Synthesis, Characterization and Magnetic Hyperthermia of Monodispersed Cobalt Ferrite Nanoparticles for Cancer Therapeutics
Abstract
1. Introduction
2. Results and Discussion
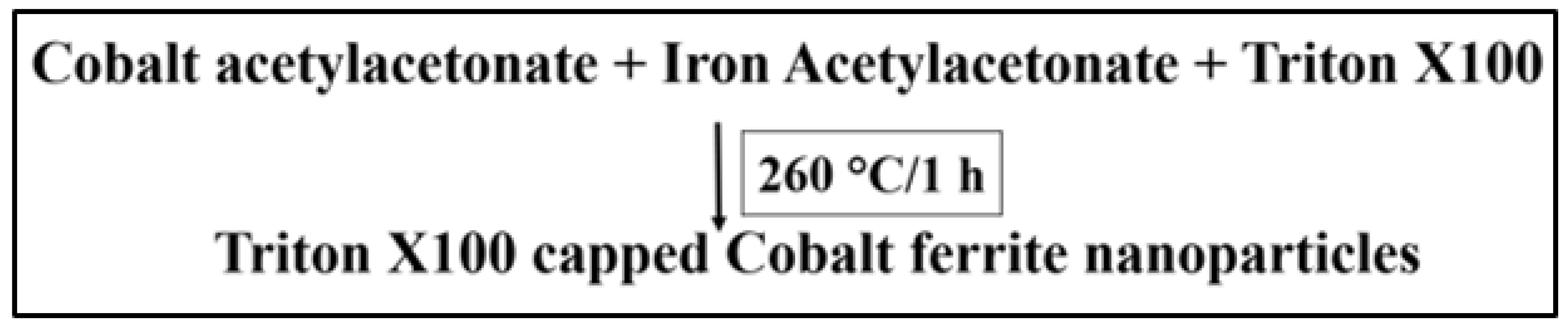
2.1. Mechanism of CFNP Synthesis
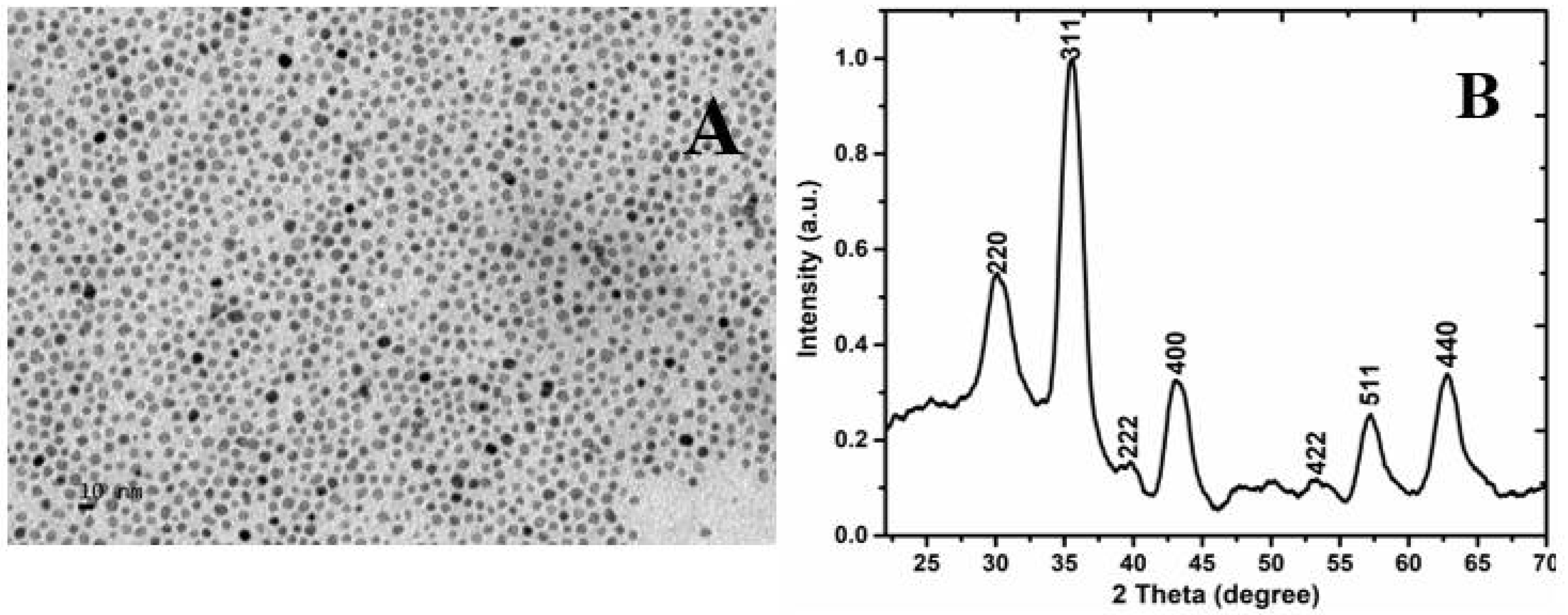
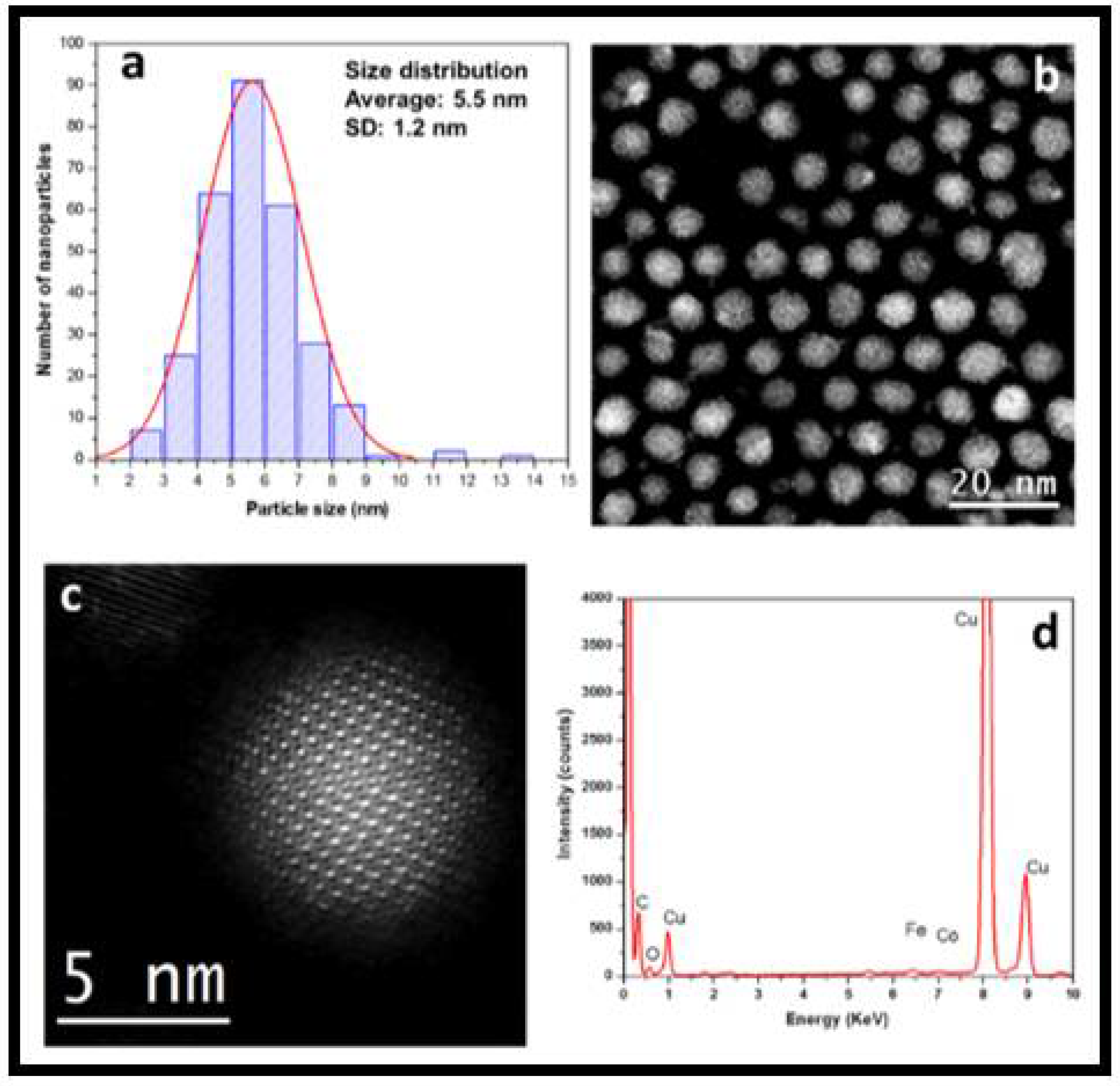
2.2. Structural Characterization
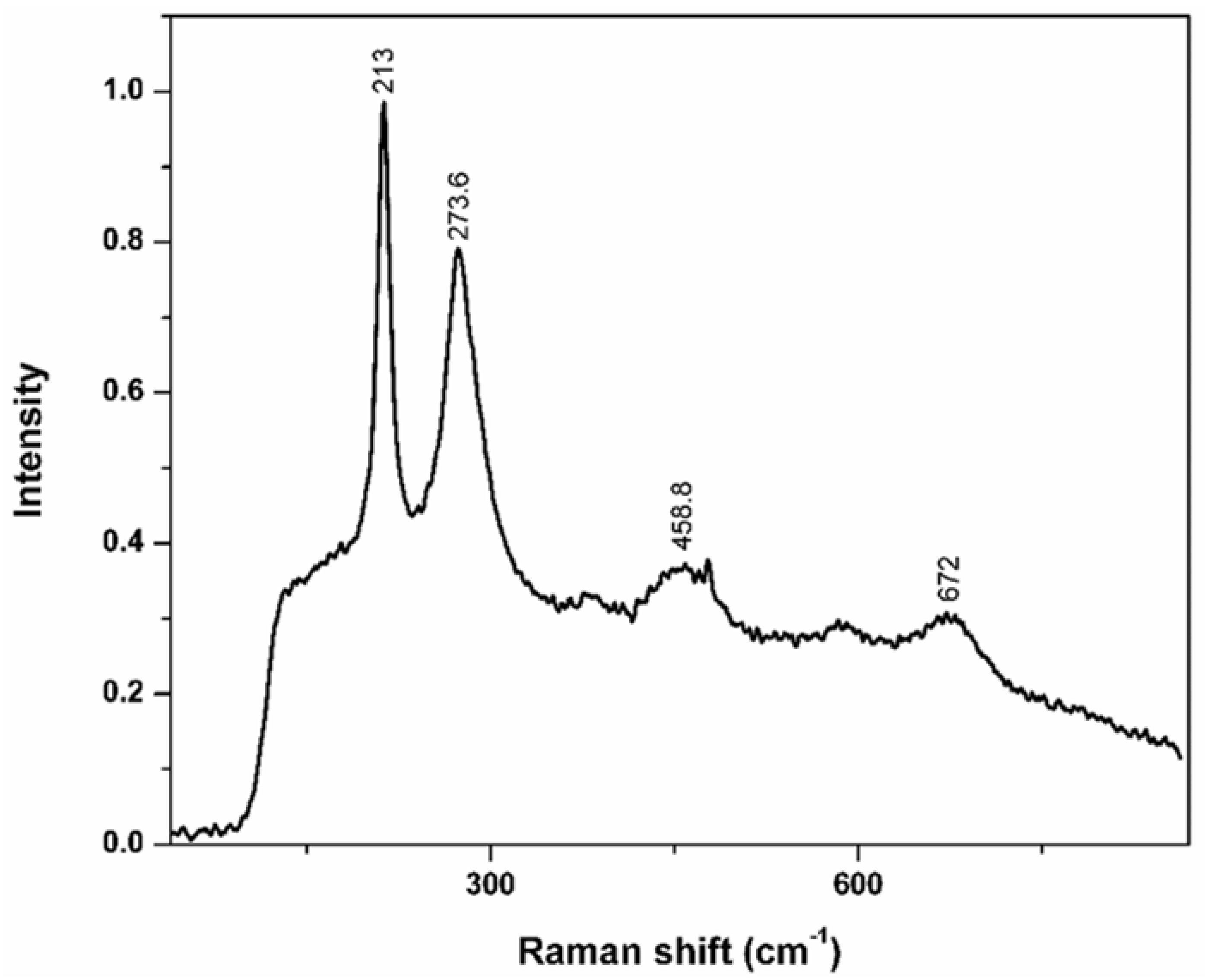
2.3. Raman Spectroscopy
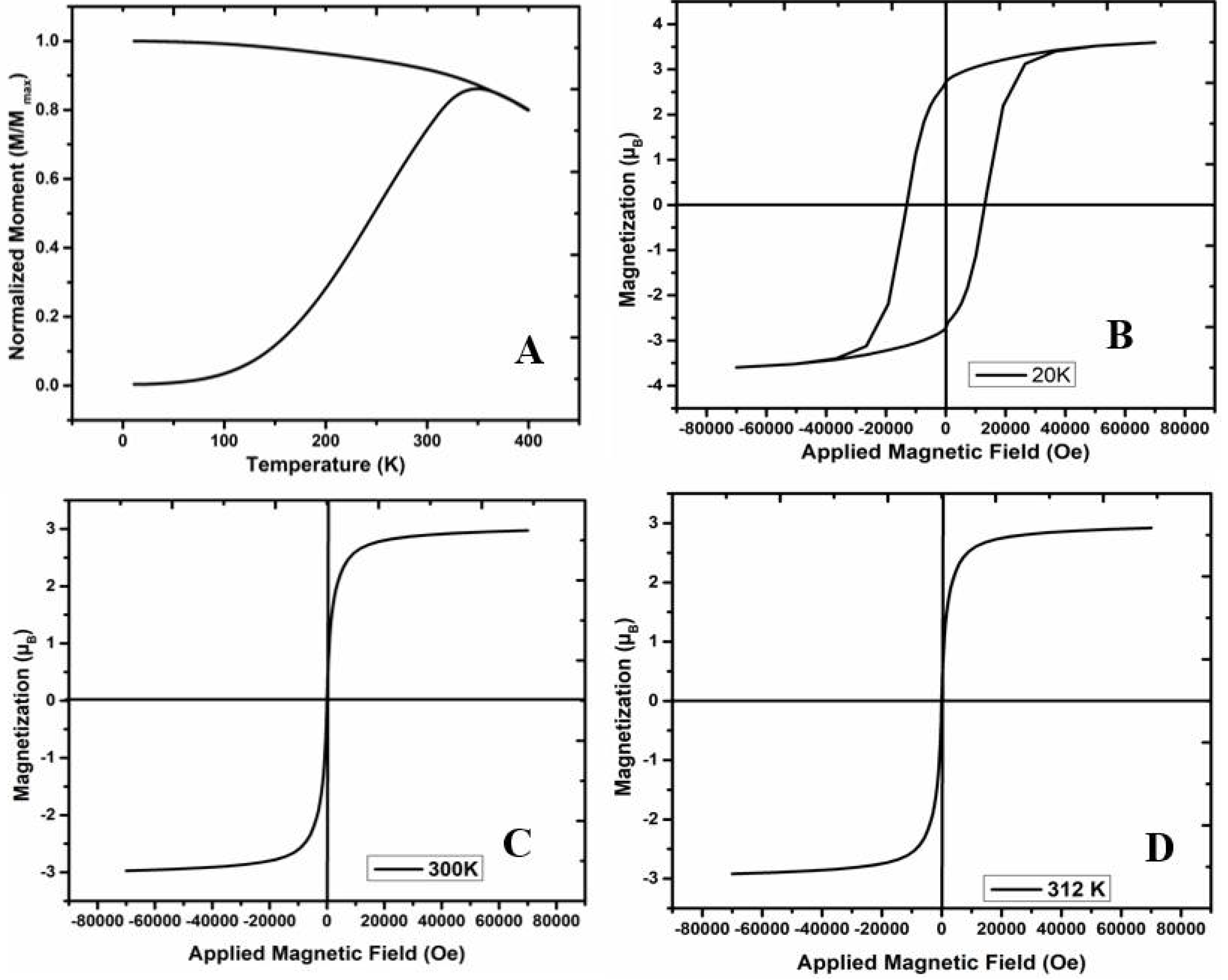
2.4. Magnetic Characterization
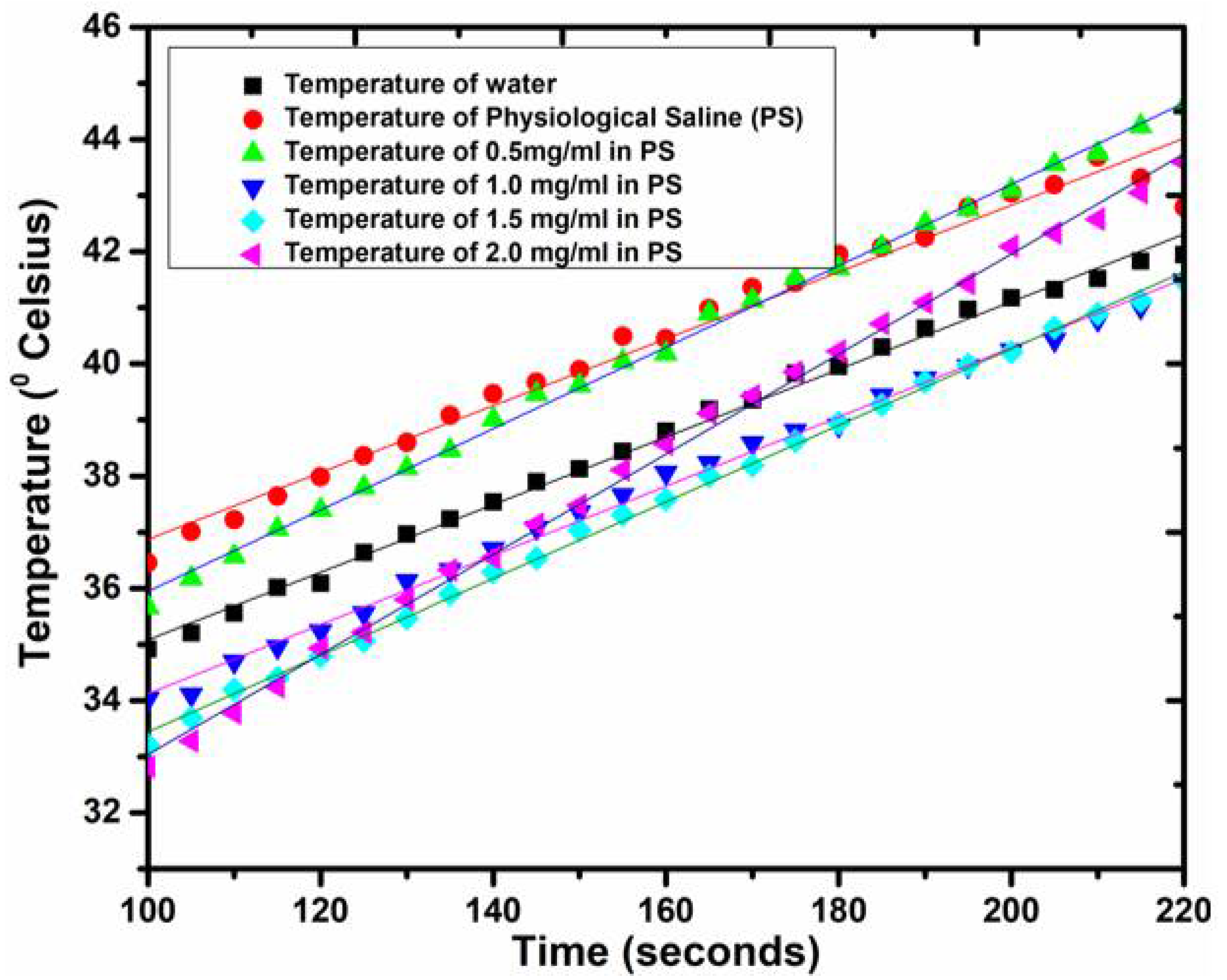
2.5. Specific Absorption Rate (SAR) of CFNPs
2.6. In Vitro Hyperthermia Studies on TNBC Cell Lines
3. Materials and Methods
3.1. Synthesis of Triton X-100 Coated CoFe2O4 Nanoparticles
3.2. Characterization of CFNPs
3.3. In-Vitro Hyperthermia Effects of Cobalt Ferrite Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanpo, N.; Berndt, C.C.; Wen, C.; Wang, J. Transition metal-substituted cobalt ferrite nanoparticles for biomedical applications. Acta Biomater. 2013, 9, 5830–5837. [Google Scholar] [CrossRef] [PubMed]
- Phadatare, M.R.; Khot, V.M.; Salunkhe, A.B.; Thorat, N.D.; Pawar, S.H. Studies on polyethylene glycol coating on NiFe2O4 nanoparticles for biomedical applications. J. Magn. Magn. Mater. 2012, 324, 770–772. [Google Scholar] [CrossRef]
- Saranya, D.; Rajan, R.; Suganthan, V.; Murugeswari, A.; Raj, N.A.N. Synthesis and Characterization of Pullulan Acetate Coated Magnetic Nanoparticle for Hyperthermic Therapy. Procedia Mater. Sci. 2015, 10, 2–9. [Google Scholar] [CrossRef]
- Karimi, Z.; Karimi, L.; Shokrollahi, H. Nano-magnetic particles used in biomedicine: Core and coating materials. Mater. Sci. Eng. C 2013, 33, 2465–2475. [Google Scholar] [CrossRef] [PubMed]
- Adireddy, S.; Lin, C.; Palshin, V.; Dong, Y.; Cole, R.; Caruntu, G. Size-controlled synthesis of quasi-monodisperse transition-metal ferrite nanocrystals in fatty alcohol solutions. J. Phys. Chem. C 2009, 113, 20800–20811. [Google Scholar] [CrossRef]
