3-(6-Phenylimidazo [2,1-b][1,3,4]thiadiazol-2-yl)-1H-Indole Derivatives as New Anticancer Agents in the Treatment of Pancreatic Ductal Adenocarcinoma
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Studies
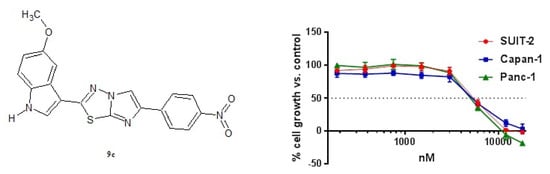
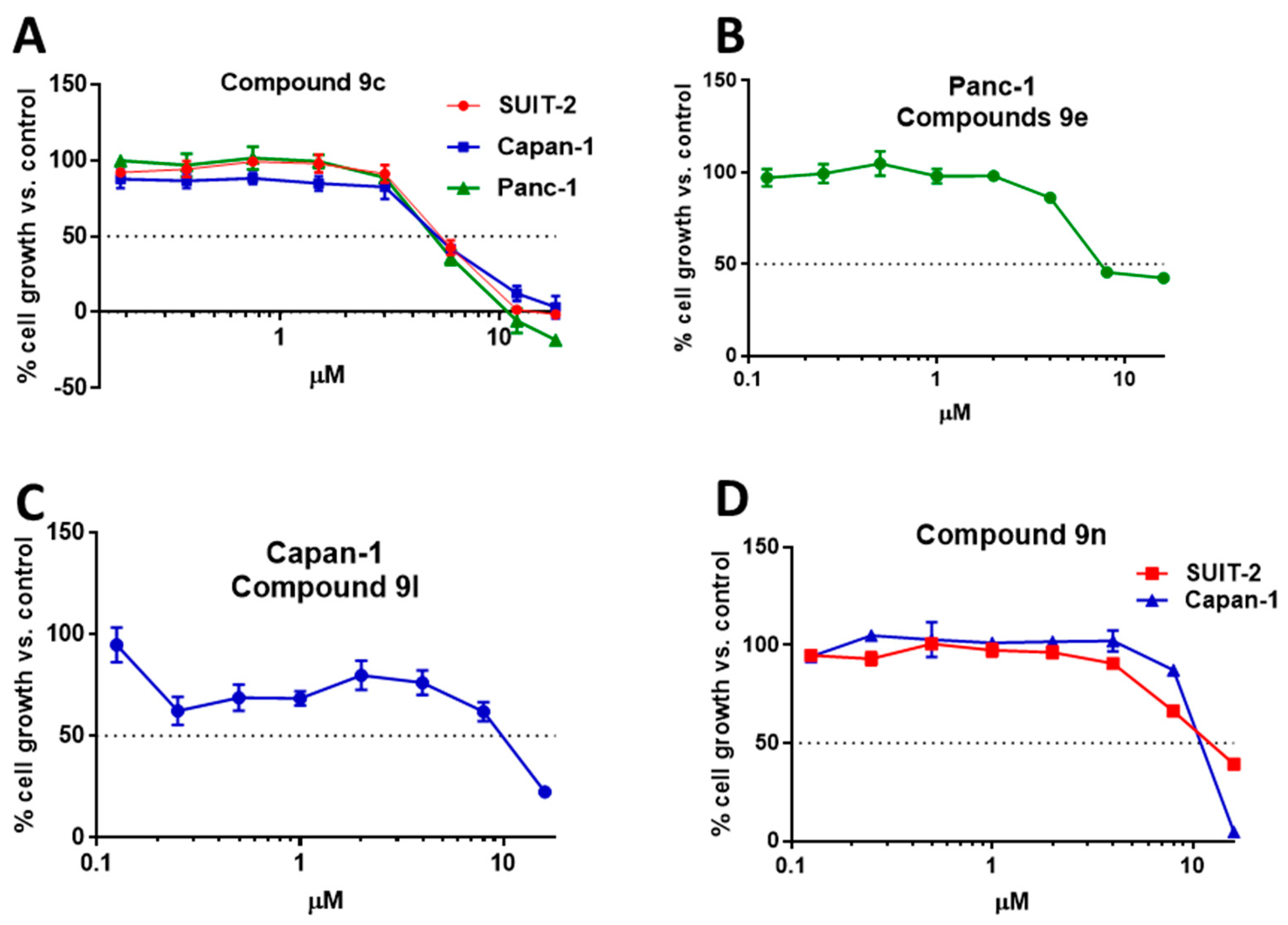
2.2.1. Antiproliferative Activity of the New Imidazothiadiazole Compounds 9a–p on SUIT-2, Capan-1 and Panc-1 Pancreatic Cancer Cells
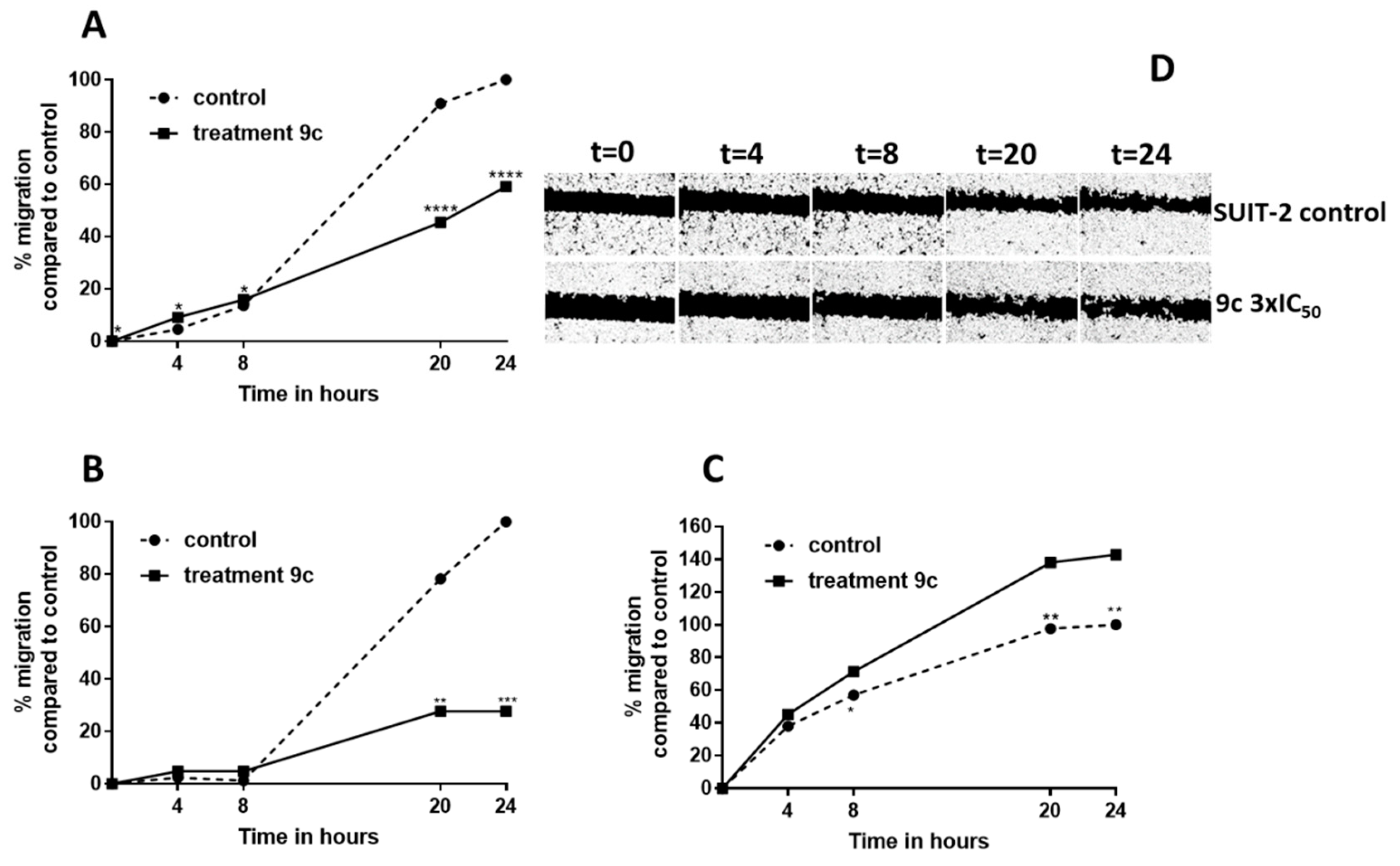
2.2.2. Compound 9c Inhibited the Migration Rate in SUIT-2, Capan-1 and Panc-1 Cells
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis of 1H-indole-3-carbonitriles (5a,b)
3.1.2. Synthesis of 1-methylindole-3-carbonitriles (6a,b)
3.1.3. Synthesis of 5-(1H-indol-3-yl)-1,3,4-thiadiazol-2-amines (7a–d)
5-(5-Methoxy-1H-indol-3-yl)-1,3,4-thiadiazol-2-amine (7a)
5-(5-Methoxy-1-methyl-1H-indol-3-yl)-1,3,4-thiadiazol-2-amine (7b)
5-(5-Fluoro-1H-indol-3-yl)-1,3,4-thiadiazol-2-amine (7c)
5-(5-Fluoro-1-methyl-1H-indol-3-yl)-1,3,4-thiadiazol-2-amine (7d)
3.1.4. Synthesis of 3-(6-phenylimidazo[2,1-b][1,3,4]thiadiazol-2-yl)-1H-indole hydrobromides (9a–p)
5-Methoxy-3-(6-phenylimidazo[2,1-b][1,3,4]thiadiazol-2-yl)-1H-indole hydrobromide 9a
5-Methoxy-3-[6-(4-fluorophenyl)imidazo[2,1-b][1,3,4]thiadiazol-2-yl]-5-methoxy-1H-indole hydrobromide 9b
5-Methoxy-3-[6-(4-nitrophenyl)imidazo[2,1-b][1,3,4]thiadiazol-2-yl]-1H-indole 9c
5-Methoxy-3-[6-(3-methoxyphenyl)imidazo[2,1-b][1,3,4]thiadiazol-2-yl]-1H-indole hydrobromide 9d
5-Methoxy-3-[6-(2,5-dimethoxyphenyl)imidazo[2,1-b][1,3,4]thiadiazol-2-yl]-5-methoxy-1H-indole 9e
5-Methoxy-3-{6-[4-(trifluoromethyl)phenyl]imidazo[2,1-b][1,3,4]thiadiazol-2-yl}-1H-indole hydrobromide 9f
5-Methoxy-1-methyl-3-(6-phenylimidazo[2,1-b][1,3,4]thiadiazol-2-yl)-1H-indole hydrobromide 9g
5-Methoxy-3-[6-(4-fluorophenyl)imidazo[2,1-b][1,3,4]thiadiazol-2-yl]-5-methoxy-1-methyl-1H-indole hydrobromide 9h
5-Methoxy-1-methyl-3-[6-(3-methoxyphenyl)imidazo[2,1-b][1,3,4]thiadiazol-2-yl]-1H-indole hydrobromide 9i
5-Methoxy-3-[6-(2,5-dimethoxyphenyl)imidazo[2,1-b][1,3,4]thiadiazol-2-yl]-5-methoxy-1-methyl-1H-indole 9j
5-Methoxy-1-methyl-3-{6-[4-(trifluoromethyl)phenyl]imidazo[2,1-b][1,3,4]thiadiazol-2-yl}-1H-indole 9k
5-Fluoro-3-(6-phenylimidazo[2,1-b][1,3,4]thiadiazol-2-yl)-1H-indole hydrobromide 9l
