Assaying Chlamydia pneumoniae Persistence in Monocyte-Derived Macrophages Identifies Dibenzocyclooctadiene Lignans as Phenotypic Switchers
Abstract
1. Introduction
2. Results
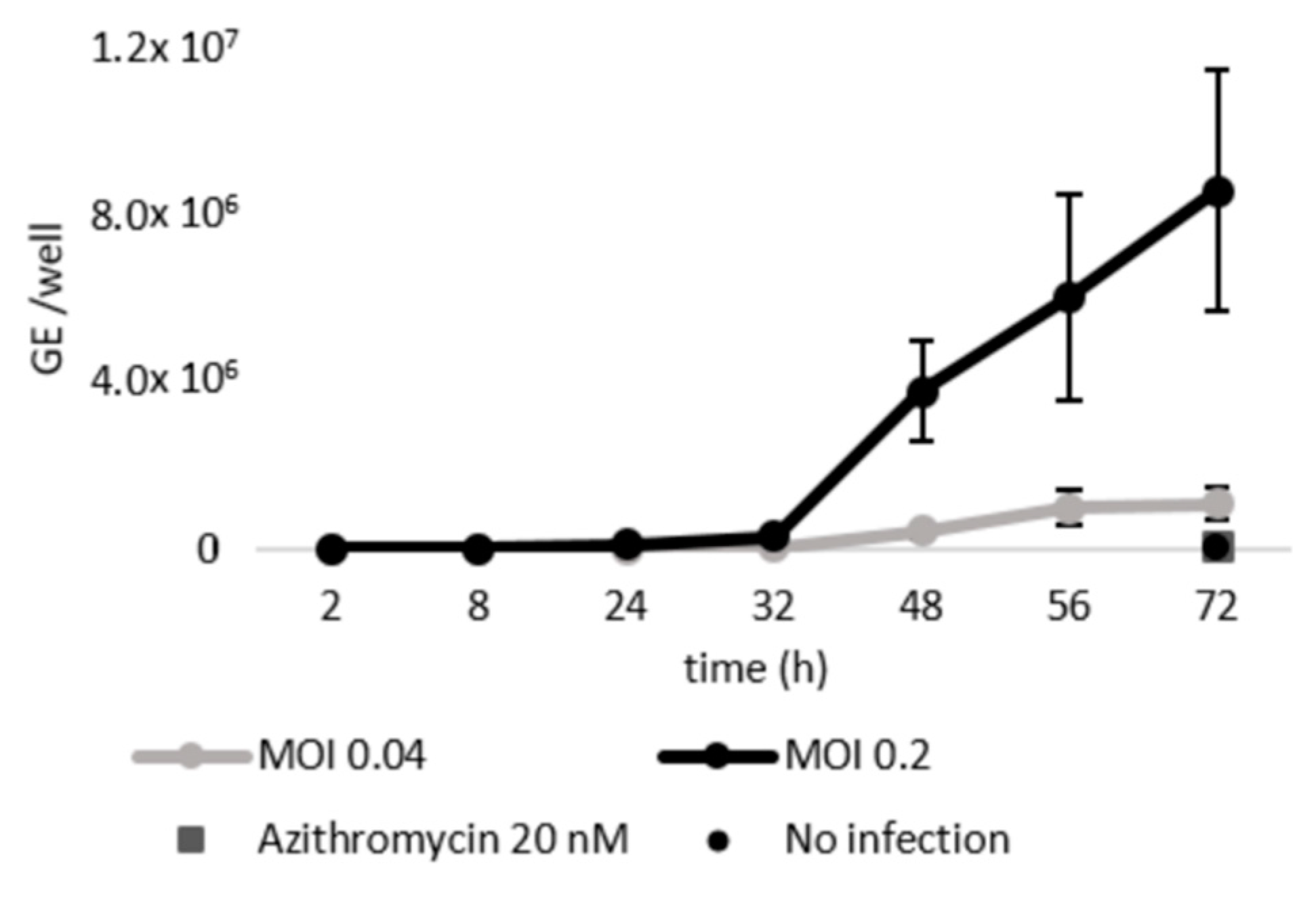
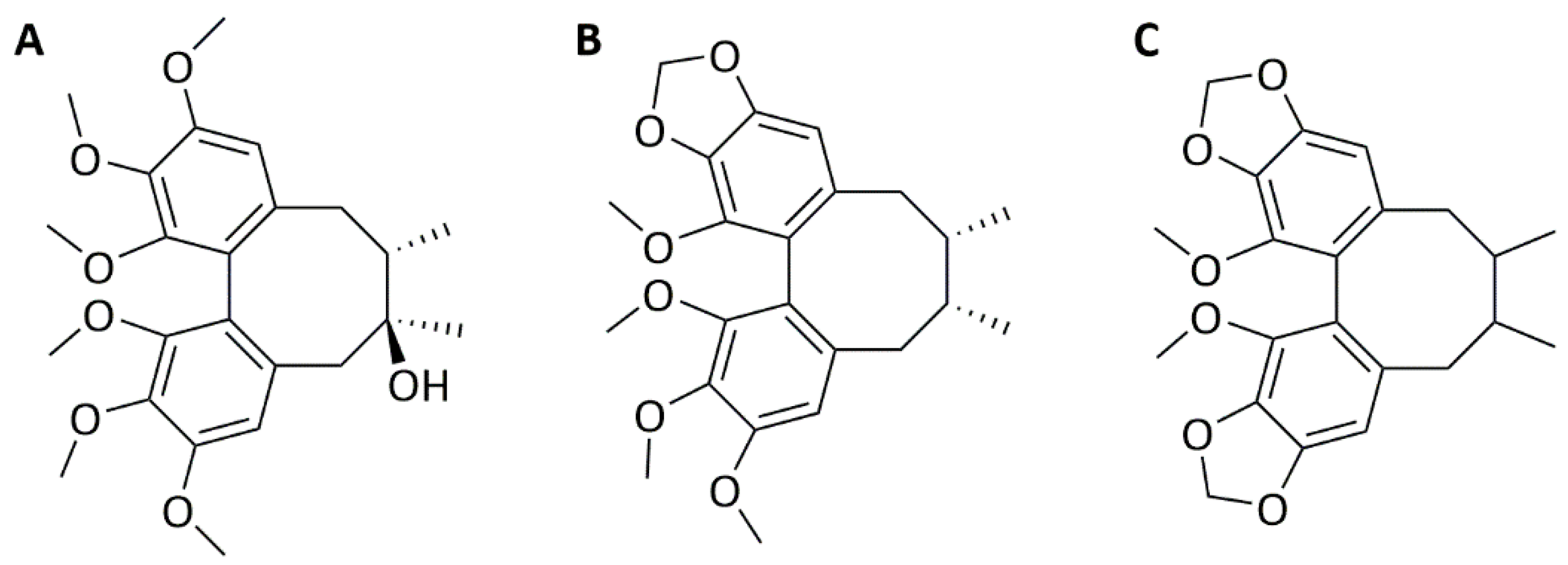
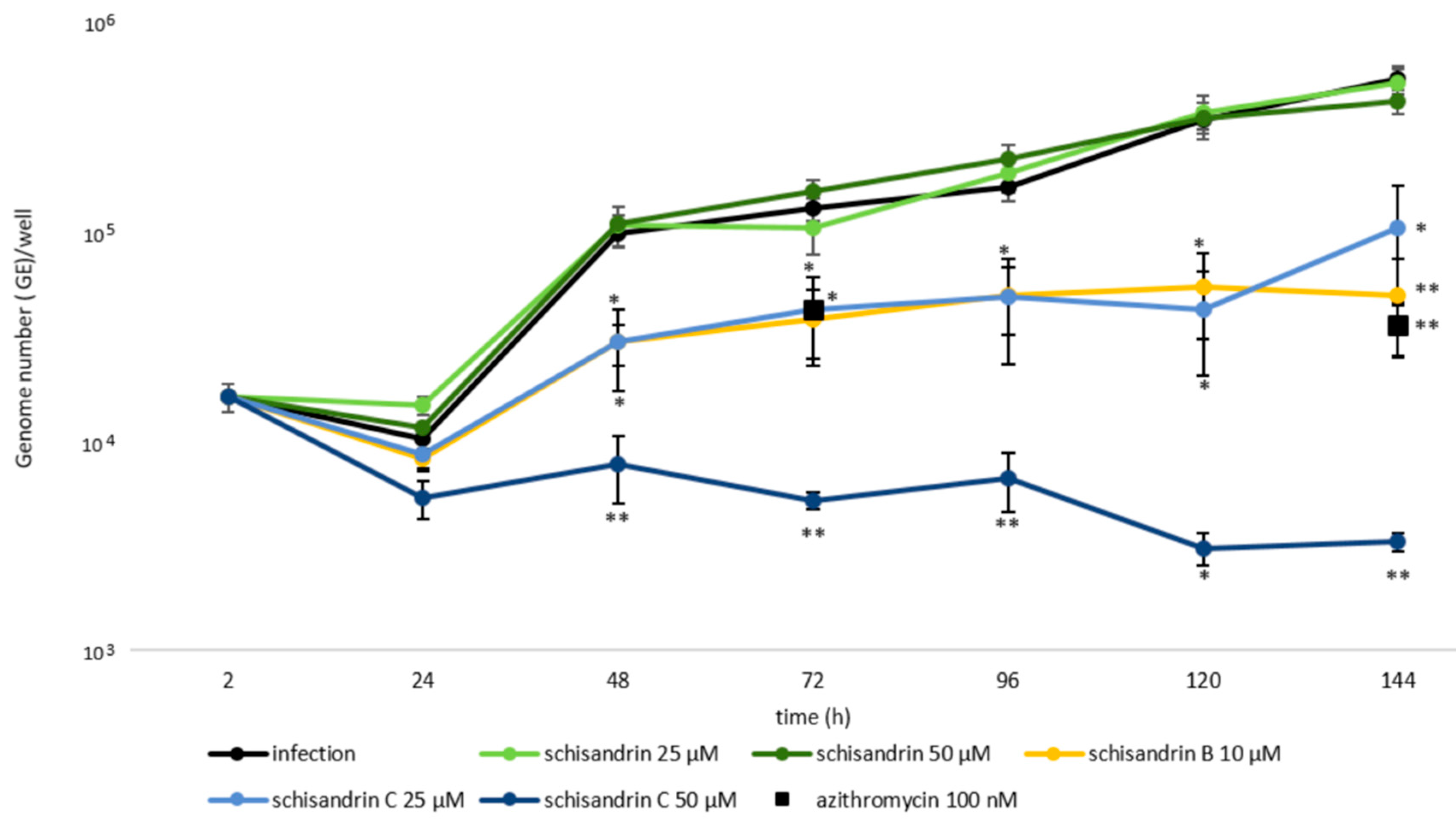
2.1. Schisandrin Lignans as Modulators of C. pneumoniae Infection
2.2. Concomitant Administration of Schisandrin and Azithromycin
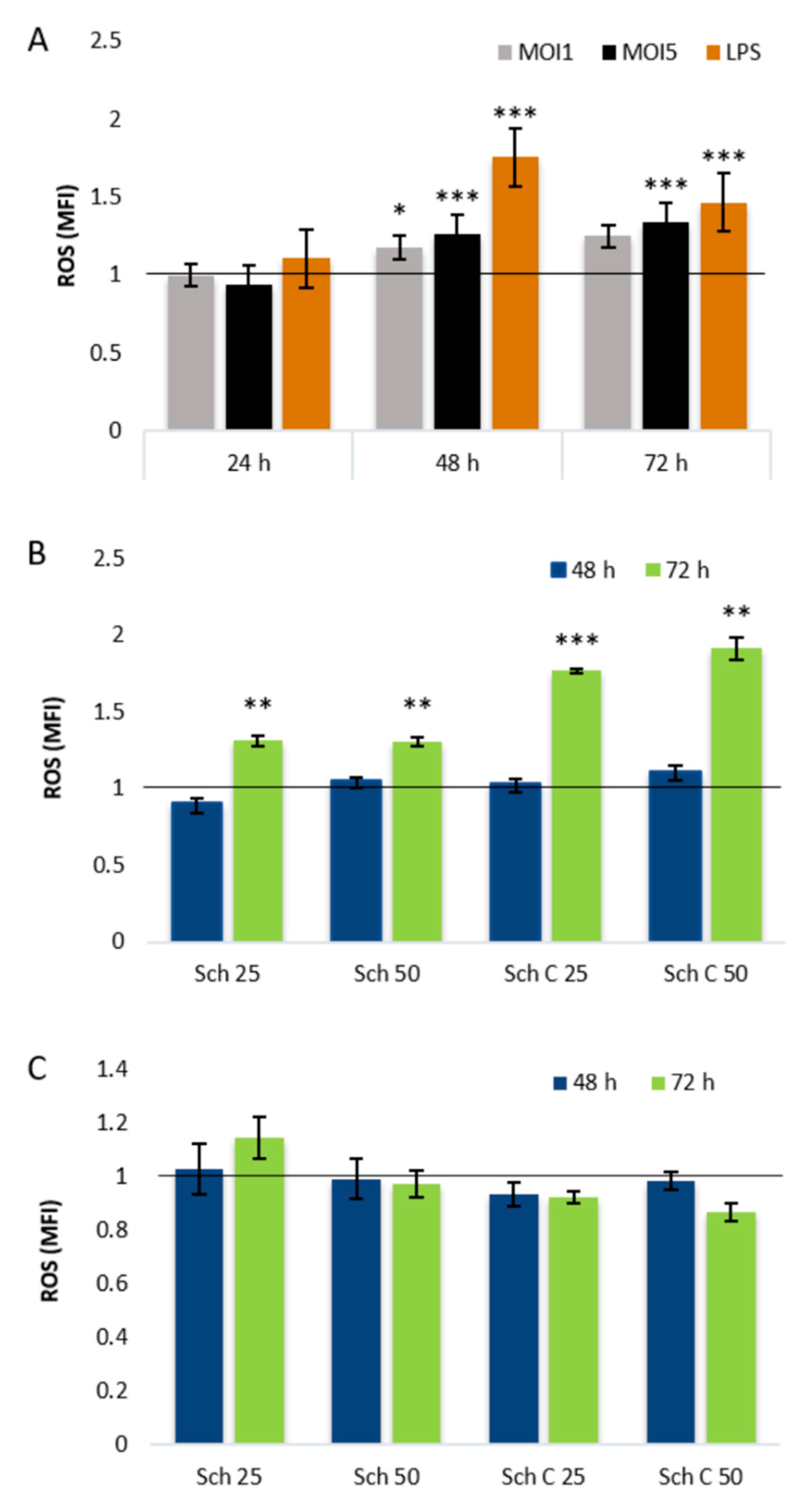
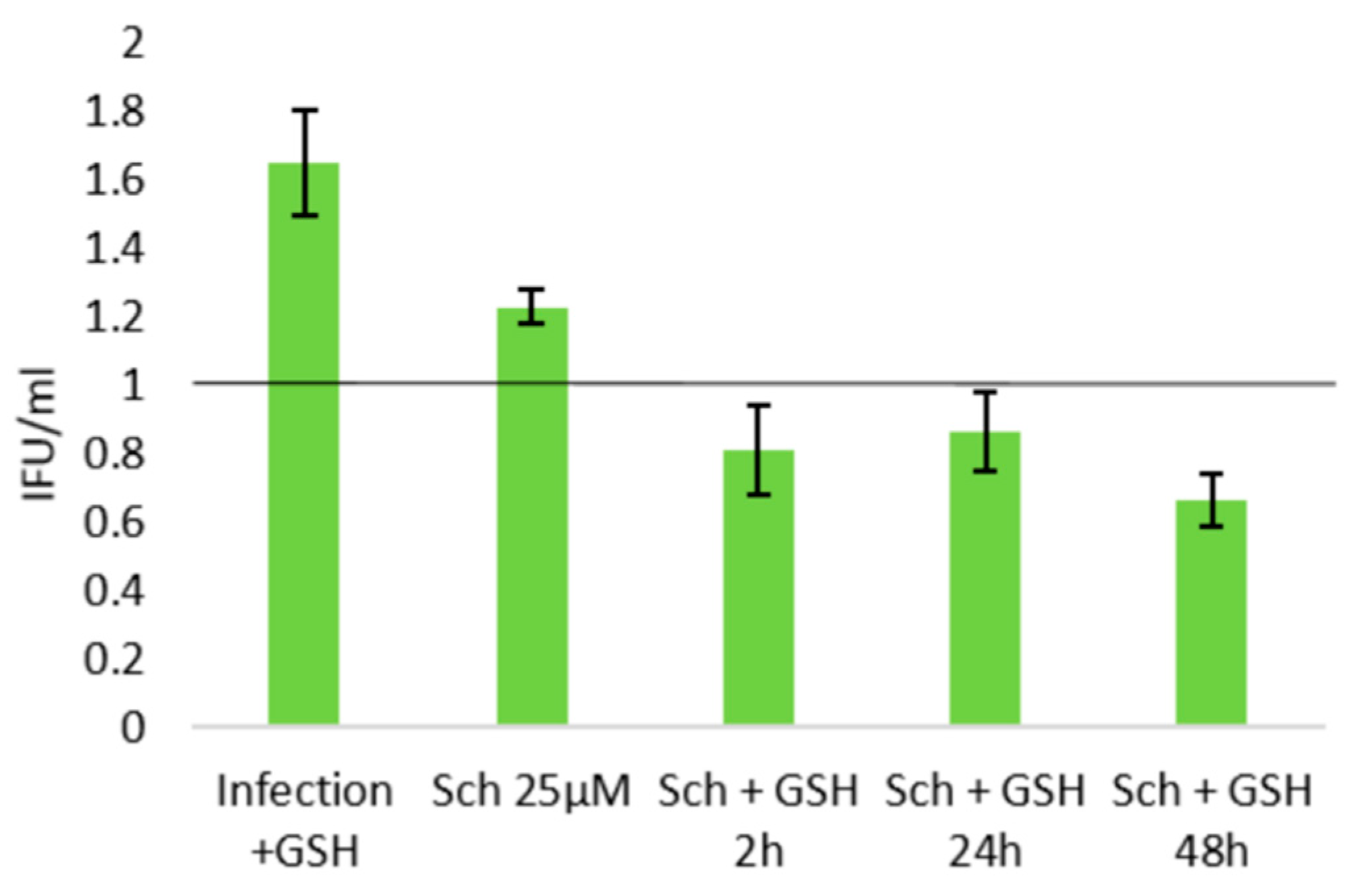
2.3. Role of Cellular Redox Status in the Effects of Schisandrin Lignans on C. pneumoniae Infection
3. Discussion
4. Materials and Methods
4.1. General
4.2. Compounds
4.3. Cell Culture
4.4. Infections
4.5. Quantitative PCR
4.6. Infectious Progeny Assay
4.7. Resazurin Assay
4.8. ATP Quantification Assay
4.9. Intracellular ROS Detection Assay
4.10. Glutathione Quantification Assay
4.11. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Cpn | Chlamydia pneumoniae |
| CHX | cycloheximide |
| DCFH-DA | 2’7′-dichlorodihydrofluorescein diacetate |
| EB | elementary body |
| GE | genome equivalents |
| GSH | glutathione |
| IFU/mL | infectious units per mL |
| iNOS | inducible nitric oxide synthase |
| LPS | lipopolysaccharide |
| MFI | mean fluorescence intensity |
| MOI | multiplicity of infection |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NMR | nuclear magnetic resonance |
| NO | nitric oxide |
| PBS | phosphate-buffered saline |
| PMA | phorbol 12-myristate 13-acetate |
| RB | reticulate body |
| ROS | reactive oxygen species |
| sch | schisandrin |
| TLR | Toll-like receptor. |
Appendix A
References
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, J.J. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Microbiol. 2019, 17, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.W.; Kingsley, L.A.; Aul, J.J.; Wadowsky, R.M.; Post, J.C.; Doyle, W.J.; Ehrlich, G.D. Comparative evaluation of culture and PCR for the detection and determination of persistence of bacterial strains and DNAs in the Chinchilla laniger model of otitis media. Ann. Otol. Rhinol. Laryngol. 1998, 107, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Borriello, G.; Werner, E.; Roe, F.; Kim, A.M.; Ehrlich, G.D.; Stewart, P.S. Oxygen Limitation Contributes to Antibiotic Tolerance of Pseudomonas aeruginosa in Biofilms. Antimicrob. Agents Chemother. 2004, 48, 2659–2664. [Google Scholar] [CrossRef] [PubMed]
