Crystallographic and NMR Investigation of Ergometrine and Methylergometrine, Two Alkaloids from Claviceps Purpurea
Abstract
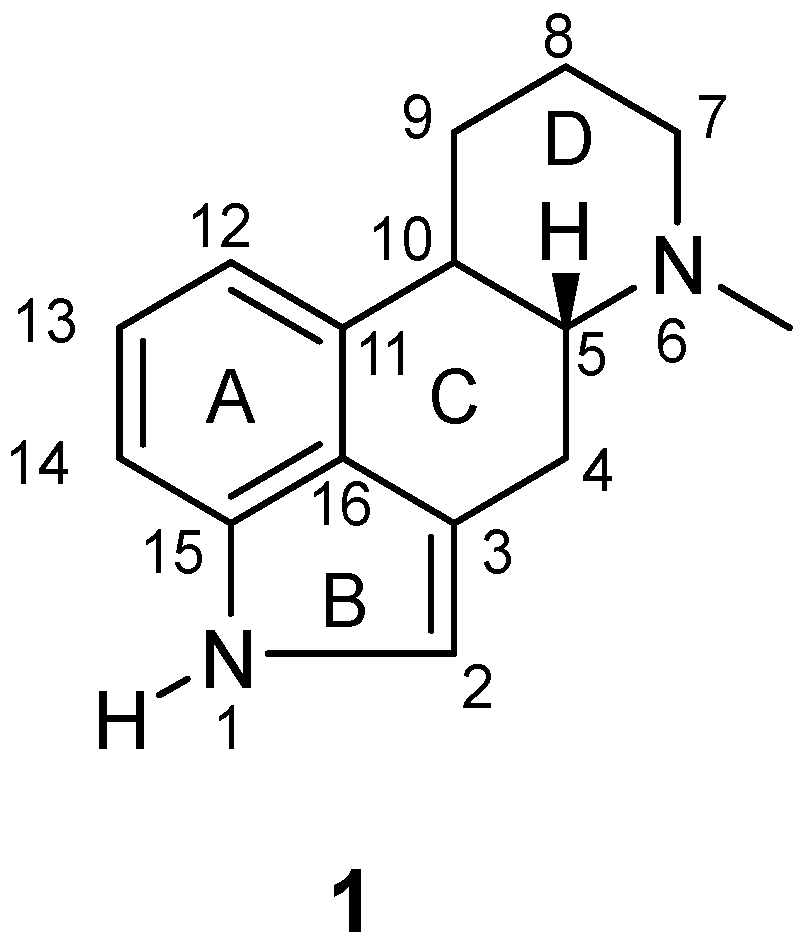
1. Introduction
2. Results and Discussion
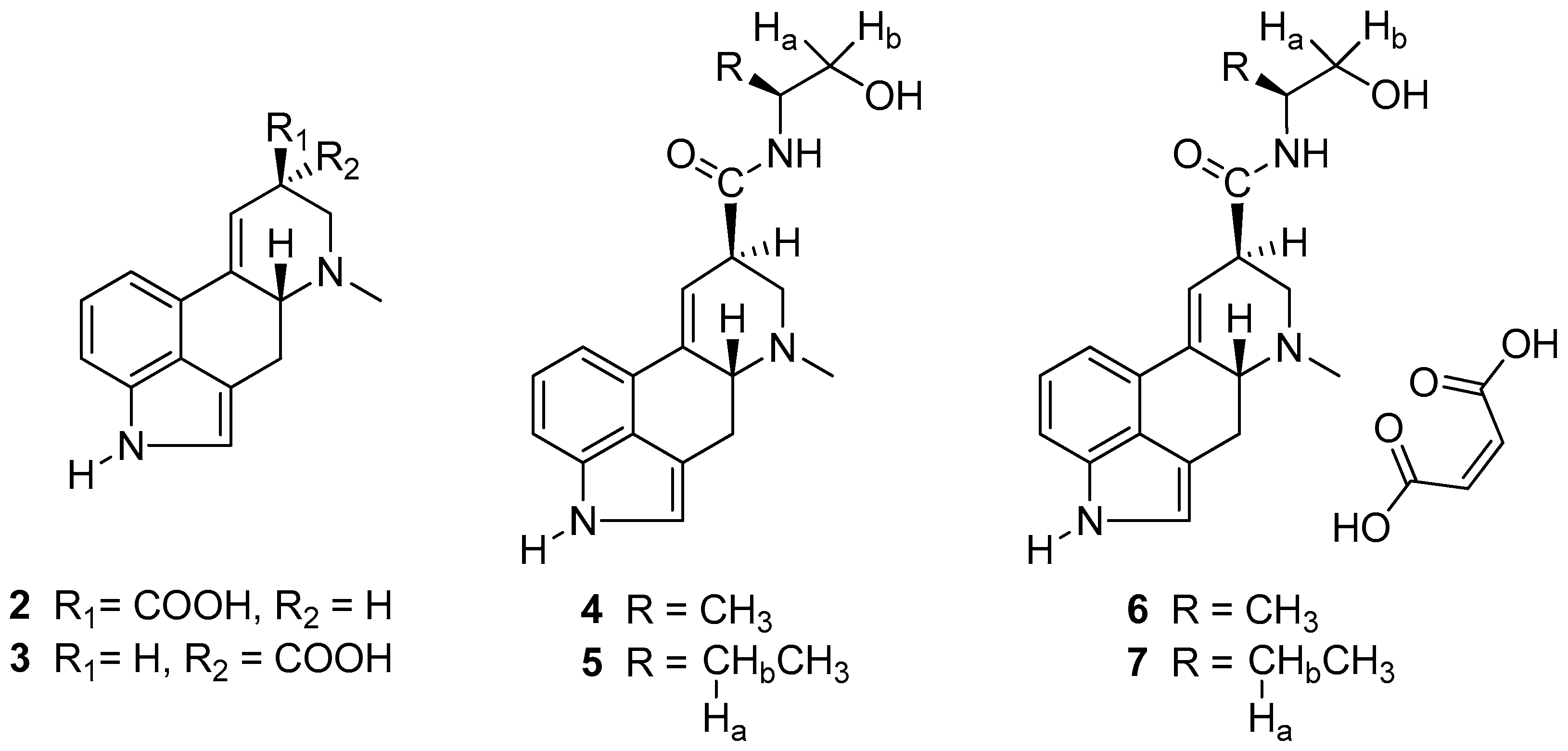
2.1. NMR Spectroscopy
2.2. X-ray Analysis
3. Materials and Methods
3.1. Chemistry
3.1.1. Ergometrine 4 from the Corresponding Maleate 6
3.1.2. Methylergometrine 5 from the Corresponding Maleate 7
3.2. NMR Spectroscopy
3.3. X-ray Crystallography
3.4. Optical Rotatory Power
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- De Groot, A.N.; van Dongen, P.W.; Vree, T.B.; Hekster, Y.A.; van Roosmalen, J. Ergot alkaloids. Current status and review of clinical pharmacology and therapeutic use compared with other oxytocics in obstetrics and gynaecology. Drugs 1998, 56, 523–535. [Google Scholar] [PubMed]
- Rutschmann, J.; Stadler, P.A. Chemical Background. In Ergot Alkaloids and Related Compounds; Berde, B.S., Ed.; Springer: Berlin, Germany, 1978; pp. 29–85. [Google Scholar]
- Moir, J.C. Ergot: From “St. Anthony’s Fire” to the isolation of its active principle, ergometrine (ergonovine). Am. J. Obstet. Gynecol. 1974, 120, 291–296. [Google Scholar] [CrossRef]
- Rall, T.W. Drug affecting uterine motility: Oxitocyn, prostaglandins, ergot alkaloids and other drugs. Tocolytic agents. In Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 8th ed.; MacMillan: New York, NY, USA, 1990; pp. 933–953. [Google Scholar]
- Dudley, H.W.; Moir, J.C. THE new active principle of ergot. Science 1935, 81, 559–560. [Google Scholar] [CrossRef] [PubMed]
- Stoll, A.; Hofmann, A. Ergot alkaloids. VI. Partial synthesis of ergobasine type alkaloids. Helv. Chim. Acta 1943, 26, 944–965. [Google Scholar] [CrossRef]
- Liu, G.; Huang, H.; Wu, X.; Chen, D.; Ren, J.; Li, Y.; Su, Z. Preparation of Ergometrine. Patent CN106866657A, 25 April 2017. [Google Scholar]
- Liu, G.; Wu, X.; Huang, H.; Li, Y.; Su, Z. Preparation of Racemic Lysergic Acid by utilizing fermn. Waste and Its Application for Preparation of Ergometrine. Patent CN106397429A, 15 February 2017. [Google Scholar]
