Anti-Cancer Effects of Green Tea Epigallocatchin-3-Gallate and Coffee Chlorogenic Acid
Abstract
1. Introduction
2. Anti-Cancer Effects of Green Tea
2.1. Human Studies on Green Tea
2.2. Basic Research on Anti-Cancer Action of Green Tea and EGCG
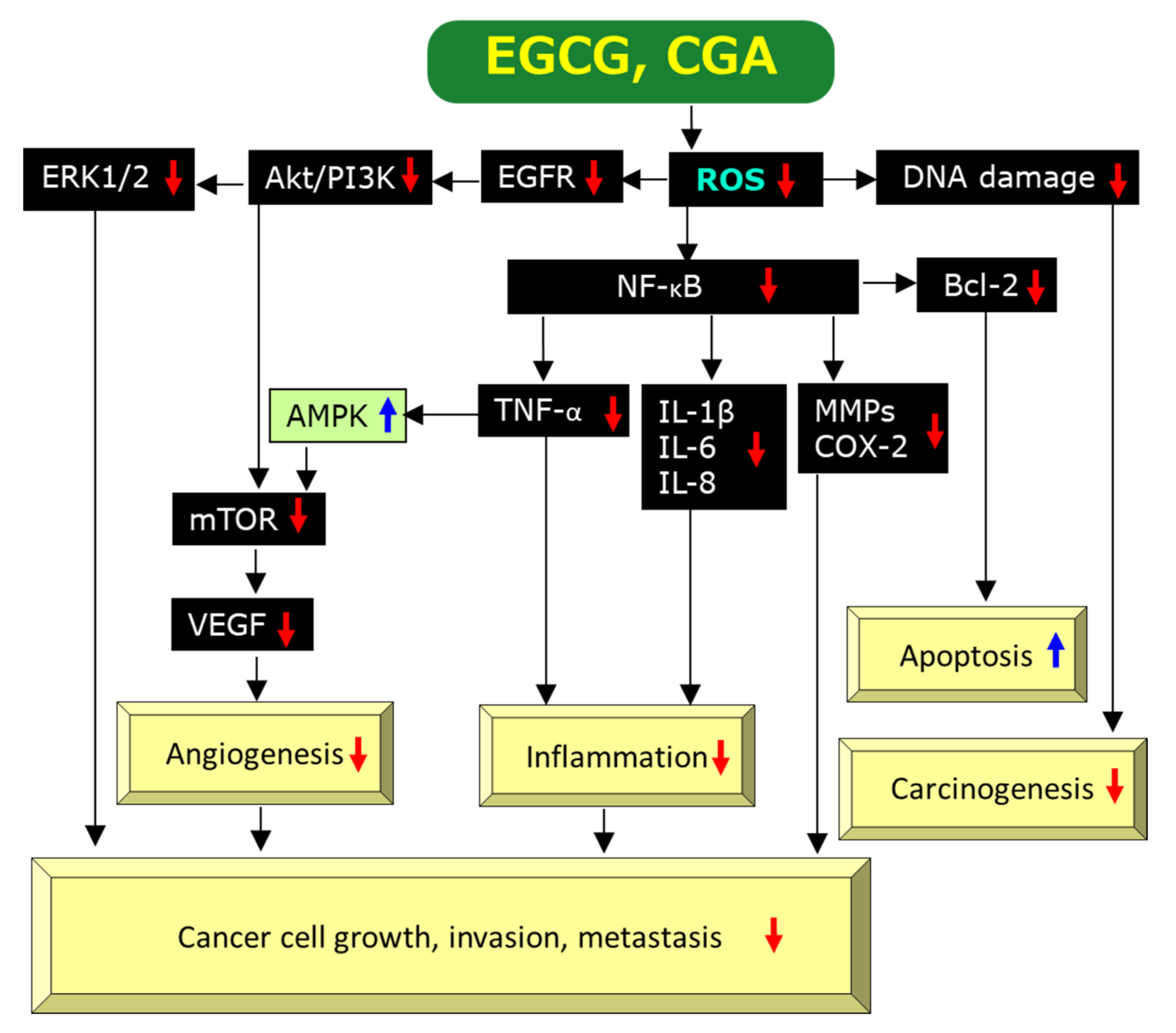
2.3. Mechanisms for Anti-Cancer Effects of EGCG
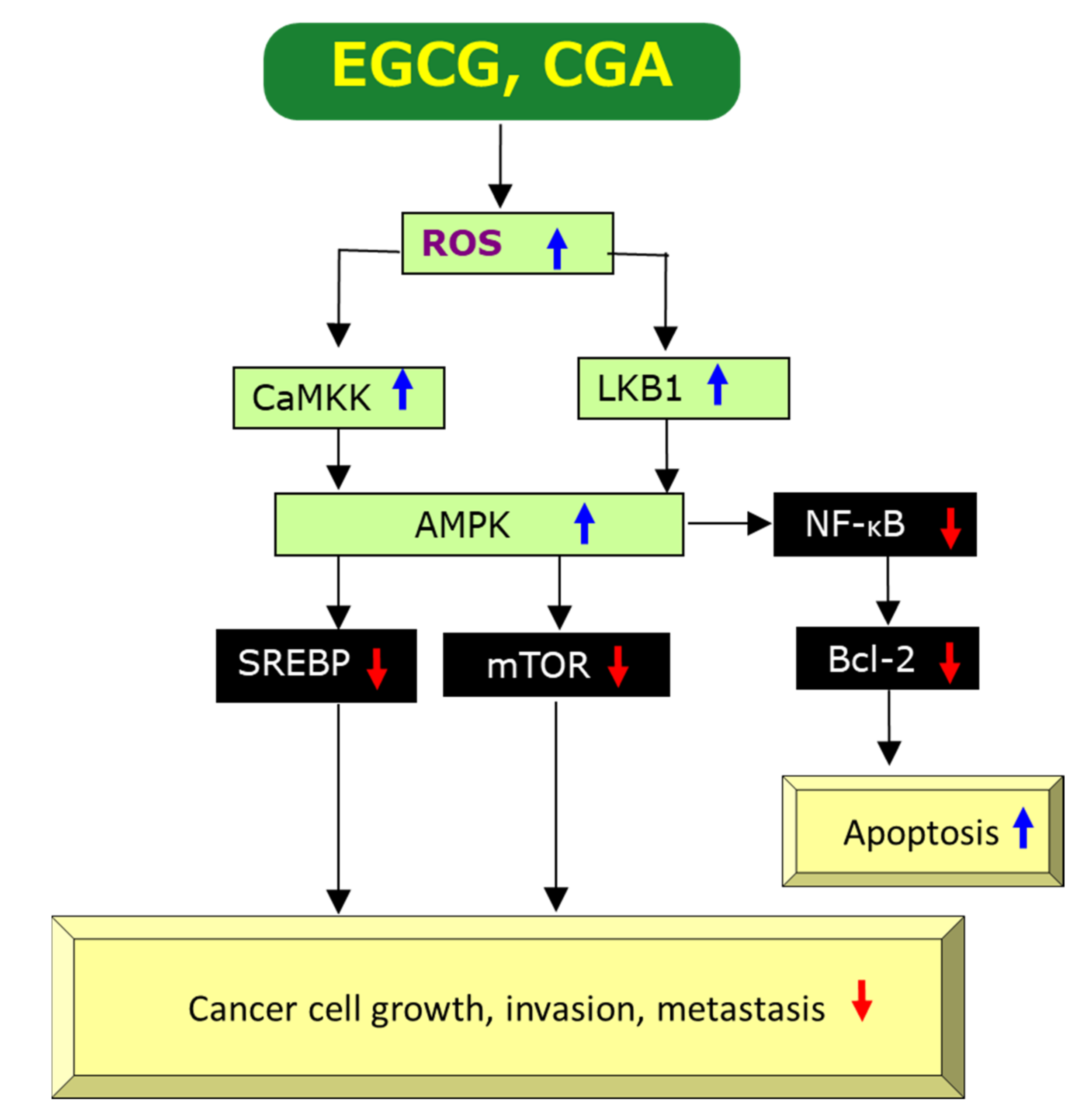
2.3.1. Anti-Oxidant and Pro-Oxidant Effects
2.3.2. Anti-Inflammatory Effects
2.3.3. Anti-Angiogenic Effects
2.3.4. Induction of Apoptosis
2.3.5. Epigenetic Modifications
2.3.6. Molecular Docking Analysis of EGCG’s Binding to Cancer-Related Proteins
2.3.7. Roles of 67LR in EGCG’s Anti-Cancer Effects
3. Anti-Cancer Effects of Coffee
3.1. Human Studies on Anti-Cancer Effects of Coffee
3.2. Comparison of Anti-Cancer Effects of Tea and Coffee in Simultaneous Human Studies
3.3. Basic Research on Anti-Cancer Action of Coffee and CGA
3.4. Mechanisms of CGA’s Action against Cancer
3.4.1. Anti-Oxidant and Pro-Oxidant Properties, Anti-Inflammatory Effects, Anti-Angiogenic Effects and Apoptosis-Inducing Activity of CGA
3.4.2. Epigenetic Modification by CGA
3.4.3. MDA of CGA’s Binding to Cancer-Related Proteins
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Suzuki, T.; Miyoshi, N.; Hayakawa, S.; Imai, S.; Isemura, M.; Nakamura, Y. Health benefits of tea consumption. In Beverage Impacts on Health and Nutrition; Springer International Publishing: Cham, Switzerland, 2016; pp. 49–67. ISBN 978-3-319-23672-8. [Google Scholar]
- Hayakawa, S.; Oishi, Y.; Tanabe, H.; Isemura, M.; Suzuki, Y. Tea, Coffee and Health Benefits. In Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–58. ISBN 978-3-319-78029-0. [Google Scholar]
- Yang, C.S.; Zhang, J.; Zhang, L.; Huang, J.; Wang, Y. Mechanisms of body weight reduction and metabolic syndrome alleviation by tea. Mol. Nutr. Food Res. 2016, 60, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Matsuo, Y.; Kouno, I. Biochemical and physicochemical characteristics of green tea polyphenols. In Green Tea Polyphenols; CRC Press: Boca Raton, FL, USA, 2013; pp. 19–38. [Google Scholar]
- Cavalli, L.; Tavani, A. Coffee consumption and its impact on health. In Beverage Impacts on Health and Nutrition; Springer International Publishing: Cham, Switzerland, 2016; pp. 29–47. [Google Scholar]
- Cano-Marquina, A.; Tarín, J.J.; Cano, A. The impact of coffee on health. Maturitas 2013, 75, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The safety of ingested caffeine: A comprehensive review. Front. Psychiatry 2017, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Bułdak, R.J.; Hejmo, T.; Osowski, M.; Bułdak, Ł.; Kukla, M.; Polaniak, R.; Birkner, E. The impact of coffee and its selected bioactive compounds on the development and progression of colorectal cancer in vivo and in vitro. Molecules 2018, 23, 3309. [Google Scholar] [CrossRef] [PubMed]
- Romualdo, G.R.; Rocha, A.B.; Vinken, M.; Cogliati, B.; Moreno, F.S.; Chaves, M.A.G.; Barbisan, L.F. Drinking for protection? Epidemiological and experimental evidence on the beneficial effects of coffee or major coffee compounds against gastrointestinal and liver carcinogenesis. Food Res. Int. 2019, 123, 567–589. [Google Scholar] [CrossRef]
- Cui, W.-Q.; Wang, S.-T.; Pan, D.; Chang, B.; Sang, L.-X. Caffeine and its main targets of colorectal cancer. World J. Gastrointest. Oncol. 2020, 12, 149–172. [Google Scholar] [CrossRef]
- Yang, C.S.; Hong, J. Prevention of chronic diseases by tea: Possible mechanisms and human relevance. Annu. Rev. Nutr. 2013, 33, 161–181. [Google Scholar] [CrossRef]
- Filippini, T.; Malavolti, M.; Borrelli, F.; Izzo, A.A.; Fairweather-Tait, S.J.; Horneber, M.; Vinceti, M. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst. Rev. 2020, 3, CD005004. [Google Scholar] [CrossRef]
- Zhang, D.; Nichols, H.B.; Troester, M.; Cai, J.; Bensen, J.T.; Sandler, D.P. Tea consumption and breast cancer risk in a cohort of women with family history of breast cancer. Int. J. Cancer 2020, 147, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Chong, F.; Song, M.; Sun, Q.; Li, T.; Xu, L.; Song, C. A dose-response meta-analysis of green tea consumption and breast cancer risk. Int. J. Food Sci. Nutr. 2020, 71, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhu, L.; Wang, K.; Yan, Y.; He, J.; Ren, Y. Green tea consumption and risk of breast cancer: A systematic review and updated meta-analysis of case-control studies. Med. Baltim. 2019, 98, e16147. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Oba, S.; Tsuji, M.; Goto, Y.; Mizuta, F.; Koda, S.; Uji, T.; Hori, A.; Tanabashi, S.; Matsushita, S.; et al. Green tea intake and colorectal cancer risk in Japan: The Takayama study. Jpn. J. Clin. Oncol. 2019, 49, 515–520. [Google Scholar] [CrossRef]
- Rafieian, N.; Azimi, S.; Manifar, S.; Julideh, H.; ShirKhoda, M. Is there any association between green tea consumption and the risk of head and neck squamous cell carcinoma: Finding from a case-control study. Arch. Oral Biol. 2019, 98, 280–284. [Google Scholar] [CrossRef]