5-Fluoro-3-[6-(3-methoxyphenyl)imidazo[2,1-b][1,3,4]thiadiazol-2-yl]-1H-indole hydrobromide 9m
5-Fluoro-3-{6-[4-(trifluoromethyl)phenyl]imidazo[2,1-b][1,3,4]thiadiazol-2-yl}-1H-indole hydrobromide 9n
5-Fluoro-3-[6-(2,5-dimethoxyphenyl)imidazo[2,1-b][1,3,4]thiadiazol-2-yl]-1H-indole hydrobromide 9o
5-Fluoro-1-methyl-3-{6-[4-(trifluoromethyl)phenyl]imidazo[2,1-b][1,3,4]thiadiazol-2-yl}-1H-indole hydrobromide 9p
3.2. Biology
3.2.1. Drugs and Chemical
3.2.2. Cell Cultures
3.2.3. Cell Growth Inhibition
3.2.4. Wound-Healing Assays
3.2.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Siegel, R.L.; Rosenberg, P.S.; Jemal, A. Emerging cancer trends among young adults in the USA: Analysis of a population-based cancer registry. Lancet Public Health 2019, 4, e137–e147. [Google Scholar] [CrossRef]
- Jadhav, V.B.; Kulkarni, M.V.; Rasal, V.P.; Biradar, S.S.; Vinay, M.D. Synthesis and anti-inflammatory evaluation of methylene bridged benzofuranyl imidazo[2,1-b][1,3,4] thiadiazoles. Eur. J. Med. Chem. 2008, 43, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Kettle, C.; Morton, D.W. A molecular approach in drug development for Alzheimer’s disease. Biomed. Pharmacother. 2018, 106, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Tahghighi, A.; Razmi, S.; Mahdavi, M.; Foroumadi, P.; Ardestani, S.K.; Emami, S.; Kobarfard, F.; Dastmalchi, S.; Shafiee, A.; Foroumadi, A. Synthesis and anti-leishmanial activity of 5-(5-nitrofuran-2-yl)-1,3,4-thiadiazol-2-amines containing N-[(1-benzyl-1H-1,2,3-triazol-4-yl) methyl] moieties. Eur. J. Med. Chem. 2012, 50, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Jakovljević, K.; Matić, I.Z.; Stanojković, T.; Krivokuća, A.; Marković, V.; Joksović, M.D.; Mihailović, N.; Nićiforović, M.; Joksović, L. Synthesis, antioxidant and antiproliferative activities of 1,3,4-thiadiazoles derived from phenolic acids. Bioorg. Med. Chem. Lett. 2017, 27, 3709–3715. [Google Scholar] [CrossRef]
- Alegaon, S.G.; Alagawadi, K.R.; Sonkusare, P.V.; Chaudhary, S.M.; Dadwe, D.H.; Shah, A.S. Novel imidazo[2,1-b] [1,3,4] thiadiazole carrying rhodanine-3-acetic acid as potential antitubercular agents. Bioorg. Med. Chem. Lett. 2012, 22, 1917–1921. [Google Scholar] [CrossRef]
- Bhongade, B.A.; Talath, S.; Gadad, R.A.; Gadad, A.K. Biological activities of imidazo[2,1-b] [1,3,4] thiadiazole derivatives: A review. J. Saudi Chem. Soc. 2016, 20, S463–S475. [Google Scholar] [CrossRef]