- Defraine, V.; Fauvart, M.; Michiels, J. Fighting bacterial persistence: Current and emerging anti-persister strategies and therapeutics. Drug Resist. Updat. 2018, 38, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.; Stephens, R.S.; Bavoil, P.M.; Kaltenboeck, B. Chlamydia. Bergey’s Man. Syst. Archaea Bact. 2015, 1–28. [Google Scholar] [CrossRef]
- Beagley, K.; Huston, W.M.; Hansbro, P.M.; Timms, P. Chlamydial infection of immune cells: Altered function and implications for disease. Crit. Rev. Immunol. 2009, 29, 275–305. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, S.; Grieshaber, N.; Yang, H.; Baxter, B.; Hackstadt, T.; Omsland, A. The Impact of Active Metabolism on Chlamydia trachomatis Elementary Body Transcript Profile and Infectivity. J. Bacteriol. 2018, 200. [Google Scholar] [CrossRef]
- Panzetta, M.E.; Valdivia, R.; Saka, H.A. Chlamydia persistence: A survival strategy to evade antimicrobial effects in-vitro and in-vivo. Front. Microbiol. 2018, 9, 3101. [Google Scholar] [CrossRef]
- Bellmann-Weiler, R.; Martinz, V.; Kurz, K.; Engl, S.; Feistritzer, C.; Fuchs, D.; Rupp, J.; Paldanius, M.; Weiss, G. Divergent modulation of Chlamydia pneumoniae infection cycle in human monocytic and endothelial cells by iron, tryptophan availability and interferon gamma. Immunobiology 2010, 215, 842–848. [Google Scholar] [CrossRef]
- Krämer, S.; Crauwels, P.; Bohn, R.; Radzimski, C.; Szaszák, M.; Klinger, M.; Rupp, J.; van Zandbergen, G. AP-1 transcription factor serves as a molecular switch between Chlamydia pneumoniae replication and persistence. Infect. Immun. 2015, 83, 2651–2660. [Google Scholar] [CrossRef]
- Ouellette, S.P.; Hatch, T.P.; AbdelRahman, Y.M.; Rose, L.A.; Belland, R.J.; Byrne, G.I. Global transcriptional upregulation in the absence of increased translation in Chlamydia during IFNγ-mediated host cell tryptophan starvation. Mol. Microbiol. 2006, 62, 1387–1401. [Google Scholar] [CrossRef] [PubMed]