- Takada, M.; Yamagishi, K.; Iso, H.; Tamakoshi, A. Green tea consumption and risk of hematologic neoplasms: The Japan collaborative cohort study for evaluation of cancer risk (JACC Study). Cancer Causes Control 2019, 30, 1223–1230. [Google Scholar] [CrossRef]
- Abe, S.K.; Saito, E.; Sawada, N.; Tsugane, S.; Ito, H.; Lin, Y.; Tamakoshi, A.; Sado, J.; Kitamura, Y.; Sugawara, Y.; et al. Green tea consumption and mortality in Japanese men and women: A pooled analysis of eight population-based cohort studies in Japan. Eur. J. Epidemiol. 2019, 34, 917–926. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Zhao, C.-N.; Cao, S.-Y.; Tang, G.-Y.; Gan, R.-Y.; Li, H.-B. Effects and mechanisms of tea for the prevention and management of cancers: An updated review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1693–1705. [Google Scholar] [CrossRef]
- Paul, P.; Koh, W.-P.; Jin, A.; Michel, A.; Waterboer, T.; Pawlita, M.; Wang, R.; Yuan, J.-M.; Butler, L.M. Soy and tea intake on cervical cancer risk: The Singapore Chinese health study. Cancer Causes Control 2019, 30, 847–857. [Google Scholar] [CrossRef]
- Tanaka, K.; Tamakoshi, A.; Sugawara, Y.; Mizoue, T.; Inoue, M.; Sawada, N.; Matsuo, K.; Ito, H.; Naito, M.; Nagata, C.; et al. Coffee, green tea and liver cancer risk: An evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn. J. Clin. Oncol. 2019, 49, 972–984. [Google Scholar] [CrossRef]
- Poorolajal, J.; Moradi, L.; Mohammadi, Y.; Cheraghi, Z.; Gohari-Ensaf, F. Risk factors for stomach cancer: A systematic review and meta-analysis. Epidemiol. Health 2020, 42, e2020004. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, N.; Tanabe, H.; Suzuki, T.; Saeki, K.; Hara, Y. Applications of a standardized green tea catechin preparation for viral warts and humanpapilloma virus-related andunrelated cancers. Molecules 2020, 25, 2588. [Google Scholar] [CrossRef] [PubMed]
- Tzellos, T.G.; Sardeli, C.; Lallas, A.; Papazisis, G.; Chourdakis, M.; Kouvelas, D. Efficacy, safety and tolerability of green tea catechins in the treatment of external anogenital warts: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Stockfleth, E.; Meyer, T. The use of sinecatechins (polyphenon E) ointment for treatment of external genital warts. Expert Opin. Biol. Ther. 2012, 12, 783–793. [Google Scholar] [CrossRef]
- Ahn, W.-S.; Yoo, J.; Huh, S.-W.; Kim, C.-K.; Lee, J.-M.; Namkoong, S.-E.; Bae, S.-M.; Lee, I.P. Protective effects of green tea extracts (polyphenon E and EGCG) on human cervical lesions. Eur. J. Cancer Prev. 2003, 12, 383–390. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Call, T.G.; Zent, C.S.; Leis, J.F.; LaPlant, B.; Bowen, D.A.; Roos, M.; Laumann, K.; Ghosh, A.K.; Lesnick, C.; et al. Phase 2 trial of daily, oral polyphenon E in patients with asymptomatic, Rai stage 0 to II chronic lymphocytic leukemia. Cancer 2013, 119, 363–370. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, H.-Y.; Fang, M.-Z.; Wang, X.-F.; Chen, H.; Huang, S.-L.; Kong, D.-S.; Li, M.; Zhang, X.; Sun, Y.; et al. Epigallocatechin gallate inhibits dimethylhydrazine-induced colorectal cancer in rats. World J. Gastroenterol. 2020, 26, 2064–2081. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Tania, M.; Srivastava, S.; Ritzer, E.E.; Pandey, A.; Aggarwal, D.; Barwal, T.S.; Jain, A.; Kaur, G.; et al. Molecular mechanisms of action of epigallocatechin gallate in cancer: Recent trends and advancement. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef]
- Gan, R.-Y.; Li, H.-B.; Sui, Z.-Q.; Corke, H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Crit. Rev. Food Sci. Nutr. 2018, 58, 924–941. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar] [CrossRef]
- Koessler, T.; Roth, A.; Cacheux, W. Early gastric cancer: Epidemiology, diagnostic and management. Rev. Med. Suisse 2014, 10, 1118–1122. [Google Scholar] [PubMed]
- Wang, Y.-Q.; Lu, J.-L.; Liang, Y.-R.; Li, Q.-S. Suppressive effects of EGCG on cervical cancer. Molecules 2018, 23, 2334. [Google Scholar] [CrossRef] [PubMed]
- Shirakami, Y.; Shimizu, M. Possible mechanisms of green tea and its constituents against cancer. Molecules 2018, 23, 2284. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Pezzani, R.; Redaelli, M.; Zorzan, M.; Imran, M.; Ahmed Khalil, A.; Salehi, B.; Sharopov, F.; Cho, W.C.; Sharifi-Rad, J. Preclinical pharmacological activities of epigallocatechin-3-gallate in signaling pathways: An update on cancer. Molecules 2020, 25, 467. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial properties of green tea catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea polyphenols in promotion of human health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef]
- Hayakawa, S.; Saito, K.; Miyoshi, N.; Ohishi, T.; Oishi, Y.; Miyoshi, M.; Nakamura, Y. Anti-cancer effects of green tea by either anti- or pro- oxidative mechanisms. Asian Pac. J. Cancer Prev. 2016, 17, 1649–1654. [Google Scholar] [CrossRef]
- Ohishi, T.; Goto, S.; Monira, P.; Isemura, M.; Nakamura, Y. Anti-inflammatory action of green tea. Antiinflamm. Antiallergy Agents Med. Chem. 2016, 15, 74–90. [Google Scholar] [CrossRef]
