Hemocyanin Modification of Chitosan Scaffolds with Calcium Phosphate Phases Increase the Osteoblast/Osteoclast Activity Ratio—A Co-Culture Study
Abstract
1. Introduction
2. Results
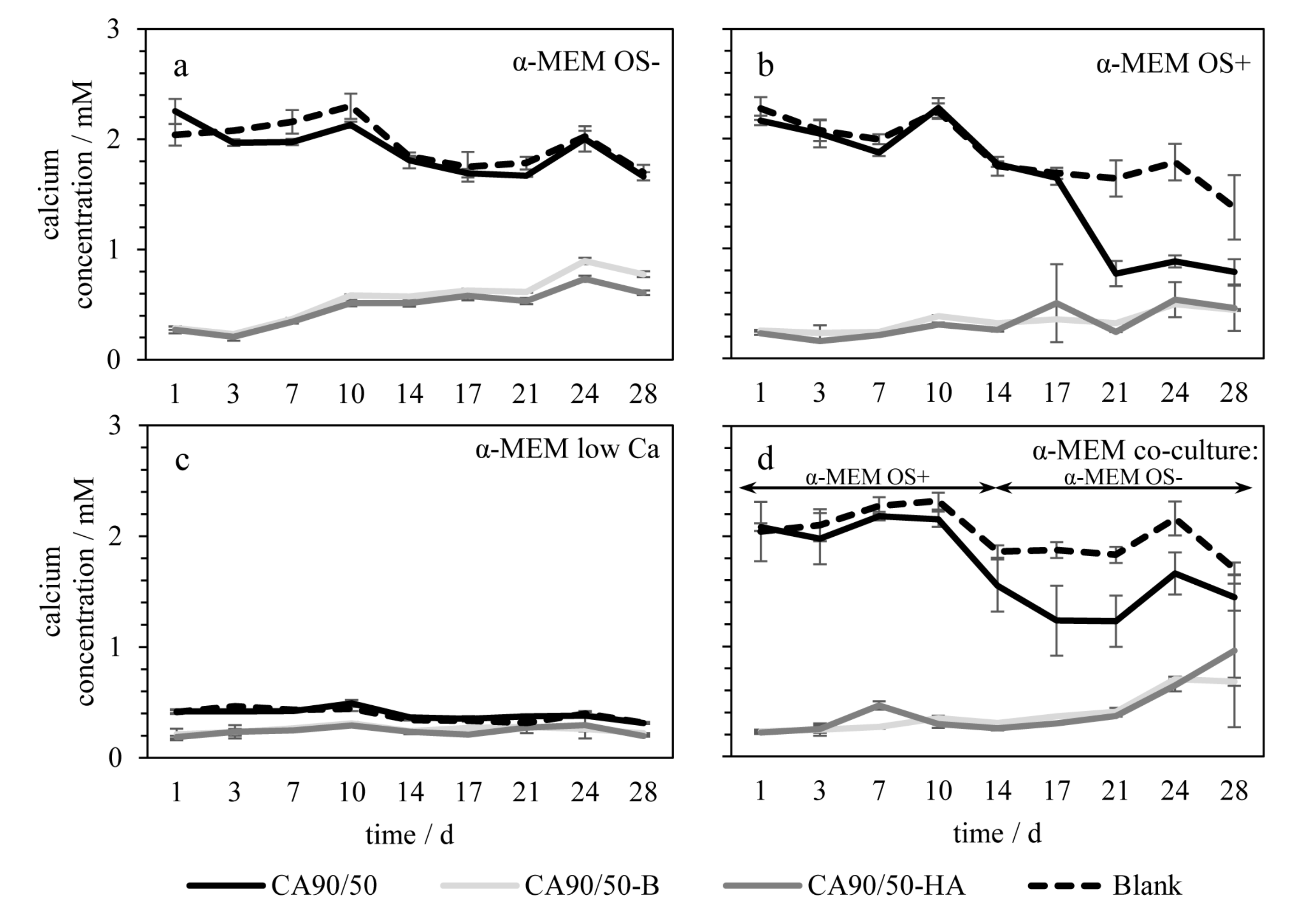
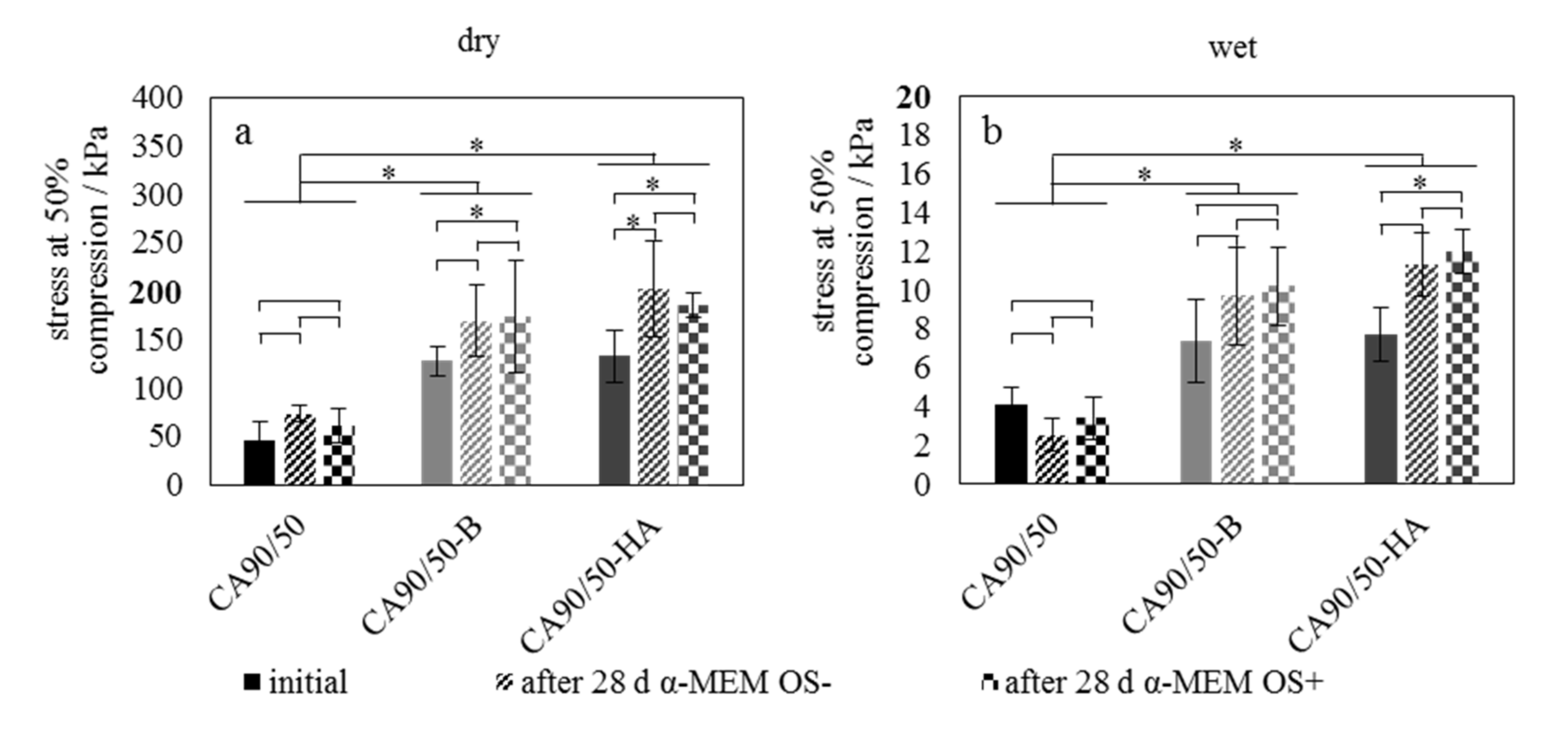
2.1. Bioactivity and Mechanical Properties
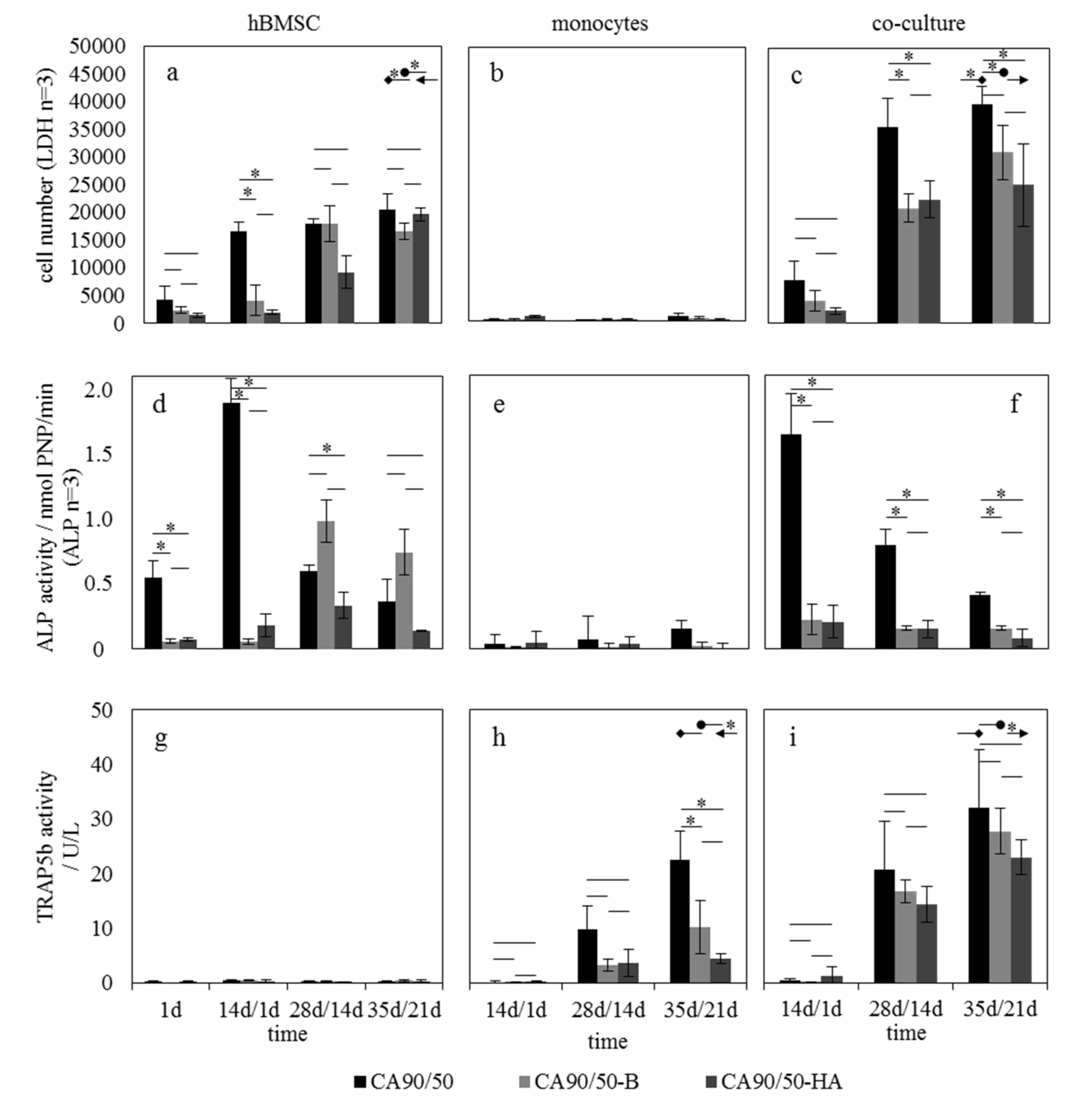
2.2. Influence of Calcium Phosphates in Scaffolds on hBMSCs and hMs in Mono- and Co-Culture
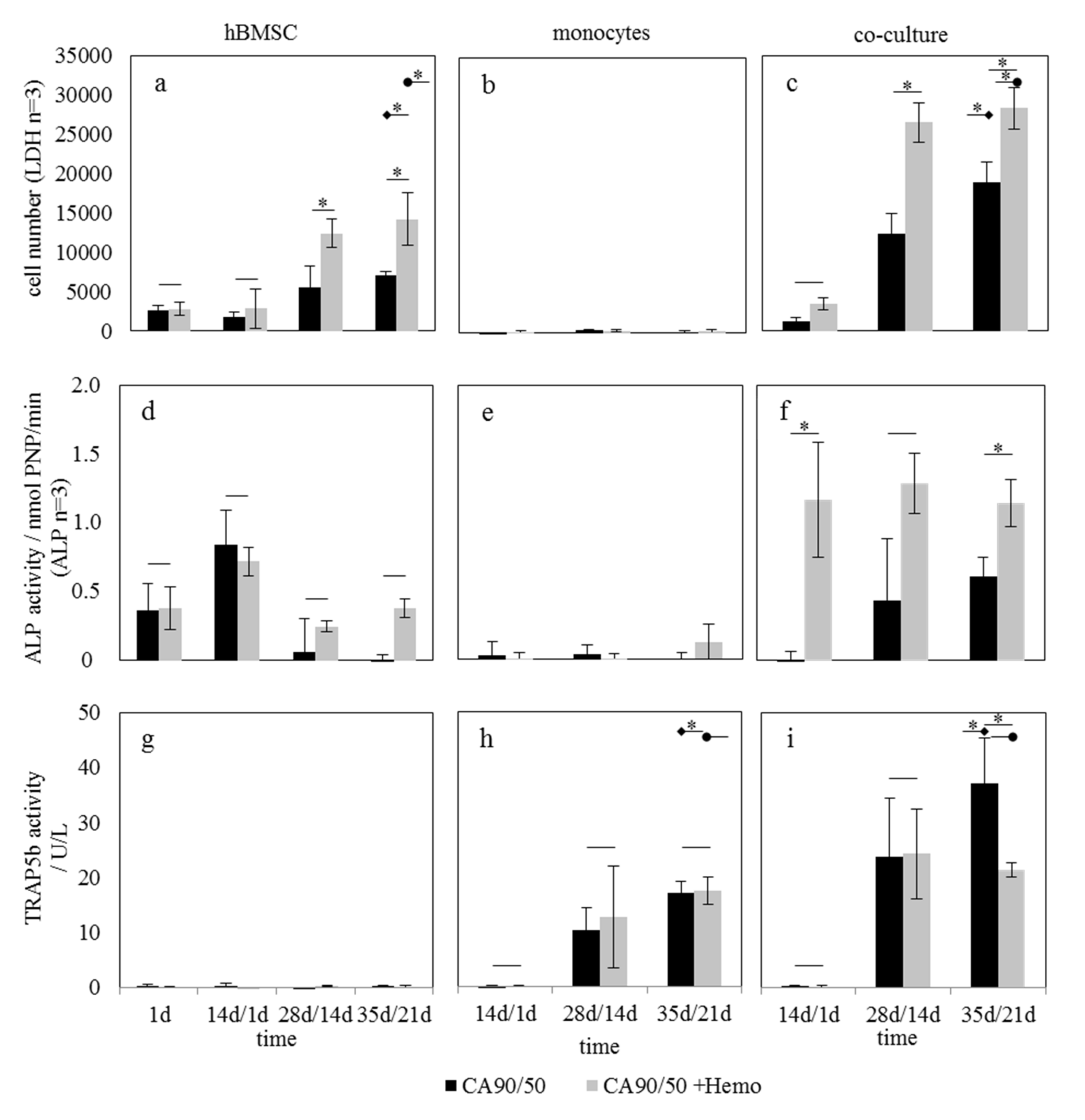
2.3. Influence of Hemocyanin-Modified Scaffolds on hBMSC and hM in Mono- and Co-Culture
3. Discussion
3.1. Bioactivity and Mechanical Properties
3.2. Influence of Scaffold Bioactivity on Osteoblast/Osteoclast Activity Ratio
3.3. Influence of Hemocyanin on Osteoblast/Osteoclast Activity Ratio
4. Materials and Methods
4.1. Preparation of Calcium-Phosphate-Modified Chitosan Scaffolds
4.2. Scaffold Characterization
4.2.1. Bioactivity
4.2.2. Mechanical Stability
4.3. Isolation of Hemocyanin from Crayfish Hemolymph and Scaffold Modification
4.4. Cell Culture Experiments
4.4.1. Human Bone Marrow Stromal Cells
4.4.2. Human Monocytes
4.4.3. Co-Culture of hBMSC/Osteoblasts and hM/Osteoclasts
4.5. Biochemical Analysis
4.5.1. ALP Activity
4.5.2. TRAP 5b Activity
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | alkaline phosphatase |
| α-MEM OS- | alpha minimum essential medium without osteogenic supplements |
| α-MEM OS+ | alpha minimum essential medium with osteogenic supplements |
| CA90/50 | scaffolds from chitosan with a degree of deacetylation of 90% and a viscosity of 50 mPas |