- Baldi, G.; Bonacchi, D.; Innocenti, C.; Lorenzi, G.; Sangregorio, C. Cobalt ferrite nanoparticles: The control of the particle size and surface state and their effects on magnetic properties. J. Magn. Magn. Mater. 2007, 311, 10–16. [Google Scholar] [CrossRef]
- Enpuku, K.; Tamai, Y.; Mitake, T.; Yoshida, T.; Matsuo, M. AC susceptibility measurement of magnetic markers in suspension for liquid phase immunoassay. J. Appl. Phys. 2010, 108, 034701–034706. [Google Scholar] [CrossRef]
- Habib, A.; Ondeck, C.; Chaudhary, P.; Bockstaller, M.; McHenry, M. Evaluation of iron-cobalt/ferrite core-shell nanoparticles for cancer thermotherapy. J. Appl. Phys. 2008, 103, 07A307. [Google Scholar] [CrossRef]
- Yun, X.; Jianmin, B.; Jian-Ping, W. High-magnetic-moment multifunctional nanoparticles for nanomedicine applications. J. Magn. Magn. Mater. 2007, 311, 131–134. [Google Scholar]
- Calero-DdelC, V.; Rinaldi, C. Synthesis and magnetic characterization of cobalt-substituted ferrite(CoxFe3-xO4) nanoparticles. J. Magn. Magn. Mater. 2007, 314, 60–67. [Google Scholar] [CrossRef]
- Cedeno-Mattei, Y.; Perales-Perez, O.; Tomar, M.S.; Roman, F.; Voyles, P.M.; Stratton, W.G. Tuning of magnetic properties in cobalt ferrite nanocrystals. J. Appl. Phys. 2008, 103, 07E512. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Ferrohydrodynamics; Dover Publications, Inc.: Mineola, NY, USA, 1985; ISBN 978-0521256247. [Google Scholar]
- Fannin, P.C. Investigating magnetic fluids by means of complex susceptibility measurements. J. Magn. Magn. Mater. 2003, 258, 446–451. [Google Scholar] [CrossRef]
