Smart Synthesis of Trimethyl Ethoxysilane (TMS) Functionalized Core–Shell Magnetic Nanosorbents Fe3O4@SiO2: Process Optimization and Application for Extraction of Pesticides
Abstract
1. Introduction
2. Results and Discussion
2.1. Resolution V Full Factorial Design (24V-FFD)
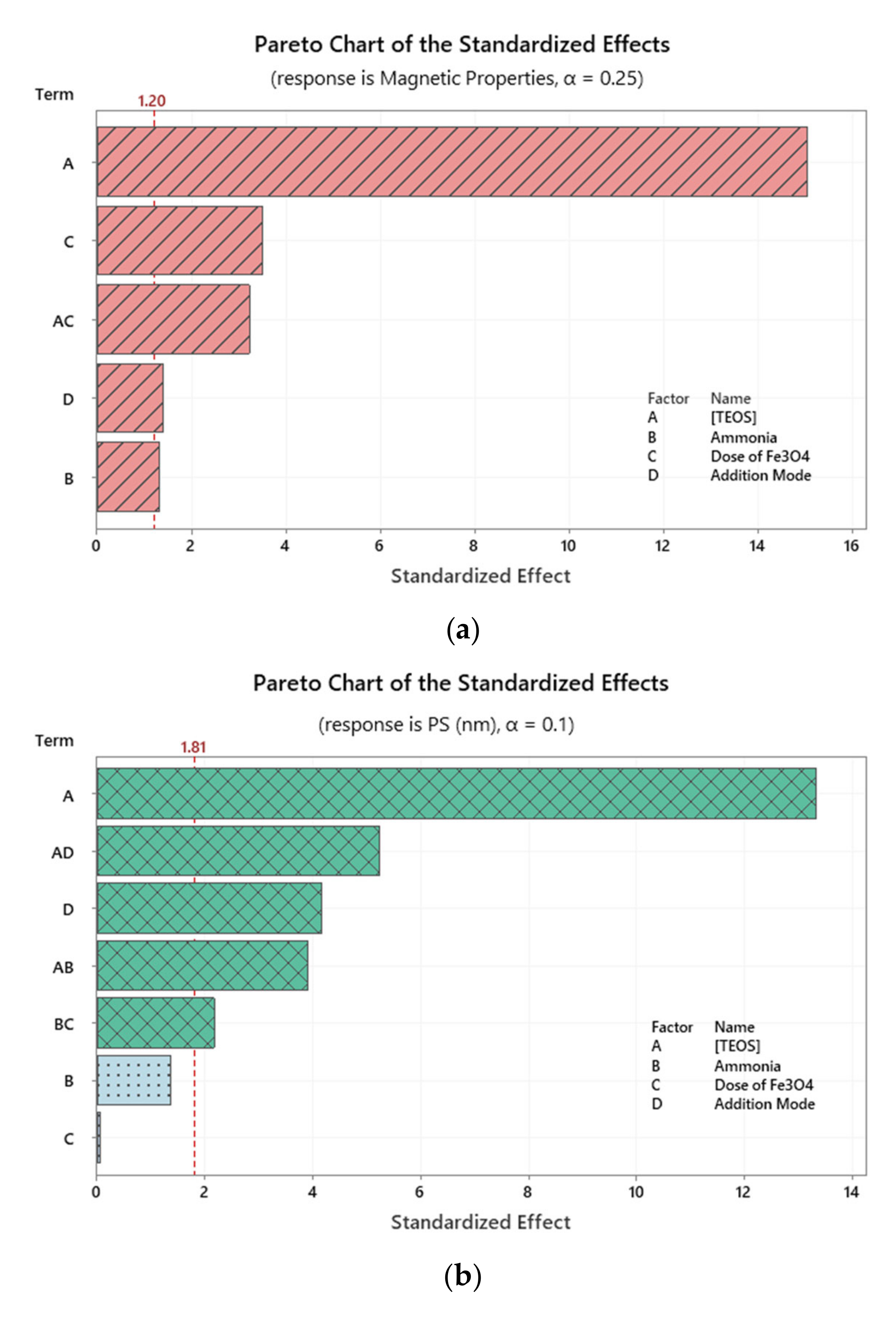
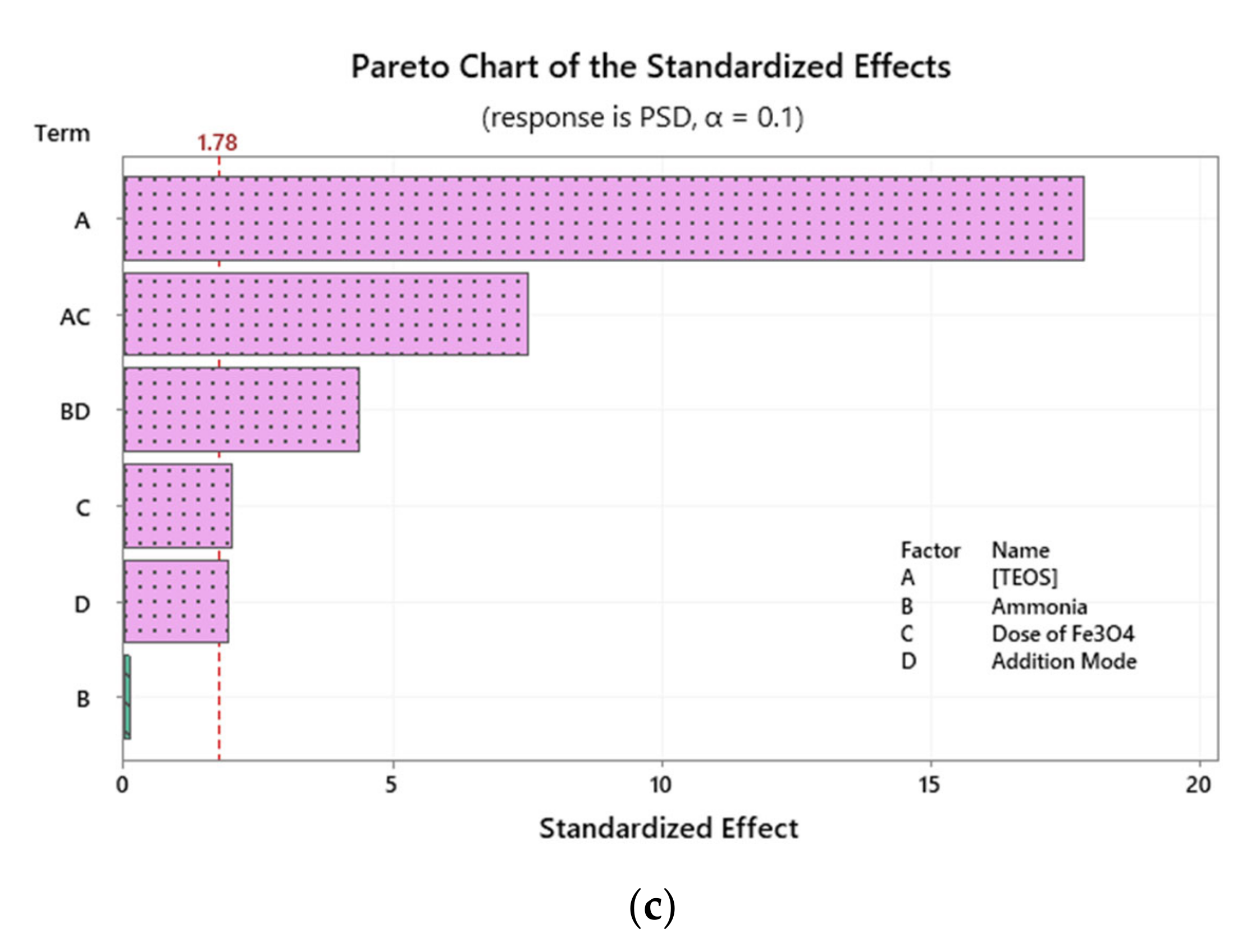
2.2. Investigation of Variables’ Significance
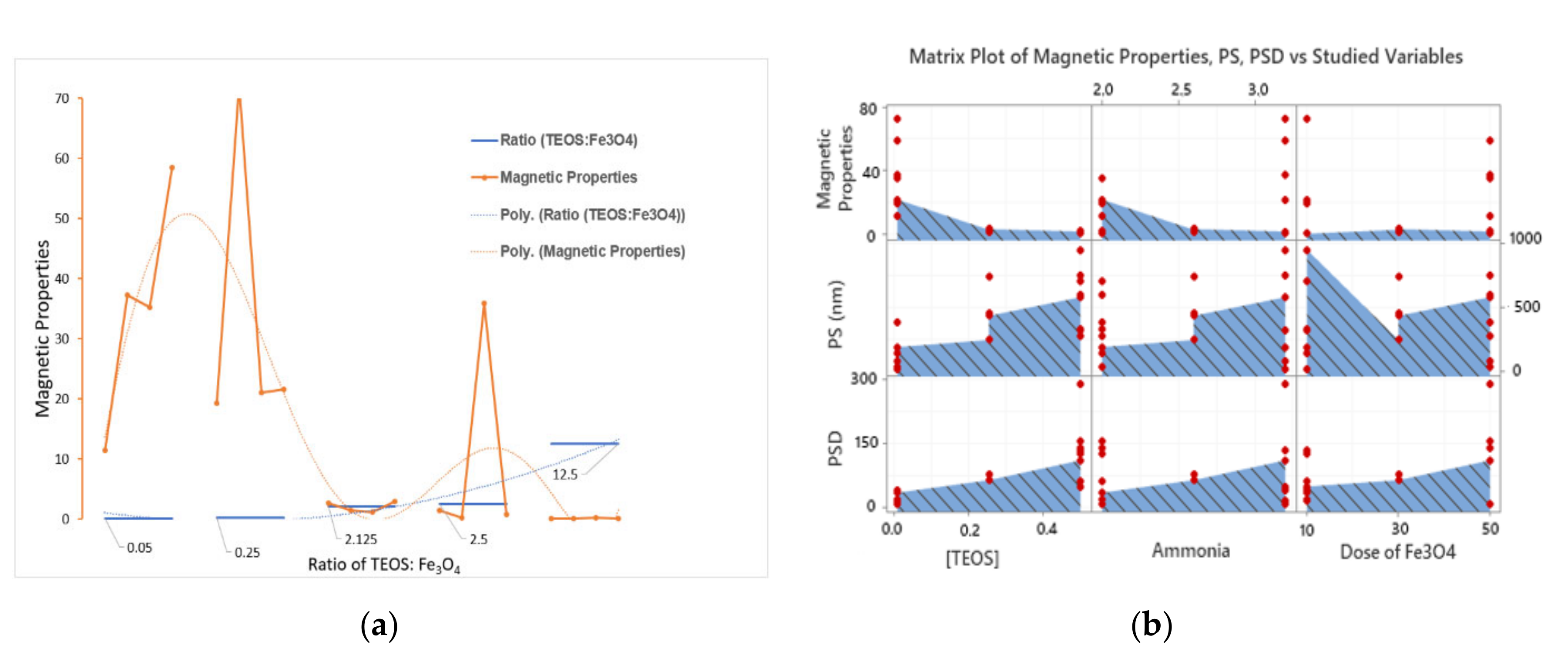
2.3. Modelling and Multiple-Response Optimization
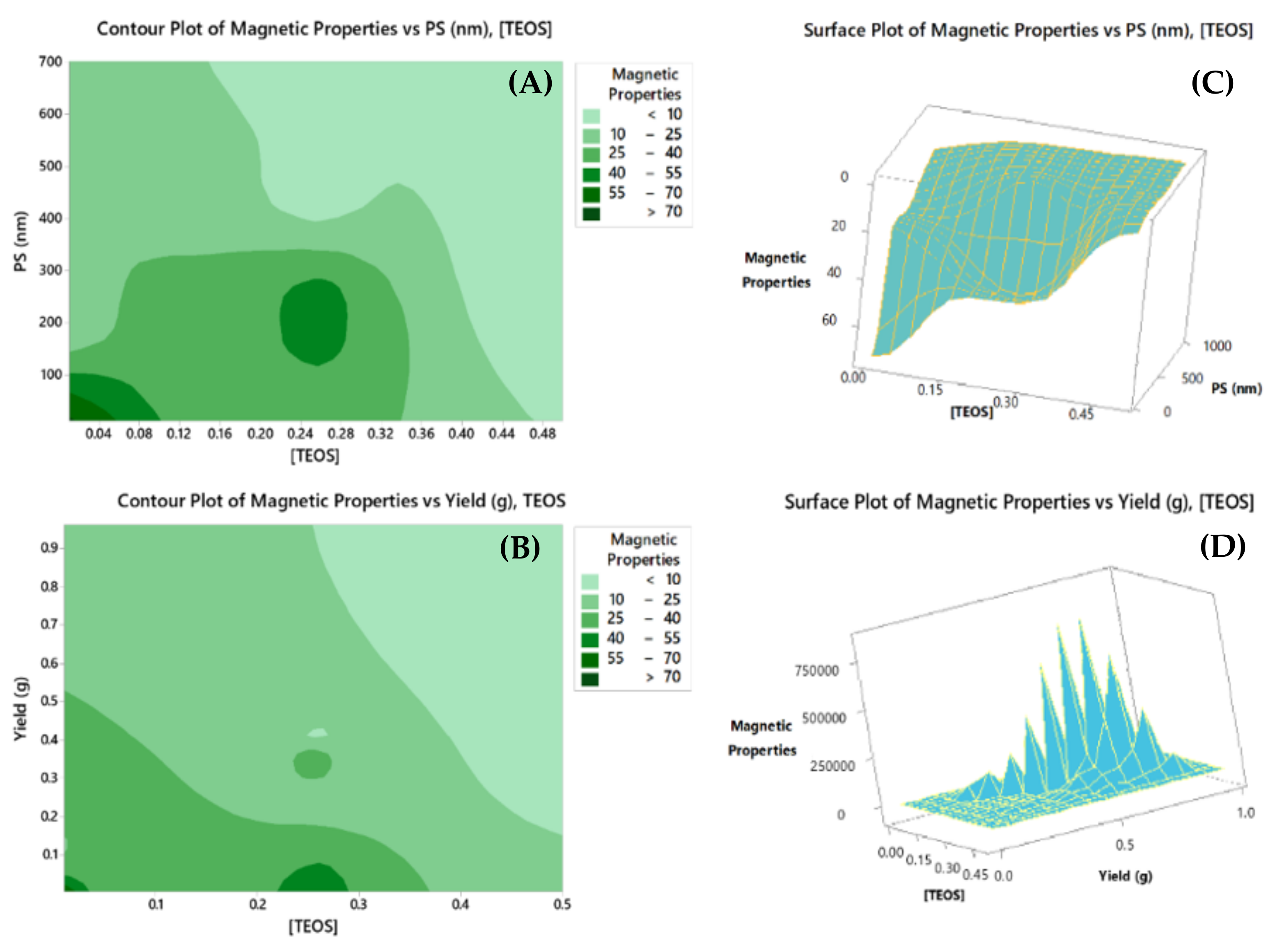
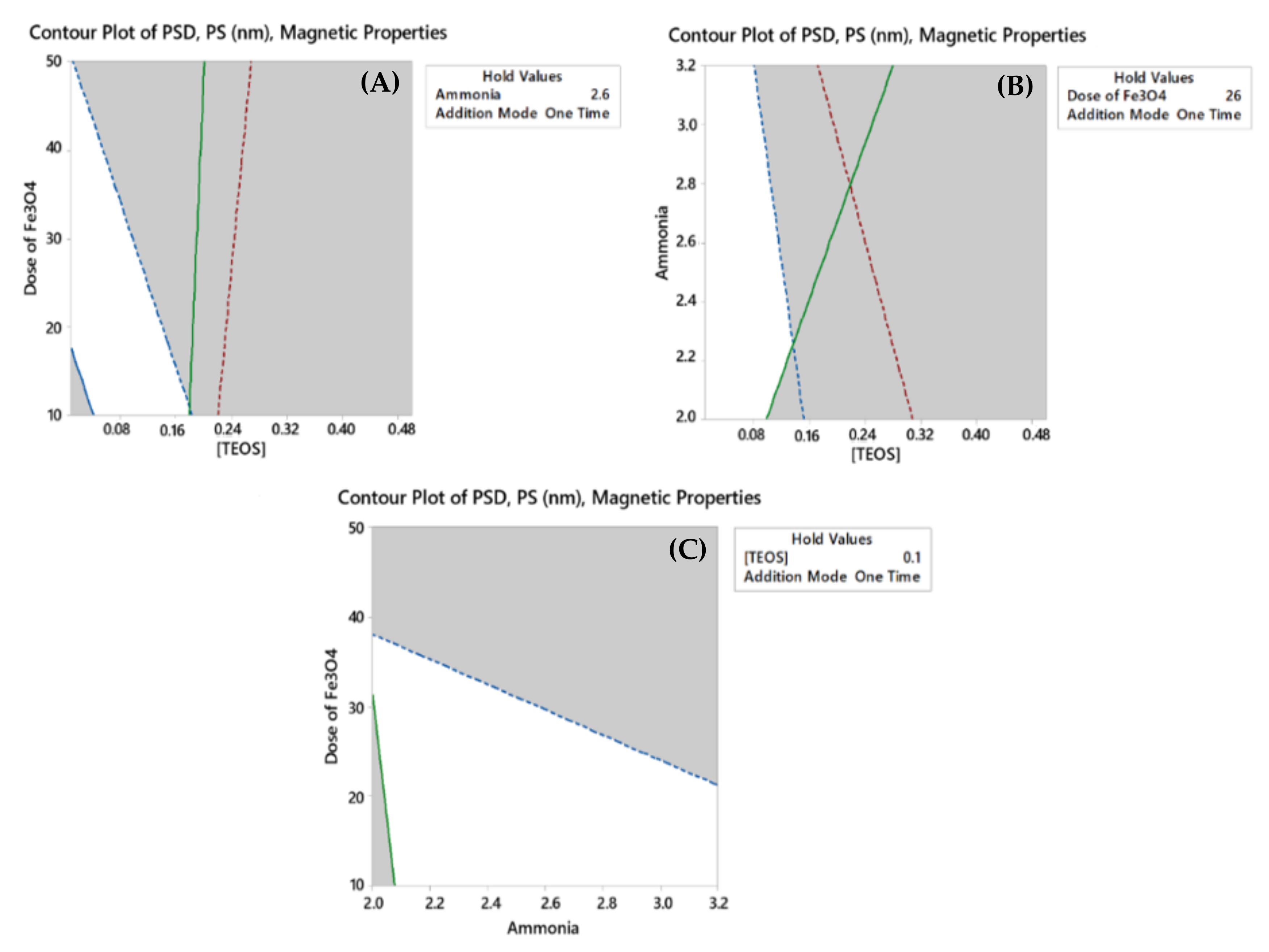
2.3.1. Overlaid Contour Plots
2.3.2. Response Optimizer
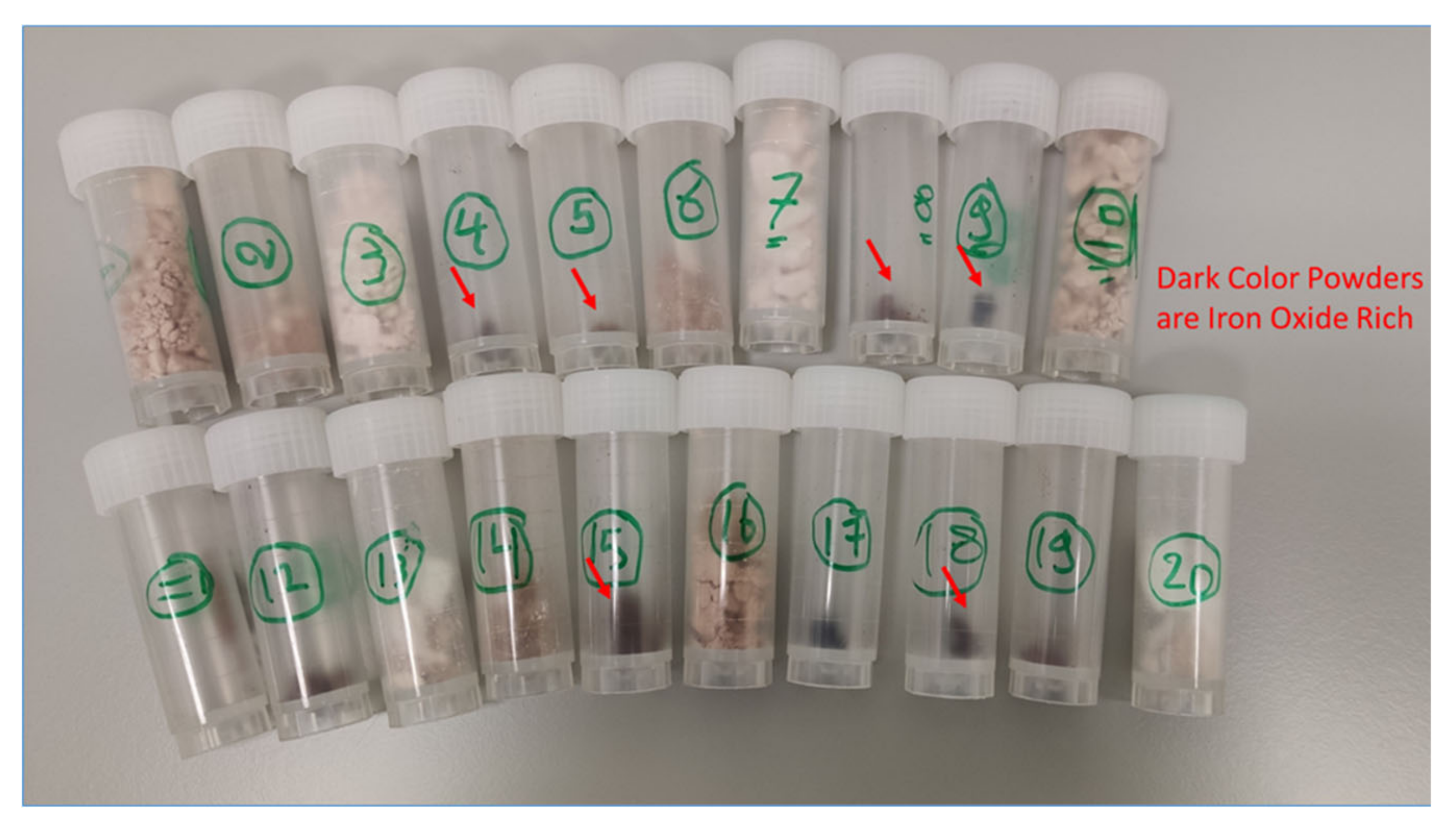
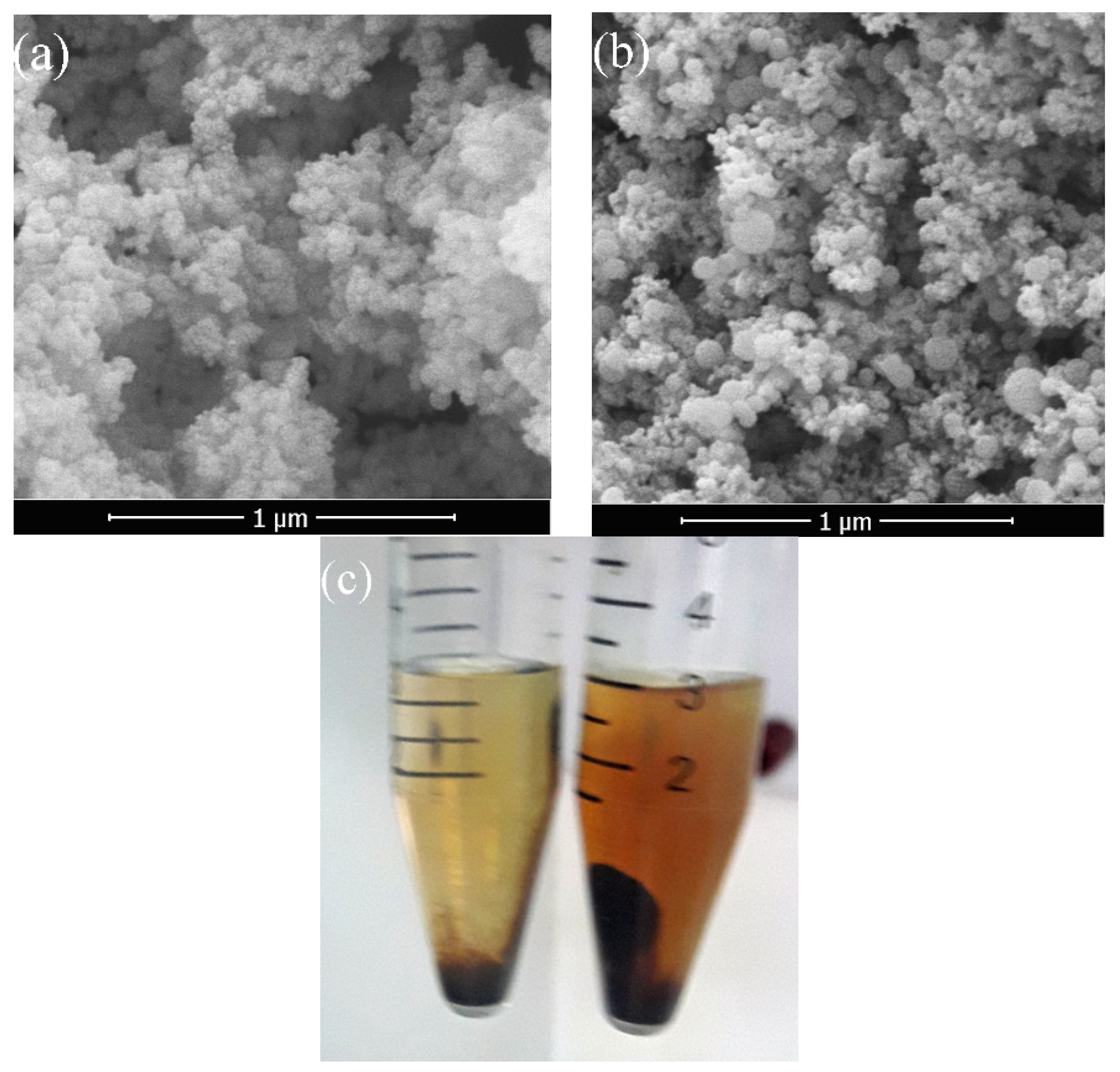
2.4. Characterization of the Produced Particles
2.4.1. Physical Characterization
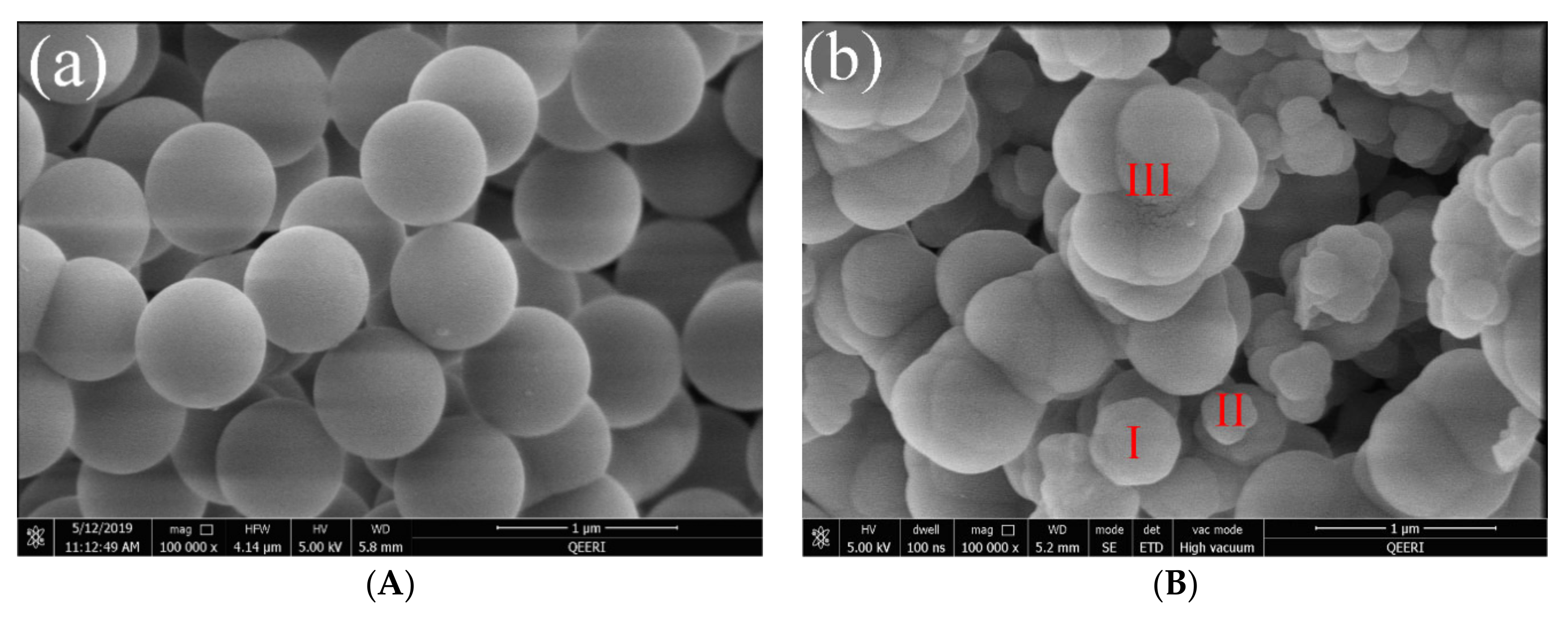
2.4.2. Morphology
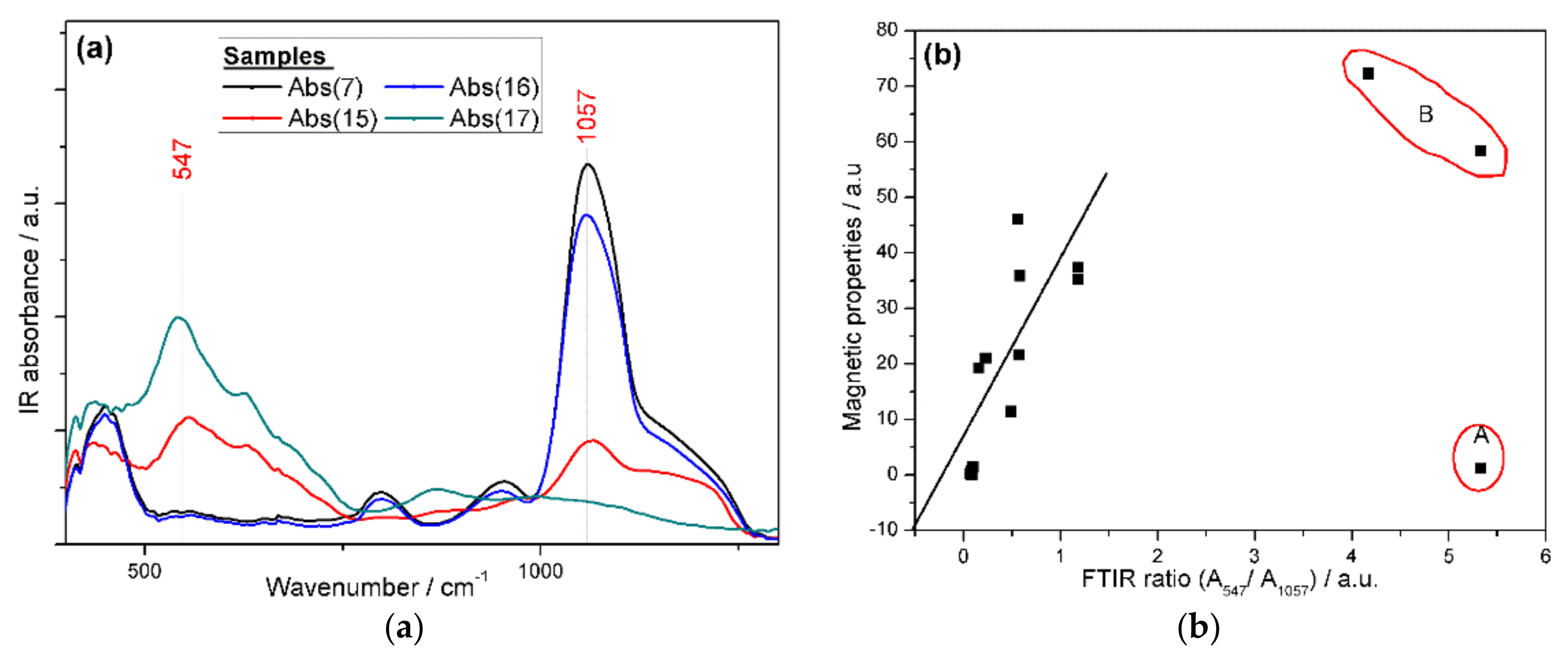
2.4.3. FTIR and TGA Analyses of the Produced Magnetic Nanoparticles (mNPs)
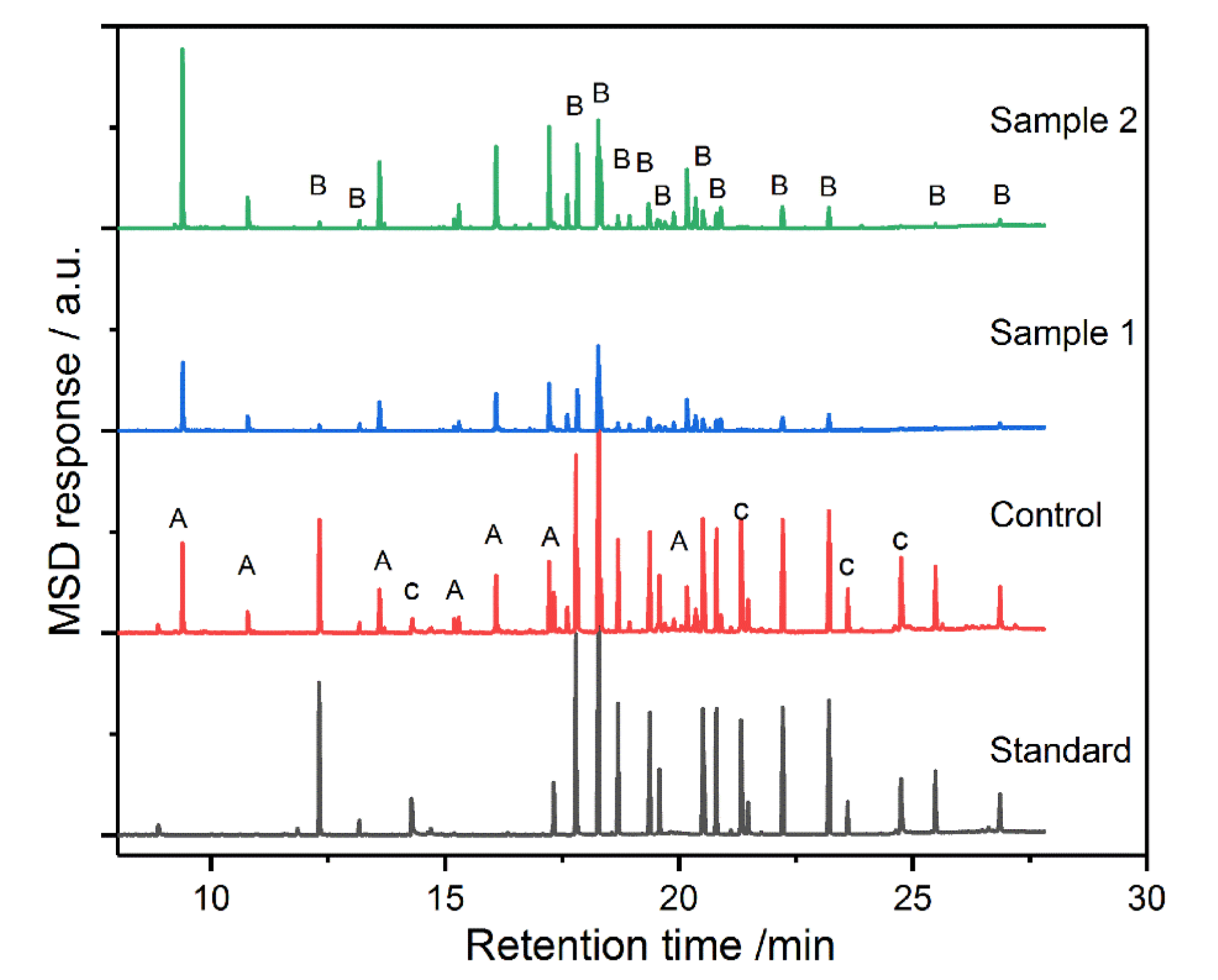