- Bach, N.J.; E Boaz, H.; Kornfeld, E.C.; Chang, C.J.; Floss, H.G.; Hagaman, E.W.; Wenkert, E. Nuclear magnetic resonance spectral analysis of the ergot alkaloids. J. Org. Chem. 1974, 39, 1272–1276. [Google Scholar] [CrossRef] [PubMed]
- Cejka, J.; Hušák, M.; Kratochvíl, B.; Jegorov, A.; Cvak, L. Crystal Structures of Ergot Alkaloid Derivatives. Ergometrine Maleate and Methylergometrine Maleate. Collect. Czechoslov. Chem. Commun. 1996, 61, 1396–1404. [Google Scholar] [CrossRef]
- Casy, A.F. Rapid identification of ergot derivatives by 1H-NMR spectroscopy. J. Pharm. Biomed. Anal. 1994, 12, 27–40. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J. Novel Methysergide Derivatives. Patent WO2014100354A1, 26 June 2014. [Google Scholar]
- He, Q.; Zhang, M.; Wu, J.; Zuo, W.; Sun, S.; Jia, C.; Xia, T. Methylergometrine Maleate Compound and Preparation Method. Patent CN105085514A, 25 November 2015. [Google Scholar]
- Rohlíček, J.; Hušák, M.; Kratochvíl, B.; Jegorov, A. Methyl-ergometrine maleate from synchrotron powder diffraction data. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, o3252–o3253. [Google Scholar] [CrossRef] [PubMed]
- Ferraboschi, P.; Ciuffreda, P.; Ciceri, S.; Grisenti, P.; Castellano, C.; Meneghetti, F. Crystallographic and spectroscopic study on a known orally active progestin. Steroids 2015, 104, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Ferraboschi, P.; Colombo, D.; Legnani, L.; Toma, L.; Grisenti, P.; Vistoli, G.; Meneghetti, F. Crystallographic, Spectroscopic, and Theoretical Investigation of the Efficiently Separated 21R and 21S-Diastereoisomers of Argatroban. Chirality 2013, 25, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Ferraboschi, P.; Sala, M.C.; Stradi, R.; Ragonesi, L.; Gagliardi, C.; Lanzarotti, P.; Ragg, E.M.; Mori, M.; Meneghetti, F. Full spectroscopic characterization of two crystal pseudopolymorphic forms of the antiandrogen cortexolone 17 alpha-propionate for topic application. Steroids 2017, 128, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Kidric, J.; Kocjan, D.; Hadzi, D. Conformational-Analysis of the D-Ring of Lysergic-Acid Amides and Its Bioactive Conformation. Croat. Chem. Acta 1985, 58, 389–397. [Google Scholar]