| CA90/50-B | chitosan scaffolds with brushite |
| CA90/50-HA | chitosan scaffolds with hydroxyapatite |
| DMEM | Dulbecco’s modified Eagle’s medium |
| EDTA | ethylenediaminetetraacetic acid |
| hBMSC | human bone marrow stromal cells |
| hM | human monocytes |
| LDH | lactate dehydrogenase |
| M-CSF | macrophage colony-stimulating factor |
| N-ASBI-P | naphthol AS-BI-phosphate |
| PBMC | peripheral blood mononuclear cell |
| PBS | phosphate-buffered saline |
| PMSF | phenylmethylsulfonyl fluoride |
| PNP | p-nitrophenol |
| RANKL | receptor activator of NF-κB ligand |
| TRAP | tartrate-resistant acid phosphatase |
References
- Zhu, S.; Ehnert, S.; Rouß, M.; Häussling, V.; Aspera-Werz, R.H.; Chen, T.; Nussler, A.K. From the Clinical Problem to the Basic Research—Co-Culture Models of Osteoblasts and Osteoclasts. Int. J. Mol. Sci. 2018, 19, 2284. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, C.; Heinemann, S.; Worch, H.; Hanke, T. Development of an osteoblast/osteoclast co-culture derived by human bone marrow stromal cells and human monocytes for biomaterials testing. Eur. Cell Mater. 2011, 21, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, C.; Heinemann, S.; Rößler, S.; Kruppke, B.; Wiesmann, H.-P.; Hanke, T. Organically modified hydroxyapatite (ormoHAP) nanospheres stimulate the differentiation of osteoblast and osteoclast precursors: A co-culture study. Biomed. Mater. 2019, 14, 035015. [Google Scholar] [CrossRef] [PubMed]
- Wenisch, S.; Stahl, J.-P.; Horas, U.; Heiss, C.; Kilian, O.; Trinkaus, K.; Hild, A.; Schnettler, R. In vivo mechanisms of hydroxyapatite ceramic degradation by osteoclasts: Fine structural microscopy. J. Biomed. Mater. Res. 2003, 67A, 713–718. [Google Scholar] [CrossRef]