- Chen, P.C.; Wheeler, D.S.; Malhotra, V.; Odoms, K.; Denenberg, A.G.; Wong, H.R. A green tea-derived polyphenol, epigallocatechin-3-gallate, inhibits IkappaB kinase activation and IL-8 gene expression in respiratory epithelium. Inflammation 2002, 26, 233–241. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Michell, B.J.; van Denderen, B.J.W.; Watt, M.J.; Carey, A.L.; Fam, B.C.; Andrikopoulos, S.; Proietto, J.; Görgün, C.Z.; Carling, D.; et al. Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab. 2006, 4, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Bulboaca, A.E.; Boarescu, P.-M.; Porfire, A.S.; Dogaru, G.; Barbalata, C.; Valeanu, M.; Munteanu, C.; Râjnoveanu, R.M.; Nicula, C.A.; Stanescu, I.C. The effect of nano-epigallocatechin-gallate on oxidative stress and matrix metalloproteinases in experimental diabetes mellitus. Antioxid. Basel 2020, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Ganapathy, S.; Hingorani, S.R.; Srivastava, R.K. EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer. Front. Biosci. 2008, 13, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, H.; Pervin, M.; Goto, S.; Isemura, M.; Nakamura, Y. Beneficial effects of plant polyphenols on obesity. Obes. Control Ther. 2017, 4, 1–16. [Google Scholar]
- Collins, Q.F.; Liu, H.-Y.; Pi, J.; Liu, Z.; Quon, M.J.; Cao, W. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5’-AMP-activated protein kinase. J. Biol. Chem. 2007, 282, 30143–30149. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Quon, M.J.; Kim, J.-A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Hyttinen, J.M.T.; Kaarniranta, K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: Impact on healthspan and lifespan. J. Mol. Med. Berl. 2011, 89, 667–676. [Google Scholar] [CrossRef]
- Xiang, H.-C.; Lin, L.-X.; Hu, X.-F.; Zhu, H.; Li, H.-P.; Zhang, R.-Y.; Hu, L.; Liu, W.-T.; Zhao, Y.-L.; Shu, Y.; et al. AMPK activation attenuates inflammatory pain through inhibiting NF-κB activation and IL-1β expression. J. Neuroinflamm. 2019, 16, 34. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of reactive oxygen species in cancer progression: Molecular mechanisms and recent advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef]
- Stark, T.; Livas, L.; Kyprianou, N. Inflammation in prostate cancer progression and therapeutic targeting. Transl. Androl. Urol. 2015, 4, 455–463. [Google Scholar]
- Chen, C.-Y.; Kao, C.-L.; Liu, C.-M. The cancer prevention, anti-inflammatory and anti-oxidation of bioactive phytochemicals targeting the TLR4 Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 2729. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, Q. Therapeutic targets of multiple angiogenic factors for the treatment of cancer and metastasis. Adv. Cancer Res. 2007, 97, 203–224. [Google Scholar] [PubMed]
- Bos, R.; Zhong, H.; Hanrahan, C.F.; Mommers, E.C.; Semenza, G.L.; Pinedo, H.M.; Abeloff, M.D.; Simons, J.W.; van Diest, P.J.; van der Wall, E. Levels of hypoxia-inducible factor-1 alpha during breast carcinogenesis. J. Natl. Cancer Inst. 2001, 93, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Koshikawa, N.; Iyozumi, A.; Gassmann, M.; Takenaga, K. Constitutive upregulation of hypoxia-inducible factor-1alpha mRNA occurring in highly metastatic lung carcinoma cells leads to vascular endothelial growth factor overexpression upon hypoxic exposure. Oncogene 2003, 22, 6717–6724. [Google Scholar] [CrossRef][Green Version]
- Fu, J.-D.; Yao, J.-J.; Wang, H.; Cui, W.-G.; Leng, J.; Ding, L.-Y.; Fan, K.-Y. Effects of EGCG on proliferation and apoptosis of gastric cancer SGC7901 cells via down-regulation of HIF-1α and VEGF under a hypoxic state. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 155–161. [Google Scholar]
- Gu, J.-W.; Makey, K.L.; Tucker, K.B.; Chinchar, E.; Mao, X.; Pei, I.; Thomas, E.Y.; Miele, L. EGCG, a major green tea catechin suppresses breast tumor angiogenesis and growth via inhibiting the activation of HIF-1α and NFκB, and VEGF expression. Vasc. Cell 2013, 5, 9. [Google Scholar] [CrossRef]
- Wu, D.; Liu, Z.; Li, J.; Zhang, Q.; Zhong, P.; Teng, T.; Chen, M.; Xie, Z.; Ji, A.; Li, Y. Epigallocatechin-3-gallate inhibits the growth and increases the apoptosis of human thyroid carcinoma cells through suppression of EGFR/RAS/RAF/MEK/ERK signaling pathway. Cancer Cell Int. 2019, 19, 43. [Google Scholar] [CrossRef]
- Kondo, T.; Ohta, T.; Igura, K.; Hara, Y.; Kaji, K. Tea catechins inhibit angiogenesis in vitro, measured by human endothelial cell growth, migration and tube formation, through inhibition of VEGF receptor binding. Cancer Lett. 2002, 180, 139–144. [Google Scholar] [CrossRef]
- Das, A.; Banik, N.L.; Ray, S.K. Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes. Cancer 2010, 116, 164–176. [Google Scholar] [CrossRef]
- Zan, L.; Chen, Q.; Zhang, L.; Li, X. Epigallocatechin gallate (EGCG) suppresses growth and tumorigenicity in breast cancer cells by downregulation of miR-25. Bioengineered 2019, 10, 374–382. [Google Scholar] [CrossRef]
