Abstract
An increasing interest in the synthesis and use of optically active pyridine N-oxides as chiral controllers for asymmetric reactions has been observed in the last few years. Chiral heteroaromatic N-oxides can work as powerful electron-pair donors, providing suitable electronic environments in the transition state formed within the reaction. The nucleophilicity of the oxygen atom in N-oxides, coupled with a high affinity of silicon to oxygen, represent ideal properties for the development of synthetic methodology based on nucleophilic activation of organosilicon reagents. The application of chiral N-oxides as efficient organocatalysts in allylation, propargylation, allenylation, and ring-opening of meso-epoxides, as well as chiral ligands for metal complexes catalyzing Michael addition or nitroaldol reaction, can also be found in the literature. This review deals with stereoselective applications of N-oxides, and how the differentiating properties are correlated with their structure. It contains more recent results, covering approximately the last ten years. All the reported examples have been divided into five classes, according to the chirality elements present in their basic molecular frameworks.
1. Introduction
Enantioselective organocatalysis is one of the more rapidly growing fields of research in modern organic chemistry. It provides the ability to effectively replace the enantioselective metal-containing catalysts with wholly organic molecules, while maintaining a high level of chemical efficiency and stereo control [1,2,3]. A key for success is the structural simplicity of the organocatalyst, which should be much more readily available than their organometallic equivalents. Among various Lewis base catalysts, those having the pyridine oxide moiety situated within a chiral environment, constitute a distinct class of highly active catalysts that are capable of high asymmetric induction, usually under mild reaction conditions. Since Nakajima’s report in 1998 [4] showing that axially chiral 2,2′- bipyridine N,N’-dioxides are effective as catalysts for the asymmetric allylation, the application of N-oxides has attracted considerable attention. This report outlines the most important and significant developments in catalytic uses of pyridine and bipyridine based N-oxides and their possible other applications, reported through the past decade. An observed increasing interest in chiral N-oxides can be explained by their advantages over metal-based catalysts, including their cost-effectiveness, low environmental harmfulness, and stability in the air [5,6,7]. In general, most of the heteroaromatic N-oxides have been obtained by oxidation. A simple and efficient procedure for the methyltrioxorhenium-mediated oxidation of pyridines has been developed by Sharpless [8] but it has several limitations. Procedures using m-chloroperoxybenzoic acid (m-CPBA) in mild conditions seem to be more common [9]. Alike preparation of various N-oxide derivatives makes it difficult to group them according to the structural features. It is easier to divide them by type of chirality they represent, defined as axial, central, helical and planar chirality. All these classes will be shown and briefly described in this work, along with the examples of catalytic applications in various enantioselective processes.
2. Chiral Heteroaromatic N-Oxides as Organocatalysts
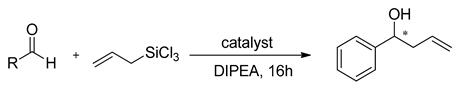
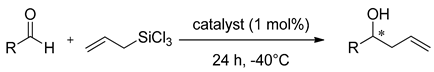
Properties of heteroaromatic N-oxides can be classified as strong Lewis bases because of N-O bond polarization [10]. They are able to activate the C-Si bond in halosilane compounds (Lewis acids), which makes them perfect mediators for allylation and crotylation of aldehydes with allyltrichlorosilanes, which are called Sakurai–Hosomi–Denmark-type reactions (see Scheme 1). This explains why allylation is the most popular testing reaction and is very well examined. It has also become a standard testing ground for new chiral Lewis basic organocatalysts [5,6,7,11]. The good selectivity is obtained when the catalyst allows the reaction to proceed via a closed cyclic chair-like transition state involving hypervalent silicates, as shown in Scheme 1. Additionally, the resulting homoallylic alcohols are considered the cornerstones of organic synthesis. Mostly, they are used in the synthesis of numerous natural products as building blocks but also in the synthesis of drug candidates and other functional molecules [11,12,13,14,15,16,17]. Apart from allylation there were also reported examples of using N-oxides in the catalytic ring-opening of meso-epoxides [18,19,20,21], propargylation [22], allenylation [23], aldol reaction [24,25] and reduction of ketoimines [26,27].
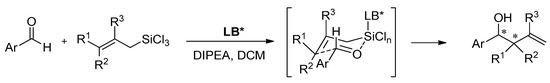
Scheme 1.
Lewis base (LB) catalyzed allylation of aldehydes with substituted allyltrichlorosilanes.
2.1. Axially Chiral N-Oxides and N,N′-Dioxides
The first mention of N-oxides and their catalytic asymmetric applications were related to those, containing axial chirality and were developed by Nakajima et al. [4]. Initially, compounds were based on 4,4’-biquinoline and 2,2’-bipyridine N,N′-dioxide backbones (1 and 2, see Figure 1) which, apart from Nakajima’s work, were also investigated by Feng and coworkers [28]. A little later, Kotora et al. synthesized N-oxides with tetrahydroisoquinoline [29] and bis(tetrahydroisoquinoline) frameworks [30,31]. It is worth to highlight that organocatalysts 1 and 2 give, so far, one of the highest results (both in terms of yield and enantioselectivity) in the allylation of benzaldehyde with allyltrichlorosilane (85%, 88% ee (R) and 95%, 84% ee (S) respectively). Chang et al. received an analog of 1, containing ethyl instead of methyl groups (3, see Figure 1) and describe their application as organocatalyst (10 mol%) in allylation of 4- metoxybenzaldehyde with allyltrichlorosilane. The chiral product was obtained with high enantiomeric purity (92% ee) with satisfactory yield 66% [32], which slightly exceeds the result obtained with the application of 1.
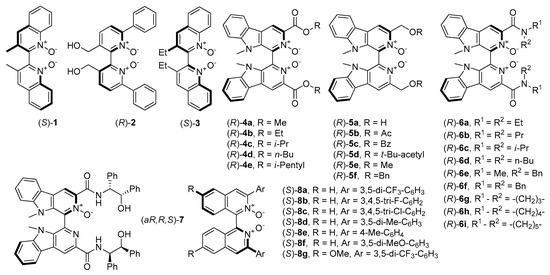
Figure 1.
N-oxides possessing an axial element of chirality ((aR,R,S)-7, which possess also central chirality is placed here due to the similarity of the structure).
Reports concerning chiral biscarboline dioxide derivatives 4 and 5 were developed by Zhu et al. [33,34]. Executed screening of these compounds as benzaldehyde allylation catalysts showed, that the reaction yield for all types of catalysts was quantitative. In the case of use 4 also enantioselectivity was high (95%) while in the case of 5, results were moderate (up to 82% ee). This implies that ester groups are involved in catalyzing this reaction. Catalysts 4a and 4c were chosen to test them in allylation of substituted benzaldehydes and aliphatic aldehydes. Independently from a substrate, using 4a or 4c (1 mol%) gave high enantioselectivity (91–97% ee or 53–90% ee respectively) and moderate to high yields (up to 90%). That makes these catalysts very versatile and applicable to different types of substrates, both these with electron-withdrawing and electron-donating groups. Furnished homoallylic alcohols (except for these ones from 2,6-dichlorobenzaldehyde and 3-phenylpropanal) had configuration S, so opposite to configuration of used catalysts (see Figure 1).
Results of allylation using 4a and 4c are shown in Table 1. Catalysts 4a and 4c were also tested in allylation of benzaldehyde with different, substituted allyltrichlorosilanes, including crotylation (R1 = Me, R2 = R3 = H, Scheme 1). Generally, high ee values were achieved (up to 96%) but low to moderate yields (19–88%). The ratio of syn/anti products was the same as the ratio of Z/E isomers in the used substrate. Except for crotylation, in all other reactions slightly higher yields were obtained using catalyst 4c but the ee values, obtained for both catalysts were similar. For catalyst 4c, the impact of solvent was also tested. Enantioselectivity was satisfactory for all used solvents (85–95% ee) but the complete conversion was obtained only with solvents such as CH2Cl2 and CH3CN. For THF, toluene, EtOAc and Et2O conversion did not exceed 10%. It is also worth to mention that the syntheses of 4 and 5, although multistep, were performed using simple transformations. Overall yield was about 40–50% for 4 and about 80% for 5.

Table 1.
Asymmetric allylation of aldehydes with allyltrichlorosilane catalyzed by different N-oxides.
Zhu and coworkers have synthesized the chiral biscarboline N,N’-dioxide derivatives possessing secondary amide groups 6 (see Figure 1) [26,35]. Checking the effectiveness of 6a–b and 6d–h as catalysts in allylation of benzaldehyde with trichlorosilane (CH2Cl2, −80° C, 20 h), the authors focused on optimization the catalyst loading and, choosing the most effective catalyst, on examination of its application range [35]. The best results were observed for catalysts 6g (84% ee) and 6h (87% ee), which contained 4- and 5-membered cyclic amides, respectively. What is interesting, the highest enantioselectivity for 6h has been obtained using 1 mol% of the catalyst. With other amounts: 0.1, 0.5, 5 or 10 mol%, the enantioselectivity was lower. The 6h was applied in a series of reactions with different substrates. Generally, the resulted yield and enantioselectivity were good to excellent (12 examples, 85–97%, 67–96% ee). The influence of the position of substitution in benzaldehyde on the course of the reaction was tested. Mostly, m- and p-substituted benzaldehydes were compared. For electron-withdrawing groups, higher enantioselectivity was observed when the substituent was in position 3 (Table 1, entries 7, 10). The different situations took place for the electron-donating methoxy group. In this case, also o-substituted benzaldehyde was applied and, in all cases the yield was good (86–90%) but enantioselectivity increased in order: orto-, meta-, para-methoxybenzaldehyde (67, 83, 94% ee respectively, Table 1, entries 2, 6, 11). All obtained homoallylic alcohols had the configuration R, the same as the used catalyst. A comparison of the results of 6h application with the described results of the use of structurally similar catalysts 4 and also with bisquinoline derivatives 8 are presented in Table 1. The catalyst 6h was not very effective for aliphatic aldehydes (entry 32, Table 1). It was found that dichloromethane (DCM) was the suitable solvent, and as it was expected, the enantiomeric excess was strongly dependent on the reaction temperature.
Axially-chiral symmetrically substituted 2,2′-biquinoline N,N’-dioxide derivatives were developed by Takenaka and Peverati. Simple transformations allowed to receive the new Lewis base catalysts 8 (see Figure 1) with yields up to 99% [36]. Their catalytic utility was tested in allylation of 4-metoxybenzaldehyde with allyltrichlorosilane. Loading of 0.1 mol% was sufficient to get 83–96% conversion with enantioselectivity up to 96%. The best catalysts 8a, 8b, and 8d were also used in the allylation of cinnamaldehyde. In this case, the most efficient was 8a (having two bis 3,5-trifluoromethylphenyl substituents), which gave respective homoallylic alcohol with 84% yield and 92% ee. The obtained results were excellent, even at loading lowered to 0.05 mol%, for substituted aromatic, heteroaromatic and aliphatic aldehydes, but only if substrates were electron-rich aldehydes. The authors noticed that 8a is less reactive and less selective for the halogen-substituted aldehydes. Considering the fact, that electron-rich Lewis base catalysts are usually more reactive in reaction with halosilane compounds, they decided to prepare compound 8g, having two phenyl rings double substituted with CF3 groups, suitable for aldehydes with halogen groups [36]. Application of 8g increased the yield, unfortunately with unchanged or only slightly increased enantioselectivity. The catalytic efficiency of 8a and 8g, compared with previously mentioned axial-chiral catalysts is listed in Table 1.