- Grayston, J.T.; Belland, R.J.; Byrne, G.I.; Kuo, C.C.; Schachter, J.; Stamm, W.E.; Zhong, G. Infection with Chlamydia pneumoniae as a cause of coronary heart disease: The hypothesis is still untested. Pathog. Dis. 2015, 73, 1. [Google Scholar] [CrossRef] [PubMed]
- Webley, W.C.; Hahn, D.L. Infection-mediated asthma: Etiology, mechanisms and treatment options, with focus on Chlamydia pneumoniae and macrolides. Respir. Res. 2017, 18, 98. [Google Scholar] [CrossRef] [PubMed]
- Hanski, L.; Vuorela, P.M. Recent advances in technologies for developing drugs against Chlamydia pneumoniae. Expert Opin. Drug Discov. 2014, 9, 791–802. [Google Scholar] [CrossRef]
- Hakala, E.; Hanski, L.L.; Yrjönen, T.; Vuorela, H.J.; Vuorela, P.M. The Lignan-containing Extract of Schisandra chinensis Berries Inhibits the Growth of Chlamydia pneumonia. Nat. Prod. Commun. 2015, 10, 1001–1004. [Google Scholar]
- Hakala, E.; Hanski, L.; Uvell, H.; Yrjönen, T.; Vuorela, H.; Elofsson, M.; Vuorela, P.M. Dibenzocyclooctadiene lignans from Schisandra spp. selectively inhibit the growth of the intracellular bacteria Chlamydia pneumoniae and Chlamydia trachomatis. J. Antibiot. 2015, 68, 609–614. [Google Scholar] [CrossRef]
- Jiang, Y.; Fan, X.; Wang, Y.; Tan, H.; Chen, P.; Zeng, H.; Huang, M.; Bi, H. Hepato-protective effects of six schisandra lignans on acetaminophen-induced liver injury are partially associated with the inhibition of CYP-mediated bioactivation. Chem. Biol. Interact. 2015, 231, 83–89. [Google Scholar] [CrossRef]
- Chen, N.A.; Ko, M. Schisandrin B-induced glutathione antioxidant response and cardioprotection are mediated by reactive oxidant species production in rat hearts. Biol. Pharm. Bull. 2010, 33, 825–829. [Google Scholar] [CrossRef]
- Stacchiotti, A.; Volti, G.L.; Lavazza, A.; Schena, I.; Aleo, M.F.; Rodella, L.F.; Rezzani, R. Different role of Schisandrin B on mercury-induced renal damage in vivo and in vitro. Toxicology 2011, 286, 48–57. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. An overview of neuroprotective and cognitive enhancement properties of lignans from Schisandra chinensis. Biomed. Pharmacother. 2018, 97, 958–968. [Google Scholar] [CrossRef]