- Kwak, T.W.; Park, S.B.; Kim, H.-J.; Jeong, Y.-I.; Kang, D.H. Anticancer activities of epigallocatechin-3-gallate against cholangiocarcinoma cells. Onco. Targets. Ther. 2017, 10, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Jian, W.; Fang, S.; Chen, T.; Fang, J.; Mo, Y.; Li, D.; Xiong, S.; Liu, W.; Song, L.; Shen, J.; et al. A novel role of HuR in -Epigallocatechin-3-gallate (EGCG) induces tumour cells apoptosis. J. Cell. Mol. Med. 2019, 23, 3767–3771. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, W.; Zhang, J.; Zheng, X.; Yao, Y.; Tu, K.; Liu, Q. SREBP-1 has a prognostic role and contributes to invasion and metastasis in human hepatocellular carcinoma. Int. J. Mol. Sci. 2014, 15, 7124–7138. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.Y.; Kwon, H.H.; Min, S.U.; Thiboutot, D.M.; Suh, D.H. Epigallocatechin-3-gallate improves acne in humans by modulating intracellular molecular targets and inhibiting P. acnes. J. Investig. Dermatol. 2013, 133, 429–440. [Google Scholar] [CrossRef]
- Zhang, L.; Valizadeh, H.; Alipourfard, I.; Bidares, R.; Aghebati-Maleki, L.; Ahmadi, M. Epigenetic modifications and therapy in chronicobstructive pulmonary disease (COPD): An update review. COPD 2020, 17, 333–342. [Google Scholar] [CrossRef]
- Sakamoto, N.; Honma, R.; Sekino, Y.; Goto, K.; Sentani, K.; Ishikawa, A.; Oue, N.; Yasui, W. Non-coding RNAs are promising targets for stem cell-based cancer therapy. Non-Coding RNA Res. 2017, 2, 83–87. [Google Scholar] [CrossRef]
- Fang, M.Z.; Wang, Y.; Ai, N.; Hou, Z.; Sun, Y.; Lu, H.; Welsh, W.; Yang, C.S. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003, 63, 7563–7570. [Google Scholar]
- Pal, D.; Sur, S.; Roy, R.; Mandal, S.; Kumar Panda, C. Epigallocatechin gallate in combination with eugenol or amarogentin shows synergistic chemotherapeutic potential in cervical cancer cell line. J. Cell. Physiol. 2018, 234, 825–836. [Google Scholar] [CrossRef]
- Khan, M.A.; Hussain, A.; Sundaram, M.K.; Alalami, U.; Gunasekera, D.; Ramesh, L.; Hamza, A.; Quraishi, U. (-)-Epigallocatechin-3-gallate reverses the expression of various tumor-suppressor genes by inhibiting DNA methyltransferases and histone deacetylases in human cervical cancer cells. Oncol. Rep. 2015, 33, 1976–1984. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, Y.; Liu, M.; Yan, Q.; Zhao, W.; Yang, P.; Gao, Q.; Wei, J.; Zhao, W.; Ma, L. Epigallocatechin gallate inhibits cell growth and regulates miRNA expression in cervical carcinoma cell lines infected with different high-risk human papillomavirus subtypes. Exp. Ther. Med. 2018, 17, 1742–1748. [Google Scholar] [CrossRef]
- Wang, H.; Bian, S.; Yang, C.S. Green tea polyphenol EGCG suppresses lung cancer cell growth through upregulating miR-210 expression caused by stabilizing HIF-1α. Carcinogenesis 2011, 32, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Tsukamoto, S.; Huang, Y.; Makio, A.; Kumazoe, M.; Yamashita, S.; Tachibana, H. Epigallocatechin-3-O-gallate up-regulates microRNA-let-7b expression by activating 67-kDa laminin receptor signaling in melanoma cells. Sci. Rep. 2016, 6, 19225. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.W.; Yan, F.; Zhong, X.; Mazumder, P.B.; Xu-Monette, Z.Y.; Zou, D.; Young, K.H.; Ramos, K.S.; Li, Y. Regulation of p53-targeting microRNAs by polycyclic aromatic hydrocarbons: Implications in the etiology of multiple myeloma. Mol. Carcinog. 2015, 54, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Hastak, K.; Gupta, S.; Ahmad, N.; Agarwal, M.K.; Agarwal, M.L.; Mukhtar, H. Role of p53 and NF-kappaB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene 2003, 22, 4851–4859. [Google Scholar] [CrossRef]
- Hu, D.-L.; Wang, G.; Yu, J.; Zhang, L.-H.; Huang, Y.-F.; Wang, D.; Zhou, H.-H. Epigallocatechin-3-gallate modulates long non-coding RNA and mRNA expression profiles in lung cancer cells. Mol. Med. Rep. 2019, 19, 1509–1520. [Google Scholar] [CrossRef]
- Sazuka, M.; Imazawa, H.; Shoji, Y.; Mita, T.; Hara, Y.; Isemura, M. Inhibition of collagenases from mouse lung carcinoma cells by green tea catechins and black tea theaflavins. Biosci. Biotechnol. Biochem. 1997, 61, 1504–1506. [Google Scholar] [CrossRef]
- Bu, W.; Tang, Z.Y.; Sun, F.X.; Ye, S.L.; Liu, K.D.; Xue, Q.; Chen, J.; Gao, D.M. Effects of matrix metalloproteinase inhibitor BB-94 on liver cancer growth and metastasis in a patient-like orthotopic model LCI-D20. Hepatogastroenterology 1998, 45, 1056–1061. [Google Scholar]
- Saeki, K.; Hayakawa, S.; Nakano, S.; Ito, S.; Oishi, Y.; Suzuki, Y.; Isemura, M. In vitro and in silico studies of the molecular interactions of epigallocatechin-3-O-gallate (EGCG) with proteins that explain the health benefits of green tea. Molecules 2018, 23, 1295. [Google Scholar] [CrossRef]
- Nakano, S.; Megro, S.-I.; Hase, T.; Suzuki, T.; Isemura, M.; Nakamura, Y.; Ito, S. Computational molecular docking and X-ray crystallographic studies of catechins in new drug design strategies. Molecules 2018, 23, 2020. [Google Scholar] [CrossRef]
- Umeda, D.; Yano, S.; Yamada, K.; Tachibana, H. Green tea polyphenol epigallocatechin-3-gallate signaling pathway through 67-kDa laminin receptor. J. Biol. Chem. 2008, 283, 3050–3058. [Google Scholar] [CrossRef]