Good activity and selectivity in the reductive aldol reaction of chalcone and benzaldehyde with trichlorosilane (Scheme 2) were obtained by employing bisquinoline N,N’-dioxide (R)-1 as a catalyst. It resulted in up to 80% ee of the product syn [25].
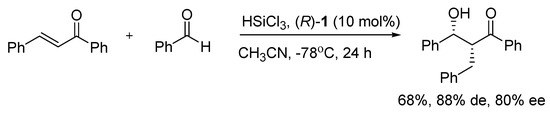
Scheme 2.
Reductive aldol reaction catalyzed with (R)-1.
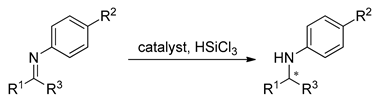
Zhu et al. have examined the catalysts 6b–e and 6h–i in the enantioselective hydrosilylation of ketoimines (Table 2) [26]. All tested N,N’-dioxides were effective; after 16 h (with 10 mol% of the catalyst) all the reactions were completed, but the enantioselectivity was moderate (6 examples, 42–77% ee). After optimization of the reaction conditions (1 mol% of catalyst in CH2Cl2 at 0 °C), catalyst 6i, as the most effective, was used in the reduction of different ketoimines (11 examples). Obtained yield, in all cases, was very high (95–99%) however the enantioselectivity was up to 85% ee. Higher enantioselectivity was observed when R1 was an aromatic ring, furnished with an electron-donating group (reaction above Table 2). If phenyl in R1 had electron-withdrawing substituents, the asymmetric induction was lower. Except for three examples (Table 2, entries 7, 11, 13), obtained products had configuration S, opposite to used catalyst.

Table 2.
Enantioselective hydrosilylation of various ketoimines catalyzed by (R)-6i and 7.
The next approach presented by Zhu’s research group was the mono N-oxide 7, with amide possessing additional stereogenic center [27], in order to improve the previously studied hydrosilylation of ketoimines. In fact, obtained results were excellent in many cases (14 examples, 94–98%, 75–96% ee), which makes the catalyst versatile and slightly more effective than 6i. A rough comparison of the catalytic efficiency of 6i and 7 are given in Table 2. Application of 7 gave comparable yields and better enantioselectivity but requires higher catalyst loading.
2.2. N-Oxides Possessing Central Chirality
Much more attention through the past decade has been given to compounds with central chirality. The report of Mlostoń and Jurczak from 2009 deserves to be mentioned [38]. Authors presented novel chiral C2-symmetric bisimidazole-N-oxides 9 (see Figure 2), derived from trans-1,2-diaminocyclohexane, thereby breaking the tendency of catalysts based on pyridine or bipyridine N-oxides. Screening of catalysts in the reaction of benzaldehyde with allyltrichlorosilane showed that the presence of phenyl group in the imidazole ring (R2 in 9b) has a positive effect on the reaction efficiency (3 examples, 84–86%). Unfortunately, the introduction of two phenyl substituents (9d), only slightly increased enantioselectivity (from 43% ee to 53% ee). However, the presence of the second phenyl substituent in 9d caused the inversion of the absolute configuration of obtained homoallyl alcohol. To improve the catalytic efficiency, different options of catalyst loading for (R,R)-9d, as well as reaction temperature was checked. The best result (90%, 64% ee) has been received for a reaction carried out at 0 °C with 10 mol% of (R,R)-9d. Also, estimation for a scope of aldehyde substrates was done. Higher enantioselectivity for m-substituted, than for o-substituted benzaldehydes was noticed. The highest asymmetric induction was observed for heteroaromatic aldehydes—furfural (76% ee) and thiophene-2-carboxyaldehyde (80% ee).
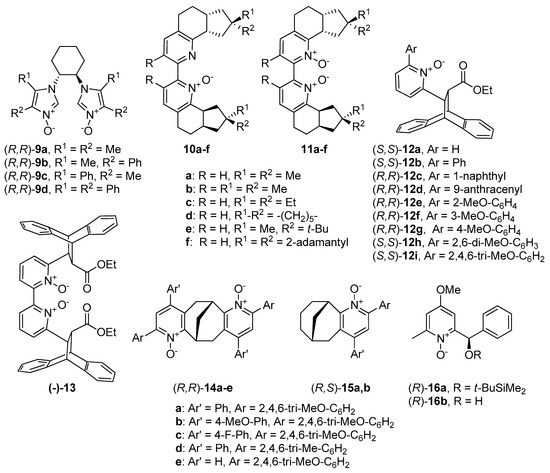
Figure 2.
N-oxides possessing a central element of chirality.
Boyd presented the synthetic pathway to obtain bipyridine N-oxide derivatives 10 and corresponding N,N’-dioxides 11 [39], showed in Figure 2. It was found that the allylation reaction is slower when using the mono N-oxides, and thus, the reactions applying them were carried out at higher temperatures (0 °C or −40 °C, 24 h), compared with those using the corresponding N,N’- dioxides (−78 °C, 12 h). In that case, although the mono- and dioxides were applied to allylation of the same aldehydes with allyltrichlorosilane, it is difficult to compare the results unambiguously. The optimal enantioselectivity was observed in the allylation of 4-methoxybenzaldehyde as a substrate, using either N-oxides 10 (56–86% ee) or corresponding N,N’-dioxides 11 (59–80% ee), compared to allylation of benzaldehyde (24–35% ee and 14–26% ee). The highest enantioselectivity (86% ee) was observed in allylation of 4-methoxybenzaldehyde using 10b as a catalyst. Despite the clear difference in the enantioselectivity of these reactions, their yields did not differ much and were mostly in the range of 30–40% (except 10a–60–72%). Better yields were observed when using 11 as the catalyst, and the compound 11a gave the highest induction (80% ee).
Conformationally rigid chiral backbone, with strong steric requirements, possessing an N-oxide unit was developed in 2012 by Ramanathan et al. [37]. Cycloaddition of anthracene and (E)-ethyl 3-(2-pyridyl)-propenoate, followed by the resolution of the enantiomers using l-(+)-tartaric acid, and then oxidation with m-chloroperoxybenzoic acid (m-CPBA) gave nine derivatives with usually good overall yields (up to 96%). All the chiral pyridine N-oxides 12 were evaluated in enantioselective allylation of 4-methoxybenzaldehyde (20 mol% of catalyst at −40 °C).
It was stated that an electron-rich catalyst causes greater enantioselectivity of the reaction using electron-rich aldehydes. The presence of alkyl or aryl groups at positions 2 and/or 6 of the pyridine ring reduces the nucleophilicity of the corresponding N-oxide. Therefore, the methoxy-substituted benzene rings (electron-rich aryl groups) have been attached at position 6 of the pyridine ring to afford the catalyst which can enhance both reactivity and selectivity. After optimizing the conditions, the reactions were carried out in a solvent mixture (CHCl3 and 1,1,2,2-tetrachloroethane in a 1:1 ratio—which enhances the yield and enantioselectivity), and the temperature was lowered from −40 °C to −78 °C. 12i was determined as the most effective catalyst, which gave 84% yield and 87% ee in the case of 4-methoxybenzaldehyde and 81%, 94% ee for 3,4-dimethoxybenzaldehyde (see Table 1). In comparison to previously described catalysts 4a, c and 8a, g, for 12i higher loading (20 mol%) was needed (results compared in Table 1). The advantage of 12i can be, that it can be used for unusual substrates. High enantioselectivity has been achieved in the allylation of heterocyclic and polycyclic aldehydes with 12i (Table 1, entries 19, 21, 25). For others, results were rather moderate. Catalyst 12i was also examined in the crotylation of 4-metoxybenaldehyde with crotyltrichlorosilane (E/Z = 82/12). The corresponding anti/syn alcohols were obtained in 81:19 ratio with 83% ee and 58% ee, respectively.
Working with various enantiopure hydroxymethyl-substituted pyridine derivatives, Reissig and Eidamshaus received compounds 16 (see Figure 2), but using them as catalysts gave rather poor results [40]. Using 5 mol% of 16a benzaldehyde was converted into homoallylic alcohol in 65% with 24% ee after 10 days at rt. Application of desilylated compound 16b caused an increase in enantioselectivity to 47% ee but together with a simultaneous drastic decrease of yield to 12%.
Interesting attempts to prepare polysaccharide (amylose and cellulose) derivatives, bearing pyridine N-oxide substituents, were made by the Ikai group [7]. A controlled number of 3- or 4-pyridine N-oxide groups have been attached to polysaccharide units by ester bonds. The great advantage of this type of compound is their non-toxicity to the environment. Unfortunately, the test applications in the reaction of asymmetric allyltrichlorosilane with benzaldehyde do not seem competitive with other described catalysts. The best results were received using amylose derivatives shown in Figure 3 (47–62%, 13–32% ee). It has been found that the amount and the position of N-oxide groups affected the reaction yield and enantioselectivity. Amylose not substituted with N-oxide groups do not show any catalytic activity. The increase of the number of N-oxide units in the catalyst from 19% to 23% caused improvement in both yield (from 47% to 62%) and enantioselectivity (from 13% to 32%). Further increase in the amount of pyridine N-oxide had a negative effect. Also, the location of the N-oxide groups was significant.
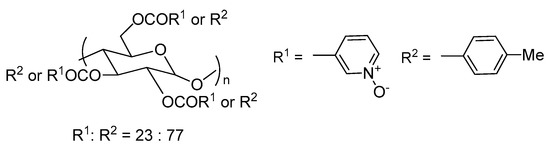
Figure 3.
N-oxide derivative of amylose.
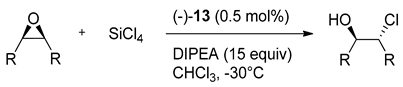
Ramanathan group has tested the versatility of prepared derivatives as Lewis basic activators in the desymmetrization reaction of meso-epoxides with silicon tetrachloride [18]. Catalysts 12 applied in the reaction with cis-stilbene epoxide were not very selective (12a gave 32% ee, 12b-5% ee and 12i-42% ee). In contrast, the C2-symmetric bipyridine dioxide 13 with similarly conformationally rigid, chiral bicyclic skeleton gave 89% ee already in the initial trials. In subsequent experiments, the reaction conditions have been optimized in terms of the solvent used, the catalyst loading, reaction time and temperature, and the quantity of DIPEA used as a base. The best conditions for ring-opening of meso-epoxides were determined as 0.5 mol% of catalyst at −30 °C for 70 min, in CHCl3, with 15 equivalents of DIPEA (see, reaction above Table 3). The results of the evaluation of (–)-13 for the enantioselective desymmetrization of meso-epoxides of various structural classes are shown in Table 3. The best effectiveness was observed for cis-stilbene epoxide opening (94% yield, 93% ee, entry 1). One of the challenging substrates, cyclooctene oxide (Table 3, entry 7) successfully furnished the corresponding chlorohydrin in 84% yield with 69% ee, which is better result compare to obtained with bis(tetrahydroisoquinoline) N,N′-dioxides as catalysts (56% ee) [19].

Table 3.
Desymmetrization of meso-epoxides with SiCl4 catalyzed by 13 [18].