2.5. Extraction of Carbamate and Organophosphorus Pesticides
2.5.1. Carbamate Pesticides
2.5.2. Organophosphorus Pesticides
3. Materials and Methods
3.1. Materials and Reagents
3.2. Software and Instrumentation
3.3. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
3.4. Determination of Magnetic Properties
3.5. Procedure
3.5.1. Preparation of Magnetic Core
3.5.2. Preparation of Fe3O4@SiO2 Nanocomposites
3.5.3. Preparation of Functionalized Fe3O4@SiO2 Nanocomposites
3.5.4. Removal of Pesticides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Filipponi, L.; Sutherland, D. Nanotechnologies—Principles, Applications, Implications and Hands-On Activities—A Compendium for Educators. 2012. Available online: https://ec.europa.eu/research/industrial_technologies/pdf/nano-hands-on-activities_en.pdf (accessed on 15 July 2020).
- Majewski, P.; Thierry, B. Functionalized Magnetite Nanoparticles—Synthesis, Properties, and Bio-Applications. Crit. Rev. Solid State Mater. Sci. 2007, 32, 203–215. [Google Scholar] [CrossRef]
- Rho, W.-Y.; Kim, H.-M.; Kyeong, S.; Kang, Y.-L.; Kim, D.-H.; Kang, H.; Jeong, C.; Lee, J.Y.; Jun, B.-H. Facile synthesis of monodispersed silica-coated magnetic nanoparticles. J. Ind. Eng. Chem. 2014, 20, 2646–2649. [Google Scholar] [CrossRef]
- Ghorbani, F.; Kamari, S. Core–shell magnetic nanocomposite of Fe3O4@SiO2@NH2 as an efficient and highly recyclable adsorbent of methyl red dye from aqueous environments. Environ. Technol. Innov. 2019, 14, 100333. [Google Scholar] [CrossRef]
- Huang, Y.; Keller, A.A. EDTA functionalized magnetic nanoparticle sorbents for cadmium and lead contaminated water treatment. Water Res. 2015, 80, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Najafpoor, A.; Ostad, R.N.; Alidadi, H.; Rohani-Bastami, T.; Davoudi, M.; Barjasteh-Askari, F.; Zanganeh, J. Effect of magnetic nanoparticles and silver-loaded magnetic nanoparticles on advanced wastewater treatment and disinfection. J. Mol. Liq. 2020, 303, 112640. [Google Scholar] [CrossRef]
