Evaluation of Fungistatic Activity of Eight Selected Essential Oils on Four Heterogeneous Fusarium Isolates Obtained from Cereal Grains in Southern Poland
Abstract
1. Introduction
2. Results
2.1. Results of Determination of Isolates of Individual Fusarium sp.
2.2. Results of the Determination of the Qualitative and Quantitative Composition of Essential Oils
- Garlic oil (C)—only organosulphur compounds (100%) were present, such as diallyl trisulphide (46.31 ± 0.37%), diallyl disulphide (22.62 ± 0.24%), allyl methyl trisulphide (21.46 ± 0.29%), diallyl monosulphide (5.22 ± 0.11%), allyl methyl disulphide (3.34 ± 0.07%) and dimethyl trisulphide (1.05 ± 0.13%).
- Lemongrass oil (L)—aliphatic monoterpenoid-citral (68.94%) was found to be a dominant constituent; other compounds were present in concentrations significantly lower than 10%, so it was established that the additional compound can be aliphatic monoterpenoid-linalool (5.73%). The ratio of monoterpenoids to monoterpenes was approximately 17:1.
- Litsea cubeba oil (LC)—aliphatic monoterpenoid-citral (61.72%) was the dominant constituent; however, an additional constituent was monocyclic monoterpene-limonene (20.94%). Other constituents occurred in low concentrations, less than 5%. The ratio of monoterpenoids to monoterpenes was approximately 2:1.
- Thyme oil (T)—the dominant constituent was monocyclic monoterpenoid-thymol (45.75%); an additional constituent was monocyclic monoterpene-limonene (15.15%). Other compounds were present in concentrations lower than 10%. The presence of aliphatic monoterpenoids-linalool (8.90%) and monocyclic monoterpene-γ terpinene (8.10%) is noteworthy. The ratio of monoterpenoids to monoterpenes was approximately 2:1.
- Tea tree oil (TTO)—monocyclic monoterpenoid-1-terpinen-4-ol (38.24%) was the dominant constituent; an additional constituent was monoterpene bi- and tricyclic—3-caren (17.04%); other compounds were present in lower concentrations, but exceeding 10%, i.e., monoterpenoid 2-3 cyclic eucalyptol (13.90%) and monocyclic monoterpene α-terpinene (10.29%). The ratio of monoterpenoids to monoterpenes was approximately 2:1.
- Cajeput oil (K)—monoterpenoids were present as dominant and auxiliary constituents; monocyclic—was the dominant α-terpineol (36.57%), and 2–3 cyclic—eucalyptol (18.50%) was the additional. The presence of the aliphatic monoterpenoid linalool (11.19%) is also worth mentioning. The ratio of monoterpenoids to monoterpenes was approximately 6:1.
- Verbena oil (V)—the dominant and additional constituents were monoterpenoids; aliphatic—citral (36.0%) (dominant) and monocyclic monoterpenoid-α-terpineol (18.26%) (additional). The presence of bi- and tricyclic monoterpenoids-eucalyptol (13.46%) is also worth mentioning. The ratio of monoterpenoids to monoterpenes was approximately 10:1.
- Grapefruit oil (G)—the dominant constituent was monocyclic monoterpene-limonene (34.6%), and aliphatic monoterpene-β myrcene (5.32%) or aliphatic monoterpenoid-linalool (4.83%) can be considered as additional constituents. Other numerous constituents were present in small quantities up to 4%. High content of auxiliary heterogeneous substances, not terpenes’ (32.09%) was noted. The ratio of monoterpenoids to monoterpenes was approximately 1:3.