- Kumazoe, M.; Sugihara, K.; Tsukamoto, S.; Huang, Y.; Tsurudome, Y.; Suzuki, T.; Suemasu, Y.; Ueda, N.; Yamashita, S.; Kim, Y.; et al. 67-kDa laminin receptor increases cGMP to induce cancer-selective apoptosis. J. Clin. Investig. 2013, 123, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Negri, A.; Naponelli, V.; Rizzi, F.; Bettuzzi, S. Molecular targets of epigallocatechin-gallate (EGCG): A special focus on signal transduction and cancer. Nutrients 2018, 10, 1936. [Google Scholar] [CrossRef] [PubMed]
- Wierzejska, R. Coffee consumption vs. cancer risk—a review of scientific data. Rocz. Panstw. Zakl. Hig. 2015, 66, 293–298. [Google Scholar] [PubMed]
- Yu, M.C.; Mack, T.M.; Hanisch, R.; Cicioni, C.; Henderson, B.E. Cigarette smoking, obesity, diuretic use, and coffee consumption as risk factors for renal cell carcinoma. J. Natl. Cancer Inst. 1986, 77, 351–356. [Google Scholar] [PubMed]
- Grubben, M.J.; Van Den Braak, C.C.; Broekhuizen, R.; De Jong, R.; Van Rijt, L.; De Ruijter, E.; Peters, W.H.; Katan, M.B.; Nagengast, F.M. The effect of unfiltered coffee on potential biomarkers for colonic cancer risk in healthy volunteers: A randomized trial. Aliment. Pharmacol. Ther. 2000, 14, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Steinkellner, H.; Hoelzl, C.; Uhl, M.; Cavin, C.; Haidinger, G.; Gsur, A.; Schmid, R.; Kundi, M.; Bichler, J.; Knasmüller, S. Coffee consumption induces GSTP in plasma and protects lymphocytes against (+/−)-anti-benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide induced DNA-damage: Results of controlled human intervention trials. Mutat. Res. 2005, 591, 264–275. [Google Scholar] [CrossRef]
- Mišík, M.; Hoelzl, C.; Wagner, K.-H.; Cavin, C.; Moser, B.; Kundi, M.; Simic, T.; Elbling, L.; Kager, N.; Ferk, F.; et al. Impact of paper filtered coffee on oxidative DNA-damage: Results of a clinical trial. Mutat. Res. 2010, 692, 42–48. [Google Scholar] [CrossRef]
- Loftfield, E.; Freedman, N.D. Coffee and digestive cancers-what do we know, and where do we go? Br. J. Cancer 2020, 122, 1273–1274. [Google Scholar] [CrossRef]
- Zhao, L.-G.; Li, Z.-Y.; Feng, G.-S.; Ji, X.-W.; Tan, Y.-T.; Li, H.-L.; Gunter, M.J.; Xiang, Y.-B. Coffee drinking and cancer risk: An umbrella review of meta-analyses of observational studies. BMC Cancer 2020, 20, 101. [Google Scholar] [CrossRef]
- Yu, E.Y.W.; Dai, Y.; Wesselius, A.; van Osch, F.; Brinkman, M.; van den Brandt, P.; Grant, E.J.; White, E.; Weiderpass, E.; Gunter, M.; et al. Coffee consumption and risk of bladder cancer: A pooled analysis of 501,604 participants from 12 cohort studies in the BLadder Cancer Epidemiology and Nutritional Determinants (BLEND) international study. Eur. J. Epidemiol. 2020, 35, 523–535. [Google Scholar] [CrossRef]
- Shaposhnikov, S.; Hatzold, T.; Yamani, N.E.; Stavro, P.M.; Lorenzo, Y.; Dusinska, M.; Reus, A.; Pasman, W.; Collins, A. Coffee and oxidative stress: A human intervention study. Eur. J. Nutr. 2018, 57, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Bamia, C.; Lagiou, P.; Jenab, M.; Trichopoulou, A.; Fedirko, V.; Aleksandrova, K.; Pischon, T.; Overvad, K.; Olsen, A.; Tjønneland, A.; et al. Coffee, tea and decaffeinated coffee in relation to hepatocellular carcinoma in a European population: Multicentre, prospective cohort study. Int. J. Cancer 2015, 136, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Hashibe, M.; Galeone, C.; Buys, S.S.; Gren, L.; Boffetta, P.; Zhang, Z.-F.; La Vecchia, C. Coffee, tea, caffeine intake, and the risk of cancer in the PLCO cohort. Br. J. Cancer 2015, 113, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Thomopoulos, T.P.; Ntouvelis, E.; Diamantaras, A.-A.; Tzanoudaki, M.; Baka, M.; Hatzipantelis, E.; Kourti, M.; Polychronopoulou, S.; Sidi, V.; Stiakaki, E.; et al. Maternal and childhood consumption of coffee, tea and cola beverages in association with childhood leukemia: A meta-analysis. Cancer Epidemiol. 2015, 39, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Milne, E.; Greenop, K.R.; Petridou, E.; Bailey, H.D.; Orsi, L.; Kang, A.Y.; Baka, M.; Bonaventure, A.; Kourti, M.; Metayer, C.; et al. Maternal consumption of coffee and tea during pregnancy and risk of childhood all: A pooled analysis from the childhood Leukemia International Consortium. Cancer Causes Control 2018, 29, 539–550. [Google Scholar] [CrossRef]
- Hashemian, M.; Sinha, R.; Murphy, G.; Weinstein, S.J.; Liao, L.M.; Freedman, N.D.; Abnet, C.C.; Albanes, D.; Loftfield, E. Coffee and tea drinking and risk of cancer of the urinary tract in male smokers. Ann. Epidemiol. 2019, 34, 33–39. [Google Scholar] [CrossRef]
- Hong, X.; Xu, Q.; Lan, K.; Huang, H.; Zhang, Y.; Chen, S.; Chi, Z.; Lin, J.; Zhou, Y.; Wu, W.; et al. The effect of daily fluid management and beverages consumption on the risk of bladder cancer: A meta-analysis of observational study. Nutr. Cancer 2018, 70, 1217–1227. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Z.; Jin, Y.; Guo, J. Association between tea and coffee consumption and brain cancer risk: An updated meta-analysis. World J. Surg. Oncol. 2019, 17, 51. [Google Scholar] [CrossRef]