- Fannin, P.C. On the high-frequency measurement of the dynamic properties of nano-particle colloids. J. Magn. Magn. Mater. 2009, 321, 850–853. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.; Dai, S.; Ma, Y.; Cui, S.; Achilefu, S.; Gu, Y. Multifunctional gold nanostar conjugates for tumor imaging and combined photothermal and chemo-therapy. Theranostics 2013, 3, 633–649. [Google Scholar] [CrossRef]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Wang, Y.; Yu, X.; Zhang, S.; Zhang, Q.; Cheng, Y. A Facile Strategy to Prepare Dendrimer-stabilized Gold Nanorods with Sub-10-nm Size for Efficient Photothermal Cancer Therapy. Sci. Rep. 2016, 6, 22764–22774. [Google Scholar] [CrossRef]
- Huang, N.; Wang, H.; Zhao, J.; Lui, H.; Korbelik, M.; Zeng, H. Single-wall carbon nanotubes assisted photothermal cancer therapy: Animal study with a murine model of squamous cell carcinoma. Lasers Surg. Med. 2010, 42, 638–648. [Google Scholar] [CrossRef]
- Burke, A.; Ding, X.; Singh, R.; Kraft, R.A.; Levi-Polyachenko, N.; Rylander, M.N.; Szot, C.; Buchanan, C.; Whitney, J.; Fisher, J.; et al. Long-term survival following a single treatment of kidney tumors with multiwalled carbon nanotubes and near-infrared radiation. Proc. Natl. Acad. Sci. USA 2009, 106, 12897–12902. [Google Scholar] [CrossRef]
- Zhou, J.; Lu, Z.; Zhu, X.; Wang, X.; Liao, Y.; Ma, Z.; Li, F. NIR photothermal therapy using polyaniline nanoparticles. Biomaterials 2013, 34, 9584–9592. [Google Scholar] [CrossRef]
- Van Landeghem, F.K.; Maier-Hauff, K.; Jordan, A.; Hoffmann, K.T.; Gneveckow, U.; Scholz, R.; Thiesen, B.; Brück, W.; von Deimling, A. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials 2009, 30, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.; Chen, B.; Ding, J.; Xia, G.; Gao, C.; Cheng, J.; Jin, N.; Zhou, Y.; Li, X.; et al. Pharmacokinetic parameters and tissue distribution of magnetic Fe3O4 nanoparticles in mice. Int. J. Nanomed. 2010, 5, 861–866. [Google Scholar]