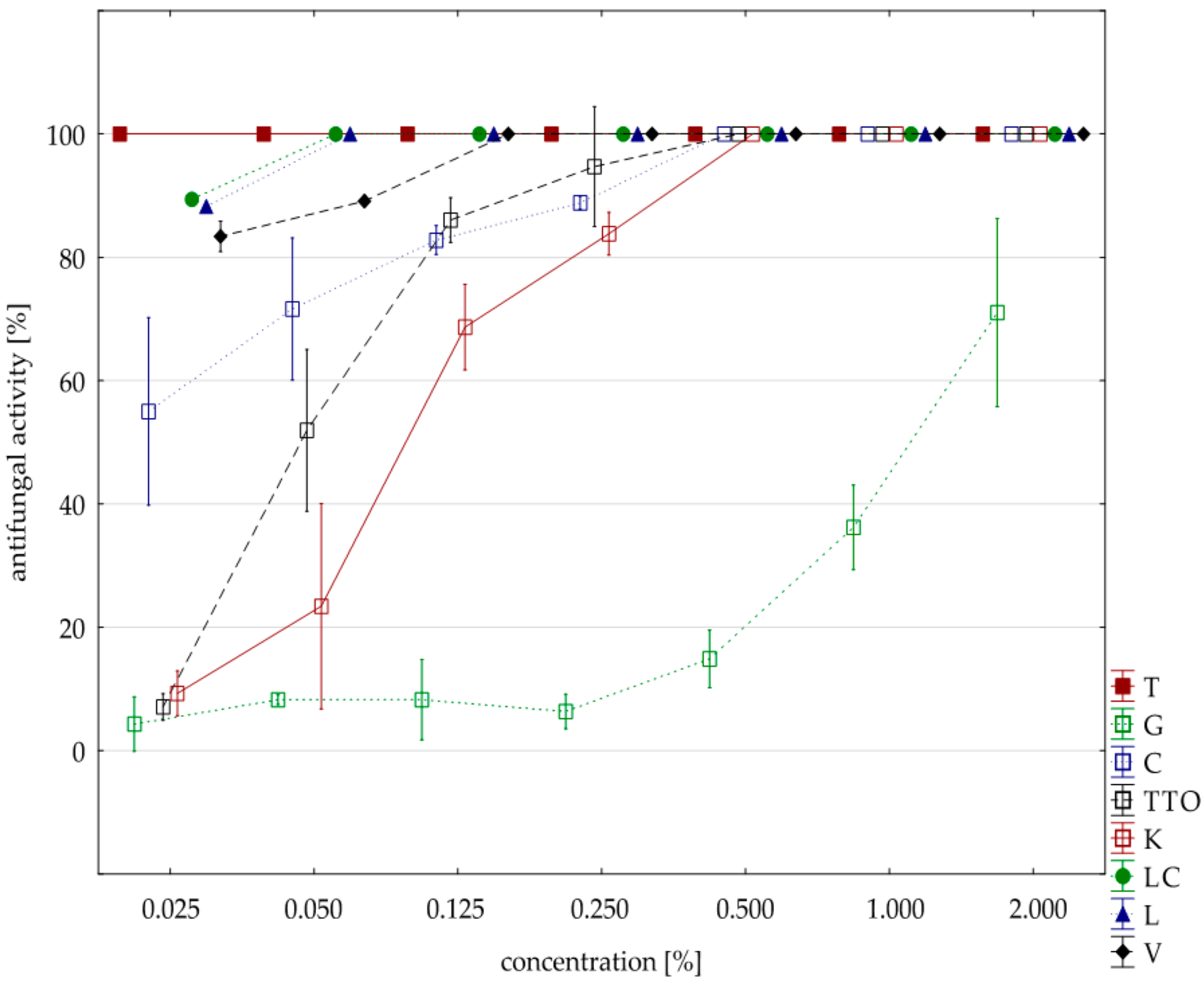
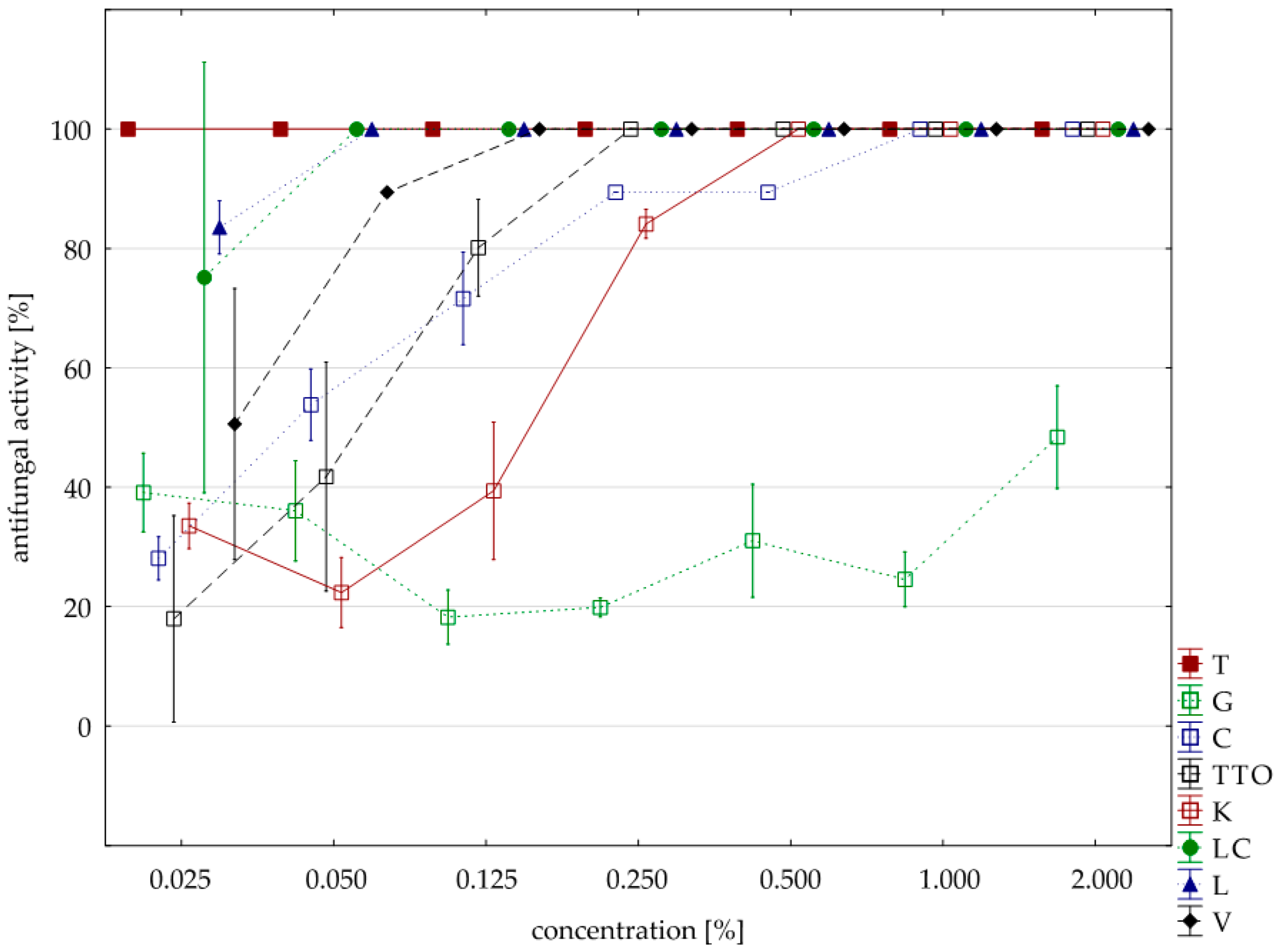
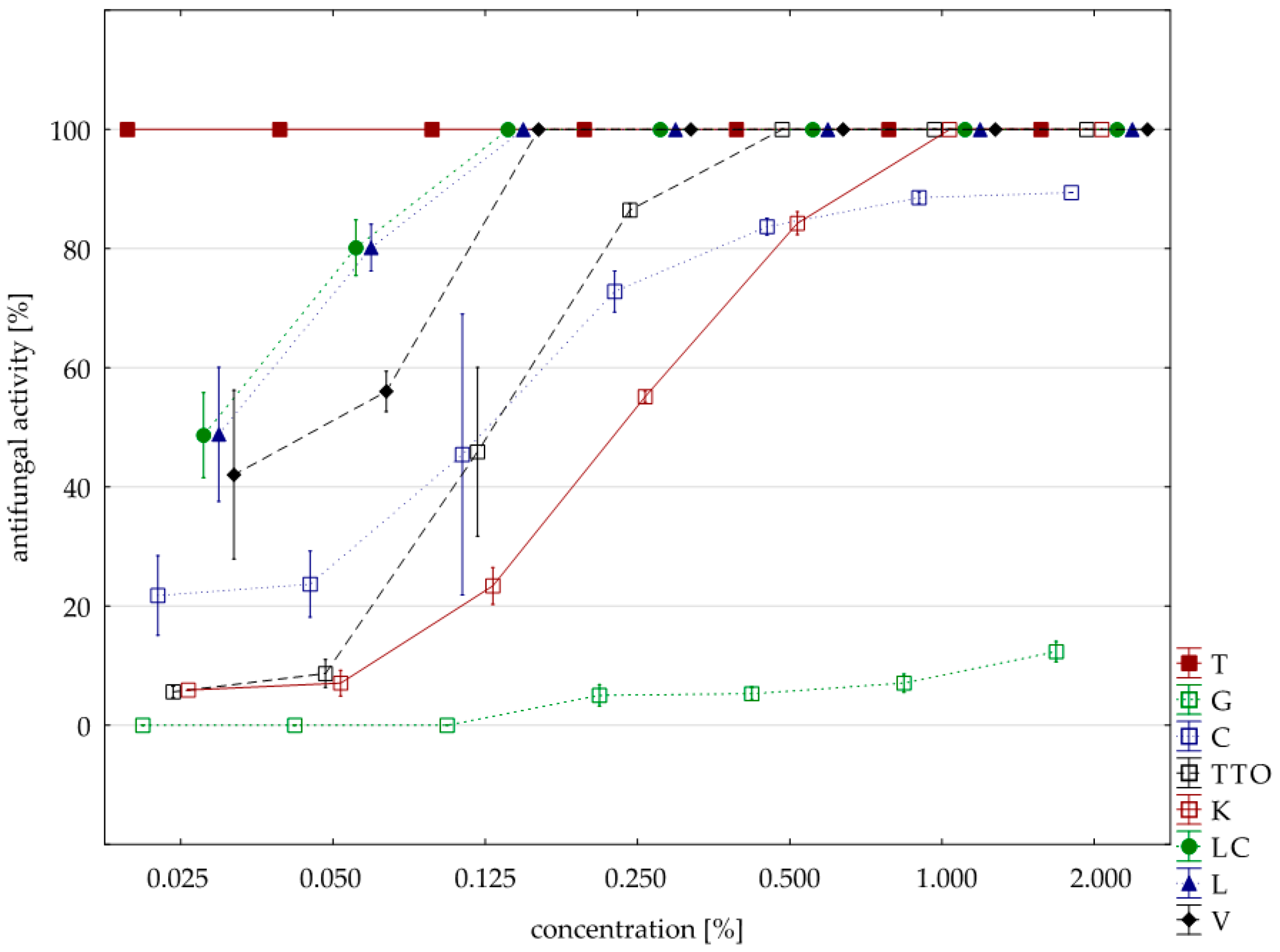
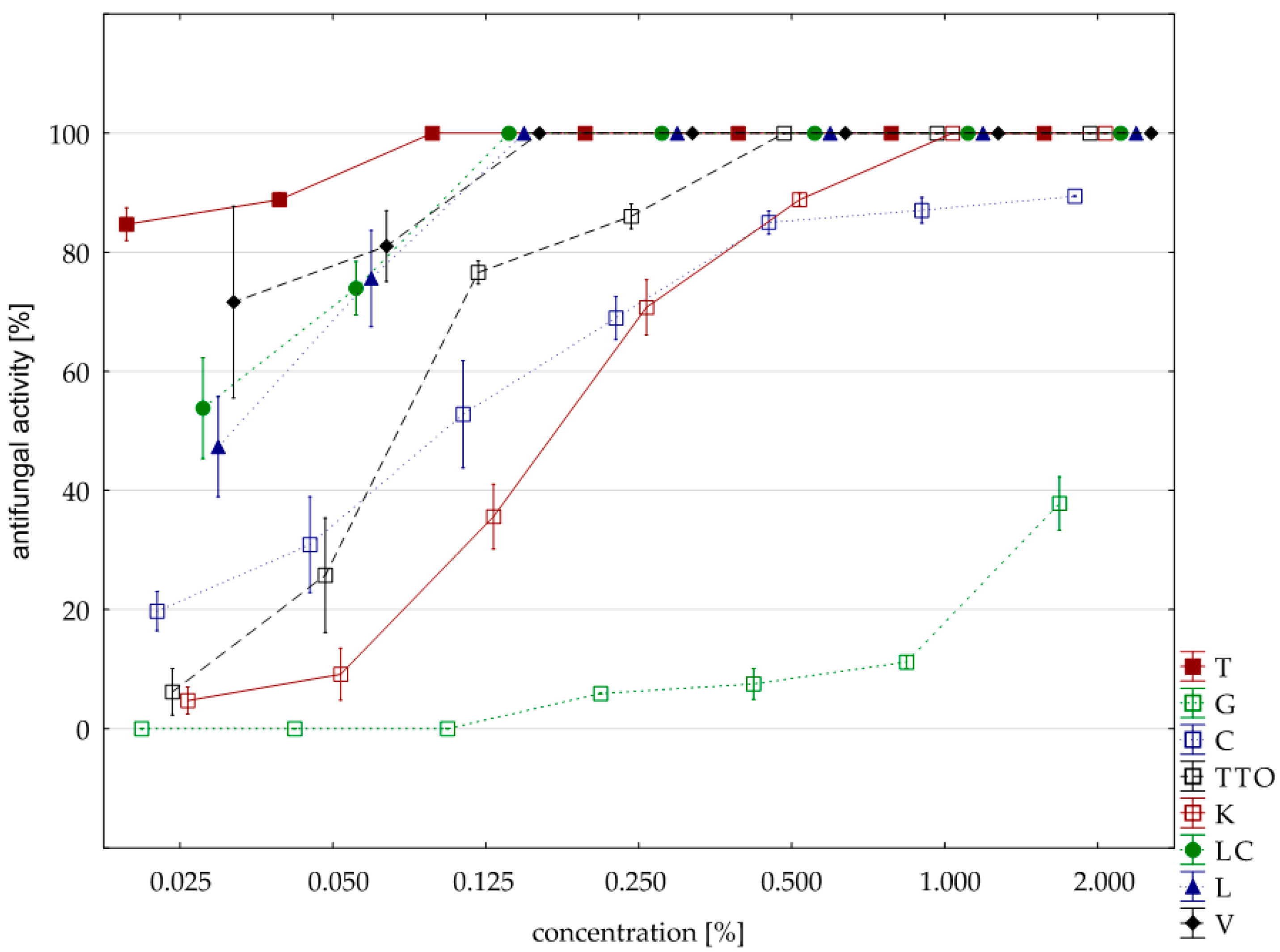
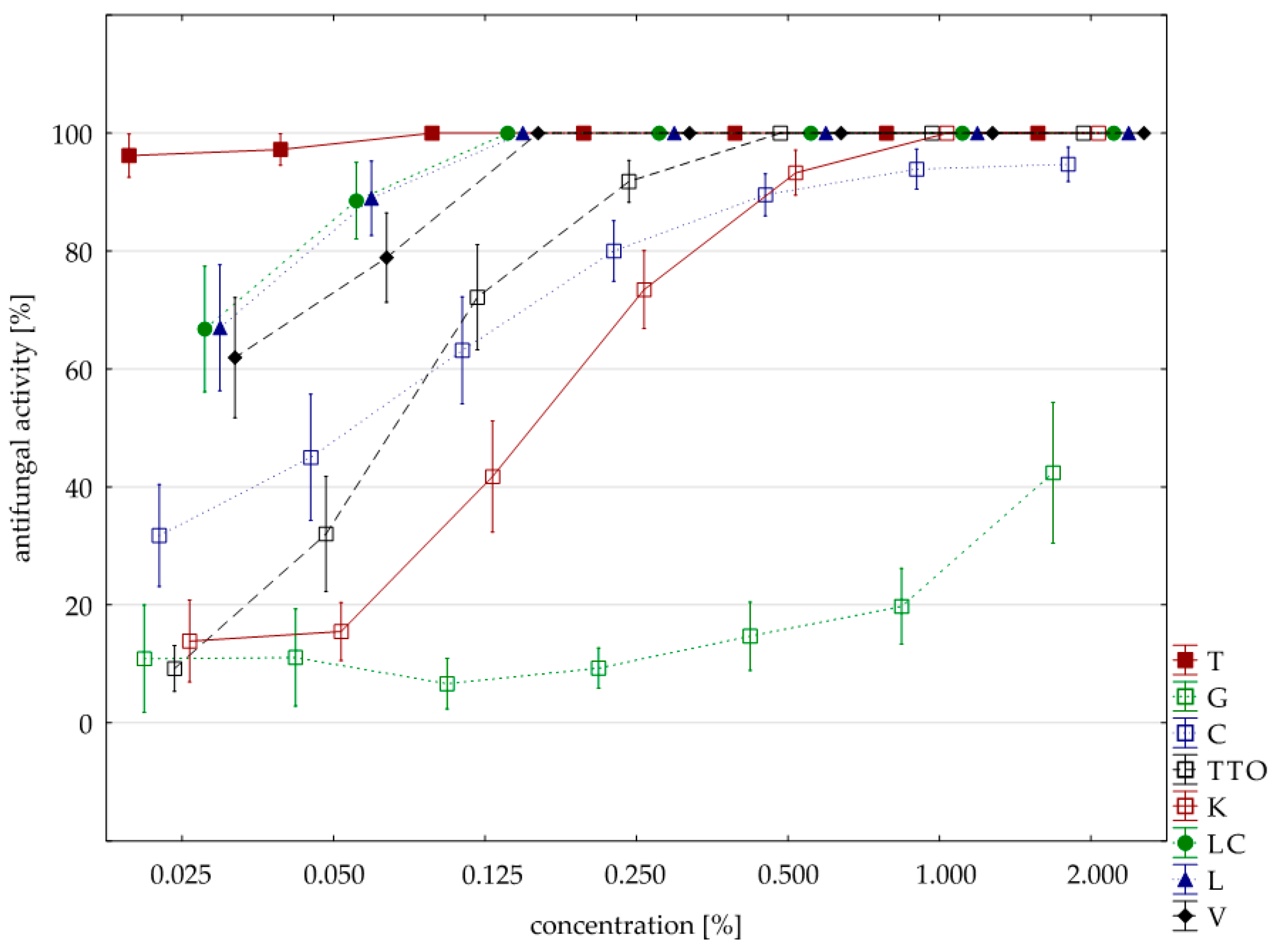
2.3. Results of the Assessment of Fungistatic Activity of Essential Oils and Their Influence on Individual Fusarium ssp.
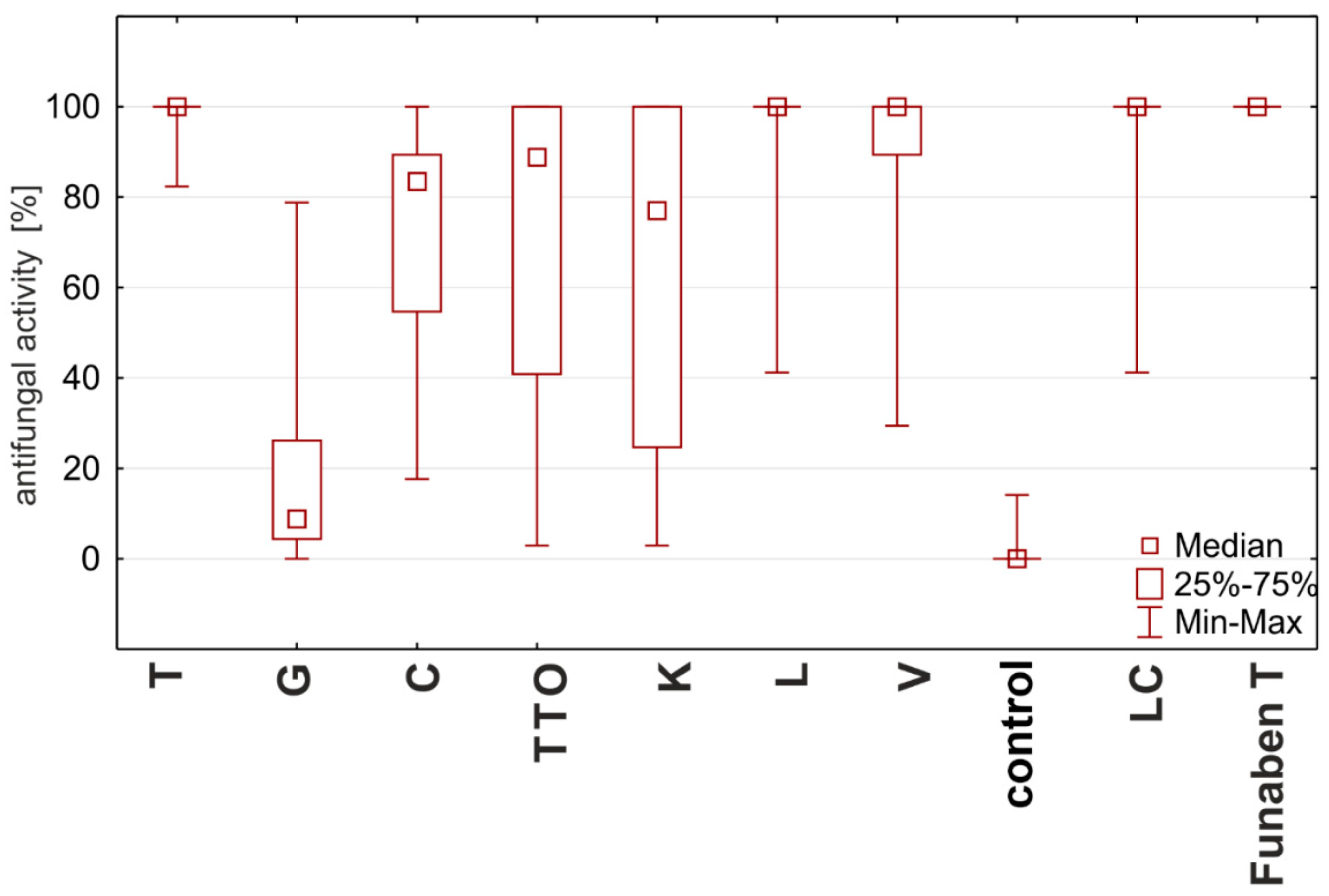
2.4. Fungistatic Activity of Oils: Combined Analysis of Fusarium Isolates
3. Discussion
3.1. Composition and Fungistatic Activity of EsO
3.2. Additional Comments
4. Materials and Methods
4.1. The Research Material
- Four isolates of Fusarium (F. avenaceum GM2, F. culmorum KP17, F. graminearum L22, and F. oxysporum P6) isolated from infected wheat grains from southern Poland (location see Table 1);
- Commercial EsO of varying chemical composition, i.e.,: thyme (T), Thymus vulgaris (produced by MELASAN, Eugendorf, Austria); lemongrass (L), Cymbopogon citratus (Lemongrass), Litsea cubeba (LC), Litsea cubeba, and grapefruit (G), Citrus paradisi (produced by TAOASIS GmbH, Berlin, Germany); verbena (V), Lippia javanica (produced by Piping Rock Health Products, LLC, Ronkonkoma, NY 11779 USA) garlic (C), Allium Sativum (produced by CAELO, Hilden, Germany); tea tree (TTO), Melaleuca alternifolia (produced by MEDESIGN IC GmbH Dietramszell—Linden, Germany); cajeput (K), Melaleuca leucadendron var. cajaputi (produced by PRIMAVERA LIFE GmbH, Oy-Mittelberg, Germany); at the following concentrations: 0.025; 0.05; 0.125; 0.25; 0.50; 1.0; and 2.0%. The oil colloid solutions were prepared in water with 0.05% Tween 80 (produced by BTL, Poland) and fed into a liquefied PDA medium (Patato Dextrose Agar (BIOCORP, Warszawa, Poland).
- Chemical seed treatment Funaben T (Zakłady Chemiczne “Organika Azot” S.A., Jaworzno, Poland), containing 20% carbendazim and 45% thiocarbamate, applied in concentrations lower, higher and recommended by the manufacturer (0.125, 0.25, and 0.5%). It was a relative control of the effectiveness of EsO.
4.2. Procedure for Obtaining Biological Material
4.3. Determination of the Quantitative Chemical Composition of Essential Oils
4.4. Determination of Fungistatic Activity of Essential Oils
- I—inhibition coefficient—growth stimulation [%]
- K—diameter of the fungus colony on the control plate [mm]
- C—diameter of the fungus colony on the plate with the given oil [mm].
4.5. Statistical Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sienkiewicz, M.; Kalemba, D.; Wasiela, M. The evaluation of the sensitivity of clinical strains of Escherichia coli to the action of thyme and lavender oil in relation to their drug resistance. Med. Exp. Microbiol. 2011, 63, 273–281. [Google Scholar]
- Cavanagh, H.M.A.; Wilkinson, J.M. Biological Activities of Lavender Essential Oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Orłowska, M. Research on Qualitative and Quantitative Composition of Chosen Thyme Species and its Biological Properties. Ph.D. Thesis, University of Silesia, Katowice, Poland, 13 December 2016. [Google Scholar]
- Kędzia, A. Garlic oil—Chemical components, pharmacological and medical activity. Adv. Phytother. 2009, 3, 198–203. [Google Scholar]
- Duke, S.O. Ecophysiological aspects of allelopathy. Planta 2003, 217, 529–539. [Google Scholar] [CrossRef]
- Azrak, S.; Karaman, S. Allelopathic effect of some essential oils and components on germination of weed species. Acta Agric. Scand. Sect. B 2008, 58, 88–92. [Google Scholar] [CrossRef]
- El-Zemity, S.R.; Ahmed, S.M. Antifungal activity of some essential oils and their major chemical constituents against some phytopathogenic fungi. J. Pest. Cont. Environ. Sci. 2005, 13, 61–72. [Google Scholar]
- Burgieł, Z.J.; Smagłowski, M. Fungistatic properties of tea tree oil. Zeszyty Problemowe Postepow Nauk Rolniczych 2008, 529, 13–18. [Google Scholar]
- Zengin, H.; Baysal, A.H. Antibacterial and Antioxidant Activity of Essential Oil Terpenes against Pathogenic and Spoilage-Forming Bacteria and Cell Structure-Activity Relationships Evaluated by SEM Microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef]
- Marei, G.I.K.; Abdelgalei, S.A.M. Antifungal potential and biochemical effects of monoterpenes and phenylpropenes on plant pathogenic fungi. Plant Prot. Sci. 2018, 54, 9–16. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernaández-López, J.; Pêrez-Ălvarez, J. Antifungal activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. Food Control 2007, 19, 1130–1138. [Google Scholar] [CrossRef]