- Sheikh, Z.; Abdallah, M.N.; Hanafi, A.A.; Misbahuddin, S.; Rashid, H.; Glogauer, M. Mechanisms of in vivo degradation and resorption of calcium phosphate based biomaterials. Materials 2015, 8, 7913–7925. [Google Scholar] [CrossRef]
- Schilling, A.F.; Filke, S.; Brink, S.; Korbmacher, H.; Amling, M.; Rueger, J.M. Osteoclasts and Biomaterials. Eur. J. Trauma 2006, 32, 107–113. [Google Scholar] [CrossRef]
- Heinemann, S.; Heinemann, C.; Wenisch, S.; Alt, V.; Worch, H.; Hanke, T. Calcium phosphate phases integrated in silica/collagen nanocomposite xerogels enhance the bioactivity and ultimately manipulate the osteoblast/osteoclast ratio in a human co-culture model. Acta Biomater. 2013, 9, 4878–4888. [Google Scholar] [CrossRef]
- Matsuo, K.; Irie, N. Osteoclast-osteoblast communication. Arch. Biochem. Biophys. 2008, 473, 201–209. [Google Scholar] [CrossRef]
- Glenske, K.; Donkiewicz, P.; Köwitsch, A.; Milosevic-Oljaca, N.; Rider, P.; Rofall, S.; Franke, J.; Jung, O.; Smeets, R.; Schnettler, R.; et al. Applications of Metals for Bone Regeneration. Int. J. Mol. Sci. 2018, 19, 826. [Google Scholar] [CrossRef]
- Malafaya, P.B.; Reis, R.L. Bilayered chitosan-based scaffolds for osteochondral tissue engineering: Influence of hydroxyapatite on in vitro cytotoxicity and dynamic bioactivity studies in a specific double-chamber bioreactor. Acta Biomater. 2009, 5, 644–660. [Google Scholar] [CrossRef]