- Schillaci, D.; Spanò, V.; Parrino, B.; Carbone, A.; Montalbano, A.; Barraja, P.; Diana, P.; Cirrincione, G.; Cascioferro, S. Pharmaceutical Approaches to Target Antibiotic Resistance Mechanisms. J. Med. Chem. 2017, 60, 8268–8297. [Google Scholar] [CrossRef]
- Cascioferro, S.; Parrino, B.; Petri, G.L.; Cusimano, M.G.; Schillaci, D.; Di Sarno, V.; Musella, S.; Giovannetti, E.; Cirrincione, G.; Diana, P. 2,6-Disubstituted imidazo[2,1-b] [1,3,4] thiadiazole derivatives as potent staphylococcal biofilm inhibitors. Eur. J. Med. Chem. 2019, 167, 200–210. [Google Scholar] [CrossRef]
- Parrino, B.; Schillaci, D.; Carnevale, I.; Giovannetti, E.; Diana, P.; Cirrincione, G.; Cascioferro, S. Synthetic small molecules as anti-biofilm agents in the struggle against antibiotic resistance. Eur. J. Med. Chem. 2019, 161, 154–178. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.S.; Panjamurthy, K.; Kumar, S.; Nambiar, M.; Ramareddy, S.A.; Chiruvella, K.K.; Raghavan, S.C. Synthesis and biological evaluation of novel 2-aralkyl-5-substituted-6-(4′-fluorophenyl)-imidazo[2,1-b] [1,3,4] thiadiazole derivatives as potent anticancer agents. Eur. J. Med. Chem. 2011, 46, 2109–2116. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Hegde, M.; Gopalakrishnan, V.; Renuka, V.K.; Ramareddy, S.A.; De Clercq, E.; Schols, D.; Gudibabande Narasimhamurthy, A.K.; Raghavan, S.C.; Karki, S.S. 2-(4-Chlorobenzyl)-6-arylimidazo[2,1-b] [1,3,4] thiadiazoles: Synthesis, cytotoxic activity and mechanism of action. Eur. J. Med. Chem. 2014, 84, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Arjomandi, O.K.; Hussein, W.M.; Vella, P.; Yusof, Y.; Sidjabat, H.E.; Schenk, G.; McGeary, R.P. Design, synthesis, and in vitro and biological evaluation of potent amino acid-derived thiol inhibitors of the metallo-β-lactamase IMP-1. Eur. J. Med. Chem. 2016, 114, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, R.; Baraldi, P.G.; Prencipe, F.; Balzarini, J.; Liekens, S.; Estévez, F. Design, synthesis and antiproliferative activity of novel heterobivalent hybrids based on imidazo[2,1-b] [1,3,4] thiadiazole and imidazo[2,1-b] [1,3] thiazole scaffolds. Eur. J. Med. Chem. 2015, 101, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Gopalakrishnan, V.; Hegde, M.; Rana, V.; Dhepe, S.S.; Ramareddy, S.A.; Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M.; et al. Synthesis and antiproliferative activity of imidazo[2,1-b] [1,3,4] thiadiazole derivatives. Bioorg. Med. Chem. Lett. 2014, 24, 4682–4688. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.M.; Sing, B.; Bhardwaj, V.; Palkar, M.; Shaikh, M.S.; Rane, R.; Alwan, W.S.; Gadad, A.K.; Noolvi, M.N.; Karpoormath, R. Design, synthesis and evaluation of small molecule imidazo[2,1-b] [1,3,4] thiadiazoles as inhibitors of transforming growth factor-β type-I receptor kinase (ALK5). Eur. J. Med. Chem. 2015, 93, 599–613. [Google Scholar] [CrossRef]
- Parrino, B.; Carbone, A.; Ciancimino, C.; Spanò, V.; Montalbano, A.; Barraja, P.; Cirrincione, G.; Diana, P.; Sissi, C.; Palumbo, M.; et al. Water-soluble isoindolo[2,1-a] quinoxalin-6-imines: In vitro antiproliferative activity and molecular mechanism(s) of action. Eur. J. Med. Chem. 2015, 94, 149–162. [Google Scholar] [CrossRef]
- Parrino, B.; Ullo, S.; Attanzio, A.; Cascioferro, S.; Spanò, V.; Carbone, A.; Montalbano, A.; Barraja, P.; Cirrincione, G.; Tesoriere, L.; et al. Synthesis of 5H-pyrido[3,2-b] pyrrolizin-5-one tripentone analogs with antitumor activity. Eur. J. Med. Chem. 2018, 158, 236–246. [Google Scholar] [CrossRef]
- Diana, P.; Stagno, A.; Barraja, P.; Montalbano, A.; Carbone, A.; Parrino, B.; Cirrincione, G. Synthesis of the new ring system pyrrolizino[2,3-b] indol-4(5H)-one. Tetrahedron 2011, 67, 3374–3379. [Google Scholar] [CrossRef]
- Parrino, B.; Carbone, A.; Spanò, V.; Montalbano, A.; Giallombardo, D.; Barraja, P.; Attanzio, A.; Tesoriere, L.; Sissi, C.; Palumbo, M.; et al. Aza-isoindolo and isoindolo-azaquinoxaline derivatives with antiproliferative activity. Eur. J. Med. Chem. 2015, 94, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Diana, P.; Stagno, A.; Barraja, P.; Carbone, A.; Parrino, B.; Dall’Acqua, F.; Vedaldi, D.; Salvador, A.; Brun, P.; Castagliuolo, I.; et al. Synthesis of triazenoazaindoles: A new class of triazenes with antitumor activity. ChemMedChem 2011, 6, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Parrino, B.; Ciancimino, C.; Carbone, A.; Spano’, V.; Montalbano, A.; Barraja, P.; Cirrincione, G.; Diana, P. Synthesis of isoindolo [1,4] benzoxazinone and isoindolo [1,5] benzoxazepine: Two new ring systems of pharmaceutical interest. Tetrahedron 2015, 71, 7332–7338. [Google Scholar] [CrossRef]