- Hanski, L.; Ausbacher, D.; Tiirola, T.M.; Strøm, M.B.; Vuorela, P.M. Amphipathic β2, 2-Amino Acid Derivatives Suppress Infectivity and Disrupt the Intracellular Replication Cycle of Chlamydia pneumoniae. PLoS ONE 2016, 11, e0157306. [Google Scholar] [CrossRef] [PubMed]
- Hanski, L.L.; Kapp, K.; Tiirola, T.M.; Orav, A.; Vuorela, H.J.; Püssa, T.; Vuorela, P.M. Mint Flavorings from Candies Inhibit the Infectivity of Chlamydia pneumoniae. Nat. Prod. Commun. 2016, 11, 1725–1728. [Google Scholar] [CrossRef] [PubMed]
- Kohlhoff, S.; Huerta, N.; Hammerschlag, M.R. In Vitro Activity of Levonadifloxacin (WCK 771) against Chlamydia pneumoniae. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Kohlhoff, S.A.; Huerta, N.; Hammerschlag, M.R. In vitro activity of omadacycline against Chlamydia pneumoniae. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Kortesoja, M.; Karhu, E.; Olafsdottir, E.S.; Freysdottir, J.; Hanski, L. Impact of dibenzocyclooctadiene lignans from Schisandra chinensis on the redox status and activation of human innate immune system cells. Free Radic. Biol. Med. 2019, 131, 309–317. [Google Scholar] [CrossRef]
- Kim, J.; Heo, P.; Yang, T.; Lee, K.; Cho, D.; Kim, B.T.; Suh, J.; Lim, H.; Shin, D.; Kim, S. Selective killing of bacterial persisters by a single chemical compound without affecting normal antibiotic-sensitive cells. Antimicrob. Agents Chemother. 2011, 55, 5380–5383. [Google Scholar] [CrossRef]
- Pan, J.; Bahar, A.A.; Syed, H.; Ren, D. Reverting antibiotic tolerance of Pseudomonas aeruginosa PAO1 persister cells by (Z)-4-bromo-5-(bromomethylene)-3-methylfuran-2 (5H)-one. PLoS ONE 2012, 7, e45778. [Google Scholar] [CrossRef]
- Li, C.; Cheng, Y.; Hsieh, C.; Tsai, T. Pharmacokinetics of schizandrin and its pharmaceutical products assessed using a validated LC–MS/MS method. Molecules 2018, 23, 173. [Google Scholar] [CrossRef]