- Hashem, M.; Moharam, A.M.; Zaied, A.A. Efficacy of essential oils in the control of cumin root rot disease caused by Fusarium spp. Crop Prot. 2010, 29, 1111–1117. [Google Scholar] [CrossRef]
- Krzyśko-Łupicka, T.; Walkowiak, W.; Białoń, M. Comparison of the fungistatic activity of selected essential oils relative to Fusarium graminearum isolates. Molecules 2019, 24, 311. [Google Scholar] [CrossRef] [PubMed]
- Pârvu, M.; Barbu-Tudoran, L.; Roşca-Casian, O.; Vlase, L.; Tripon, S. Ultrastructural Changes in Fusarium oxysporum f. sp Tulipae hyphae treated in vitro with Allium fistolosum plant extract. Ann. RSCB 2008, 15, 65–72. [Google Scholar]
- Salas-Campos, I.; Camacho-Umana, E.; Hernandez-Chavarria, F. Ultrastructural alteration induced by the essential oil of cinnamon in Fusarium solani isolated from onychomycosis. Rev. Biomed. 2013, 24, 21–23. [Google Scholar]
- Danielewicz, B.; Gwiazdowski, R.; Bednarek-Bartsch, A. Influence of some selected fungicides on Fusarium genus cultures growth limitation. Prog. Plant Prot. 2013, 53, 759–761. [Google Scholar]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 1–47. [Google Scholar] [CrossRef]
- Kwon, H.W.; Yoon, J.H.; Kim, S.H.; Hong, S.B.; Cheon, Y.; Ko, S.J. Detection of Extracellular Enzyme Activities in Various Fusarium sp. Mycobiology 2007, 35, 162–165. [Google Scholar] [CrossRef]
- Wang, J.L.; Yu, J.H.; Liu, J.; Yuan, Y.Z.; Li, N.; He, M.Q.; Qi, T.; Hui, G.; Li, X.; Liu, D.L. Novel mutations in CYP51B from Penicillium digitatum involved in prochloraz resistance. J. Microbiol. 2014, 52, 762–770. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Prajapati, V.; Verma, N.; Bahl, J.R.; Bansal, R.P.; Khanuja, S.P.S. Bioactivities of the leaf essential oil of Curcuma longa (var. ch-66) on three species of stored-product beetles (Coleoptera). J. Econ. Entomol. 2002, 95, 183–189. [Google Scholar] [CrossRef]
- Świerczyńska, I. Influence of selected biopreparations on the growth of several species of Fusarium in laboratory conditions. J. Res. Appl. Agric. Engin. 2010, 55, 158–161. [Google Scholar]
- Gao, T.; Zhou, H.; Zhou, W.; Hu, L.; Chen, J.; Shi, Z. The Fungicidal Activity of Thymol against Fusarium graminearum via Inducing Lipid Peroxidation and Disrupting Ergosterol Biosynthesis. Molecules 2016, 21, 770. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Q.; Tao, N.; Jing, G. Transcriptional profiling analysis of Penicillium digitatum, the causal agent of citrus green mold, unravels an inhibited ergosterol biosynthesis pathway in response to citral. BMC Genom. 2016, 17, 599. [Google Scholar] [CrossRef] [PubMed]
- Shreaz, S.; Shiekh, R.A.; Raja, V.; Wani, W.A.; Behbehani, J.M. Impaired ergosterol biosynthesis mediated fungicidal activity of Co(II) complex with ligand derived from cinnamaldehyde. Chem. Biol. Interact. 2016, 247, 64–74. [Google Scholar] [CrossRef]
- Sahab, A.F.; Aly, S.; Hathout, A.S.; Ziedan, E.S.H.; Sabry, B.A. Application of Some Plant Essential Oils to Control Fusarium Isolates Associated with Freshly Harvested Maize in Egypt. J. Essent. Oil Bear. Plants 2014, 17, 1146–1155. [Google Scholar] [CrossRef]
- Cakir, A.; Kordali, S.; Kilic, H.; Kaya, E. Antifungal properties of essential oil and crude extracts of Hypericum linarioides Bosse. Biochem. Syst. Ecol. 2005, 33, 245–256. [Google Scholar] [CrossRef]
- Terzi, V.; Morcia, C.; Faccioli, P.; Vale, G.; Tacconi, G.; Malnati, M. In vitro antifungal activity of the tea tree (Melaleuca alternifolia) essential oil and its major components against plant pathogens. Lett. Appl. Microbiol. 2007, 44, 613–618. [Google Scholar] [CrossRef]
- Uwineza, M.S.; El-Yousfi, B.; Lamiri, A. Antifungal activities of essential oils of Mentha pulegium, Eugenia aromatica and Cedrus atlantica on Fusarium culmorum and Bipolaris sorokiniana in vitro. Rev. Maroc. Prot. Plantes 2018, 12, 19–32. [Google Scholar]
- Ferreira, F.M.D.; Hirooka, E.Y.; Ferreira, F.D.; Silva, M.V.; Mossini, S.A.G.; Machinski, M., Jr. Effect of Zingiber officinale Roscoe essential oil in fungus control and deoxynivalenol production of Fusarium graminearum Schwabe in vitro. Food Addit. Contam. Part A 2018, 35, 2168–2174. [Google Scholar] [CrossRef]