- Lee, J.H.; Jang, J.T.; Choi, J.S.; Moon, S.H.; Noh, S.H.; Kim, J.W.; Kim, J.G.; Kim, I.S.; Park, K.I.; Cheon, J. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat. Nanotechnol. 2011, 6, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Fantechi, E.; Innocenti, C.; Zanardelli, M.; Fittipaldi, M.; Falvo, E.; Carbo, M.; Shullani, V.; Di Cesare Mannelli, L.; Ghelardini, C.; Ferretti, A.M.; et al. A smart platform for hyperthermia application in cancer treatment: Cobalt-doped ferrite nanoparticles mineralized in human ferritin cages. ACS Nano 2014, 8, 4705–4719. [Google Scholar] [CrossRef] [PubMed]
- Le Renard, P.E.; Jordan, O.; Faes, A.; Petri-Fink, A.; Hofmann, H.; Rüfenacht, D.; Bosman, F.; Buchegger, F.; Doelker, E. The in vivo performance of magnetic particle-loaded injectable, in situ gelling, carriers for the delivery of local hyperthermia. Biomaterials 2010, 31, 691–705. [Google Scholar] [CrossRef]
- Hergt, R.; Andra, W.; D’Ambly, C.G.; Hilger, I.; Kaiser, W.A.; Richter, U.; Schmidt, H.-G. Physical limits of hyperthermia using magnetite fine particles. IEEE Trans. Magn. 1998, 34, 3745–3754. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Johnson, R.H.; Robinson, M.P.; Preece, A.W.; Green, J.L.; Pothecary, N.M.; Railton, C.J. Effect of frequency and conductivity on field penetration of electromagnetic hyperthermia applicators. Phys. Med. Biol. 1993, 38, 1023–1034. [Google Scholar] [CrossRef]
- Van den Berg, C.A.T.; Bartels, L.W.; De Leeuw, A.A.C.; Lagendijk, J.J.W.; van de Kamer, J.B. Experimental validation of hyperthermia SAR treatment planning using MR B1+ imaging. Phys. Med. Biol. 2004, 49, 5029–5042. [Google Scholar] [CrossRef]
- Briceño, S.; Suarez, J.; Gonzalez, G. Solvothermal synthesis of cobalt ferrite hollow spheres with chitosan. Mater. Sci. Eng. C 2017, 78, 842–846. [Google Scholar] [CrossRef]