Stončius and Neniškis presented a completely different approach. The Lewis bases were invented, in which the pyridine N-oxide unit has been attached to a chiral bicyclo [3.3.1] nonane backbone [18]. Authors designed the structures, that contain two 2,4-diaryl-substituted pyridine N-oxide moieties 14 and corresponding monofunctional congeners 15 (see Figure 2). Catalyst precursors were prepared in two steps: initial Michael addition was followed by the subsequent cyclization reactions to furnish corresponding bis(pyridines). Oxidation of them with m-CPBA produced expected N-oxides. Another synthetic strategy was taken for 14e, which the authors briefly explained in the published work [9a]. The obtained catalysts were examined in the allylation of benzaldehyde with allyltrichlorosilane, but the results were mediocre, so they were tested also in the enantioselective ring-opening of meso-epoxides with silicon tetrachloride. Cyclohexene oxide ring-opening reaction was used as a model. The resulting yields were good, but enantioselectivity was far below expectations (7 examples, 72–85% yield, 3–47% ee). Better results obtained with the use of 14a (72%, 32% ee), compared to 14d (73%, 3% ee) suggest a beneficial effect of electron-rich 2,4,6-trimethoxyphenyl substituents on enantioselectivity. Catalysts 14a and 14e were also used in ring-opening of other epoxides, both cyclic and aromatic. Noteworthy is the result of opening the cyclopentene oxide with 14e (85%, 88% ee)–the highest reported to date for the Lewis base-catalyzed desymmetrization of cyclic substrates. Any increase in ring size resulted in decreasing in selectivity (e.g., for cyclohexene oxide 77%, 47% ee). Interesting results have been obtained in the catalytic desymmetrization reaction of an epoxy norbornene derivative, known for that furnish syn, exo-chloroalcohol 17 as a major product, rather than vicinal chlorohydrin 18 (Scheme 3). For catalysts 14a–c enantioselectivities of p-nitrobenzoate (PNB) 17 were excellent (90–96% ee) but yields were not higher than 50%. In other cases, the yield of reaction and asymmetric induction were low. Scale-up (for 14b) or increase of catalyst loading (for 15b) did not affect the yield or ee values but it was possible to isolate p-nitrobenzoate 19. In turn, the reduction of catalyst loading (for 14b) had a negative effect on reaction yield. Reduction from 10 mol% to 5 mol% caused a decrease in yield from 50% to 30%.
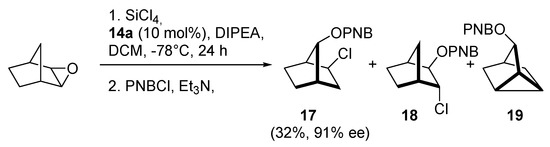
Scheme 3.
Catalytic ring-opening of norbornene oxide with SiCl4 [18].
N-Oxides Having the Terpene Unit
Naturally occurring and easily available terpenes and alkaloids are established chiral scaffolds for asymmetric organocatalysts. They were applied for the construction of chiral pyridine N-oxides, designed as a powerful Lewis-basic catalyst (Figure 4) already in the first years of the 21st century [41,42,43]. The latest reports about such catalysts come from 2008. Malkov and Kočovský [44] have prepared derivatives, lacking the C2 symmetry and having the chirality ‘‘concentrated’’ on one side of the molecule, such as dioxide 20-a quinoline analog with an isomeric terpene unit, the benzoquinoline analog 21 with similar chiral motif, and a series of bipyridine N,N’-dioxides with phenyl group 22 or pyridine N-oxide having (α-pyridyl-N-oxide)phenyl group 23. All these compounds (in 10 mol% loading) were tested in standard benzaldehyde allylation reaction.
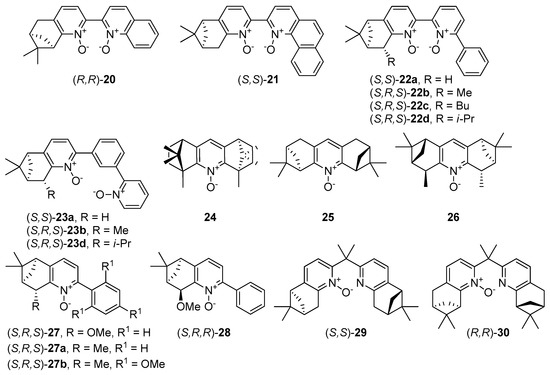
Figure 4.
N-oxides possessing fragment from terpenes and alkaloids.
Chelucci et al. [45] presented pyridine N-oxide derivatives as a polycyclic structure with terpene fragments (24–28) and dipyridine monoxide with spacer (29,30), showed in Figure 4. Compounds 24–26 possess C2-symmetry. The catalytic activity and stereo-differentiating ability of the new compounds, tested in the allyltrichlorosilane addition to benzaldehyde, appeared to be poor. The yield did not exceed 65% and enantiomeric excesses were not higher than 48%. Application of compound 24 resulted in 65% yield, but isolated homoallylic alcohol was a racemate, while in the case of 25, the obtained ee was 48%, but benzaldehyde was converted into alcohol in 31% yield. Epimers 27 and 28 behave as pseudo enantiomers and gave the opposite configuration of an allylic alcohol. Similar results of asymmetric induction have been achieved for both organocatalysts, 27 and its methyl analog 27a. Due to the previously observed relationship in similar C2-symmetric structures, where enantioselectivity for mono N-oxide was higher than for N,N’-dioxide, compounds 29 and 30 were designed. Next to 29 and 30, analogous N,N’-dioxides were also reported [46]. However, in these cases, the advantage of mono-oxides has not been observed. Compounds 24–26 and 29–30 were used also in the enantioselective opening of cis-stilbene. The yield achieved for all examined compounds was moderate to high (47–95%). Unfortunately, obtained halohydrin in most experiments was a racemate. Only for catalyst 25 asymmetric induction occurred (37% ee).
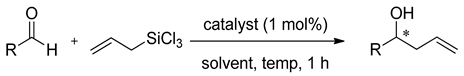
METHOX (27b), developed and successfully used in allylation of aromatic aldehydes with allyltrichlorosilane a few years earlier [47], in 2011 was tested in similar reaction but using allyldisilane (Scheme 4) [48]. Catalytic reactions of allyldisilane with aldehydes proceeded at very low rates and required a week or more to reach completion (10–15 mol% of (+)-27b, at −35 °C, in CH3CN). Nevertheless, METHOX exhibited excellent diastereo- and enantioselectivities for benzaldehyde and its derivatives (up to 97% ee). Further, this methodology was extended to the group of α,β-unsaturated aldehydes [48,49]. Different α,β-unsaturated aldehydes 31, incorporating aromatic, aliphatic substituents and cyclic ones were examined, and the results are shown in Figure 5. All of the aldehydes, with or without the α-branching, were found to react with good conversion within 3–5 days, providing high enantioselectivity, up to 93% ee. However, slightly better effectiveness was observed for 31a–c and 31f. In other cases, obtained yields varied between 37–60% and measured asymmetric induction from 66% ee to 89% ee. Received products were levorotary (except for (S)-31j use) when (+)-METHOX was employed. Aldehyde 31d, crotylated under the same conditions, gave rise to the expected anti-diastereoisomer as practically the only product, with the highest enantiomeric excess (96% ee). METHOX has been found to exhibit a high tolerance to aldehyde electronics, and the reactions require low catalyst loading (even 5 mol%).
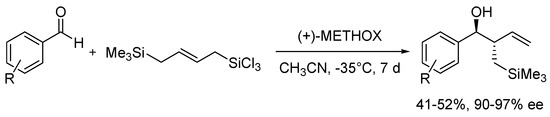
Scheme 4.
Allylation of aldehydes with allyldisilane catalyzed by (+)-METHOX.
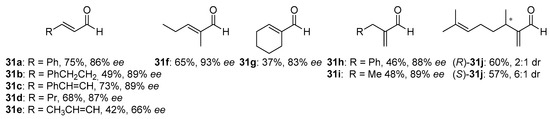
Figure 5.
The scope of the reaction employing (+)-METHOX for α,β-unsaturated aldehydes.
The observed difference in the reactivity and selectivity of dioxide and monoxide catalysts suggests that these two catalyst types can operate via different mechanisms [44]. In the case of bidentate N,N’-dioxides, cationic transition state A can be envisioned (see Figure 6). On this basis, a significant impact on the observed enantioselectivity of both, the unsymmetrical catalyst substitution and the huge effect of axial chirality of the catalyst can be explained (determining the absolute configuration of the product). Alternatively, the reaction proceeds via an associative pathway, involving the neutral octahedral silicon complex B. Transition state B provides high enantiocontrol in the allylation of aromatic aldehydes, however, it can be sensitive to the electronic effects of substituents in a substrate and any variation in the catalyst structure proximal to the coordinating center.
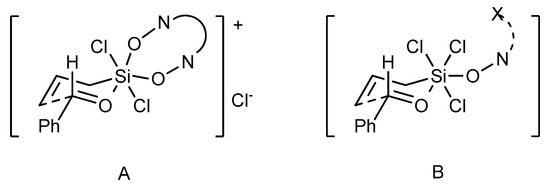
Figure 6.
Envisioned transition states in the N-oxide catalyzed allylation of aldehydes [44].
2.3. N-Oxides with Central and Axial Chirality
An interesting direction in the exploration of catalytically useful N-oxides is the combination of two types of chirality. Researches mainly concentrate on symmetrically or unsymmetrically substituted chiral bis(tetrahydroisoquinoline) N,N’-dioxides (Figure 7), which with their structure resemble compound 8. In 2008 Kotora et al. synthesized symmetrically substituted by (R)-tetrahydrofuran-2-yl dioxides 32 (see Figure 7), which were tested in the allylation of aromatic [50] as well as aliphatic aldehydes [30]. Catalysts synthesis was based on cyclotrimerization of tetrayne with (R)-tetrahydrofuran-2-carbonitrile, followed by oxidation of received bipyridines by m-CPBA. At the end separation of a resulted mixture of diastereoisomers was necessary. A simple column chromatography on alumina, gave isolated yields 48% for (aR,R,R)-32 and 28% for (aS,R,R)-32. The configuration was assigned by X-ray crystallographic analysis. In allylation of benzaldehyde, 4-trifluoromethylbenzaldehyde or 4-methoxybenzaldehyde performed in MeCN, at −40 °C for 1 h, with 1 mol% catalyst loading, both diastereomeric catalysts were effective [50]. Aldehydes were converted into corresponding homoallylic alcohols almost quantitatively (Table 4, entries 1, 4, 10). Only for allylation of 4-trifluoromethylbenzaldehyde using (aR,R,R)-32 the yield was slightly lower (82%, entry 10). Applying the same catalyst, the enantioselectivity was lower for aldehyde with an electron-withdrawing group (15% ee in comparison to 48% ee for benzaldehyde, Table 4, entry 10) and higher for aldehyde substituted with an electron-donating group (60% ee, Table 4, entry 4). When (aS,R,R)-32 was used decrease of asymmetric induction for 4-trifluoromethylbenzaldehyde was observed again (Table 4, entry 10), but for 4-methoxybenzaldehyde obtained alcohol was a racemate (Table 4, entry 4).
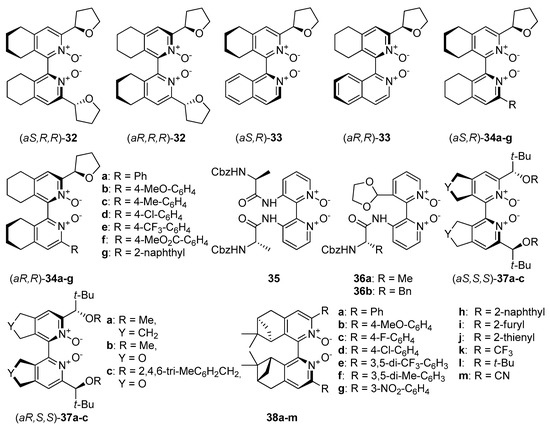
Figure 7.
N-Oxides possessing two types of chirality—central and axial.