- Szopa, A.; Ekiert, R.; Ekiert, H. Current knowledge of Schisandra chinensis (Turcz.) Baill (Chinese magnolia vine) as a medicinal plant species: A review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochem. Rev. 2017, 16, 195–218. [Google Scholar] [CrossRef]
- Noubade, R.; Wong, K.; Ota, N.; Rutz, S.; Eidenschenk, C.; Valdez, P.A.; Ding, J.; Peng, I.; Sebrell, A.; Caplazi, P. NRROS negatively regulates reactive oxygen species during host defence and autoimmunity. Nature 2014, 509, 235. [Google Scholar] [CrossRef]
- West, A.P.; Brodsky, I.E.; Rahner, C.; Woo, D.K.; Erdjument-Bromage, H.; Tempst, P.; Walsh, M.C.; Choi, Y.; Shadel, G.S.; Ghosh, S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 2011, 472, 476. [Google Scholar] [CrossRef] [PubMed]
- Itoh, R.; Murakami, I.; Chou, B.; Ishii, K.; Soejima, T.; Suzuki, T.; Hiromatsu, K. Chlamydia pneumoniae harness host NLRP3 inflammasome-mediated caspase-1 activation for optimal intracellular growth in murine macrophages. Biochem. Biophys. Res. Commun. 2014, 452, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.S.; Kaufmann, B.B.; Chand, N.S.; Haseley, N.; Hung, D.T. Eradication of bacterial persisters with antibiotic-generated hydroxyl radicals. Proc. Natl. Acad. Sci. USA 2012, 109, 12147–12152. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.T.; Call, D.R.; Beyenal, H. Eradication of Pseudomonas aeruginosa biofilms and persister cells using an electrochemical scaffold and enhanced antibiotic susceptibility. NPJ Biofilms Microbiomes 2016, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.Y.; Tang, M.H.; Mak, D.H.; Poon, M.K.; Ko, K.M. Hepatoprotective mechanism of schisandrin B: Role of mitochondrial glutathione antioxidant status and heat shock proteins. Free Radic. Biol. Med. 2003, 35, 368–380. [Google Scholar] [CrossRef]
- Takanche, J.S.; Lee, Y.; Kim, J.; Kim, J.; Han, S.; Lee, S.; Yi, H. Anti-inflammatory and antioxidant properties of Schisandrin C promote mitochondrial biogenesis in human dental pulp cells. Int. Endod. J. 2018, 51, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Azenabor, A.A.; Muili, K.; Akoachere, J.; Chaudhry, A. Macrophage antioxidant enzymes regulate Chlamydia pneumoniae chronicity: Evidence of the effect of redox balance on host–pathogen relationship. Immunobiology 2006, 211, 325–339. [Google Scholar] [CrossRef]