- González-Vázquez, A.; Planell, J.A.; Engel, E. Extracellular calcium and CaSR drive osteoinduction in mesenchymal stromal cells. Acta Biomater. 2014, 10, 2824–2833. [Google Scholar] [CrossRef] [PubMed]
- Maeno, S.; Niki, Y.; Matsumoto, H.; Morioka, H.; Yatabe, T.; Funayama, A.; Toyama, Y.; Taguchi, T.; Tanaka, J. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials 2005, 26, 4847–4855. [Google Scholar] [CrossRef] [PubMed]
- Glenske, K.; Wagner, A.-S.; Hanke, T.; Cavalcanti-Adam, E.A.; Heinemann, S.; Heinemann, C.; Kruppke, B.; Arnhold, S.; Moritz, A.; Schwab, E.H.; et al. Bioactivity of xerogels as modulators of osteoclastogenesis mediated by connexin 43. Biomaterials 2014, 35, 1487–1495. [Google Scholar] [CrossRef]
- Kruppke, B.; Farack, J.; Weil, S.; Aflalo, E.D.; Poláková, D.; Sagi, A.; Hanke, T. Crayfish hemocyanin on chitin bone substitute scaffolds promotes the proliferation and osteogenic differentiation of human mesenchymal stem cells. J. Biomed. Mater. Res. Part. A 2020, 108, 694–708. [Google Scholar] [CrossRef]
- Glazer, L.; Tom, M.; Weil, S.; Roth, Z.; Khalaila, I.; Mittelman, B.; Sagi, A. Hemocyanin with phenoloxidase activity in the chitin matrix of the crayfish gastrolith. J. Exp. Biol. 2013, 216, 1898–1904. [Google Scholar] [CrossRef] [PubMed]
- Coates, C.J.; Nairn, J. Diverse immune functions of hemocyanins. Dev. Comp. Immunol. 2014, 45, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Endo, H.; Watanabe, T.; Nishioka, T.; Hirata, T. Hemocyanin in the exoskeleton of crustaceans: Enzymatic properties and immunolocalization. Pigment. Cell Res. 2005, 18, 136–143. [Google Scholar] [CrossRef]