- Manganyi, M.C.; Regnier, T.; Olivier, E.I. Antimicrobial activities of selected essential oils against Fusarium oxysporum isolates and their biofilms. S. Afr. J. Bot. 2015, 99, 115–121. [Google Scholar] [CrossRef]
- Sharma, A.; Rajendran, S.; Srivastava, A.; Sharma, S.; Kundu, B. Antifungal activities of selected essential oils against Fusarium oxysporum f. sp. Lycopersici 1322, with emphasis on Syzygium aromaticum essential oil. J. Biosci. Bioeng. 2017, 123, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, K.; Otlewska, A.; Kunicka-Styczyńska, A.; Krajewska, A. Candida albicans Impairments Induced by Peppermint and Clove Oils at Sub-Inhibitory Concentrations. Int. J. Mol. Sci. 2017, 18, 1307. [Google Scholar] [CrossRef] [PubMed]
- Oussalah, M.; Caillet, S.; Lacroix, M. Mechanism of action of Spanish oregano, Chinese cinnamon, and savory essential oils against cell membranes and walls of Escherichia coli O157:H7 and Listeria monocytogenes. J. Food Prot. 2006, 69, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Espina, L.; Somolinos, M.; Lorán, S.; Conchello, P.; García, D.; Pagan, R. Chemical composition of commercial citrus fruit essential oils and evaluation of their antimicrobial activity acting alone in combined processes. Food Control 2011, 22, 896–902. [Google Scholar] [CrossRef]
- Settanni, L.; Palazzolo, E.; Guarrasi, V.; Aleo, A.; Mammina, C.; Moschetti, G.; Germanà, M.A. Inhibition of foodborne pathogen bacteria by essential oils extracted from citrus fruits cultivated in Sicily. Food Control 2012, 26, 326–330. [Google Scholar] [CrossRef]
- Białoń, M.; Krzyśko-Łupicka, T.; Koszałkowska, M.; Wieczorek, P. Chemical composition of lemon essential oils and their fungicidal activity against Candida yeasts. Mycopathologia 2014, 177, 29–39. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Koutsoudaki, C.; Krsek, M.; Rodger, A. Chemical composition and antibacterial activity of the essential oil and the gum of Pistacia lentiscus Var. chia. J. Agric. Food Chem. 2005, 53, 7681–7685. [Google Scholar] [CrossRef]
- Rao, A.; Zhang, Y.; Muend, S.; Rao, R. Mechanism of antifungal activity of terpenoid phenols resembles calcium stress and inhibition of the TOR pathway. Antimicrob. Agents Chemother. 2010, 54, 5062–5069. [Google Scholar] [CrossRef]
- Mehani, M.; Salhi, N.; Valeria, T.; Ladjel, S. Antifungal effects of essential oil of Eucalyptus camaldulensis plant on Fusarium graminearum and Fusarium sporotrichioides. Int. J. Curr. Res. 2014, 6, 10795–10797. [Google Scholar]
- Rai, M.K.; Qureshi, S.; Pandey, A.K. In vitro susceptibility of opportunistic Fusarium spp. to essential oils. Mycoses 1999, 42, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakci, R.; Kesdek, M.; Mete, E. Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour. Technol. 2008, 99, 8788–8795. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Sharifzadeh, A.; Shokri, H.; Khosravi, A.R.; Abbaszadeh, A. Antifungal efficacy of thymol, carvacrol, eugenol and menthol as alternative agents to control the growth of food-relevant fungi. J. Mycol. Med. 2014, 24, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Campos-Requena, V.H.; Rivas, B.L.; Pérez, M.A.; Figueroa, C.R.; Sanfuentes, E.A. The synergistic antimicrobial effect of carvacrol and thymol in clay/polymer nanocomposite films over strawberry gray mold. LWT-Food Sci. Technol. 2015, 64, 390–396. [Google Scholar] [CrossRef]
- Marei, G.I.K.; Abdelgaleil, S.A.M.; Rasoul, M.A.A. Comparative antifungal activities and biochemical effects of monoterpenes on plant pathogenic fungi. Pestic. Biochem. Physiol. 2012, 103, 56–61. [Google Scholar] [CrossRef]
- Pattnaik, S.; Subramanyam, V.R.; Bapaji, M.; Kole, C.R. Antibacterial and antifungal activity of aromatic constituents of essential oils. Microbios 1997, 89, 39–46. [Google Scholar]
- Fisher, K.; Phillips, C. Potential antimicrobial uses of essential oils in food: Is citrus the answer? Trends Food Sci. Technol. 2008, 19, 156–164. [Google Scholar] [CrossRef]