- Prusty, B.K.; Böhme, L.; Bergmann, B.; Siegl, C.; Krause, E.; Mehlitz, A.; Rudel, T. Imbalanced oxidative stress causes chlamydial persistence during non-productive human herpes virus co-infection. PLoS ONE 2012, 7, e47427. [Google Scholar] [CrossRef]
- Azenabor, A.A.; Chaudhry, A.U. Chlamydia pneumoniae survival in macrophages is regulated by free Ca2 dependent reactive nitrogen and oxygen species. J. Infect. 2003, 46, 120–128. [Google Scholar] [CrossRef]
- Di Pietro, M.; Filardo, S.; De Santis, F.; Mastromarino, P.; Sessa, R. Chlamydia pneumoniae and oxidative stress in cardiovascular disease: State of the art and prevention strategies. Int. J. Mol. Sci. 2015, 16, 724–735. [Google Scholar] [CrossRef]
- Mao, X.; Liao, Z.; Guo, L.; Xu, X.; Wu, B.; Xu, M.; Zhao, X.; Bi, K.; Jia, Y. Schisandrin C Ameliorates Learning and Memory Deficits by Aβ1–42-induced Oxidative Stress and Neurotoxicity in Mice. Phytother. Res. 2015, 29, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Alkhuder, K.; Meibom, K.L.; Dubail, I.; Dupuis, M.; Charbit, A. Glutathione provides a source of cysteine essential for intracellular multiplication of Francisella tularensis. PLoS Pathog. 2009, 5. [Google Scholar] [CrossRef] [PubMed]
- Lazarev, V.N.; Borisenko, G.G.; Shkarupeta, M.M.; Demina, I.A.; Serebryakova, M.V.; Galyamina, M.A.; Levitskiy, S.A.; Govorun, V.M. The role of intracellular glutathione in the progression of Chlamydia trachomatis infection. Free Radic. Biol. Med. 2010, 49, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Meury, J.; Robin, A. Glutathione-gated K channels of Escherichia coli carry out K efflux controlled by the redox state of the cell. Arch. Microbiol. 1990, 154, 475–482. [Google Scholar] [CrossRef]
- Oberley-Deegan, R.E.; Rebits, B.W.; Weaver, M.R.; Tollefson, A.K.; Bai, X.; McGibney, M.; Ovrutsky, A.R.; Chan, E.D.; Crapo, J.D. An oxidative environment promotes growth of Mycobacterium abscessus. Free Radic. Biol. Med. 2010, 49, 1666–1673. [Google Scholar] [CrossRef]