- Cascioferro, S.; Attanzio, A.; Di Sarno, V.; Musella, S.; Tesoriere, L.; Cirrincione, G.; Diana, P.; Parrino, B. New 1,2,4-Oxadiazole Nortopsentin Derivatives with Cytotoxic Activity. Mar. Drugs 2019, 17, 35. [Google Scholar] [CrossRef]
- Parrino, B.; Attanzio, A.; Spanò, V.; Cascioferro, S.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Diana, P.; Cirrincione, G.; Carbone, A. Synthesis, antitumor activity and CDK1 inhibiton of new thiazole nortopsentin analogues. Eur. J. Med. Chem. 2017, 138, 371–383. [Google Scholar] [CrossRef]
- Spanò, V.; Attanzio, A.; Cascioferro, S.; Carbone, A.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Cirrincione, G.; Diana, P.; Parrino, B. Synthesis and Antitumor Activity of New Thiazole Nortopsentin Analogs. Mar. Drugs 2016, 14, 226. [Google Scholar] [CrossRef]
- Carbone, A.; Parrino, B.; Di Vita, G.; Attanzio, A.; Spanò, V.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Livrea, M.A.; Diana, P.; et al. Synthesis and antiproliferative activity of thiazolyl-bis-pyrrolo [2,3-b] pyridines and indolyl-thiazolyl-pyrrolo [2,3-c] pyridines, nortopsentin analogues. Mar. Drugs 2015, 13, 460–492. [Google Scholar] [CrossRef]
- Parrino, B.; Carbone, A.; Di Vita, G.; Ciancimino, C.; Attanzio, A.; Spanò, V.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Livrea, M.A.; et al. 3-[4-(1H-indol-3-yl)-1,3-thiazol-2-yl]-1H-pyrrolo[2,3-b] pyridines, nortopsentin analogues with antiproliferative activity. Mar. Drugs 2015, 13, 1901–1924. [Google Scholar] [CrossRef]
- Meijer, L.L.; Garajova, I.; Caparello, C.; Le Large, T.Y.; Frampton, A.; Vasile, E.; Fune, N.; Kazemier, G.; Giovannetti, E. Plasma miR-181a-5p Down-Regulation Predicts Response and Improved Survival After FOLFIRINOX in Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2019. [Google Scholar] [CrossRef]
- El Hassouni, B.; Li Petri, G.; Liu, D.; Cascioferro, S.; Parrino, B.; Hassan, W.; Diana, P.; Ali, A.; Frampton, A.E.; Giovannetti, E. Pharmacogenetics of treatments for pancreatic cancer. Expert Opin. Drug Metab. Toxicol. 2019, 15, 437–447. [Google Scholar] [CrossRef]