- Lowenstam, H.A.; Weiner, S. On Biomineralization; Oxford University Press on Demand: Oxford, UK, 1989. [Google Scholar]
- Shechter, A.; Glazer, L.; Cheled, S.; Mor, E.; Weil, S.; Berman, A.; Bentov, S.; Aflalo, E.D.; Khalaila, I.; Sagi, A. A gastrolith protein serving a dual role in the formation of an amorphous mineral containing extracellular matrix. Proc. Natl. Acad. Sci. USA 2008, 105, 7129–7134. [Google Scholar] [CrossRef]
- Johnsson, M.S.; Nancollas, G.H. The Role of Brushite and Octacalcium Phosphate in Apatite Formation. Crit. Rev. Oral Biol. Med. 1992, 3, 61–82. [Google Scholar] [CrossRef]
- Nancollas, G.H.; Tomažič, B. Growth of calcium phosphate on hydroxyapatite crystals. Effect of supersaturation and ionic medium. J. Phys. Chem. 1974, 78, 2218–2225. [Google Scholar] [CrossRef]
- Bellows, C.G.; Heersche, J.N.M.; Aubin, J.E. Inorganic phosphate added exogenously or released from β-glycerophosphate initiates mineralization of osteoid nodules in vitro. Bone Miner. 1992, 17, 15–29. [Google Scholar] [CrossRef]
- Khouja, H.I.; Bevington, A.; Kemp, G.J.; Russell, R.G.G. Calcium and orthophosphate deposits in vitro do not imply osteoblast-mediated mineralization: Mineralization by betaglycerophosphate in the absence of osteoblasts. Bone 1990, 11, 385–391. [Google Scholar] [CrossRef]
- Schoenfeld, C.M.; Lautenschlager, E.P.; Meyer, P.R. Mechanical properties of human cancellous bone in the femoral head. Med. Biol. Eng. 1974, 12, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Freier, T.; Koh, H.S.; Kazazian, K.; Shoichet, M.S. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials 2005, 26, 5872–5878. [Google Scholar] [CrossRef]