- Reniere, M.L.; Whiteley, A.T.; Hamilton, K.L.; John, S.M.; Lauer, P.; Brennan, R.G.; Portnoy, D.A. Glutathione activates virulence gene expression of an intracellular pathogen. Nature 2015, 517, 170. [Google Scholar] [CrossRef]
- Blunder, M.; Pferschy-Wenzig, E.M.; Fabian, W.M.; Hüfner, A.; Kunert, O.; Saf, R.; Schühly, W.; Bauer, R. Derivatives of schisandrin with increased inhibitory potential on prostaglandin E2 and leukotriene B4 formation in vitro. Bioorg. Med. Chem. 2010, 18, 2809–2815. [Google Scholar] [CrossRef]
- Kuo, C.C.; Grayston, J.T. A sensitive cell line, HL cells, for isolation and propagation of Chlamydia pneumoniae strain TWAR. J. Infect. Dis. 1990, 162, 755–758. [Google Scholar] [CrossRef]
- Alvesalo, J.; Vuorela, H.; Tammela, P.; Leinonen, M.; Saikku, P.; Vuorela, P. Inhibitory effect of dietary phenolic compounds on Chlamydia pneumoniae in cell cultures. Biochem. Pharmacol. 2006, 71, 735–741. [Google Scholar] [CrossRef]
- Tondella, M.L.; Talkington, D.F.; Holloway, B.P.; Dowell, S.F.; Cowley, K.; Soriano-Gabarro, M.; Elkind, M.S.; Fields, B.S. Development and evaluation of real-time PCR-based fluorescence assays for detection of Chlamydia pneumoniae. J. Clin. Microbiol. 2002, 40, 575–583. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nature Protoc. 2006, 1, 3159. [Google Scholar] [CrossRef] [PubMed]
- Rhen, M. Salmonella and Reactive Oxygen Species: A Love-Hate Relationship. J. Innate Immun. 2019, 11, 216–226. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the key compounds are available from the authors on request. |


| 24 h | 48 h | 72 h | 144 h | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound (µM) | Res | ATP | Res | ATP | Res | ATP | Res | |||
| Cpn - | Cpn - | Cpn + | Cpn - | Cpn - | Cpn + | Cpn - | Cpn - | Cpn + | Cpn - | |
| sch 25 | 118 | 113 | 120 * | 105 | 172 | 122 | 93 | 103 | 99 | 127 |
| sch 50 | 106 | 107 | 117 | 119 | 151 | 96 | 92 | 92 | 87 | 120 |
| sch B 10 | - | 101 | 111 | - | 156 | 89 | - | 103 | 88 | 130 |
| sch B 25 | 80 | 97 | 105 | 70 | 113 | 74 | 80 * | 85 | 73 | 52 * |