- Patil, R.M.; Shete, P.B.; Thorat, N.D.; Otari, S.V.; Barick, K.C.; Prasad, A.; Ningthoujamb, R.S.; Tiwalea, B.M.; Pawar, S.H. Superparamagnetic iron oxide/chitosan core/shells for hyperthermia application: Improved colloidal stability and biocompatibility. J. Magn. Magn. Mater. 2014, 355, 22–30. [Google Scholar] [CrossRef]
- Long, J.; Jiao, A.; Wei, B.; Wu, Z.; Zhang, Y.; Xu, X.; Jin, Z. A novel method for pullulanase immobilized onto magnetic chitosan/Fe3O4 composite nanoparticles by in situ preparation and evaluation of the enzyme stability. J. Mol. Catal. B Enzym. 2014, 109, 53–61. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, L. Synthesis and magnetic properties of CoFe2O4 nanoparticles by using PEG as surfactant additive. Mater. Sci. Eng. B 2007, 141, 82–86. [Google Scholar] [CrossRef]
- Suharyadi, E.; Setiadi, E.A.; Shabrina, N.; Kato, T.; Iwata, S. Magnetic Properties and Microstructures of Polyethylene Glycol (PEG)-Coated Cobalt Ferrite Nanoparticles Synthesized by Coprecipitation Method. Adv. Mater. Res. 2014, 896, 126–133. [Google Scholar] [CrossRef]
- Pansailom, N.; Rattanamai, S.; Leepheng, P.; Rattanawarinchai, P.; Chattrairat, K.; Phromyothin, D. Surface modification: PEG/Dextran encapsulation of SPIONs. Mater. Today Proc. 2017, 4, 6306–6310. [Google Scholar] [CrossRef]
- Herrera, A.P.; Polo-Corrales, L.; Chavez, E.; Cabarcas-Bolivar, J.; Uwakweh, O.N.C.; Rinaldi, C. Influence of aging time of oleate precursor on the magnetic relaxation of cobalt ferrite nanoparticles synthesized by the thermal decomposition method. J. Magn. Magn. Mater. 2013, 328, 41–52. [Google Scholar] [CrossRef]
- Unni, M.; Uhl, A.M.; Savliwala, S.; Savitzky, B.H.; Dhavalikar, R.; Garraud, N.; Arnold, D.P.; Kourkoutis, L.F.; Andrew, J.S.; Rinaldi, C. Thermal Decomposition Synthesis of Iron Oxide Nanoparticles with Diminished Magnetic Dead Layer by Controlled Addition of Oxygen. ACS Nano 2017, 11, 2284–2303. [Google Scholar] [CrossRef]