- Kruppke, B.; Wagner, A.S.; Heinemann, C.; Farack, J.; Wenisch, S.; Wiesmann, H.-P.; Hanke, T. Strontium ions promote in vitro human bone marrow stromal cell proliferation and differentiation in calcium-lacking media. Dev. Growth Differ. 2018, 61, 166–175. [Google Scholar] [CrossRef]
- Eklou-Kalonji, E.; Denis, I.; Lieberherr, M.; Pointillart, A. Effects of extracellular calcium on the proliferation and differentiation of porcine osteoblasts in vitro. Cell Tissue Res. 1998, 292, 163–171. [Google Scholar] [CrossRef]
- Saldaña, L.; Sánchez-Salcedo, S.; Izquierdo-Barba, I.; Bensiamar, F.; Munuera, L.; Vallet-Regí, M.; Vilaboa, N. Calcium phosphate-based particles influence osteogenic maturation of human mesenchymal stem cells. Acta Biomater. 2009, 5, 1294–1305. [Google Scholar] [CrossRef]
- Li, Z.; Kong, K.; Qi, W. Osteoclast and its roles in calcium metabolism and bone development and remodeling. Biochem. Biophys. Res. Commun. 2006, 343, 345–350. [Google Scholar] [CrossRef]
- Heinemann, C.; Heinemann, S.; Vater, C.; Worch, H.; Hanke, T. Influence of osteogenic supplements on the osteoclastogenesis of human monocytes. J. Dev. Biol. Tissue Eng. 2011, 3, 56–61. [Google Scholar]
- Kruger, T.E.; Miller, A.H.; Wang, J. Collagen scaffolds in bone sialoprotein-mediated bone regeneration. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef]