- Farrugia, L.J. ORTEP -3 for Windows—A version of ORTEP -III with a Graphical User Interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]
- Husak, M.; Kratochvíl, B.; Jegorov, A. Crystal structure of ergometrine ethyl acetate solvate (2:1), (C19H23N3O2)2(C4H8O2). Z. Krist. New Cryst. Struct 1998, 213, 195–196. [Google Scholar]
- Cremer, D.; Pople, J.A. General definition of ring puckering coordinates. J. Am. Chem. Soc. 1975, 97, 1354–1358. [Google Scholar] [CrossRef]
- Merkel, S.; Koppen, R.; Koch, M.; Emmerling, F.; Nehls, I. Ergometrinine. Acta Crystallogr. Sect. E Struct. Rep. Online 2010, 66, o2275. [Google Scholar] [CrossRef] [PubMed]
- Bruker. SAINT Software Reference Manual, version 6; Bruker AXS Inc.: Madison, WI, USA, 2003. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 4–7 are available from the authors. |



| 1H | 4 (DMSO-d6) | 5 (DMSO-d6) | 6 (D2O) | 7 (D2O) |
|---|---|---|---|---|
| H-1 | 10.69 (brs) | 10.70 (brs) | − | − |
| H-2 | 7.02 (brs) | 7.02 (brs, partially overlapped) | 7.15 (brs) | 7.16 (brs) |
| H-4α | 2.44 (overlapped) | 2.45 (overlapped) | 2.89 (brt, J = 13.1) | 2.91 (brt, J = 13.1) |
| H-4β | 3.46 (dd, J = 14.9, 5.7) | 3.46 (dd, J = 14.7, 5.5) | 3.69 (overlapped) | 3.73 (overlapped) |
| H-5β | 3.00–2.93 (m) | 3.00–2.93 (m) | 3.92 (br) | 3.99 (br) |
| H-7β | 2.50 (overlapped with DMSO) | 2.50 (overlapped with DMSO) | 3.47 (brt, 10.8) | 3.49 (brt, J = 11.7) |
| H-7α | 3.00 (dd, J = 11.0, 5.0) | 3.02 (dd, J = 11.2, 5.3) | 3.77 (br) | 3.79 (br) |
| H-8α | 3.40–3.36 (m, overlapped) | 3.47–3.42 (m, overlapped) | 3.83 (br) | 3.89 (overlapped) |
| H-9 | 6.34 (brs) | 6.35 (brs) | 6.46 (brs) | 6.49 (brs) |
| H-12 | 7.04 (overlapped) | 7.03 (brdd, J = 8.2, 0.8, partially overlapped) | 7.22 (d, J = 7.3) | 7.23 (d, J = 7.1) |
| H-13 | 7.06 (tripletoid m) | 7.06 (tripletoid m) | 7.26 (tripletoid m) | 7.27 (tripletoid m) |
| H-14 | 7.18 (dd, J = 6.9, 1.1) | 7.18 (dd, J = 7.4, 0.9) | 7.42 (d, J = 7.8) | 7.43 (d, J = 7.6) |
