Level (ng/mL) | Matrix | Precision, RSD% 1 | Process Efficiency ± SD, % | Matrix Effect ± SD, % | |
| Intraday | Interday | |||||
| COC | 2.0 | Blood VAMS | 5.2 | 5.3 | 91 ± 3 | 93 ± 2 |
| Plasma VAMS | 4.9 | 5.0 | 93 ± 2 | 94 ± 3 | ||
| 50 | Blood VAMS | 5.0 | 5.2 | 96 ± 3 | 93 ± 2 | |
| Plasma VAMS | 4.6 | 4.9 | 96 ± 4 | 95 ± 1 | ||
| 500 | Blood VAMS | 4.8 | 5.1 | 95 ± 1 | 95 ± 4 | |
| Plasma VAMS | 4.3 | 4.7 | 98 ± 2 | 97 ± 2 | ||
| BEG | 1.0 | Blood VAMS | 5.4 | 5.4 | 88 ± 3 | 89 ± 1 |
| Plasma VAMS | 5.1 | 5.4 | 90 ± 4 | 91 ± 4 | ||
| 50 | Blood VAMS | 5.2 | 5.3 | 91 ± 2 | 89 ± 3 | |
| Plasma VAMS | 4.9 | 5.0 | 94 ± 1 | 92 ± 4 | ||
| 500 | Blood VAMS | 4.9 | 5.3 | 93 ± 3 | 91 ± 1 | |
| Plasma VAMS | 4.5 | 4.8 | 94 ± 2 | 91 ± 2 | ||
| EME | 2.5 | Blood VAMS | 5.3 | 5.8 | 87 ± 4 | 90 ± 4 |
| Plasma VAMS | 5.3 | 5.6 | 91 ± 3 | 91 ± 2 | ||
| 50 | Blood VAMS | 5.6 | 5.6 | 92 ± 2 | 91 ± 3 | |
| Plasma VAMS | 5.1 | 5.4 | 93 ± 3 | 93 ± 3 | ||
| 500 | Blood VAMS | 5.0 | 5.2 | 92 ± 2 | 92 ± 3 | |
| Plasma VAMS | 4.8 | 5.1 | 95 ± 2 | 93 ± 4 | ||
| CET | 2.0 | Blood VAMS | 5.3 | 5.9 | 86 ± 3 | 89 ± 1 |
| Plasma VAMS | 5.1 | 5.6 | 89 ± 3 | 92 ± 2 | ||
| 50 | Blood VAMS | 4.9 | 5.4 | 89 ± 2 | 91 ± 2 | |
| Plasma VAMS | 4.9 | 5.2 | 94 ± 2 | 93 ± 4 | ||
| 500 | Blood VAMS | 5.0 | 5.4 | 91 ± 3 | 91 ± 1 | |
| Plasma VAMS | 4.6 | 4.9 | 94 ± 2 | 94 ± 3 | ||
| COC-D3 | 50 | Blood VAMS | 4.8 | 4.9 | 95 ± 2 | 94 ± 1 |
| Plasma VAMS | 4.3 | 4.5 | 96 ± 3 | 96 ± 1 | ||
| BEG-D3 | 50 | Blood VAMS | 5.0 | 5.0 | 92 ± 2 | 91 ± 2 |
| Plasma VAMS | 4.6 | 4.7 | 95 ± 2 | 93 ± 2 | ||
| EME-D3 | 50 | Blood VAMS | 5.2 | 5.3 | 93 ± 2 | 94 ± 3 |
| Plasma VAMS | 4.7 | 5.0 | 95 ± 2 | 95 ± 2 | ||
| CET-D3 | 50 | Blood VAMS | 4.5 | 4.7 | 92 ± 3 | 94 ± 2 |
| Plasma VAMS | 4.6 | 4.9 | 95 ± 1 | 96 ± 2 | ||
| Subject | Matrix | Concentration Found ± SD (ng/mL) 1 | |||
|---|---|---|---|---|---|
| COC | BEG | EME | CET | ||
| 1 | Capillary blood VAMS | 216 ± 8 | 584 ± 12 | 156 ± 6 | / |
| 2 | Capillary blood VAMS | 153 ± 4 | 376 ±9 | 94 ± 5 | 28 ± 5 |
| 3 | Capillary blood VAMS | 322 ± 9 | 312 ± 8 | 106 ± 5 | / |
| 4 | Plasma VAMS | 108 ± 4 | 407 ± 10 | 88 ± 4 | 57 ± 6 |
| 5 | Plasma VAMS | 19 ± 3 | 193 ± 4 | 13 ± 2 | 39 ± 5 |
| 6 | Plasma VAMS | 63 ± 3 | 234 ± 8 | 54 ± 3 | / |
| Analyte | Q1 (m/z) | Q3 (m/z)1 | Dwell Time (ms) | Cone Voltage (V) | Collision Energy (eV) |
|---|---|---|---|---|---|
| COC | 304.27 | 82.1 | 200 | 40 | 40 |
| 182.0 | 35 | ||||
| BEG | 290.16 | 82.1 | 200 | 60 | 35 |
| 168.1 | 25 | ||||
| EME | 200.13 | 182.0 | 200 | 35 | 30 |
| 82.1 | 25 | ||||
| CET | 318.24 | 196.1 | 200 | 60 | 35 |
| 150.1 | 30 | ||||
| COC-D3 | 307.26 | 185.1 | 200 | 60 | 30 |
| BEG-D3 | 293.25 | 85.1 | 200 | 50 | 30 |
| EME-D3 | 203.25 | 185.1 | 200 | 60 | 25 |
| CET-D3 | 321.26 | 199.0 | 200 | 60 | 30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandrioli, R.; Mercolini, L.; Protti, M. Blood and Plasma Volumetric Absorptive Microsampling (VAMS) Coupled to LC-MS/MS for the Forensic Assessment of Cocaine Consumption. Molecules 2020, 25, 1046. https://doi.org/10.3390/molecules25051046
Mandrioli R, Mercolini L, Protti M. Blood and Plasma Volumetric Absorptive Microsampling (VAMS) Coupled to LC-MS/MS for the Forensic Assessment of Cocaine Consumption. Molecules. 2020; 25(5):1046. https://doi.org/10.3390/molecules25051046
Chicago/Turabian StyleMandrioli, Roberto, Laura Mercolini, and Michele Protti. 2020. "Blood and Plasma Volumetric Absorptive Microsampling (VAMS) Coupled to LC-MS/MS for the Forensic Assessment of Cocaine Consumption" Molecules 25, no. 5: 1046. https://doi.org/10.3390/molecules25051046
APA StyleMandrioli, R., Mercolini, L., & Protti, M. (2020). Blood and Plasma Volumetric Absorptive Microsampling (VAMS) Coupled to LC-MS/MS for the Forensic Assessment of Cocaine Consumption. Molecules, 25(5), 1046. https://doi.org/10.3390/molecules25051046