- Yadav, R.S.; Kuřitka, I.; Vilcakova, J.; Havlica, J.; Masilko, J.; Kalina, L.; Tkacz, J.; Švec, J.; Enev, V.; Hajdúchová, M. Impact of grain size and structural changes on magnetic, dielectric, electrical, impedance and modulus spectroscopic characteristics of CoFe2O4 nanoparticles synthesized by honey mediated sol-gel. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 045002. [Google Scholar] [CrossRef]
- Murray, C.B.; Sun, S.H.; Doyle, H.; Betley, T. Monodisperse 3d Transition-Metal (Co,Ni,Fe) Nanoparticles and Their Assembly into Nanoparticle Superlattices. MRS Bull. 2001, 26, 985–991. [Google Scholar] [CrossRef]
- Noh, S.-H.; Na, W.; Jang, J.-T.; Lee, J.-H.; Lee, E.J.; Moon, S.H.; Lim, Y.; Shin, J.-S.; Cheon, J. Nanoscale Magnetism Control via Surface and Exchange Anisotropy for Optimized Ferrimagnetic Hysteresis. Nano Lett. 2012, 12, 3716–3721. [Google Scholar] [CrossRef]
- Yun, H.; Liu, X.; Paik, T.; Palanisamy, D.; Kim, J.; Vogel, W.D.; Viescas, A.J.; Chen, J.; Papaefthymiou, G.C.; Kikkawa, J.M.; et al. Size- and Composition-Dependent Radio Frequency Magnetic Permeability of Iron Oxide Nanocrystals. ACS Nano 2014, 8, 12323–12337. [Google Scholar] [CrossRef] [PubMed]
- Knobel, M.; Nunes, W.C.; Winnischofer, H.; Rocha, T.C.R.; Socolovsky, L.M.; Mayorga, C.L.; Zanchet, D. Effects of magnetic interparticle coupling on the blocking temperature of ferromagnetic nanoparticle arrays. J. Non Cryst. Solids 2007, 353, 743–747. [Google Scholar] [CrossRef]
- Yamamoto, K.; Majetich, S.A.; McCartney, M.R.; Sachan, M.; Yamamuro, S.; Hirayama, T. Direct visualization of dipolar ferromagnetic domain structures in Co nanoparticle monolayers by electron holography. Appl. Phys. Lett. 2008, 93, 082502. [Google Scholar] [CrossRef]
- Morup, S.; Hansen, M.F.; Frandsen, C. Magnetic interactions between nanoparticles. Beilstein J. Nanotechnol. 2010, 1, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Cullity, B.D. Introduction to Magnetic Materials, 1st ed.; Addison-Wesley Publishing Company, Inc.: Reading, MA, USA, 1972; ISBN 9780201012187. [Google Scholar]