Table 4.
Asymmetric allylation of aldehydes by 32 [50] and 34a [31].
In addition, a strong solvent effect on stereoselectivity has been observed. For (aR,R,R)-32 used in chlorobenzene no reaction was observed, but using (aS,R,R)-32 the enantioselectivity was significantly higher (Table 4, entries 2, 5, 11), for all three substrates. For benzaldehyde yield did not change, while for substituted substrates it decreases by half. Inversion of product configuration was observed in dependence of solvent used–R in MeCN into S in PhCl. Kotora also mentioned in the same publication about isoquinolyl-tetrahydroisoquinoline 33 unsymmetrically substituted with tetrahydrofuran-2-yl group. The catalysts themselves were obtained in low yields (4–17%), and their use for allylation of benzaldehyde gave rather mediocre results (86% with 49% ee using (R,R)-33 and 84% with 48% ee using (S,R)-33). However, configurations of received homoallylic alcohols (S with (R,R)-33 and R with (S,R)-33) indicates that configuration of the product is controlled by the axial chirality of the catalyst (the opposite is obtained). The catalytic activity of 32 was also studied in the allylation of aliphatic aldehydes. Different solvents e.g., acetonitrile, dichloromethane, chloroform, and acetone have been tested in allylation of cyclohexanecarboxaldehyde as the model substrate and there is no doubt that the solvent effect controls the reaction mechanism. Using (aR,R,R)-32 enantioselectivity was low, regardless of the solvent used (10–19% ee, Table 5, entries 1–4), but the yield proved to be highly dependent on the solvent. The highest yield was obtained when MeCN was applied (85%, Table 5, entry 1). For the remaining solvents, the yields were similar and amounted to 34–40%. Application of (aS,R,R)-32 resulted in an increase of both the yield (52–79%, Table 5, entries 5–8) and the reaction enantioselectivity (39–68% ee). Configuration of obtained alcohol was inverse to those obtained with (aR,R,R)-32, which confirms prior observation about the decisive influence of the axial chirality of the catalyst. (aS,R,R)-32 employed toward other aliphatic substrates gave generally high yield (79–91%, Table 5, entries 9–12) except for pivaloyl aldehyde (10%, Table 5, entry 13) but enantioselectivity was rather moderate (22–68% ee). For cyclic substrates, products have configuration R (Table 5, entries 11–12), while straight-chain aldehydes resulted in alcohols with configuration S (Table 5, entries 9, 10, 13).

Table 5.
Asymmetric allylation of aliphatic aldehydes by 32 [30] and 34a [31].
The unsymmetrically substituted bis(tetrahydroisoquinoline) N,N’-dioxides 34a were also synthesized and employed in catalytic allylation of aromatic aldehydes (see Table 4) [31]. Again, the huge influence of the solvent on the stereochemical result of the reaction was observed. The catalyst (1 mol% loading) with S-axial chirality gave R-product in MeCN (Table 4, entry 1) and S-product in PhCl and THF (Table 4, entries 2,3). Also, in MeCN the enantioselectivity was about two times lower than in PhCl and THF, for which results were similar. Therefore, reactions with other substrates were carried in THF. Generally, for both isomers, 34a results were very good, but (aS,R)-34a worked better as a catalyst for highly enantioselective allylation of benzaldehydes, bearing electron-withdrawing as well as electron-donating groups. Slightly better yield can be observed in the case of p-substituted benzaldehydes than for m-substituted (Table 4, entries 5–9, 11–14). A drastic decrease in the yield and asymmetric induction was observed for o-chlorobenzaldehyde (Table 4, entry 15). Results depicted in Table 4 clearly show, that unsymmetrically substituted bis(tetrahydroisoquinoline) N,N’-dioxides 34a exhibit higher catalytic activity, than symmetrically substituted derivatives 32. Yields of obtained products are comparable, but much better enantioselectivity gives the use of 34 (both epimers). The enantioselectivity was highly solvent-dependent. It seems, that THF enables the reaction mechanism to proceed through the sterically more crowded neutral six-coordinate silicon species, leading to higher enantioselectivity [30]. The scope of application of 34a for aliphatic aldehydes was tested in the reactions with n-octanal or cyclohexylcarbaldehyde (Table 5, entries 14–17) [31]. The reaction in both cases was characterized by good to excellent yields (88–95%) and moderate enantioselectivity (38–67% ee).
Higher results were detected for (aR,R)-34a. (aR,R)-34a was also applied in allylation of benzaldehyde with E- crotyltrichlorosilane (6:1 E/Z mixture in PhCl, for 24 h, at −40 °C with 1 mol% of catalyst), giving 2.4/1 anti/syn diastereoisomer mixture, with good yield 82% and satisfactory enantioselectivity 91% and 87% ee, respectively. The scope of application in the asymmetric allylation reaction of both, symmetric catalysts 32 was studied [51]. Under optimized conditions (1 mol% of the catalyst in THF at −40 °C) a number of different, aromatic and aliphatic α,β-unsaturated aldehydes were checked, testing both epimers of 32 (Scheme 5). Better enantioselectivity was achieved using (aS,R,R) diastereomer but slightly higher yields were obtained for (aR,R,R) diastereomer–respectively, 52–82% yield, 63–96% ee and 40–95% yield, 4–68% ee. The best enantioselectivity was achieved using (aS,R,R)-32 with substrates having both groups: R1 (as phenyl) and R2 (as methyl group or chlorine atom)-96% ee in both cases. By using (aR,R,R)-32 it was found that, that higher enantioselectivity (62–68% ee) was obtained when α-substitution (R2) was present independently of whether R1 was aromatic or aliphatic. Better yields for (aR,R,R)-32 (82–97%) were observed when R1 was phenyl substituted with an electron-withdrawing group or unsubstituted phenyl group, but then the presence of electron-donating group as R2 was necessary. For both catalysts employed, the configuration of obtained aliphatic alcohols was R, whereas it was opposite for products having an aromatic substituent. In most applications, both catalysts appear to be similarly effective to the METHOX described earlier.
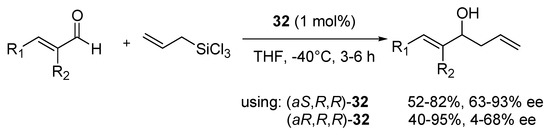
Scheme 5.
Allylation of α,β-unsaturated aldehydes with allyltrichlorosilane catalyzed by 32 [51].
Interesting comparative studies have been done by Kotora’s research group for different enantioselective allylation procedures and different catalysts applied N-oxides 34a-Lewis basic catalyst were compared with (S)-BINOL-Lewis acid catalyst (Keck protocol) and (R)-TRIP PA-Brønsted acid catalyst in enantioselective allylation of o-substituted benzaldehydes (Scheme 6) [52].
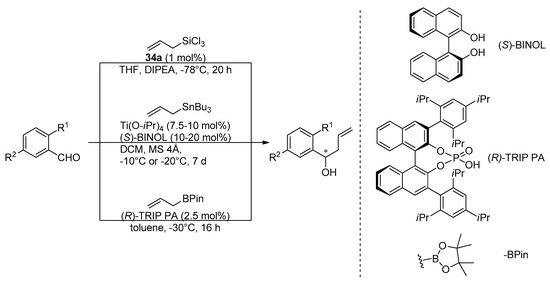
Scheme 6.
Enantioselective allylations of o-substituted benzaldehydes carried out in comparative studies [52].
The reactions performed needed different catalyst loadings and different reaction times. Despite this, an attempt to compare the results was made. Chiral catalytic systems have rarely been used for allylation of o-substituted benzaldehydes, so the effect of substitution in the ortho position of the aromatic aldehydes was tested using the three procedures shown in Scheme 6. Definitely, the best yields were obtained using phosphorous catalyst (R)-TRIP PA (93–99% in comparison to results up to 70% for 34a and up to 80% for (S)-BINOL), but achieving high enantioselectivity was problematic in all cases. In some cases the use of N,N’-dioxide catalysts gives better optical purity, in others, Keck or Brønsted acid allylation seems to be more effective. Also, none of the catalysts were versatile. Among tested N,N’-dioxides, again better yields gave (aR,R)-34a and higher asymmetric induction was observed using (aR,S)-34a. The best results were obtained for o- fluorobenzaldehyde (48%, 82% ee for (aR,R)-dioxide and 34%, 66% ee for (aR,S)-dioxide), o-vinylbenzaldehyde (40%, 72% ee and 40%, 76% ee, written in the same order) and m- methoxybenzaldehyde (60%, 85% ee and 60%, 88% ee), which are rather moderate. The effect of the solvent was examined in allylation of 2-iodo-5-methoxybenzaldehyde. For (R,R)-epimer reaction proceeded better in dichloromethane than THF—70%, 41% ee in comparison to 5%, 56% ee and reversely, in case (R,S)-enantiomer-40%, 4% ee versus 53%, 80% ee, whereas in toluene reaction did not proceed at all, in both cases. When the reaction was performed in THF, the configuration of all products was the same as the axial configuration of the catalyst. Inversion of product configuration was observed when dichloromethane was applied. N-oxide catalysts were successfully applied to the synthesis of a few natural products [12,13,14,15,16,17]. For example, duloxetine, which is used in the treatment of major depression, was synthesized involving the allylation of 2-thiophenecarboxaldehyde with (aR,S)-34a as catalyst (see Scheme 7) [12].
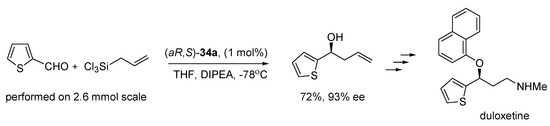
Scheme 7.
Allylation of 2-thiophenecarboxaldehyde using (aR,S)-34a in total synthesis of duloxetine [12].
The advantage of the N,N’-dioxide catalysts is in their rather low loading, (only 1 mol%) being sufficient to bring about the desired allylation. In the case of phosphorous catalyst TRIP PA, the level of asymmetric induction depends on the catalyst loading, optimally it is 10 mol%, which is rather high (Scheme 8) [15].
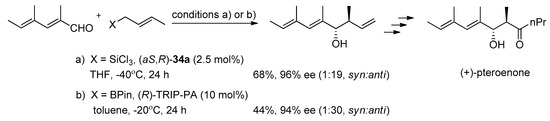
Scheme 8.
Comparison of the crotylation involving N,N’-dioxide 34a and TRIP PA used as a catalyst in the total synthesis of (+)-pteroenone [15].
Malkov and Kočovský [48] have compared the effectiveness of catalyst 34a and METHOX in the reaction of different aldehydes with allyldisilane. Generally, obtained results were high and similar for both catalysts, however, reactions applying METHOX needed higher loading (15–20 mol% versus 5–10 mol%) and much longer time (7 days versus 12 h) to be completed. Optimal solvent for use of 34a as catalyst was THF, while CH3CN for METHOX (temp. −35 °C for both). At the enantioselectivity of a similar order, the yields obtained were slightly higher for 34a (for both isomers 71–83% yield and 73–98% ee). In all cases, only anti isomers were obtained. For both catalyst types decrease of efficiency and enantioselectivity was observed in case benzaldehydes substituted by electron-donating group (methoxy group) in para-position. For (aR,R)-34a two non-aromatic aldehydes were used–hexanal and α,β-unsaturated hex-2-enal. Great enantioselectivity 98% ee and good yield 83% has been received for the second one. For (aS,R)-34a observed that decreasing the amount from 10 mol% to 1 mol% with simultaneous elongation of the reaction time from 12 h to 24 h caused a slight decrease of both the yield and enantioselectivity (from 82%, 96% ee to 70%, 91% ee).