- Parashar, A.; Sikarwar, S.; Jain, R. Removal of pharmaceuticals from wastewater using magnetic iron oxide nanoparticles (IOPs). Int. J. Environ. Anal. Chem. 2020, 1–17. [Google Scholar] [CrossRef]
- Kristianto, H.; Tanuarto, M.Y.; Prasetyo, S.; Sugih, A.K. Magnetically assisted coagulation using iron oxide nanoparticles-Leucaena leucocephala seeds’ extract to treat synthetic Congo red wastewater. Int. J. Environ. Sci. Technol. 2020, 17, 3561–3570. [Google Scholar] [CrossRef]
- Davies, G.-L.; Barry, A.; Gun’Ko, Y.K. Preparation and size optimisation of silica nanoparticles using statistical analyses. Chem. Phys. Lett. 2009, 468, 239–244. [Google Scholar] [CrossRef]
- Park, S.K.; Kim, K.D.; Kim, H.T. Preparation of silica nanoparticles: Determination of the optimal synthesis conditions for small and uniform particles. Colloids Surfaces A: Physicochem. Eng. Asp. 2002, 197, 7–17. [Google Scholar] [CrossRef]
- Elazazy, M.S.; Issa, A.A.; Al-Mashreky, M.; Al-Sulaiti, M.; Al-Saad, K.; Al-Sulaiti, M. Application of fractional factorial design for green synthesis of cyano-modified silica nanoparticles: Chemometrics and multifarious response optimization. Adv. Powder Technol. 