- Le Large, T.Y.S.; El Hassouni, B.; Funel, N.; Kok, B.; Piersma, S.R.; Pham, T.V.; Olive, K.P.; Kazemier, G.; van Laarhoven, H.W.M.; Jimenez, C.R.; et al. Proteomic analysis of gemcitabine-resistant pancreatic cancer cells reveals that microtubule-associated protein 2 upregulation associates with taxane treatment. Ther. Adv. Med. Oncol. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, E.; van der Borden, C.L.; Frampton, A.E.; Ali, A.; Firuzi, O.; Peters, G.J. Never let it go: Stopping key mechanisms underlying metastasis to fight pancreatic cancer. Semin. Cancer Biol. 2017, 44, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Le Large, T.Y.S.; Bijlsma, M.F.; Kazemier, G.; van Laarhoven, H.W.M.; Giovannetti, E.; Jimenez, C.R. Key biological processes driving metastatic spread of pancreatic cancer as identified by multi-omics studies. Semin. Cancer Biol. 2017, 44, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Iwamura, T.; Caffrey, T.C.; Kitamura, N.; Yamanari, H.; Setoguchi, T.; Hollingsworth, M.A. P-selectin expression in a metastatic pancreatic tumor cell line (SUIT-2). Cancer Res. 1997, 57, 1206–1212. [Google Scholar] [PubMed]
- Deer, E.L.; Gonzalez-Hernandez, J.; Coursen, J.D.; Shea, J.E.; Ngatia, J.; Scaife, C.L.; Firpo, M.A.; Mulvihill, S.J. Phenotype and Genotype of Pancreatic Cancer Cell Lines. Pancreas 2010, 39, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Maftouh, M.; Avan, A.; Funel, N.; Frampton, A.E.; Fiuji, H.; Pelliccioni, S.; Castellano, L.; Galla, V.; Peters, G.J.; Giovannetti, E. miR-211 modulates gemcitabine activity through downregulation of ribonucleotide reductase and inhibits the invasive behavior of pancreatic cancer cells. Nucleosides Nucleotides Nucleic Acids 2014, 33, 384–393. [Google Scholar] [CrossRef]
- Avan, A.; Crea, F.; Paolicchi, E.; Funel, N.; Galvani, E.; Marquez, V.E.; Honeywell, R.J.; Danesi, R.; Peters, G.J.; Giovannetti, E. Molecular Mechanisms Involved in the Synergistic Interaction of the EZH2 Inhibitor 3-Deazaneplanocin A with Gemcitabine in Pancreatic Cancer Cells. Mol. Cancer Ther. 2012, 11, 1735–1746. [Google Scholar] [CrossRef]
- Avan, A.; Caretti, V.; Funel, N.; Galvani, E.; Maftouh, M.; Honeywell, R.J.; Lagerweij, T.; Van Tellingen, O.; Campani, D.; Fuchs, D.; et al. Crizotinib inhibits metabolic inactivation of gemcitabine in c-Met-driven pancreatic carcinoma. Cancer Res. 2013, 73, 6745–6756. [Google Scholar] [CrossRef]