- Bessa, P.C.; Casal, M.; Reis, R.L. Bone morphogenetic proteins in tissue engineering: The road from laboratory to clinic, part II (BMP delivery). J. Tissue Eng. Regen. Med. 2008, 2, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Decker, H.; Tuczek, F. Tyrosinase/catecholoxidase activity of hemocyanins: Structural basis and molecular mechanism. Trends Biochem. Sci. 2000, 25, 392–397. [Google Scholar] [CrossRef]
- Mody, N.; Parhami, F.; Sarafian, T.A.; Demer, L.L. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic. Biol. Med. 2001, 31, 509–519. [Google Scholar] [CrossRef]
- Marrazzo, P.; O’Leary, C. Repositioning natural antioxidants for therapeutic applications in tissue engineering. Bioengineering 2020, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Kruppk, B.; Farack, J.; Sommer, F.; Weil, S.; Aflalo, E.D.; Wiesmann, H.-P.; Sagi, A.; Hanke, T. In Situ Crosslinking of Highly Porous Chitosan Scaffolds for Bone Regeneration: Production Parameters and In Vitro Characterization. Macromol. Mater. Eng. 2017, 302, 1700147. [Google Scholar] [CrossRef]
- Nwe, N.; Furuike, T.; Tamura, H. The mechanical and biological properties of chitosan scaffolds for tissue regeneration templates are significantly enhanced by chitosan from Gongronella butleri. Materials (Basel) 2009, 2, 374–398. [Google Scholar] [CrossRef]
- Hattersley, G.; Owens, J.; Flanagan, A.M.; Chambers, T.J. Macrophage colony stimulating factor (M-CSF) is essential for osteoclast formation in vitro. Biochem. Biophys. Res. Commun. 1991, 177, 526–531. [Google Scholar] [CrossRef]
- Allen, M.; Millett, P.; Dawes, E.; Rushton, N. Lactate dehydrogenase activity as a rapid and sensitive test for the quantification of cell numbers in vitro. Clin. Mater. 1994, 16, 189–194. [Google Scholar] [CrossRef]