Zhao et al. have designed symmetrically and unsymmetrically substituted, axially chiral 2,2’- bipyridine N,N’-dioxides combined with central chirality introduced with α-amino acid residues: C1-symmetric 36 and C2-symmetric 35 (see Figure 7) [53]. Using 10 mol% of catalyst for allylation of p-nitrobenzaldehyde in CH2Cl2 at −78 °C (with the addition of DIPEA) rather mediocre results were obtained 41–66% yield, 27–40% ee. It was observed that the levorotary catalyst gave comparable or even slightly better results in shorter reaction times. Also, levorotary catalyst gave (R)-product, while dextrorotary catalyst gave (S)-product. Nine different polar and nonpolar solvents were tested in allylation using (−)- 35 as catalyst and CH2Cl2 (48%, 33% ee), next to MeCN (35%, 41% ee), seemed to be better solvents for the allylation reactions. But in MeCN, nearly 3 times longer reaction time was needed to achieve shown results (11 h versus 4 h). Catalyst (–)-36b showed similar enantioselectivity in the same test reaction, giving 66% yield and 35% ee, Table 6, entry 1). Both, C2-symmetric catalyst 35 and C1-symmetric catalyst 36b have been evaluated in the allylation of other substituted benzaldehydes (see Table 6). The best results were obtained with 4-methylbenzaldehyde and 1,4-benzodioxane-6-carboxaldehyde–61–65% ee and 53–63% ee, respectively (Table 6, entries 6 and 8). By increasing the catalyst loading (for 35, Table 6, entry 7), a bit higher yield was obtained, but a decrease in enantioselectivity was observed. The allylation of 4-methoxybenzaldehyde was also performed using (–)-36b and, despite that methoxy group is electron-donating (similar to methyl group), in this case, the obtained product was racemic.

Table 6.
Asymmetric allylation of benzaldehydes with allyltrichlorosilane catalyzed by 35 and (–)-36b [53].
Kotora et al. synthesized a number of derivatives 34 (see Figure 7), with variously modified aromatic substituents at the 3’ position [54]. All these dioxide derivatives have been tested, as Lewis base catalysts, in allylation of aromatic aldehydes (0.5 mol%) in THF and CH2Cl2. Again, THF was a better reaction medium. As observed, the substitution of catalysts in the aryl group by EDG or EWG did not significantly change the catalytic activity of 34a. Slightly better results were observed for all (S,R) isomers, except the results of cinnamaldehyde allylation, which were similar for both epimers of 34b–g. Asymmetric induction was not observed in the allylation of 2-thiophenecarboxaldehyde. Gradual increase of catalyst loading showed, that both the yield and enantioselectivity increased up to 74%, 80% ee for 5 mol% of (aR,R)-34a and 90%, 88% ee for 5 mol% of (aS,R)-34a. The allylations of benzaldehyde, p-methoxybenzaldehyde and p- trifluoromethylbenzaldehyde using 34, described in this report, were performed using 0.5 mol% of the catalyst [54]. Comparing them with the same reactions applying 1mol% of the catalyst [36] allows the conclusion that reducing the loading does not affect enantioselectivity. Only in case of p-trifluorobenzaldehyde the yield slightly decreased using (S,R)-enantiomer, while with use (R,R)-enantiomer, the yield apparently decreased, when half amount of catalyst has been used.
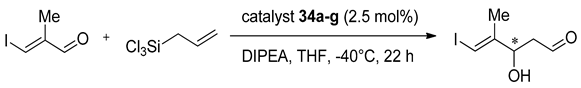
The predictable utility of the expected product as a chiral building block in the synthesis of natural products [54,55] was the reason for the application of 34a–g in allylation of E-3-iodomethacrylaldehyde (Table 7). For this reaction, the influence of modification of the phenyl group in catalyst turned out to be significant. The advantage of (aR,R)-catalysts 34b–g over (aR,R)-34a (with unsubstituted phenyl group in 3′ position) was especially noticeable in term of enantioselectivity (80–97% in comparison to 57%). When it comes to the yield, the highest result was observed for (aR,R)-34e, having 4-trifluoromethylphenyl group (Table 7, entry 5), while by using (aR,R)-34b, having 4-methoxyphenyl group (Table 7, entry 2), the lowest conversion was obtained. In all other cases, yields were comparable (approx. 70%). In the case of the (aS,R)-isomers, for all catalysts, including (aS,R)-34a, the obtained enantioselectivity was excellent (98–99% ee). For compounds with EWG or EDG substituent present at the phenyl group, an increase of yield was observed–from 49% to 66–89%. The best and the worst results coincided with those obtained for (aR,R)-enantiomers (Table 7, entries 5 and 2, respectively). The configuration of the obtained product was the same as the axial configuration in the catalyst.

Table 7.
Allylation of E-3-iodomethacrylaldehyde catalyzed by 34a–g [54].
Kotora and Lamaty presented bipyridine N,N’-dioxides 37, with C2-symmetry (see Figure 7) [24,25]. Their synthesis was based on catalytic [2+2+2] cyclotrimerization either hepta-1,6-diyne or propargyl ether as starting material with various nitriles. A detailed synthetic pathway, including two different approaches, was described. Obtained compounds 37a,b were examined in the allylation of benzaldehyde. Although the use of acetonitrile as solvent notably improved the yields, (93% with (aR,S,S)-37b and 96% with (aR,S,S)-37a), compared to results in THF, the enantioselectivity remained moderate (45% ee and 33% ee, respectively). On the other hand, using DCM rise in enantioselectivity (72% ee using (aR,S,S)-37b and 46% ee using (aR,S,S)-37a) with noticeably decreased the yield. In both cases, slightly better enantioselectivity was observed for (aS,S,S)-isomers of 37a,b, but it was still rather moderate (51% ee and 40% ee respectively).
A recent report by Rubtsov and Malkov et al. [56] presents the synthesis of atropoisomeric bipyridine N,N′-dioxides with terpene derived moieties 38 (see Figure 7). The combination of axial chirality and terpene-derived structures seemed to be a promising direction. Especially compound 38e turned out to be an extremely efficient catalyst producing excellent enantio- and diastereoselectivities in the asymmetric crotylation over a whole range of aldehydes tested [14]. Catalyst (−)-38e proved particularly efficient with unsaturated aldehydes, though with aliphatic enantioselectivity dropped representing a common trend (see Scheme 9).
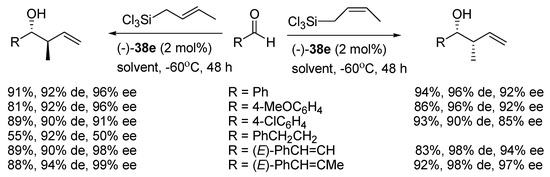
Scheme 9.
Asymmetric crotylation of aldehydes with (-)-38e.
Applying (−)-38e in the same reaction conditions to a larger scale (5 mmol), provides excellent yield and enantioselectivity retained paving the way for the asymmetric synthesis of (−)-elisabethadione (see Scheme 10) [14].
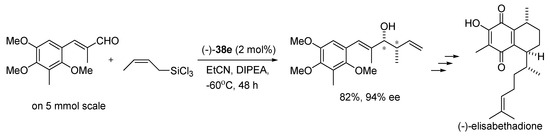
Scheme 10.
Crotylation with 38e in the total synthesis of (-)-elisabethandione [14].
Compounds 37 were also applied in aldol reaction of acetophenone with trichlorosilyl ketene acetal (Scheme 11) [24]. Good to excellent yields were achieved using 37a,b catalysts, independently of their configuration (87–96%). For 37a, enantioselectivity was higher when (aS,S,S)-isomer was used and for 37b,c with (aR,S,S)-isomer. The difference between the structure of catalysts is the presence of oxygen in the five-membered ring of compounds. The more selective were catalysts bearing the oxygen atom in the five-membered ring (37b,c). The best result received for (aR,S,S)-37b was 96% yield and 78% ee, which is better than obtained with the catalyst (aR,R)-34a in the same conditions.
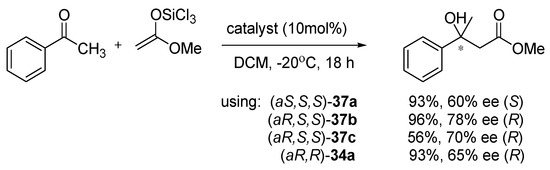
Scheme 11.
Catalytic aldol reaction of acetophenone with trichlorosilyl ketene acetal [24].
2.4. Helical-Chiral N-Oxides
A completely different group of N-oxide catalysts introduced Takenaka [21,22,23]. Seminal report from 2008, presented synthetic route to helical-chiral pyridine N-oxides 39–41 (see Figure 8) and results of their application in desymmetrization of meso-epoxides [21]. The catalytic reaction was performed for two epoxides possessing aromatic groups and two alkyl epoxides. In all cases the efficiency of the reaction was good (68–80%). Desymmetrization of aromatic meso-epoxides was characterized by higher enantioselectivity than for aliphatic ones (73–94% ee versus 22–65% ee). Introduction of two additional aromatic rings to 39, creating branched structure 41, allowed to obtain in all cases the highest ee values. For 1,5-cyclooctadiene oxide, the growth of enantioselectivity was the most visible–33% ee with 41, in comparison to racemate obtained using 39. Epoxide ring opening of several variously substituted cis-stilbene derivatives catalyzed by 41 was also examined. It could be concluded, that the presence of the electron-withdrawing group in cis-stilbene has no effect on enantioselectivity (2 examples, 92–94% ee) but the presence of electron-donating group slightly decreases the enantioselectivity (87% ee).
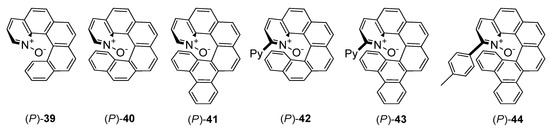
Figure 8.
Helically chiral N-oxides.
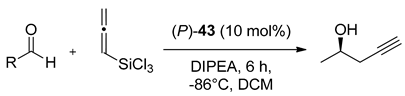
The same helical catalysts have been used in the propargylation of aldehydes with allenyltrichlorosilane [22]. Applying 10 mol% of catalyst in CH2Cl2 at −20 °C for 24 h, provided the products with good yield (80–90%) and moderate enantioselectivity (34–48% ee). Additionally, three new helically chiral N-oxides 42–44 were presented [22]. A slight modification of 40 and 41, consisting of the insertion 2-pyridine group close to N-oxide bond (42, 43, respectively) allowed to achieve complete conversion of aldehyde in 3 h but enhancement of the enantioselectivity (84% ee) was observed only for 43. In comparison, using 44, obtained by substitution of 4-methylphenyl group into 41 in position 2, the catalytic reaction did not proceed even at room temperature after 24 h. Compound 43 was applied to carry out the evaluation of the utility of different aromatic aldehydes as substrates [22]. For meta-substituted aldehydes, better enantioselectivity was obtained when substituents were electron-deficient. For ortho-substituted aldehydes, electron effects had no influence, but generally, the yield and enantioselectivity were slightly higher than for meta-substituted aldehydes, what is shown in Table 8. Catalyst 43 was also employed in propargylation of N-acylhydrazone (Scheme 12) with a satisfactory result–53% yield and 78% ee [23]. Results proved that helical chiral 2,2′-bipyridine N-monoxides that exhibited high activity towards the relatively unreactive allenyltrichlorosilane. The appropriate structural modification to the rings beneath the plane of the pyridine N-oxide can serve as a powerful means for tuning the catalyst enantioselectivity.