- Pandey, A.K.; Rai, M.K.; Acharya, D. Chemical Composition and Antimycotic Activity of the Essential Oils of Corn Mint (Mentha arvensis) and Lemon Grass (Cymbopogon flexuosus) Against Human Pathogenic Fungi. Pharm. Biol. 2003, 41, 421–424. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. Chapter 5. Investigating the effects of plant essential oils on post-harvest fruit decay. In Fungal Pathogenicity; Sultan, S., Ed.; Publ. Intech: Rijeka, Croatia, 2016; pp. 83–98. [Google Scholar] [CrossRef]
- Adebayo, O.; Dang, T.; Bélanger, A.; Khanizadeh, S. Antifungal Studies of Selected Essential Oils and a Commercial Formulation against Botrytis cinerea. J. Food Res. 2013, 2, 217–226. [Google Scholar] [CrossRef]
- Giweli, A.A.; Džamić, A.M.; Soković, M.D.; Ristić, M.S.; Marin, P.D. Chemical composition, antioxidant and antimicrobial activities of essential oil of Thymus algeriensis wild-growing in Libya. Cent. Eur. J. Biol. 2013, 8, 504–511. [Google Scholar] [CrossRef]
- Segvić-Klarić, M.; Kosalec, I.; Mastelić, J.; Piecková, E.; Pepeljnak, S. Antifungal activity of thyme (Thymus vulgaris L.) essential oil and thymol against moulds from damp dwellings. Lett. Appl. Microbiol. 2007, 44, 36–42. [Google Scholar] [CrossRef]
- Sadowska, K.; Łukaszewska-Skrzypniak, N.; Wojczyńska, J.; Stępniewska-Jarosz, S.; Tyrakowska, M.; Rataj-Guranowska, M. Evaluation of susceptibility of potential rape pathogens to selected essential oils. Prog. Plant Prot. 2017, 57, 201–205. [Google Scholar]
- Morcia, C.; Malanati, M.; Terzi, V. In vitro activity of terpinen- 4-ol, eugenol, carvone, 1,8-cineole (eucalyptol) and thymol against mycotoxigenic plant pathogens. Food Addit. Contam. 2012, 29, 415–422. [Google Scholar] [CrossRef][Green Version]
- Orlikowski, L.B. Effect of grapefruit extract on development of Phytophthora cryptogea and control of foot rot of gerbera. J. Plant Prot. Res. 2011, 41, 288–294. [Google Scholar]
- Seseni, L.; Regnier, T.; Roux-van der Merwe, M.P.; Mogale, E.; Badenhorst, J. Control of Fusarium spp. causing damping-off of pine seedlings by means of selected essential oils. Ind. Crops Prod. 2015, 76, 329–332. [Google Scholar] [CrossRef]
- Zabka, M.; Pavela, R.; Slezakova, L. Antifungal effect of Pimenta dioica essential oil against dangerous pathogenic and toxinogenic fungi. Ind. Crops Prod. 2009, 30, 250–253. [Google Scholar] [CrossRef]
- Daferera, D.J.; Ziogas, B.N.; Polissiou, M.G. The effectiveness of plant essential oils on the growth of Botrytis cinerea, Fusarium sp. and Clavibacter michiganensis subsp. michiganensis. Crop Prot. 2003, 22, 39–44. [Google Scholar] [CrossRef]
- Tousson, T.A.; Nelson, P.E. Fusarium: A Pictorial Guide to the Identification of Fusarium Species According to the Taxonomic System of Snyder and Hansen; Pennsylvania State University Press: University Park, PA, USA, 1976. [Google Scholar]
- Leslie, J.F.; Summerell, B.A. The Fusarium. Laboratory Manual; Blackwell Publishing: Hoboken, NJ, USA, 2006. [Google Scholar]
- Watanabe, T. Pictorial Atlas of Soil and Seed Fungi: Morphologies of Cultured Fungi and Key to Species, 3rd ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2010. [Google Scholar]
- Breitmaier, E. Terpenes; Wiley-VCH Verlag GmbH&Co.: Weinheim, Germany, 2006. [Google Scholar]
- Kohlmunzer, S. Farmakognozja; Wyd. Lekarskie PZWL: Warszawa, Poland, 1993. [Google Scholar]
- Soliman, K.M.; Badeaa, R.I. Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Food Chem. Toxicol. 2002, 40, 1669–1675. [Google Scholar] [CrossRef]
- Feng, W.; Chen, J.; Zheng, X.; Liu, Q. Thyme oil to control Alternaria alternata in vitro and in vivo as fumigant and contact treatments. Food Control 2011, 22, 78–81. [Google Scholar] [CrossRef]
- Wagle, B.; Budathoki, U. Antifungal Activities of Essential Oils and Crude Extracts of Some Aromatic Plants Against Fusarium Rot of Trichosanthes dioica. Nepal J. Sci. Technol. 2013, 13, 97–102. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
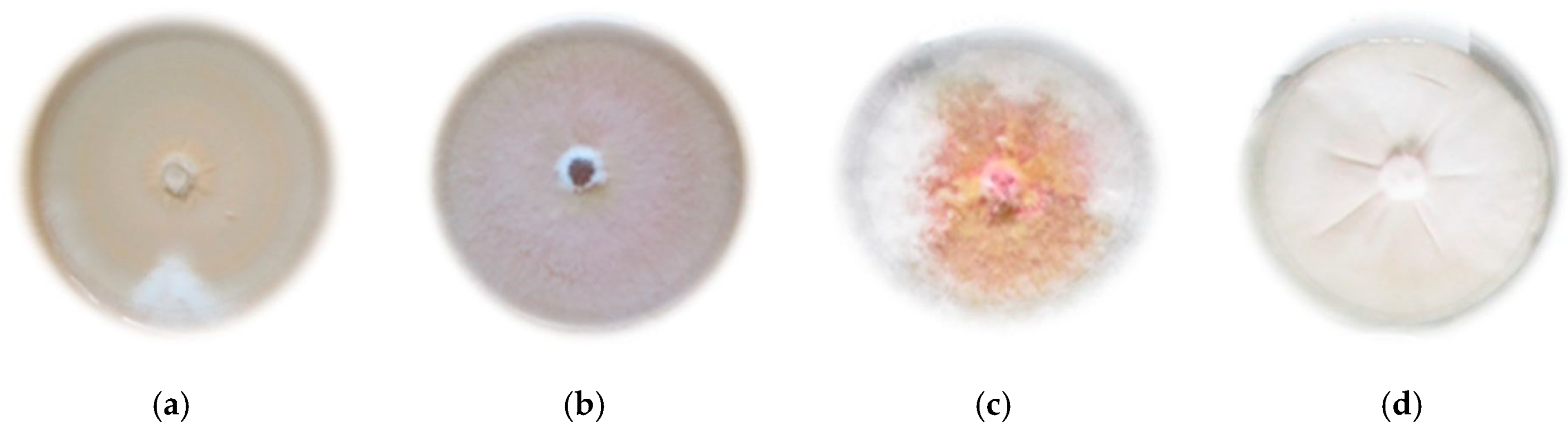
| No. | Symbol | Place of Isolation | Number of Isolates | Classic Identification | Value of the MALDI-TOF Identification Indicator |
|---|---|---|---|---|---|
| 1 | GM2 | Głubczyce | 5 | F. avenaceum | 2.49 |
| 2 | P6 | Kietrz | 4 | F. oxysporum | 2.38 |
| 3 | KP17 | Kędzierzyn | 6 | F. culmorum | 2.61 |
| 4 | L22 | Łosiów | 4 | F. graminearum | 2.53 |
| Compound | RI | Etheric Oils | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Lit * | Cal * | T | L | LC | V | TTO | K | G | |