| sch B 50 | 67 ** | 79 *** | 87 | 50 ** | 92 | 57 | 69 | 53 *** | 44 *** | 1 ** |
| sch C 25 | 89 | 94 | 100 | 78 | 139 | 96 | 111 | 102 | 90 | 122 |
| sch C 50 | 79 | 90 | 108 | 69 | 121 | 81 | 101 | 93 | 82 * | 81 |
| Cell Lysate (IFU/mL Index) | Supernatant (IFU/mL Index) | |||
|---|---|---|---|---|
| 72 h | 144 h | 72 h | 144 h | |
| sch 25 µM | 1.53 ± 0.06 * | 3.11 ± 0.62 | 1.52 ± 0.33 | 2.82 ± 0.27 |
| sch 50 µM | 2.28 ± 0.38 | 1.03 ± 0.34 | 2.56 ± 0.64 | 0.93 ± 0.18 |
| sch B 10 µM | 0.10 ± 0.03 ** | 0.01 ± 0.00 *** | 0.28 ± 0.18 | 0.04 ± 0.01 *** |
| sch C 25 µM | 0.00 ± 0.00 ** | 0.00 ± 0.00 *** | 0.00 ± 0.00 ** | 0.00 ± 0.00 *** |
| sch C 50 µM | 0.00 ± 0.00 ** | 0.00 ± 0.00 *** | 0.00 ± 0.00 ** | 0.00 ± 0.00 *** |
| azithromycin 100 nM | 0.18 ± 0.20 ** | 0.05 ± 0.03 *** | 0.30 ± 0.21 | 0.00 ± 0.00*** |
| infection (IFU/mL) | 49,700 ± 16,000 | 140,200 ± 22,800 | 5400 ± 2100 | 12,300 ± 700 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taavitsainen, E.; Kortesoja, M.; Bruun, T.; Johansson, N.G.; Hanski, L. Assaying Chlamydia pneumoniae Persistence in Monocyte-Derived Macrophages Identifies Dibenzocyclooctadiene Lignans as Phenotypic Switchers. Molecules 2020, 25, 294. https://doi.org/10.3390/molecules25020294
Taavitsainen E, Kortesoja M, Bruun T, Johansson NG, Hanski L. Assaying Chlamydia pneumoniae Persistence in Monocyte-Derived Macrophages Identifies Dibenzocyclooctadiene Lignans as Phenotypic Switchers. Molecules. 2020; 25(2):294. https://doi.org/10.3390/molecules25020294
Chicago/Turabian StyleTaavitsainen, Eveliina, Maarit Kortesoja, Tanja Bruun, Niklas G. Johansson, and Leena Hanski. 2020. "Assaying Chlamydia pneumoniae Persistence in Monocyte-Derived Macrophages Identifies Dibenzocyclooctadiene Lignans as Phenotypic Switchers" Molecules 25, no. 2: 294. https://doi.org/10.3390/molecules25020294
APA StyleTaavitsainen, E., Kortesoja, M., Bruun, T., Johansson, N. G., & Hanski, L. (2020). Assaying Chlamydia pneumoniae Persistence in Monocyte-Derived Macrophages Identifies Dibenzocyclooctadiene Lignans as Phenotypic Switchers. Molecules, 25(2), 294. https://doi.org/10.3390/molecules25020294