- Arthur, R.; Kirsh, V.A.; Rohan, T.E. Associations of coffee, tea and caffeine intake with risk of breast, endometrial and ovarian cancer among Canadian women. Cancer Epidemiol. 2018, 56, 75–82. [Google Scholar] [CrossRef]
- Bradbury, K.E.; Murphy, N.; Key, T.J. Diet and colorectal cancer in UK Biobank: A prospective study. Int. J. Epidemiol. 2020, 49, 246–258. [Google Scholar] [CrossRef]
- Quang, L.N.; Hien, N.Q.; Quang, N.T.; Chung, N.T. Active lifestyle patterns reduce the risk of colorectal cancer in the north of Vietnam: A hospital-based case-control study. Cancer Control 2019, 26, 1073274819864666. [Google Scholar] [CrossRef] [PubMed]
- Cote, D.J.; Bever, A.M.; Wilson, K.M.; Smith, T.R.; Smith-Warner, S.A.; Stampfer, M.J. A prospective study of tea and coffee intake and risk of glioma. Int. J. Cancer 2020, 146, 2442–2449. [Google Scholar] [CrossRef] [PubMed]
- Malmir, H.; Shayanfar, M.; Mohammad-Shirazi, M.; Tabibi, H.; Sharifi, G.; Esmaillzadeh, A. Tea and coffee consumption in relation to glioma: A case-control study. Eur. J. Nutr. 2019, 58, 103–111. [Google Scholar] [CrossRef]
- Ugai, T.; Matsuo, K.; Sawada, N.; Iwasaki, M.; Yamaji, T.; Shimazu, T.; Goto, A.; Inoue, M.; Kanda, Y.; Tsugane, S.; et al. Coffee and green tea consumption and subsequent risk of acute myeloid leukemia and myelodysplastic syndromes in Japan. Int. J. Cancer 2018, 142, 1130–1138. [Google Scholar] [CrossRef]
- Karalexi, M.A.; Dessypris, N.; Clavel, J.; Metayer, C.; Erdmann, F.; Orsi, L.; Kang, A.Y.; Schüz, J.; Bonaventure, A.; Greenop, K.R.; et al. Coffee and tea consumption during pregnancy and risk of childhood acute myeloid leukemia: A Childhood Leukemia International Consortium (CLIC) study. Cancer Epidemiol. 2019, 62, 101581. [Google Scholar] [CrossRef]
- Tamura, T.; Wada, K.; Konishi, K.; Goto, Y.; Mizuta, F.; Koda, S.; Hori, A.; Tanabashi, S.; Matsushita, S.; Tokimitsu, N.; et al. Coffee, green tea, and caffeine intake and liver cancer risk: A prospective cohort study. Nutr. Cancer 2018, 70, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Seow, W.J.; Koh, W.-P.; Jin, A.; Wang, R.; Yuan, J.-M. Associations between tea and coffee beverage consumption and the risk of lung cancer in the Singaporean Chinese population. Eur. J. Nutr. 2019, 59, 3083–3091. [Google Scholar] [CrossRef]
- Mirtavoos-Mahyari, H.; Salehipour, P.; Parohan, M.; Sadeghi, A. Effects of coffee, black tea and green tea consumption on the risk of non-Hodgkin’s lymphoma: A systematic review and dose-response meta-analysis of observational studies. Nutr. Cancer 2019, 71, 887–897. [Google Scholar] [CrossRef]
- Fortes, C. Are anti-inflammatory foods associated with a protective effect for cutaneous melanoma? Eur. J. Cancer Prev. 2020. [Google Scholar] [CrossRef]
- Sen, A.; Papadimitriou, N.; Lagiou, P.; Perez-Cornago, A.; Travis, R.C.; Key, T.J.; Murphy, N.; Gunter, M.; Freisling, H.; Tzoulaki, I.; et al. Coffee and tea consumption and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2019, 144, 240–250. [Google Scholar] [CrossRef]
- Oh, C.C.; Jin, A.; Yuan, J.-M.; Koh, W.-P. Coffee, tea, caffeine, and risk of nonmelanoma skin cancer in a Chinese population: The Singapore Chinese health study. J. Am. Acad. Dermatol. 2019, 81, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Alghamdi, M.A.; Cayssials, V.; Franceschi, S.; Almquist, M.; Hennings, J.; Sandström, M.; Tsilidis, K.K.; Weiderpass, E.; Boutron-Ruault, M.-C.; et al. Coffee and tea drinking in relation to the risk of differentiated thyroid carcinoma: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Nutr. 2019, 58, 3303–3312. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Yoshimi, N.; Yamada, Y.; Matsunaga, K.; Kawabata, K.; Hara, A.; Moriwaki, H.; Mori, H. Suppressive effects of chlorogenic acid on N-methyl-N-nitrosourea-induced glandular stomach carcinogenesis in male F344 rats. J. Toxicol. Sci. 1999, 24, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Salomone, F.; Galvano, F.; Li Volti, G. Molecular bases underlying the hepatoprotective effects of coffee. Nutrients 2017, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Furuse, T.; Yagasaki, K. Inhibitory effect of serum from rats administered with coffee on the proliferation and invasion of rat ascites hepatoma cells. Cytotechnology 1997, 25, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Hou, N.; Liu, N.; Han, J.; Yan, Y.; Li, J. Chlorogenic acid induces reactive oxygen species generation and inhibits the viability of human colon cancer cells. Anticancer Drugs 2017, 28, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Lu, Y.; Bowman, L.L.; Qian, Y.; Castranova, V.; Ding, M. Inhibition of activator protein-1, NF-kappaB, and MAPKs and induction of phase 2 detoxifying enzyme activity by chlorogenic acid. J. Biol. Chem. 2005, 280, 27888–27895. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef]
- Cha, J.W.; Piao, M.J.; Kim, K.C.; Yao, C.W.; Zheng, J.; Kim, S.M.; Hyun, C.L.; Ahn, Y.S.; Hyun, J.W. The polyphenol chlorogenic acid attenuates UVB-mediated oxidative stress in human HaCaT keratinocytes. Biomol. Ther. 2014, 22, 136–142. [Google Scholar] [CrossRef]