- Li, D.; Yun, H.; Diroll, B.T.; Doan-Nguyen, V.V.T.; Kikkawa, J.M.; Murray, C.B. Synthesis and Size-Selective Precipitation of Monodisperse Nonstoichiometric MxFe3–xO4 (M = Mn, Co) Nanocrystals and Their DC and AC Magnetic Properties. Chem. Mater. 2016, 28, 480–489. [Google Scholar] [CrossRef]
- Sharifi Dehsari, H.; Asadi, K. Impact of Stoichiometry and Size on the Magnetic Properties of Cobalt Ferrite Nanoparticles. J. Phys. Chem. C 2018, 122, 29106–29121. [Google Scholar] [CrossRef]
- Lavorato, G.; Alzamora, M.; Contreras, C.; Burlandy, G.; Litterst, F.J.; Baggio-Saitovitch, E. Internal Structure and Magnetic Properties in Cobalt Ferrite Nanoparticles: Influence of the Synthesis Method. Part. Part. Syst. Charact. 2019, 36, 1900061. [Google Scholar] [CrossRef]
- Aslibeiki, B.; Kameli, P.; Salamati, H.; Concas, G.; Salvador Fernandez, M.; Talone, A.; Muscas, G.; Peddis, D. Co-doped MnFe2O4 nanoparticles: Magnetic anisotropy and interparticle interactions. Beilstein J. Nanotechnol. 2019, 10, 856–865. [Google Scholar] [CrossRef]
- Usov, N.A.; Peschany, S.E. Theoretical hysteresis loops for single-domain particles with cubic anisotropy. J. Magn. Magn. Mater. 1997, 174, 247–260. [Google Scholar] [CrossRef]
- Tanaka, M.; Sato, M. Microwave heating of water, ice, and saline solution: Molecular dynamics study Citation. J. Chem. Phys. 2007, 126, 034509. [Google Scholar] [CrossRef]
- Chen, C.T.A. Specific Heat Capacities of Aqueous Sodium Chloride Solutions at High Pressures. J. Chem. Eng. Data 1982, 27, 356–358. [Google Scholar] [CrossRef]
- Kim, D.K.; Amin, M.S.; Elborai, S.; Lee, S.; Koseoglu, Y.; Zahn, M.; Muhammed, M. Energy absorption of superparamagnetic iron oxide nanoparticles by microwave irradiation. J. Appl. Phys. 2005, 97, 10J510. [Google Scholar] [CrossRef]