Table 8.
Asymmetric propargylation of aldehydes catalyzed by (P)-43 [23].
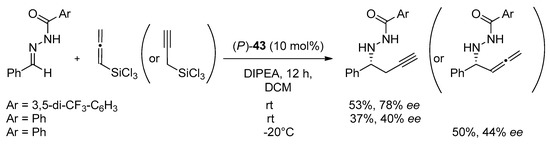
Scheme 12.
Propargylation and allenylation of N-acylhydrazones [23].
Pyridine N-oxides have been also used as a functional group in helically chiral organocatalysts supported on polymers. For example, pyridyl N-oxide substituted poly(methacrylate)s [57], poly(biphenylylacetylene)s [58] and D-glucose-linked biphenyl polymers [59] were reported. All these catalysts: monomers, polymers without N-O bond and polymers bearing N-oxide group, were examined in allylation of a few chosen aromatic aldehydes. Results in both, yields and asymmetric induction were rather low but, as a brief summary it should be emphasized, that presence of the N-O bond as well as the application of polymer-supported catalysts increase the enantioselectivity of the reaction.
2.5. N-Oxides Possessing Planar Chirality
Compounds bearing [2.2] paracyclophane moiety 45–48 (see Figure 9), which represents planar chiral N-oxides should also be briefly mentioned. Rowlands et al. presented a facile synthesis of the mentioned compounds in two steps from [2.2] paracyclophane, based on Fagnou’s direct arylation [60]. Then, obtained compounds were applied in the allylation of benzaldehyde with allyltrichlorosilane. For catalysts 45b and 46b, the presence of methoxy group in their structure caused decrease of the yield and enantioselectivity and also inversion of configuration from R to S, in comparison to corresponding unsubstituted structures 45a and 46a–from 65%, 38% ee to 52%, 36% ee and from 72%, 38% ee to 58%, 28% ee, respectively. Unsubstituted, mixed pyridine/pyridine N-oxide catalyst 47 was less effective (55% yield and 30% ee) compare to 46a and inversion of product configuration was also observed, which might indicate that presence of N-oxide group plays a crucial role. It is also worth to compare the results obtained by Rowlands and coworkers with those obtained by Andrus and coworkers [61]. The latter group synthesized aza-paracyclophane N-oxide catalysts 48. Among them (S)-48a turned out to be a brilliant catalyst for allylation of various aromatic and aliphatic aldehydes with allyltrichlorosilane, giving 87–95% yield and 87–96% ee [61]. It is puzzling whether a structure containing aza-paracyclophane N-oxide would be effective catalysts or the presence of an oxazoline group is essential.
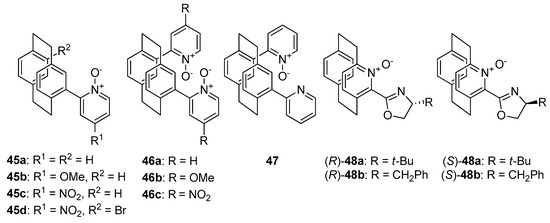
Figure 9.
Planar chiral N-oxides.
3. Chiral Heteroaromatic N-Oxides as Ligands for Metal Catalysis
Over the past decade, relatively little attention has been paid to the use of chiral heteroaromatic N-oxides as ligands for metal complexes catalyzed reactions. Several mentions come from 2002–2003 and include (S)-1-CdI2 complex as a catalyst for conjugate addition of thiol to enone or enal, providing 70–78% ee [62], and (R)-1-Sc(OTf)3 complex as a catalyst for Michael addition of β-keto ester to methyl vinyl ketone or acrolein, giving almost quantitative yield with moderate enantioselectivity of 38–84% ee [48]. Chiral copper (II)-terpyridine mono-N-oxide and di-N-oxide complexes were used in asymmetric cyclopropanation of styrene [63]. Enantiomeric excess up to 83% and yield up to 97% were achieved. Currently, rather the applications of chiral alkyl amine N-oxides (mostly proline N-oxide derivatives) as ligands for metal complexes, in various types of asymmetric reactions, can be found in the literature [64,65,66]. The different direction was presented by Wolińska, who used chiral pyridine N-oxide derivatives possessing oxazoline moiety 49–51 (see Figure 10) for the asymmetric nitroaldol reaction, catalyzed by a copper complex. Catalysts 49, used in Henry reaction of 3-nitrobenzaldehyde [67], gave high efficiency (80–88%) but unfortunately, the enantioselectivity was low (11–14% ee). Chiral 3-oxazoline pyridine N-oxides substituted by with 1,2,4-triazine ring (50, 51) [68] were also examined in nitroaldol reaction of m-nitrobenzaldehyde but all attempts resulted in racemic nitroalcohol. An external base addition was tried and some improvement has been observed [69]. However, enantioselectivity grows (from racemate to 41% ee) only for bases with low pKa value. The reason for the low catalytic effectiveness may be the relatively large distance between the complexation site (N-O) and the stereogenic center.
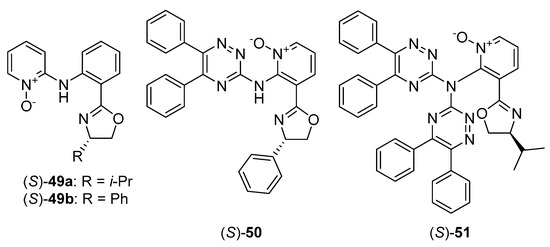
Figure 10.
N-oxide ligands used in Cu-catalyzed nitroaldol reaction.
4. Other Applications of Pyridine N-Oxides
Without any doubt, N-oxides are used in asymmetric organocatalysis, but also their applications in different branches of science are of great importance. They have significant synthetic value as intermediates in multi-step syntheses. They are widely used to various functionalization of N-heteroaromatic compounds-this mainly concerns the C-H bond in position 2. The examples of the application of N-oxides as synthetic intermediates in the industrial synthesis of some pharmaceuticals are also described, e.g., pranoprofen or omeprazole [70]. Compounds containing in their structure the 2-mercaptopyridine-N-oxide moiety have anti-cancer, bactericidal, and fungicidal activity [71,72]. N-oxides are also a crucial component in personal care products such as soaps, toothpaste, washing agents, shampoos and cosmetics [73]. Interesting properties of the N-O bond caused that N-oxides are used also in materials engineering. They consist of a wide group of polymer additives, e.g., crosslinkers, vulcanization accelerators, epoxy resin hardeners, UV absorbers or additives for stereospecific polymerization of polypropylene [74]. The most attention is focused on polymers with N-oxide groups e.g., hyperbranched polyimide N-oxide, which is used as photocatalyst [75]. Their greatest advantages, in comparison to photocatalysts based on inorganic compounds, are easy and cost-efficient synthesis and, particularly, the possibility of visible light absorption without the necessity of structural modifications. Another example of the photocatalyst is light crosslinked polymers, based on triazine N-oxide fragment. It has been shown that they are effective photocatalysts, causing degradation of methyl orange, an azo dye employed as a pH indicator [76]. Most dyes have a very stable structure, which makes their degradation especially difficult and uncontrolled entry of these compounds into water affects flora and fauna. In the case of water reservoirs where there is no flow of water, it might cause eutrophication. N-oxides are also used in coordination polymers, among which semiconductor luminescent materials with tunable luminescence are sought. This type of material can be applied in lighting and displays, as well as in-memory devices and sensors. As an example can be mentioned coordination polymers with symmetric and unsymmetrical ligands-4,4′- and 2,2′-bipyridine N,N’-dioxides and N-oxides [77]. Recent reports concern also pH-responsive polystyrene-b-poly(4-vinylpyridine-N-oxide) membranes [78] and the possibility of applying the coatings from a solution of cellulose-N-methylmorpholine-N-oxide to paper [79]. In the first case, at low pH the pores open (the solution flow increases), and at high pH, the pores close (the solution flow is reduced). The membrane is synthesized by oxidation of polystyrene-b-poly(4-vinylpyridine), which shows an inverse pH response and the presence of both forms in membrane opens up an attractive way for pH-based separations [78]. In other cases, depending on the composition of the coating and whether is it continuous or porous, it is possible to improve the tear strength, print quality as well as the adhesive or antibacterial properties of the paper. It also affects fire resistance, thermal and electrical conductivity, and the friction coefficient. Paper with a coating from a cellulose-N-oxide solution was characterized above all by higher hydrophobicity and smoothness of the surface as well as better tear resistance [79].
5. Conclusions
Chiral pyridine N-oxides and 2,2’-bipyridine N,N’-dioxides are very well suited as Lewis base organocatalysts. They are able to activate trichlorosilanes and catalyze a number of reactions such as allylation of aldehydes (furnishing homoallylic alcohols—main field), hydrosilylation, ring-opening of epoxides and aldol condensations. Their stereo-differentiating properties largely depend on the type of chirality represented by the molecules, but they can also be modified by the presence of substituents that slightly change the electron properties of the catalysts. However, the results reported in this paper clearly show that fine-tuning of the properties of the catalysts may not be as straightforward and simple as expected. The stereogenic axis in the catalyst appears to have the most pronounced effect on both, the product configuration and its optical purity. However, this effect also highly depends on the solvent used. Generally, N-oxides do not require the application of large amounts to have an acceptable effect compared to other organocatalysts. On average, loading ranges from 5–10 mol%, although sometimes 1 mol% is enough. Extensive optimization of the stereoselective features of the catalysts and reaction conditions has resulted in the high level of stereocontrol and yields in allylation of aromatic or unsaturated aldehydes, although good results have been obtained also for aliphatic, e.g., cyclic aldehydes. Allylation procedures employing N-oxide organocatalysts have found practical applications in the synthesis of natural products.
Although a variety of chiral pyridine N-oxide derivatives are able to perform reactions efficiently and with high enantioselectivity, they often need synthesis according to tedious procedures, sometimes involving a resolution step. Therefore, the developing of new structures, readily available and efficient as chiral organocatalysts for the reaction of trichlorosilyl compounds is still very active.