- Rakshit, S.; Mandal, L.; Pal, B.C.; Bagchi, J.; Biswas, N.; Chaudhuri, J.; Chowdhury, A.A.; Manna, A.; Chaudhuri, U.; Konar, A.; et al. Involvement of ROS in chlorogenic acid-induced apoptosis of Bcr-Abl+ CML cells. Biochem. Pharmacol. 2010, 80, 1662–1675. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, Y.-W.; Park, Y.; Lee, H.-J.; Kim, K.-W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, Y.M.; Jung, W.; Park, S.-B.; Kim, C.-S.; Kim, J.S. Aster koraiensis extract and chlorogenic acid inhibit retinal angiogenesis in a mouse model of oxygen-induced retinopathy. Evid. Based Complement. Alternat. Med. 2018, 2018, 6402650. [Google Scholar] [CrossRef] [PubMed]
- Lukitasari, M.; Nugroho, D.A.; Widodo, N. Chlorogenic acid: The conceivable chemosensitizer leading to cancer growth suppression. J. Evid. Based Integr. Med. 2018, 23, 2515690X18789628. [Google Scholar] [CrossRef] [PubMed]
- Mitrea, D.R.; Malkey, R.; Florian, T.L.; Filip, A.; Clichici, S.; Bidian, C.; Moldovan, R.; Hoteiuc, O.A.; Toader, A.M.; Baldea, I. Daily oral administration of chlorogenic acid prevents the experimental carrageenan-induced oxidative stress. J. Physiol. Pharmacol. 2020, 71. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Xie, Z.; Rao, J.; Xu, G.; Huang, K.; Li, W.; Yin, Z. Chlorogenic acid inhibits proliferation and induces apoptosis in A498 human kidney cancer cells via inactivating PI3K/Akt/mTOR signalling pathway. J. Pharm. Pharmacol. 2019, 71, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jin, M.L.; Yi, E.H.; Kim, Y.; Park, G. Neochlorogenic acid inhibits against LPS-activated inflammatory responses through up-regulation of Nrf2/HO-1 and involving AMPK pathway. Environ. Toxicol. Pharmacol. 2018, 62, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feng, Y.; Li, Y.; Hu, Y.; Zhang, Q.; Huang, Y.; Shi, K.; Ran, C.; Hou, J.; Zhou, G.; et al. Chlorogenic acid decreases malignant characteristics of hepatocellular carcinoma cells by inhibiting DNMT1 expression. Front. Pharmacol. 2020, 11, 867. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Zhu, B.T. Inhibition of DNA methylation by caffeic acid and chlorogenic acid, two common catechol-containing coffee polyphenols. Carcinogenesis 2006, 27, 269–277. [Google Scholar] [CrossRef]
- Mira, A.; Shimizu, K. In vitro cytotoxic activities and molecular mechanisms of angelica shikokiana extract and its isolated compounds. Pharmacogn. Mag. 2015, 11, S564–S569. [Google Scholar]
- Liu, H.; Guo, X.; Liu, J.; Xue, F.; Bai, G.; Liang, Y. Chlorogenic-induced inhibition of non-small cancer cells occurs through regulation of histone deacetylase 6. Cell. Mol. Biol. 2018, 64, 134–139. [Google Scholar]
- Bora-Tatar, G.; Dayangaç-Erden, D.; Demir, A.S.; Dalkara, S.; Yelekçi, K.; Erdem-Yurter, H. Molecular modifications on carboxylic acid derivatives as potent histone deacetylase inhibitors: Activity and docking studies. Bioorg. Med. Chem. 2009, 17, 5219–5228. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Luo, L.; Zhu, Z.-D.; Zhou, X.; Wang, Y.; Xue, J.; Zhang, J.; Cai, X.; Chen, Z.-L.; Ma, Q.; et al. Chlorogenic acid inhibits liver fibrosis by blocking the miR-21-regulated TGF-β1/Smad7 signaling pathway in vitro and in vivo. Front. Pharmacol. 2017, 8, 929. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, F.; Xue, J.; Zhou, X.; Luo, L.; Ma, Q.; Chen, Y.-F.; Zhang, J.; Zhang, S.-L.; Zhao, L. Antischistosomiasis liver fibrosis effects of chlorogenic acid through IL-13/miR-21/Smad7 signaling interactions in vivo and in vitro. Antimicrob. Agents Chemother. 2017, 61, e01347:1–e01347:16. [Google Scholar] [CrossRef] [PubMed]
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver fibrosis: Mechanistic concepts and therapeutic perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, L.-L.; Xue, N.-N.; Li, C.; Guo, H.-H.; Ren, T.-K.; Zhan, Y.; Li, W.-B.; Zhang, J.; Chen, X.-G.; et al. Chlorogenic acid effectively treats cancers through induction of cancer cell differentiation. Theranostics 2019, 9, 6745–6763. [Google Scholar] [CrossRef]
- Dong, K.F.; Huo, M.Q.; Sun, H.Y.; Li, T.K.; Li, D. Mechanism of Astragalus membranaceus in the treatment of laryngeal cancer based on gene co-expression network and molecular docking. Sci. Rep. 2020, 10, 11184. [Google Scholar] [CrossRef]
- Orlando, G.; Recinella, L.; Chiavaroli, A.; Brunetti, L.; Leone, S.; Carradori, S.; Di Simone, S.; Ciferri, M.C.; Zengin, G.; Ak, G.; et al. Water extract from inflorescences of industrial hemp futura 75 variety as a source of anti-inflammatory, anti-proliferative and antimycotic agents: Results from in silico, in vitro and ex vivo studies. Antioxidants 2020, 9, 437. [Google Scholar] [CrossRef]
- Elfiky, A.A. Natural products may interfere with SARS-CoV-2 attachment to the host cell. J. Biomol. Struct. Dyn. 2020, 1–10. [Google Scholar] [CrossRef]
- Ahmad, B.; Rizwan, M.; Rauf, A.; Raza, M.; Bashir, S.; Molnar, J.; Csonka, A.; Szabo, D.; Mubarak, M.S.; Noor, M.; et al. Isolation of chlorogenic acid from soil borne fungi Screlotium rolfsii, their reversal of multidrug resistance and anti-proliferative in mouse lymphoma Cells. Med. Chem. 2017, 13, 721–726. [Google Scholar] [CrossRef]