- Ebos, J.M.L.; Lee, C.R.; Cruz-Munoz, W.; Bjarnason, G.A.; Christensen, J.G.; Kerbel, R.S. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 2009, 15, 232–239. [Google Scholar] [CrossRef]
- Carbone, A.; Parrino, B.; Cusimano, M.G.; Spanò, V.; Montalbano, A.; Barraja, P.; Schillaci, D.; Cirrincione, G.; Diana, P.; Cascioferro, S. New Thiazole Nortopsentin Analogues Inhibit Bacterial Biofilm Formation. Mar. Drugs 2018, 16, 274. [Google Scholar] [CrossRef]
- Boosa, V.; Bilakanti, V.; Velisoju, V.K.; Gutta, N.; Inkollu, S.; Akula, V. An insight on the influence of surface Lewis acid sites for regioselective CH bond C3-cyanation of indole using NH4I and DMF as combined cyanide source over Cu/SBA-15 catalyst. Mol. Catal. 2018, 445, 43–51. [Google Scholar] [CrossRef]
- Sciarrillo, R.; Wojtuszkiewicz, A.; Kooi, I.E.; Gómez, V.E.; Boggi, U.; Jansen, G.; Kaspers, G.-J.; Cloos, J.; Giovannetti, E. Using RNA-sequencing to Detect Novel Splice Variants Related to Drug Resistance in In Vitro Cancer Models. J. Vis. Exp. 2016, 118, 54714. [Google Scholar] [CrossRef] [PubMed]
- Massihnia, D.; Avan, A.; Funel, N.; Maftouh, M.; van Krieken, A.; Granchi, C.; Raktoe, R.; Boggi, U.; Aicher, B.; Minutolo, F.; et al. Phospho-Akt overexpression is prognostic and can be used to tailor the synergistic interaction of Akt inhibitors with gemcitabine in pancreatic cancer. J. Hematol. Oncol. 2017, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Caparello, C.; Meijer, L.L.; Garajova, I.; Falcone, A.; Le Large, T.Y.; Funel, N.; Kazemier, G.; Peters, G.J.; Vasile, E.; Giovannetti, E. FOLFIRINOX and translational studies: Towards personalized therapy in pancreatic cancer. World J. Gastroenterol 2016, 22, 6987–7005. [Google Scholar] [CrossRef]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
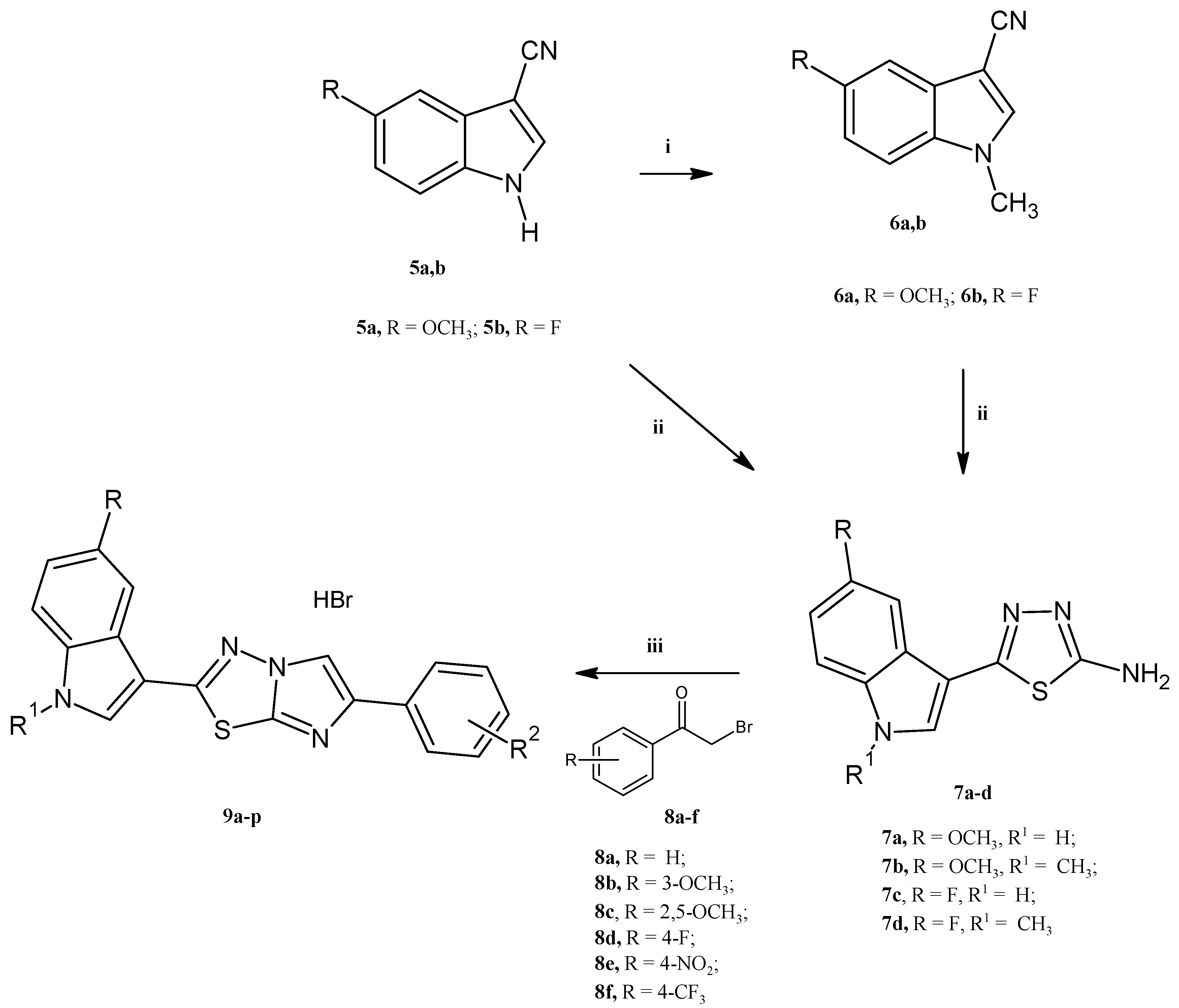
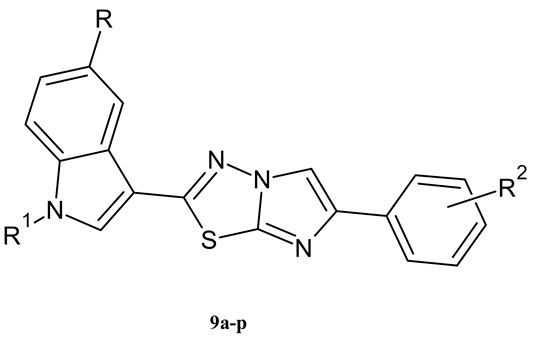
| Compound | R | R1 | R2 | Yield (%) |
|---|---|---|---|---|
| 9a | OCH3 | H | H | 80% |
| 9b | OCH3 | H | 4-F | 75% |
| 9c | OCH3 | H | 4-NO2 | 73% |
| 9d | OCH3 | H | 3-OCH3 | 78% |
| 9e | OCH3 | H | 2,5-OCH3 | 78% |
| 9f | OCH3 | H | 4-CF3 | 68% |
| 9g | OCH3 | CH3 | H | 81% |
| 9h | OCH3 | CH3 | 4-F | 72% |
| 9i | OCH3 | CH3 | 3-OCH3 | 60% |
| 9j | OCH3 | CH3 | 2,5-OCH3 | 65% |
| 9k | OCH3 | CH3 | 4-CF3 | 67% |