2018, 29, 1204–1215. [Google Scholar] [CrossRef]
- El-Gendy, A.; El-Shafie, A.S.; Issa, A.; Al-Meer, S.; Al-Saad, K.; Elazazy, M. Carbon-Based Materials (CBMs) for Determination and Remediation of Antimicrobials in Different Substrates: Wastewater and Infant Foods as Examples. In Carbon-Based Material for Environmental Protection and Remediation; Bartoli, M., Frediani, M., Rosi, L., Eds.; IntechOpen: London, UK, 2020; Available online: https://www.intechopen.com/books/carbon-based-material-for-environmental-protection-and-remediation/carbon-based-materials-cbms-for-determination-and-remediation-of-antimicrobials-in-different-substrates (accessed on 25 August 2020). [CrossRef]
- Wang, L.; Shen, C.; Cao, Y. PVP modified Fe3O4@SiO2 nanoparticles as a new adsorbent for hydrophobic substances. J. Phys. Chem. Solids 2019, 133, 28–34. [Google Scholar] [CrossRef]
- Tadyszak, K.; Coy, E.; Coy, E.; Andruszkiewicz, R.; Milewski, S.; Kardava, I.; Scheibe, B.; Jurga, S.; Chybczyńska, K. Spectroscopic and magnetic studies of highly dispersible superparamagnetic silica coated magnetite nanoparticles. J. Magn. Magn. Mater. 2017, 433, 254–261. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, C.; Zhang, Z.; Wu, W.; Wang, X.; Yu, Z. Synthesis, functionalization, and nanomedical applications of functional magnetic nanoparticles. Chin. Chem. Lett. 2018, 29, 1601–1608. [Google Scholar] [CrossRef]
- Jadhav, S.A.; Scalarone, D.; Brunella, V.; Ugazio, E.; Sapino, S.; Berlier, G. Thermoresponsive copolymer-grafted SBA-15 porous silica particles for temperature-triggered topical delivery systems. Express Polym. Lett. 2017, 11, 96–105. [Google Scholar] [CrossRef]
- Itoh, H.; Sugimoto, T. Systematic control of size, shape, structure, and magnetic properties of uniform magnetite and maghemite particles. J. Colloid Interface Sci. 2003, 265, 283–295. [Google Scholar] [CrossRef]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.