Author Contributions
Conceptualization, R.S.; collecting the literature data, Z.W.; preliminary writing, Z.W.; tables and schemes, Z.W. + R.S.; original draft preparation, R.S.; writing—review and editing, R.S.; supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dalko, P.I.; Moisan, L. In the golden age of organocatalysis. Angew. Chem. Int. Ed. 2004, 43, 5138–5175. [Google Scholar] [CrossRef]
- Bolm, C. Catalysis with Organic Molecules: A Success Story in Modern Catalytic Chemistry. Adv. Synth. Catal. 2004, 346, 1022. [Google Scholar] [CrossRef]
- Pavel Kočovskỳ, P.; Malkov, A.V. Asymmetric synthesis: From transition metals to organocatalysis. Pure Appl. Chem. 2008, 80, 953–966. [Google Scholar]
- Nakajima, M.; Saito, M.; Shiro, M.; Hashimoto, S. (S)-3,3′-Dimethyl-2,2′-biquinoline N,N′-Dioxide as an Efficient Catalyst for Enantioselective Addition of Allyltrichlorosilanes to Aldehydes. J. Am. Chem. Soc. 1998, 120, 6419–6420. [Google Scholar] [CrossRef]
- Koukal, P.; Ulč, J.; Nečas, D.; Kotora, M. Pyridine N-Oxides and Derivatives Thereof in Organocatalysis. In Heterocyclic N-Oxides. Topics in Heterocyclic Chemistry; Larionov, O., Ed.; Springer: Cham, Switzerland, 2017; Volume 53, pp. 29–58. [Google Scholar]
- Chelucci, G.; Murineddu, G.; Pinna, G.A. Chiral pyridine N-oxides: Useful ligands for asymmetric catalysis. Tetrahedron: Asymmetry 2004, 15, 1373–1389. [Google Scholar] [CrossRef]
- Ikai, T.; Moro, M.; Maeda, K.; Kanoh, S. Synthesis of polysaccharide derivatives bearing pyridine N-oxide groups and their use as asymmetric organocatalysts. React. Funct. Polym. 2011, 71, 1055–1058. [Google Scholar] [CrossRef]
- Copéret, C.; Adolfsson, H.; Khuong, T.-A.V.; Yudin, A.K.; Sharpless, K.B. A Simple and Efficient Method for the Preparation of Pyridine N-Oxides. J. Org. Chem. 1998, 63, 1740–1741. [Google Scholar] [CrossRef]
- Shaker, Y. Recent trends in the chemistry of pyridine N-oxides. Arkivoc 2001, 1, 242–268. [Google Scholar]
- Váňa, J.; Roithová, J.; Kotora, M.; Beran, P.; Rulíšek, L.; Kočovský, P. Proton Affinities of Organocatalysts Derived from Pyridine N-oxide. Croat. Chem. Acta 2014, 87, 349–356. [Google Scholar]
- Malkov, A.V.; Stončius, S.; Bell, M.; Castelluzzo, F.; Ramírez-López, P.; Biedermannová, L.; Langer, V.; Rulišek, L.; Kočovský, P. Mechanistic Dichotomy in the Asymmetric Allylation of Aldehydes with Allyltrichlorosilanes Catalyzed by Chiral Pyridine N-Oxides. Chem. Eur. J. 2013, 19, 9167–9185. [Google Scholar] [CrossRef]
- Motloch, P.; Valterová, I.; Kotora, M. Enantioselective Allylation of Thiophene-2-carbaldehyde: Formal Total Synthesis of Duloxetine. Adv. Synth. Catal. 2014, 356, 199–204. [Google Scholar] [CrossRef]
- Hessler, F.; Korotvička, A.; Nečas, D.; Valterová, I.; Kotora, M. Syntheses of a Flobufen Metabolite and Dapoxetine Based on Enantioselective Allylation of Aromatic Aldehydes. Eur. J. Org. Chem. 2014, 2543–2548. [Google Scholar] [CrossRef]
- O’Hora, P.S.; Incerti-Pradillos, C.A.; Kabeshov, M.A.; Shipilovskikh, S.A.; Rubstov, A.E.; Elsegood, M.R.J.; Malkov, A.V. Catalytic Asymmetric Crotylation of Aldehydes: Application in Total Synthesis of (−)-Elisabethadione. Chem. Eur. J. 2015, 21, 4551–4555. [Google Scholar]
- Koukal, P.; Kotora, M. Enantioselective Allylation of (2E,4E)-2,4-Dimethylhexadienal: Synthesis of (5R,6S)-(+)-Pteroenone. Chem. Eur. J. 2015, 21, 7408–7412. [Google Scholar] [CrossRef] [PubMed]
- Kadlčίková, A.; Valterová, I.; Ducháčková, L.; Roithová, J.; Kotora, M. Lewis Base Catalyzed Enantioselective Allylation of α,β-Unsaturated Aldehydes. Chem. Eur. J. 2010, 16, 9442–9944. [Google Scholar] [CrossRef]
- Vlašaná, K.; Betík, R.; Valterová, I.; Nečas, D.; Kotora, M. Enantioselective Allylations of Selected α,β,γ,δ-Unsaturated Aldehydes by Axially Chiral, N,N′-dioxides. Synthesis of the Left-hand Part of Papulacandin D. Curr. Organocatal. 2016, 3, 301–305. [Google Scholar]
- Neniškis, A.; Stončius, S. Enantioselective Ring Opening of meso-Epoxides with Silicon Tetrachloride Catalyzed by Pyridine N-Oxides Fused with the Bicyclo[3.3.1]nonane Framework. Eur. J. Org. Chem. 2015, 6359–6369. [Google Scholar]
- Kadlčíková, A.; Vlašaná, K.; Kotora, M. Enantioselective epoxide ring opening catalyzed by bis(tetrahydroisoquinoline) N,N′-dioxides. Collect. Czech. Chem. Commun. 2011, 76, 415–422. [Google Scholar] [CrossRef]
- Gnanamani, E.; Someshwar, N.; Sanjeevi, J.; Ramanathan, C.R. Conformationally Rigid Chiral Bicyclic Skeleton-Tethered Bipyridine N,N’-Dioxide as Organocatalyst: Asymmetric Ring Opening of meso-Epoxides. Adv. Synth. Catal. 2014, 356, 2219–2223. [Google Scholar] [CrossRef]
- Takenaka, N.; Saranghtem, R.S.; Captain, B. Helical chiral pyridine N-oxides: A new family of asymmetric catalysts. Angew. Chem. Int. Ed. 2008, 47, 9708–9710. [Google Scholar] [CrossRef]
- Chen, J.; Captain, B.; Takenaka, N. Helical Chiral 2,2′-Bipyridine N-Monoxides as Catalysts in the Enantioselective Propargylation of Aldehydes with Allenyltrichlorosilane. Org. Lett. 2011, 13, 1654–1657. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Takenaka, N. Applications of helical-chiral pyridines as organocatalysts in asymmetric synthesis. Chem. Rec. 2013, 13, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Bednářová, E.; Nečas, D.; Cisařová, I.; Dušek, M.; Lamaty, F.; Kotora, M. Synthesis of new bipyridine N,N’-dioxides and their application in asymmetric allylation of benzaldehyde and aldol addition to acetophenone. Monatsh. Chem. 2019, 150, 29–48. [Google Scholar] [CrossRef]
- Sugiura, M.; Sato, N.; Sonoda, Y.; Kotani, S.; Nakajima, M. Diastereo- and Enantioselective Reductive Aldol Reaction with Trichlorosilane Using Chiral Lewis Bases as Organocatalysts. Chem. Asian J. 2010, 5, 478–481. [Google Scholar] [CrossRef]
- Pei, Y.-N.; Deng, Y.; Li, J.-L.; Liu, L.; Zhu, H.-J. New chiral biscarboline N,N′-dioxide derivatives as catalyst in enantioselective reduction of ketoimines with trichlorosilane. Tetrahedron Lett. 2014, 55, 2948–2952. [Google Scholar] [CrossRef]
- Pan, W.; Deng, Y.; He, J.B.; Bai, B.; Zhu, H.J. Highly efficient asymmetric-axle-supported N-O amides in enantioselective hydrosilylation of ketimines with trichlorosilane. Tetrahedron 2013, 69, 7253–7257. [Google Scholar] [CrossRef]
- Jiao, Z.; Feng, X.; Liu, B.; Chen, F.; Zhang, G.; Jiang, Y. Enantioselective Strecker Reactions between Aldimines and Trimethylsilyl Cyanide Promoted by Chiral N,N′-Dioxides. Eur. J. Org. Chem. 2003, 3818–3826. [Google Scholar] [CrossRef]
- Hrdina, R.; Valterová, I.; Hodačová, J.; Cisařová, I.; Kotora, M. A Simple Approach to Unsymmetric Atropoisomeric Bipyridine N,N’-Dioxides and Their Application in Enantioselective Allylation of Aldehydes. Adv. Synth. Catal. 2007, 349, 822–826. [Google Scholar] [CrossRef]
- Hrdina, R.; Boyd, T.; Valterová, I.; Hodačová, J.; Kotora, M. Catalytic Asymmetric Allylation of Aliphatic Aldehydes by Chiral Bipyridine N,N′-Dioxides. Synlett 2008, 20, 3141–3144. [Google Scholar]
- Kadlčíková, A.; Hrdina, R.; Valterová, I.; Kotora, M. Simple and Fast Synthesis of New Axially Chiral Bipyridine N,N’-Dioxides for Highly Enantioselective Allylation of Aldehydes. Adv. Synth. Catal. 2009, 351, 1279–1283. [Google Scholar] [CrossRef]
- Kwak, J.; Ohk, J.; Jung, Y.; Chang, S. Rollover Cyclometalation Pathway in Rhodium Catalysis: Dramatic NHC Effects in the C–H Bond Functionalization. J. Am. Chem. Soc. 2012, 134, 17778–17788. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Shen, L.; Ren, J.; Zhu, H.-J. Chiral Biscarboline N,N’-Dioxide Derivatives: Highly Enantioselective Addition of Allyltrichlorosilane to Aldehydes. Adv. Synth. Catal. 2012, 354, 354–358. [Google Scholar] [CrossRef]
- Bai, B.; Zhu, H.-J.; Pan, W. Structure influence of chiral 1,1′-biscarboline-N,N′-dioxide on the enantioselective allylation of aldehydes with allyltrichlorosilanes. Tetrahedron 2012, 68, 6829–6836. [Google Scholar] [CrossRef]
- Deng, Y.; Pan, W.; Pei, Y.-N.; Li, J.-L.; Bai, B.; Zhu, H.-J. Addition of aldehydes with allyltrichlorosilane catalyzed by chiral bis-N–O secondary amides. Tetrahedron 2013, 69, 10431–10437. [Google Scholar] [CrossRef]
- Reep, C.; Morgante, P.; Peverati, R.; Takenaka, N. Axial-Chiral Biisoquinoline N,N′-Dioxides Bearing Polar Aromatic C-H Bonds as Catalysts in Sakurai-Hosomi-Denmark Allylation. Org. Lett. 2018, 20, 5757–5761. [Google Scholar] [CrossRef]
- Gnanamani, E.; Someshwar, N.; Ramanathan, C.R. Conformationally Rigid Chiral Pyridine N-Oxides as Organocatalyst: Asymmetric Allylation of Aldehydes. Adv. Synth. Catal. 2012, 354, 2101–2106. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Mucha, P.; Mlostoń, G.; Jurczak, J. Novel Chiral C2-Symmetric Bisimidazole-N-oxide s as Promising Organocatalysts for Enantioselective Allylation of Aromatic Aldehydes. Synlett. 2009, 11, 1757–1760. [Google Scholar]
- Boyd, D.R.; Sharma, N.D.; Sbircea, L.; Murphy, D.; Malone, J.F.; James, S.L.; Allen, C.C.R.; Hamilton, J.T.G. Chemoenzymatic synthesis of chiral 2,2′-bipyridine ligands and their N-oxide derivatives: Applications in the asymmetric aminolysis of epoxides and asymmetric allylation of aldehydes. Org. Biomol. Chem. 2010, 8, 1081–1090. [Google Scholar] [CrossRef]
- Eidamshaus, C.; Reissig, H.-U. Application of novel enantiopure hydroxymethyl-substituted pyridine derivatives in asymmetric catalysis. Tetrahedron: Asymmetry 2011, 22, 1644–1652. [Google Scholar] [CrossRef]