- Janckila, A.J.; Takahashi, K.; Sun, S.Z.; Yam, L.T. Tartrate-resistant acid phosphatase isoform 5b as serum marker for osteoclastic activity. Clin. Chem. 2001, 47, 74–80. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds CA90/50, CA90/50-B, CA90/50-HA and CA90/50+Hemo are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruppke, B.; Heinemann, C.; Farack, J.; Weil, S.; Aflalo, E.D.; Sagi, A.; Hanke, T. Hemocyanin Modification of Chitosan Scaffolds with Calcium Phosphate Phases Increase the Osteoblast/Osteoclast Activity Ratio—A Co-Culture Study. Molecules 2020, 25, 4580. https://doi.org/10.3390/molecules25194580
Kruppke B, Heinemann C, Farack J, Weil S, Aflalo ED, Sagi A, Hanke T. Hemocyanin Modification of Chitosan Scaffolds with Calcium Phosphate Phases Increase the Osteoblast/Osteoclast Activity Ratio—A Co-Culture Study. Molecules. 2020; 25(19):4580. https://doi.org/10.3390/molecules25194580
Chicago/Turabian StyleKruppke, Benjamin, Christiane Heinemann, Jana Farack, Simy Weil, Eliahu David Aflalo, Amir Sagi, and Thomas Hanke. 2020. "Hemocyanin Modification of Chitosan Scaffolds with Calcium Phosphate Phases Increase the Osteoblast/Osteoclast Activity Ratio—A Co-Culture Study" Molecules 25, no. 19: 4580. https://doi.org/10.3390/molecules25194580
APA StyleKruppke, B., Heinemann, C., Farack, J., Weil, S., Aflalo, E. D., Sagi, A., & Hanke, T. (2020). Hemocyanin Modification of Chitosan Scaffolds with Calcium Phosphate Phases Increase the Osteoblast/Osteoclast Activity Ratio—A Co-Culture Study. Molecules, 25(19), 4580. https://doi.org/10.3390/molecules25194580