- Asano, M.; Sakaguchi, M.; Tanaka, S.; Kashimura, K.; Mitani, T.; Kawase, M.; Matsumura, H.; Yamaguchi, T.; Fujita, Y.; Tabuse, K. Effects of Normothermic Conditioned Microwave Irradiation on Cultured Cells Using an Irradiation System with Semiconductor Oscillator and Thermo-regulatory Applicator. Sci. Rep. 2017, 7, 41244. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina, M.A.; Oza, G.; Ángeles-Pascual, A.; González M., M.; Antaño-López, R.; Vera, A.; Leija, L.; Reguera, E.; Arriaga, L.G.; Hernández Hernández, J.M.; et al. Synthesis, Characterization and Magnetic Hyperthermia of Monodispersed Cobalt Ferrite Nanoparticles for Cancer Therapeutics. Molecules 2020, 25, 4428. https://doi.org/10.3390/molecules25194428
Medina MA, Oza G, Ángeles-Pascual A, González M. M, Antaño-López R, Vera A, Leija L, Reguera E, Arriaga LG, Hernández Hernández JM, et al. Synthesis, Characterization and Magnetic Hyperthermia of Monodispersed Cobalt Ferrite Nanoparticles for Cancer Therapeutics. Molecules. 2020; 25(19):4428. https://doi.org/10.3390/molecules25194428
Chicago/Turabian StyleMedina, Mauricio A., Goldie Oza, A. Ángeles-Pascual, Marlene González M., R. Antaño-López, A. Vera, L. Leija, Edilso Reguera, L. G. Arriaga, José Manuel Hernández Hernández, and et al. 2020. "Synthesis, Characterization and Magnetic Hyperthermia of Monodispersed Cobalt Ferrite Nanoparticles for Cancer Therapeutics" Molecules 25, no. 19: 4428. https://doi.org/10.3390/molecules25194428
APA StyleMedina, M. A., Oza, G., Ángeles-Pascual, A., González M., M., Antaño-López, R., Vera, A., Leija, L., Reguera, E., Arriaga, L. G., Hernández Hernández, J. M., & Ramírez, J. T. (2020). Synthesis, Characterization and Magnetic Hyperthermia of Monodispersed Cobalt Ferrite Nanoparticles for Cancer Therapeutics. Molecules, 25(19), 4428. https://doi.org/10.3390/molecules25194428