| N-CH3 | 2.44 (s) | 2.45 (s) | 3.11 (s) | 3.13 (s) |
| CH | 3.86–3.76 (m) | 3.71–3.62 (m) | 4.10-4.02 (m) | 3.94–3.84 (m) |
| CH3 | 1.05 (d, J = 6.6) | 0.85 (t, J = 7.4) | 1.21 (d, J = 6.9) | 0.95 (t, J = 7.1) |
| CHaCH3 | − | 1.39–1.26 (m) | − | 1.54–1.42 (m) |
| CHbCH3 | − | 1.66–1.53 (m) | − | 1.73–1.62 (m) |
| CHaOH | 3.32–3.25 (m) | 3.37–3.30 (m) | 3.61 (dd, J = 11.5, 6.6) | 3.63 (dd, J = 11.7, 6.6) |
| CHbOH | 3.43–3.36 (m) | 3.42–3.38 (m) | 3.71 (dd, J = 11.7, 6.0) | 3.73 (dd, J = 11.5, 4.6) |
| CH2OH | 4.72 (brs) | 4.68 (t, J = 5.7) | − | − |
| CONH | 7.76 (d, J=7.8) | 7.69 (d, J = 8.5) | − | − |
| CH=CH maleate | − | − | 6.21 (s) | 6.23 (s) |
| 13C | 4 (DMSO-d6) | 5 (DMSO-d6) | 6 (D2O) | 7 (D2O) |
|---|---|---|---|---|
| C-2 | 119.2 | 119.2 | 121.5 | 121.5 |
| C-3 | 109.0 | 109.0 | 106.4 | 106.4 |
| C-4 | 26.8 | 26.8 | 24.8 | 24.7 |
| C-5 | 62.6 | 62.6 | 62.8 | 62.7 |
| C-7 | 55.5 | 55.7 | 54.1 | 54.1 (overlapped) |
| C-8 | 42.8 | 42.9 | 41.4 | 41.5 |
| C-9 | 120.3 | 120.4 | 118.7 | 118.7 |
| C-10 | 135.1 | 135.0 | 132.6 | 132.6 |
| C-11 | 127.4 | 127.5 | 125.1 | 125.2 |
| C-12 | 111.0 | 111.0 | 113.2 | 113.2 |
| C-13 | 122.2 | 122.3 | 124.0 | 124.0 |
| C-14 | 109.7 | 109.7 | 112.2 | 112.2 |
| C-15 | 133.8 | 133.9 | 134.3 | 134.3 |
| C-16 | 125.8 | 125.8 | 125.7 | 125.7 |
| N-CH3 | 43.4 | 43.4 | 42.1 | 42.0 |
| CH | 46.5 | 52.1 | 48.3 | 54.2 (overlapped) |
| CH3 | 17.2 | 10.5 | 16.5 | 10.3 |
| CH2CH3 | − | 23.7 | − | 24.0 |
| CH2OH | 64.4 | 63.0 | 65.0 | 63.8 |
| CH=CH maleate | − | − | 134.9 | 134.9 |
| COOH | − | − | 171.5 | 171.6 |
| CONH | 171.2 | 171.7 | 171.8 | 172.4 |
| 4 (DMSO-d6) | 5 (DMSO-d6) | 6 (D2O) | 7 (D2O) | |
|---|---|---|---|---|
| N-1 | −251.1 (128.4) | −251.0 (128.5) | −253.3 (126.2) | −253.3 (126.2) |
| N-6 | −340.3 (39.2) | −340.2 (39.3) | −332.8 (46.7) | −332.7 (46.8) |
| CONH | −255.5 (124.0) | −258.1 (121.4) | −248.3 (131.2) | −251.4 (128.1) |
| Quaternary Carbon | HMBC (C→H) | |
|---|---|---|
| 4 and 5 (DMSO-d6) | 6 and 7 (D2O) | |
| C-3 | H-4α, H-4β, H-2, H-1 | H-4α, H-2 |