| Monoterpenes | |||||||||
| tricyclene | 923 | 920 | 0.17 ± 0.01 | 0.44 ± 0.08 | 0 | 0 | |||
| α-thujene | 928 | 928 | 0.44 ± 0.05 | 0.83 ± 0.07 | |||||
| α-pinene | 936 | 933 | 2.75 ± 0.09 | 0.49 ± 0.11 | 2.86 ± 0.16 | 0 | 3.42 ± 0.06 | 5.37 ± 0.01 | 3.27 ± 0.01 |
| camphene | 950 | 947 | 1.93 ± 0.07 | 3.71 ± 0.06 | 0.58 ± 0.04 | 0.80 ± 0.02 | 0 | ||
| β-pinene | 978 | 974 | 0.65 ± 0.02 | 0 | 3.95 ± 0.08 | 1.08 ± 0.05 | 0.81 ± 0.02 | 3.93 ± 0.15 | |
| β-myrcene | 989 | 991 | 2.44 ± 0.03 | 0.38 ± 0.11 | 3.01 ± 0.07 | 5.32 ± 0.01 | |||
| α-phellandrene | 1004 | 1002 | 0.87 ± 0.03 | 0.05 ± 0.02 | 0.15 ± 0.08 | ||||
| sabinene (4,10-thujene) | 1004 | 1009 | 0.27 ± 0.04 | 0.17 ± 0.03 | 1.56 ± 0.03 | ||||
| 3-carene | 1011 | 1005 | 17.04 ± 0.15 | ||||||
| α-terpinene | 1017 | 1018 | 2.32 ± 0.10 | 10.29 ± 0.09 | |||||
| p-cymene | 1024 | 1020 | 3.62 ± 0.03 | ||||||
| limonene | 1029 | 1026 | 15.15 ± 0.18 | 20.94 ± 0.13 | 34.63 ± 0.73 | ||||
| γ-terpinene | 1060 | 1061 | 8.10 ± 0.07 | 2.02 ± 0.07 | 0.37 ± 0.04 | 0.78 ± 0.01 | |||
| terpinolene | 1087 | 1087 | 0.45 ± 0.01 | 3.87 ± 0.05 | 0.27 ± 0.02 | 0.08 ± 0.01 | |||
| β-patchulene | 1457 | 1455 | 0.16 ± 0.04 | ||||||
| Sum monoterpenes | 34.38 | 4.64 | 28.33 | 8.73 | 37.12 | 12.95 | 45.64 | ||
| Monoterpenoids | |||||||||
| α and β citral (geranial and neral) | - | - | 68.94 ± 0.10 | 61.72 ± 0.43 | 36.00 ± 0.08 | ||||
| trifluorolavandulol | 1999 | 2.19 ± 0.07 | |||||||
| eucalyptol | 1031 | 1027 | 13.46 ± 0.17 | 13.90 ± 0.15 | 18.50 ± 0.05 | ||||
| linalool oxide | 1065 | 1064 | 0,12 ± 0,03 | ||||||
| linalool | 1099 | 1105 | 8.90 ± 0.18 | 5.73 ± 0.22 | 2.58 ± 0.04 | 8.53 ± 0.01 | 11.19 ± 0.17 | 4.83 ± 0.039 | |
| 1-terpineol | 1137 | 1135 | 1.19 ± 0.05 | 1.39 ± 0.06 | 0.47 ± 0.01 | 0.87 ± 0.10 | |||
| p-menth-3-en-9-ol | 1141 | 1140 | 0.71 ± 0.02 | ||||||
| camphor | 1143 | 1141 | 4.62 ± 0.03 | ||||||
| verbenol | 1145 | 1145 | 0.18 ± 0.014 | ||||||
| β-citronellal | 1154 | 1152 | 1.87 ± 0.13 | 0.42 ± 0.0.03 | |||||
| borneol | 1166 | 1168 | 3.07 ± 0.09 | 2.93 ± 0.07 | 1.32 ± 0.02 | ||||
| 1-terpinen-4-ol | 1177 | 1181 | 4.51 ± 0.05 | 38.24 ± 0.38 | 4.41 ± 0.35 | 0.09 ± 0.003 | |||
| α-terpineol | 1190 | 1197 | 1.14 ± 0.09 | 1.02 ± 0.06 | 18.26 ± 0.150 | 6.88 ± 0.04 | 36.57 ± 0.21 | 1.83 ± 0.048 | |
| α-pinene oxide | 1197 | 1195 | 0.51 ± 0.029 | ||||||
| cis-geraniol | 1238 | 1234 | 0.55 ± 0.046 | ||||||
| β citral (neral) | 1242 | 1231 | 0.92 ± 0.058 | ||||||
| trans-geraniol | 1255 | 1252 | 0.45 ± 0.04 | ||||||
| linalyl acetate | 1255 | 1260 | 0.93 ± 0.06 | 1.87 ± 0.028 | |||||
| geranial | 1270 | 1269 | 1.36 ± 0.022 | ||||||
| thymol | 1290 | 1298 | 45.75 ± 0.18 | ||||||
| α-terpinyl acetate | 1347 | 0.23 ± 0.021 | |||||||
| nerol acetate | 1363 | 1366 | 1.68 ± 0.01 | ||||||
| geraniol acetate | 1380 | 1385 | 2.26 ± 0.045 | ||||||
| Sum momoterpenoids | 60.98 | 79.99 | 68.58 | 87.18 | 59.02 | 71.66 | 17.69 | ||
| Sesquiterpenes | |||||||||
| α-cubebene | 1351 | 1350 | 0.52 ± 0.04 | 0.37 ± 0.004 | |||||
| α-longipinene | 1352 | 1350 | 0.67 ± 0.08 | ||||||
| ylangene | 1370 | 1370 | 0.51 ± 0.01 | ||||||
| β-cubebene | 1387 | 1390 | 0.49 ± 0.028 | ||||||
| β-elemene | 1388 | 1387 | 0.14 ± 0.05 | ||||||
| longifolene | 1407 | 1408 | 1.12 ± 0.02 | ||||||
| α-gurjunene | 1409 | 1410 | 0.23 ± 0.02 | 1.19 ± 0.09 | |||||
| caryophyllene | 1419 | 1423 | 4.31 ± 0.02 | 3.76 ± 0.012 | 2.45 ± 0.018 | 0.55 ± 0.06 | 0.28 ± 0.003 | 2.60 ± 0.19 | 0.98 ± 0.061 |
| α-caryophyllene | 1420 | 1408 | 0.33 ± 0.03 | 0.45 ± 0.01 | 0.20 ± 0.004 | 1.70 ± 0.03 | 0.14 ± 0.014 | ||
| β-gurjunene | 1431 | 1430 | 1.15 ± 0.05 | 1.23 ± 0.05 | 0.57 ± 0.03 | ||||
| (+)aromadendrene | 1441 | 1440 | 0.94 ± 0.10 | ||||||
| γ-elemene | 1449 | 1445 | 0.05 ± 0.01 | ||||||
| allo-aromadendrene | 1460 | 1458 | 0.23 ± 0.03 | 3.63 ± 0.06 | |||||
| γ-muurolene | 1476 | 1478 | 0.12 ± 0.06 | ||||||
| germacene D | 1481 | 1496 | 0 | 0.18 ± 0.01 | |||||
| (+)-valencene | 1491 | 1499 | 0 | 0.14 ± 0.09 | |||||
| β-selinene | 1493 | 1490 | 1.62 ± 0.03 | ||||||
| γ-cadinene | 1513 | 1517 | 4.83 ± 0.10 | ||||||
| σ-cadinene | 1523 | 1526 | 0.83 ± 0.04 | 0.48 ± 0.009 | |||||
| cadinene | 1533 | 1530 | 0.37 ± 0.05 | ||||||
| Sum sesquiterpenes | 4.64 | 10.86 | 2.65 | 1.67 | 3.86 | 12.90 | 2.83 | ||