- Malkov, A.V.; Orsini, M.; Pernazza, D.; Muir, W.K.; Langer, V.; Meghani, P.; Kočovský, P. Chiral 2,2‘-Bipyridine-Type N-Monoxides as Organocatalysts in the Enantioselective Allylation of Aldehydes with Allyltrichlorosilane. Org. Lett. 2002, 4, 1047–1049. [Google Scholar] [CrossRef]
- Malkov, A.V.; Bell, M.; Orsini, M.; Pernazza, D.; Massa, A.; Herrmann, P.; Meghani, P.; Kočovský, P. New Lewis-Basic N-Oxides as Chiral Organocatalysts in Asymmetric Allylation of Aldehydes. J. Org. Chem. 2003, 68, 9659–9668. [Google Scholar] [CrossRef] [PubMed]
- Malkov, A.V.; Bell, M.; Vassieu, M.; Bugatti, V.; Kočovský, P. New pyridine-derived N-oxides as chiral organocatalysts in asymmetric allylation of aldehydes. J. Mol. Catal. A: Chem. 2003, 196, 179–186. [Google Scholar] [CrossRef]
- Malkov, A.V.; Westwater, M.-M.; Gutnov, A.; Ramírez-López, P.; Friscourt, F.; Kadlčiková, A.; Hodačová, J.; Rankovic, Z.; Kotora, M.; Kočovský, P. New pyridine N-oxides as chiral organocatalysts in the asymmetric allylation of aromatic aldehydes. Tetrahedron 2008, 64, 11335–11348. [Google Scholar] [CrossRef]
- Chelucci, G.; Baldino, S.; Pinna, G.A.; Benaglia, M.; Buffa, L.; Guizzetti, S. Chiral pyridine N-oxides derived from monoterpenes as organocatalysts for stereoselective reactions with allyltrichlorosilane and tetrachlorosilane. Tetrahedron 2008, 64, 7574–7582. [Google Scholar] [CrossRef]
- Chelucci, G.; Belmonte, N.; Benaglia, M.; Pignataro, L. Enantioselective allylation of aldehydes with allyltrichlorosilane promoted by new chiral dipyridylmethane N-oxides. Tetrahedron Lett. 2007, 48, 4037–4041. [Google Scholar] [CrossRef]
- Malkov, A.V.; Bell, M.; Catelluzzo, F.; Kočovský, P. METHOX: A New Pyridine N-Oxide Organocatalyst for the Asymmetric Allylation of Aldehydes with Allyltrichlorosilanes. Org. Lett. 2005, 7, 3219–3222. [Google Scholar] [CrossRef]
- Malkov, A.V.; Kysilka, O.; Edgar, M.; Kadlčiková, A.; Kotora, M.; Kočovský, P. A Novel Bifunctional Allyldisilane as a Triple Allylation Reagent in theStereoselective Synthesis of Trisubstituted Tetrahydrofurans. Chem. Eur. J. 2011, 17, 7162–7166. [Google Scholar] [CrossRef]
- Malkov, A.V.; Barłóg, M.; Jewkes, Y.; Míkušek, J.; Kočovský, P. Enantioselective Allylation of α,β-Unsaturated Aldehydes with Allyltrichlorosilane Catalyzed by METHOX. J. Org. Chem. 2011, 76, 4800–4804. [Google Scholar] [CrossRef]
- Hrdina, R.; Dračínský, M.; Valterová, I.; Hodačová, J.; Císařová, I.; Kotora, M. New Pathway to C2-Symmetric Atropoisomeric Bipyridine N,N’-Dioxides and Solvent Effect in Enantioselective Allylation of Aldehydes. Adv. Synth. Catal. 2008, 350, 1449–1456. [Google Scholar] [CrossRef]
- Vlašaná., K.; Hrdina, R.; Valterová, I.; Kotora, M. 51. Vlašaná. K.; Hrdina, R.; Valterová, I.; Kotora, M. Enantioselective Allylation of Aldehydes Catalyzed by Diastereoisomeric Bis(tetrahydroisoquinoline) N,N′-Dioxides. Eur. J. Org. Chem. 2010, 7040–7044. [Google Scholar]
- Hessler, F.; Betík, R.; Kadlčiková, A.; Belle, R.; Kotora, M. Enantioselective Allylation of Selected ortho-Substituted Benzaldehydes: A Comparative Study. Eur. J. Org. Chem. 2014, 7245–7252. [Google Scholar] [CrossRef]
- Zhao, H.; Yue, Y.; Li, H.; Sheng, Z.; Yang, Z.; Meng, W. Asymmetric Synthesis of Novel Axially Chiral 2,2′-Bipyridine N,N′-Dioxides Bearing α-Amino Acid Residues and Their Applications in Enantioselective Allylation of Aromatic Aldehydes with Allyltrichlorosilane. Chin. J. Chem. 2013, 31, 485–493. [Google Scholar] [CrossRef]
- Ulč, J.; Nečas, D.; Koukal, P.; Havlíček, V.; Tošner, Z.; Hybelbauerová, S.; Kotora, M. Chiral Unsymmetrically Substituted Bipyridine N,N′-Dioxides as Catalysts for the Allylation of Aldehydes. Eur. J. Org. Chem. 2018, 5109–5116. [Google Scholar] [CrossRef]
- Koukal, P.; Ulč, J.; Nečas, D.; Kotora, M. Enantioselective Allylation of β-Haloacrylaldehydes: Formal Total Syntheses of Pteroenone and Antillatoxin. Eur. J. Org. Chem. 2016, 2110–2114. [Google Scholar] [CrossRef]
- Fukazawa, Y.; Vaganov, V.Y.; Shipilovskikh, S.A.; Rubstov, A.E.; Malkov, A.V. Stereoselective Synthesis of Atropisomeric Bipyridine N,N′-Dioxides by Oxidative Coupling. Org. Lett. 2019, 21, 4798–4802. [Google Scholar] [CrossRef]
- Müller, C.A.; Hoffart, T.; Holbach, M.; Reggelin, M. Pyridyl N-Oxide Substituted Helically Chiral Poly(methacrylate)s in Asymmetric Organocatalysis. Macromolecules 2005, 38, 5375–5380. [Google Scholar] [CrossRef]
- Ando, M.; Ishidate, R.; Ikai, T.; Maeda, K.; Yashima, E. Helicity Induction and Its Static Memory of Poly(biphenylylacetylene)s Bearing Pyridine N-Oxide Groups and Their Use as Asymmetric Organocatalysts. J. Polym. Sci. 2019. [Google Scholar] [CrossRef]
- Ikai, T.; Yoshida, T. Synthesis of helical π-conjugated polymers bearing pyridine N-oxide pendants and asymmetric allylation of aldehydes in the helical cavity. Org. Biomol. Chem. 2019, 17, 8537–8540. [Google Scholar] [CrossRef]
- Fulton, J.R.; Glover, J.E.; Kamara, L.; Rowlands, G.J. Facile synthesis of planar chiral N-oxides and their use in Lewis base catalysis. Chem. Commun. 2011, 47, 433–435. [Google Scholar] [CrossRef]
- Chai, Q.; Song, C.; Sun, Z.; Ma, Y.; Ma, C.; Dai, Y.; Andrus, M.B. Asymmetric allylation of aldehydes with allyltrichlorosilane using aza-paracyclophane-oxazoline-N-oxide catalysts. Tetrahedron Lett. 2006, 47, 8611–8615. [Google Scholar] [CrossRef]
- Nakajima, M. Chiral N-Oxides as Catalysts or Ligands in Enantioselective Reactions. J. Synth. Org. Chem. Jpn. 2003, 61, 1081–1087. [Google Scholar] [CrossRef]
- Wong, W.-L.; Lee, W.-S.; Kwong, H.-L. Synthesis of new chiral terpyridine mono-N-oxide and di- N-oxide ligands and their applications in copper-catalyzed asymmetric cyclopropanation. Tetrahedron: Asymmetry 2002, 13, 1485–1492. [Google Scholar] [CrossRef]
- Liu, X.; Lin, L.; Feng, X. Chiral N,N′-Dioxides: New Ligands and Organocatalysts for Catalytic Asymmetric Reactions. ACC Chem. Res. 2011, 44, 574–587. [Google Scholar] [CrossRef]
- Liu, X.; Lin, L.; Feng, X. Chiral N,N′-dioxide ligands: Synthesis, coordination chemistry and asymmetric catalysis. Org. Chem. Front. 2014, 1, 298–302. [Google Scholar] [CrossRef]
- Wang, J.; Zuo, Y.; Hu, C.; Su, Z. heoretical and experimental studies on the structure–property relationship of chiral N,N′-dioxide–metal catalysts probed by the carbonyl–ene reaction of isatin. Catal. Sci. Technol. 2017, 7, 2183–2193. [Google Scholar] [CrossRef]
- Wolińska, E. A study of chiral oxazoline ligands with a 1,2,4-triazine and other six-membered aza-heteroaromatic rings and their application in Cu-catalysed asymmetric nitroaldol reactions. Tetrahedron Asymmetry 2014, 25, 1478–1487. [Google Scholar] [CrossRef]
- Wolińska, E. Chiral oxazoline ligands with two different six-membered azaheteroaromatic rings - synthesis and application in the Cu-catalyzed nitroaldol reaction. Heterocycl. Commun. 2016, 22, 85–94. [Google Scholar] [CrossRef]
- Wolińska, E.; Karczmarczyk, Z.; Wysocki, W. Structural characterization of copper complexes with chiral 1,2,4-triazine-oxazoline ligands. Heterocycl. Commun. 2016, 22, 265–274. [Google Scholar] [CrossRef]
- Larionov, O.V.; Stephens, D.; Mfuh, A.M.; Arman, H.D.; Naumova, A.S.; Chavez, G.; Skenderi, B. Insights into the mechanistic and synthetic aspects of the Mo/P-catalyzed oxidation of N-heterocycles. Org. Biomol. Chem. 2014, 12, 3026–3036. [Google Scholar] [CrossRef]
- Mfuh, A.M.; Larionov, O.V. Heterocyclic N-Oxides—An Emerging Class of Therapeutic Agents. Curr. Med. Chem. 2015, 22, 2819–2857. [Google Scholar] [CrossRef]
- Herrmann, A.; Hedman, H.; Rosén, J.; Jansson, D.; Haraldsson, B.; Hellenäs, K.-E. Analysis of the Mushroom Nephrotoxin Orellanine and Its Glucosides. J. Nat. Prod. 2012, 75, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Limnios, D.; Kokotos, C.G. 2,2,2-Trifluoroacetophenone as an Organocatalyst for the Oxidation of Tertiary Amines and Azines to N-Oxides. Chem. Eur. J. 2014, 20, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Pietra, S. Heterocyclic N-Oxides, 1st ed.; CRC Press: Boca Raton, FL, USA, 1991; pp. 285–287. [Google Scholar]
- Yang, J.; Chu, S.; Guo, Y.; Luo, L.; Kong, F.; Wang, Y.; Zou, Z. Hyperbranched polymeric N-oxide: A novel kind of metal-free photocatalyst. Chem. Commun. 2012, 48, 3533–3535. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W.; Wang, J.; Lu, H.; Liu, Y.; Liu, Z.; Xie, Z. Light-induced synthesis of triazine N-oxide-based cross-linked polymers for effective photocatalytic degradation of methyl orange. RSC Adv. 2017, 7, 9309–9315. [Google Scholar] [CrossRef]
- Toma, O.; Mercier, N.; Allain, M.; Meinardi, F.; Botta, C. Lead(II) 4,4’-Bipyridine N-Oxide Coordination Polymers—Highly Phosphorescent Materials with Mechanochromic Luminescence Properties. Eur. J. Inorg. Chem. 2017, 844–850. [Google Scholar]
- Shevate, R.; Karunakaran, M.; Kumar, M.; Peinemann, K.-V. Polyanionic pH-responsive polystyrene-b-poly(4-vinyl pyridine-N-oxide) isoporous membranes. J. Membrane Sci. 2016, 501, 161–168. [Google Scholar] [CrossRef]
- Krysztof, M.; Olejnik, K.; Kulpinski, P.; Stanislawska, A.; Khadzhynova, S. Regenerated cellulose from N-methylmorpholine N-oxide solutions as a coating agent for paper materials. Cellulose 2018, 25, 3595–3607. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).