| C-10 | H-13, H-12, H-5, H-4α, H-4β | H-13, H-12, H-4α |
| C-11 | H-13, H-12, H-9 | H-13, H-9 |
| C-15 | H-14, H-13, H-2, H-1 | H-13, H-12, H-2 |
| C-16 | H-14, H-12, H-4β, H-2, H-1 | H-14, H-12, H-4α, H-2 |
| 6 | 7 | |||||
|---|---|---|---|---|---|---|
| H-Bond | H···A Distance (Å) | D···A Distance (Å) | D-H···A Angle (°) | H···A Distance (Å) | D···A Distance (Å) | D-H···A Angle (°) |
| N2-H···O3 | 1.8(1) | 2.7(1) | 176(1) | 1.8(1) | 3.2(1) | 168(1) |
| N1-H···O2 I | 2.9(1) | 3.5(1) | 140(1) | - | - | |
| C22-H···O6 II | 2.7(1) | 3.3(1) | 134(1) | 2.7(1)V | 3.3(1) | 130(1) |
| C21-H···O4 II | 2.8 (1) | 3.7(1) | 171(1) | - | - | |
| C21-H···O6 II | 2.7(1) | 3.4(1) | 132(1) | 2.6 (1) V | 3.3(1) | 133(1) |
| O2-H···O1 II | 2.2(1) | 2.8(1) | 131(1) | - | - | |
| N1-H···O1 III | 2.1(1) | 2.8(1) | 155(1) | - | - | |
| N3-H···O5 IV | 2.1(1) | 2.9(1) | 163(1) | 2.1(1) VI | 2.(1) | 172(1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meneghetti, F.; Ferraboschi, P.; Grisenti, P.; Reza Elahi, S.; Mori, M.; Ciceri, S. Crystallographic and NMR Investigation of Ergometrine and Methylergometrine, Two Alkaloids from Claviceps Purpurea. Molecules 2020, 25, 331. https://doi.org/10.3390/molecules25020331
Meneghetti F, Ferraboschi P, Grisenti P, Reza Elahi S, Mori M, Ciceri S. Crystallographic and NMR Investigation of Ergometrine and Methylergometrine, Two Alkaloids from Claviceps Purpurea. Molecules. 2020; 25(2):331. https://doi.org/10.3390/molecules25020331
Chicago/Turabian StyleMeneghetti, Fiorella, Patrizia Ferraboschi, Paride Grisenti, Shahrzad Reza Elahi, Matteo Mori, and Samuele Ciceri. 2020. "Crystallographic and NMR Investigation of Ergometrine and Methylergometrine, Two Alkaloids from Claviceps Purpurea" Molecules 25, no. 2: 331. https://doi.org/10.3390/molecules25020331
APA StyleMeneghetti, F., Ferraboschi, P., Grisenti, P., Reza Elahi, S., Mori, M., & Ciceri, S. (2020). Crystallographic and NMR Investigation of Ergometrine and Methylergometrine, Two Alkaloids from Claviceps Purpurea. Molecules, 25(2), 331. https://doi.org/10.3390/molecules25020331