| Sesquiterpenoids | |||||||||
| trans-nerolidol | 1524 | 1522 | 0.02 ± 0.006 | ||||||
| elemol | 1536 | 1540 | 0.05 ± 0.01 | ||||||
| caryophyllene oxide | 1581 | 1572 | 1.14 ± 0.03 | 0.44 ± 0.05 | 0.42 ± 0.08 | 0.23 ± 0.003 | |||
| guaiol | 1589 | 1590 | 0.55 ± 0.05 | ||||||
| eudesmol | 1616 | 1611 | 1.52 ± 0.03 | ||||||
| farnesol | 1722 | 1718 | 0.05 ± 0.011 | ||||||
| nootkatone | 1813 | 1818 | 1.37 ± 0.069 | ||||||
| farnesyl acetate | 1818 | 1820 | 0.03 ± 0.002 | ||||||
| Sum sesquiterpenoids | 1.14 | 0.44 | 2.49 | 1.75 | |||||
| Sum other chemical compounds | 3.37 | 26.81 | |||||||
| Main Groups of Compounds | Etheric Oils | |||||||
|---|---|---|---|---|---|---|---|---|
| T | L | LC | V | TTO | K | C | G | |
| monoterpenes | 34.38 | 4.64 | 28.33 | 8.73 | 37.12 | 12.95 | 0.00 | 45.64 |
| monoterpenoids | 60.98 | 79.99 | 68.58 | 87.17 | 59.02 | 71.66 | 0.00 | 17.69 |
| sesquiterpenes | 4.64 | 10.86 | 2.65 | 1.67 | 3.86 | 12.9 | 0.00 | 2.83 |
| sesquiterpenoids | 0.00 | 1.14 | 0.44 | 0.00 | 0.00 | 2.49 | 0.00 | 1.75 |
| sulfur-organic compounds | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 100.00 | 0.00 |
| other chemical compounds | 0.00 | 3.37 | 0.00 | 2.42 | 0.00 | 0.00 | 0.00 | 32.09 |
| Detailed Division into Groups of Compounds | T | L | LC | V | TTO | K | G |
|---|---|---|---|---|---|---|---|
| aliphatic monoterpenes | 0 | 0 | 0 | 0 | 0.38 | 3.01 | 5.32 |
| monocyclic monoterpenes | 26.44 | 0 | 20.94 | 6.14 | 14.31 | 0.64 | 35.49 |
| bi- and tricyclic monoterpenes | 5.5 | 4.64 | 7.39 | 2.59 | 22.43 | 9.3 | 4.83 |
| aliphatic monoterpenoids | 9.83 | 76.35 | 66.17 | 44.53 | 0 | 11.19 | 14.85 |
| monocyclic monoterpenoids | 48.08 | 0.71 | 2.41 | 23.24 | 45.12 | 41.85 | 2.15 |
| bi- and tricyclic monoterpenoids | 3.07 | 2.93 | 0 | 19.40 | 13.90 | 18.62 | 0.69 |
| aliphatic sesquiterpenes | 0 | 0 | 0 | 0 | 0 | 0 | 0.05 |
| monocyclic sesquiterpenes | 4.64 | 4.21 | 2.65 | 0.55 | 0.28 | 4.49 | 1.30 |
| bi- and tricyclic sesquiterpenes | 0 | 6.65 | 0 | 1.12 | 3.58 | 8.41 | 1.48 |
| aliphatic sesquiterpenoids | 0 | 0 | 0 | 0 | 0 | 0 | 0.10 |
| monocyclic sesquiterpenoids | 0 | 0 | 0 | 0 | 0 | 0 | 0.05 |
| bi- and tricyclic sesquiterpenoids | 0 | 1.14 | 0.44 | 0 | 0 | 2.49 | 1.60 |
| Oil | Concentration | N | Mean | Median | Min | Max | SD |
|---|---|---|---|---|---|---|---|
| thyme | 0.025 | 4.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| grapefruit | 2.000 | 4.00 | 71.03 | 73.82 | 57.65 | 78.82 | 9.60 |
| garlic | 0.500 | 4.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| tea tree | 0.500 | 4.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| cajeput | 0.500 | 4.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| Litsea cubeba | 0.050 | 4.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| lemongrass | 0.050 | 4.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| verbena | 0.125 | 4.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| control | 4.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Funaben T | 0.125 | 1.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| Oil | Concentration | N | Mean | Median | Min | Max | SD |
|---|---|---|---|---|---|---|---|
| thyme | 0.025 | 4.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| grapefruit | 2.000 | 4.00 | 48.38 | 47.35 | 43.53 | 55.29 | 5.40 |
| garlic | 1.000 | 4.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| tea tree | 0.250 | 4.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| cajeput | 0.500 | 3.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| Litsea cubeba | 0.050 | 4.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| lemongrass | 0.050 | 4.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| verbena | 0.125 | 4.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| control | 4.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Funaben T | 0.125 | 1.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| Oil | Concentration | N | Mean | Median | Min | Max | SD |
|---|---|---|---|---|---|---|---|
| thyme | 0.025 | 4.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| grapefruit | 2.000 | 4.00 | 12.35 | 12.35 | 11.18 | 13.53 | 1.07 |
| garlic | 2.000 | 4.00 | 89.41 | 89.41 | 89.41 | 89.41 | 0.00 |