| 9l | F | H | H | 72% |
| 9m | F | H | 3-OCH3 | 74% |
| 9n | F | H | 4-CF3 | 68% |
| 9o | F | H | 2,5-OCH3 | 76% |
| 9p | F | CH3 | 4-CF3 | 78% |
| IC50 a (µM) ± SEM b Cell Lines | |||
|---|---|---|---|
| Comp | SUIT-2 | Capan-1 | Panc-1 |
| 9a | >16 | >16 | >16 |
| 9b | >16 | >16 | >16 |
| 9c | 5.5 ± 0.19 | 5.11 ± 0.29 | 5.18 ± 0.12 |
| 9d | >16 | >16 | >16 |
| 9e | >16 | >16 | 10.26 ± 0.20 |
| 9f | >16 | >16 | >16 |
| 9g | >16 | >16 | >16 |
| 9h | >16 | >16 | >16 |
| 9i | >16 | >16 | >16 |
| 9j | >16 | >16 | >16 |
| 9k | >16 | >16 | >16 |
| 9l | 10.4 ± 0.07 | 8.57 ± 0.51 | 10.8 ± 0.13 |
| 9m | >16 | >16 | >16 |
| 9n | 11.8 ± 0.54 | 10.49 ±0.16 | >16 |
| 9o | >16 | >16 | >16 |
| 9p | >16 | >16 | >16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cascioferro, S.; Li Petri, G.; Parrino, B.; El Hassouni, B.; Carbone, D.; Arizza, V.; Perricone, U.; Padova, A.; Funel, N.; Peters, G.J.; et al. 3-(6-Phenylimidazo [2,1-b][1,3,4]thiadiazol-2-yl)-1H-Indole Derivatives as New Anticancer Agents in the Treatment of Pancreatic Ductal Adenocarcinoma. Molecules 2020, 25, 329. https://doi.org/10.3390/molecules25020329
Cascioferro S, Li Petri G, Parrino B, El Hassouni B, Carbone D, Arizza V, Perricone U, Padova A, Funel N, Peters GJ, et al. 3-(6-Phenylimidazo [2,1-b][1,3,4]thiadiazol-2-yl)-1H-Indole Derivatives as New Anticancer Agents in the Treatment of Pancreatic Ductal Adenocarcinoma. Molecules. 2020; 25(2):329. https://doi.org/10.3390/molecules25020329
Chicago/Turabian StyleCascioferro, Stella, Giovanna Li Petri, Barbara Parrino, Btissame El Hassouni, Daniela Carbone, Vincenzo Arizza, Ugo Perricone, Alessandro Padova, Niccola Funel, Godefridus J. Peters, and et al. 2020. "3-(6-Phenylimidazo [2,1-b][1,3,4]thiadiazol-2-yl)-1H-Indole Derivatives as New Anticancer Agents in the Treatment of Pancreatic Ductal Adenocarcinoma" Molecules 25, no. 2: 329. https://doi.org/10.3390/molecules25020329
APA StyleCascioferro, S., Li Petri, G., Parrino, B., El Hassouni, B., Carbone, D., Arizza, V., Perricone, U., Padova, A., Funel, N., Peters, G. J., Cirrincione, G., Giovannetti, E., & Diana, P. (2020). 3-(6-Phenylimidazo [2,1-b][1,3,4]thiadiazol-2-yl)-1H-Indole Derivatives as New Anticancer Agents in the Treatment of Pancreatic Ductal Adenocarcinoma. Molecules, 25(2), 329. https://doi.org/10.3390/molecules25020329