- Deka, S.J.; Gorai, S.; Manna, D.; Trivedi, V. Evidence of PKC binding and translocation to explain the anticancer mechanism of chlorogenic acid in breast cancer cells. Curr. Mol. Med. 2017, 17, 79–89. [Google Scholar] [CrossRef]
- Taha, K.F.; Khalil, M.; Abubakr, M.S.; Shawky, E. Identifying cancer-related molecular targets of Nandina domestica Thunb. by network pharmacology-based analysis in combination with chemical profiling and molecular docking studies. J. Ethnopharmacol. 2020, 249, 112413. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Masuda, A.; Imaida, K.; Kagawa, M.; Tsuda, H.; Ito, N. Induction of forestomach lesions in rats by oral administrations of naturally occurring antioxidants for 4 weeks. Jpn. J. Cancer Res. 1987, 78, 317–321. [Google Scholar] [PubMed]
- Hirose, M.; Fukushima, S.; Shirai, T.; Hasegawa, R.; Kato, T.; Tanaka, H.; Asakawa, E.; Ito, N. Stomach carcinogenicity of caffeic acid, sesamol and catechol in rats and mice. Jpn. J. Cancer Res. 1990, 81, 207–212. [Google Scholar] [CrossRef] [PubMed]
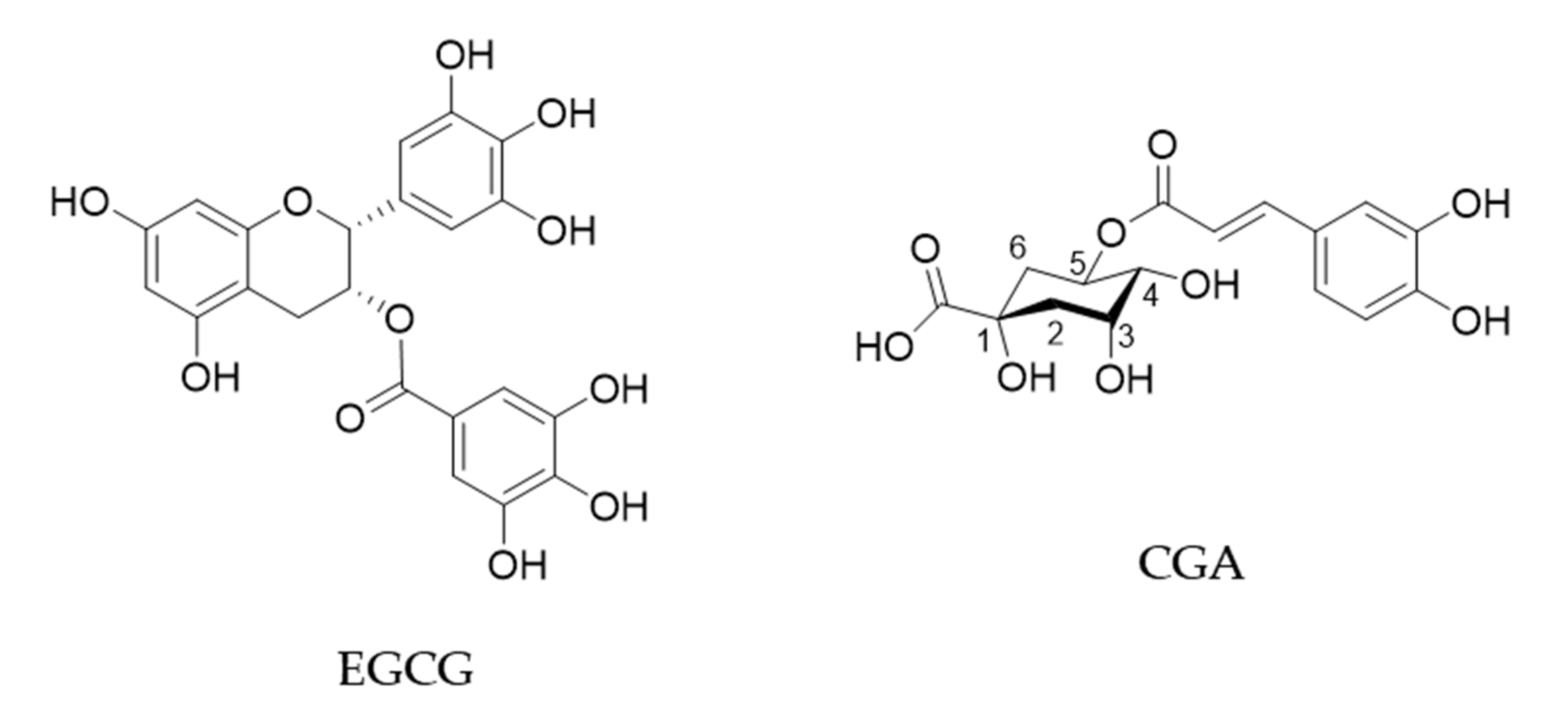
| Cancer Type | Evaluation: Decrease (↓) or No Effect (+/−) in Cancer Risk | Hazard Risk (HR) or Odds Ratio (OR) or Relative Risk (RR) [Confidence Interval] | Note | Reference |
|---|---|---|---|---|
| Breast cancer | ↓ | HR = 0.82 [0.70–0.95] for ≥5 vs. 0 cups/day | Cohort study onwomen with family history of breast cancer | [15] |
| Breast cancer | ↓ | HR = 0.86 [0.75–0.99] for highest vs. lowest intake | Meta-analysis of 16 cohort and case-control studies | [16] |
| Breast cancer | ↓ | OR = 0.83 [0.72–0.96] | Meta-analysis of 14 case-control studies | [17] |
| Colorectal cancer | +/− | Cohort study on men and women | [18] | |
| Colon cancer | ↓ | RR = 1.32 [0.90–1.94] for once/day vs. less than once/day RR = 0.76 [0.57–1.02] for 2–3 times/day RR = 0.78 [0.49–1.22] for ≥4 times/day | Cohort study on men | [18] |
| Head and neck squamous cell carcinoma | ↓ | OR = 0.29 [0.16–0.52] for <1 cup/day vs. no intake OR = 0.38 [0.17–0.86] for ≥1 cup/day vs. no intake | Case-control studyon men and women | [19] |
| Hematologic neoplasms | ↓ | HR = 0.65 [0.42–1.00] for ≤2 cups/day vs. no intake HR = 0.73 [0.47–1.13] for 3–4 cups/day vs. no intake HR = 0.63 [0.42–0.96] for ≥5 cups/day vs. no intake | Cohort studyon men and women | [20] |
| Total cancer | ↓ | HR = 0.89 [0.83–0.96] for 1–2 cups/day vs. <1 cup/day HR = 0.91 [0.85–0.98], for 3–4 cups/day vs. <1 cup/day | Meta-analysis on 8 cohort study on women | [21] |
| Cancer Type | Tea/Green Tea/Black Tea * | Coffee/Caffeinated Coffee/Decaffeinated Coffee * | Type of Epidemiological Study [Reference] |
|---|---|---|---|
| Bladder | ↓ | +/− | Cohort study [100] |
| Bladder | +/− | ↑ | Meta-analysis of cohort study and case-control study [101] |
| Brain | ↓ | ↓ | Meta-analysis of cohort study and case-control study [102] |
| Breast | +/− | +/− | Cohort study [103] |
| Colorectal | +/− | +/− | Cohort study [104] |
| Colorectal | ↓ | +/− | Case-control study [105] |
| Endometrial | +/− | ↓ | Case-control study [103] |
| Glioma | ↓ | +/− | Cohort study [106] |
| Glioma | ↓ | ↓ | Case-control study [107] |
| Leukemia, acute myeloid | +/− | +/− | Cohort study [108] |
| Leukemia, childhood acute myeloid | +/− | ↑ | Meta-analysis of case-control study [109] |
| Leukemia, childhood acute lymphoblastic | +/− | ↑ | Meta-analysis of case-control study [99] |
| Liver | +/− | ↓ | Cohort study [110] |
| Liver | +/− | ↓ | Meta-analysis of cohort study and case-control study [24] |
| Lung | ↓ | ↑ | Cohort study [111] |
| Lymphoma, non-Hodgikin’s | ↓ | +/− | Meta-analysis of cohort study and case-control study [112] |
| Melanoma, cutaneous | +/− | ↓ | Meta-analysis of cohort study [113] |
| Ovarian | +/− | +/− | Cohort study [103] |
| Prostate | +/− | +/− | Cohort study [114] |
| Renal cell carcinoma | +/− | +/− | Cohort study [100] |
| Skin cancer, non-melanoma | ↓ | ↓ | Cohort study [115] |
| Stomach | +/− | +/− | Meta-analysis of cohort study and case-control study [25] |
| Thyroid | +/− | +/− | Cohort [116] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayakawa, S.; Ohishi, T.; Miyoshi, N.; Oishi, Y.; Nakamura, Y.; Isemura, M. Anti-Cancer Effects of Green Tea Epigallocatchin-3-Gallate and Coffee Chlorogenic Acid. Molecules 2020, 25, 4553. https://doi.org/10.3390/molecules25194553
Hayakawa S, Ohishi T, Miyoshi N, Oishi Y, Nakamura Y, Isemura M. Anti-Cancer Effects of Green Tea Epigallocatchin-3-Gallate and Coffee Chlorogenic Acid. Molecules. 2020; 25(19):4553. https://doi.org/10.3390/molecules25194553
Chicago/Turabian StyleHayakawa, Sumio, Tomokazu Ohishi, Noriyuki Miyoshi, Yumiko Oishi, Yoriyuki Nakamura, and Mamoru Isemura. 2020. "Anti-Cancer Effects of Green Tea Epigallocatchin-3-Gallate and Coffee Chlorogenic Acid" Molecules 25, no. 19: 4553. https://doi.org/10.3390/molecules25194553
APA StyleHayakawa, S., Ohishi, T., Miyoshi, N., Oishi, Y., Nakamura, Y., & Isemura, M. (2020). Anti-Cancer Effects of Green Tea Epigallocatchin-3-Gallate and Coffee Chlorogenic Acid. Molecules, 25(19), 4553. https://doi.org/10.3390/molecules25194553