| tea tree | 0.500 | 4.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| cajeput | 1.000 | 4.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| Litsea cubeba | 0.125 | 4.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| lemongrass | 0.125 | 4.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| verbena | 0.125 | 4.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| control | 4.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Funaben T | 0.125 | 1.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| Oil | Concentration | N | Mean | Median | Min | Max | SD |
|---|---|---|---|---|---|---|---|
| thyme | 0.125 | 4.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| grapefruit | 2.000 | 4.00 | 37.79 | 37.06 | 35.29 | 41.77 | 2.82 |
| garlic | 2.000 | 4.00 | 89.41 | 89.41 | 89.41 | 89.41 | 0.00 |
| tea tree | 0.500 | 4.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| cajeput | 1.000 | 4.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| Litsea cubeba | 0.125 | 4.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| lemongrass | 0.125 | 4.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| verbena | 0.125 | 4.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| control | 4.00 | 3.53 | 0.00 | 0.00 | 14.12 | 7.06 | |
| Funaben T | 0.125 | 1.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| Oil | Concentration | N | Mean | Median | Min | Max | SD |
|---|---|---|---|---|---|---|---|
| thyme | 0.125 | 16.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| grapefruit | 2.000 | 16.00 | 42.39 | 42.65 | 11.18 | 78.82 | 22.37 |
| garlic | 2.000 | 16.00 | 94.71 | 94.71 | 89.41 | 100.00 | 5.47 |
| tea tree | 0.500 | 16.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| cajeput | 1.000 | 16.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| Litsea cubeba | 0.125 | 16.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| lemongrass | 0.125 | 16.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| verbena | 0.125 | 16.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| control | 16.00 | 0.88 | 0.00 | 0.00 | 14.12 | 3.53 | |
| Funaben T | 0.125 | 4.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.00 |
| Oil | Assessment of Fungistatic Activity [%] |
|---|---|
| thyme | 0.22 |
| grapefruit | 0.61 |
| garlic | 0.64 |
| tea tree | 0.59 |
| cayeput | 0.72 |
| Litsea cubeba | 0.35 |
| lemongrass | 0.35 |
| verbena | 0.33 |
| b * | SE of b * | b | SE of b | t (887) | p Value | |
|---|---|---|---|---|---|---|
| Intercept | 61.601 | 2.158 | 28.539 | 0.000 * | ||
| concentration | 0.336 | 0.021 | 1740.112 | 109.352 | 15.913 | 0.000 * |
| monoterpenes | −0.107 | 0.026 | −0.233 | 0.056 | −4.119 | 0.000 * |
| monoterpenoids | 0.257 | 0.029 | 0.309 | 0.035 | 8.778 | 0.000 * |
| sesquiterpenes | 0.196 | 0.053 | 1.595 | 0.427 | 3.736 | 0.000 * |
| sesquiterpenoids | −0.467 | 0.053 | −17.827 | 2.030 | −8.783 | 0.000 * |
| other chemical compounds | −0.219 | 0.040 | −0.724 | 0.132 | −5.481 | 0.000 * |
| Statistic | Value |
|---|---|
| R | 0.777 |
| R2 | 0.604 |
| Adjusted R2 | 0.601 |
| F (6887) | 225.15 |
| p | 0.000 * |
| Std. Error of estimate | 21.775 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzyśko-Łupicka, T.; Sokół, S.; Piekarska-Stachowiak, A. Evaluation of Fungistatic Activity of Eight Selected Essential Oils on Four Heterogeneous Fusarium Isolates Obtained from Cereal Grains in Southern Poland. Molecules 2020, 25, 292. https://doi.org/10.3390/molecules25020292
Krzyśko-Łupicka T, Sokół S, Piekarska-Stachowiak A. Evaluation of Fungistatic Activity of Eight Selected Essential Oils on Four Heterogeneous Fusarium Isolates Obtained from Cereal Grains in Southern Poland. Molecules. 2020; 25(2):292. https://doi.org/10.3390/molecules25020292
Chicago/Turabian StyleKrzyśko-Łupicka, Teresa, Sławomir Sokół, and Anna Piekarska-Stachowiak. 2020. "Evaluation of Fungistatic Activity of Eight Selected Essential Oils on Four Heterogeneous Fusarium Isolates Obtained from Cereal Grains in Southern Poland" Molecules 25, no. 2: 292. https://doi.org/10.3390/molecules25020292
APA StyleKrzyśko-Łupicka, T., Sokół, S., & Piekarska-Stachowiak, A. (2020). Evaluation of Fungistatic Activity of Eight Selected Essential Oils on Four Heterogeneous Fusarium Isolates Obtained from Cereal Grains in Southern Poland. Molecules, 25(2), 292. https://doi.org/10.3390/molecules25020292