-M.; Mahapatra, C.; Kim, H.-W.; Knowles, J.C. Sol–gel based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Moreno, Y.; Cardoso, M.; Ferrão, M.F.; Moncada, E.; dos Santos, J.H.Z.; Ruiz, Y.P.M. Effect of SiCl4 on the preparation of functionalized mixed-structure silica from monodisperse sol–gel silica nanoparticles. Chem. Eng. J. 2016, 292, 233–245. [Google Scholar] [CrossRef]
- Peng, S.; Wang, C.; Xie, A.J.; Sun, S. Synthesis and Stabilization of Monodisperse Fe Nanoparticles. J. Am. Chem. Soc. 2006, 128, 10676–10677. [Google Scholar] [CrossRef]
- Viota, J.; Durán, J.; Gonzalez-Caballero, F.; Delgado, A. Magnetic properties of extremely bimodal magnetite suspensions. J. Magn. Magn. Mater. 2007, 314, 80–86. [Google Scholar] [CrossRef]
- Bang, J.H.; Suslick, K.S. Applications of Ultrasound to the Synthesis of Nanostructured Materials. Adv. Mater. 2010, 22, 1039–1059. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Afzaal, M.; O’Brien, K. The synthesis of amine-capped magnetic (Fe, Mn, Co, Ni) oxide nanocrystals and their surface modification for aqueous dispersibility. J. Mater. Chem. 2006, 16, 2175. [Google Scholar] [CrossRef]
- Arriagada, F.; Osseo-Asare, K. Controlled hydrolysis of tetraethoxysilane in a nonionic water-in-oil microemulsion: A statistical model of silica nucleation. Colloids Surfaces A: Physicochem. Eng. Asp. 1999, 154, 311–326. [Google Scholar] [CrossRef]
- Dubertret, B.; Skourides, P.; Norris, D.J.; Noireaux, V.; Brivanlou, A.H.; Libchaber, A. In Vivo Imaging of Quantum Dots Encapsulated in Phospholipid Micelles. Sci. 2002, 298, 1759–1762. [Google Scholar] [CrossRef] [PubMed]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Tan, B.; Xiong, J.; Li, H.; You, J. Simultaneous analysis of current-use pesticides and their transformation products in water using mixture-sorbent solid phase extraction and high-performance liquid chromatography–tandem mass spectrometry. J. Sep. Sci. 2020, 43, 2409–2418. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, Y.; Yan, M.; Wang, Q.; Xu, H.; Wang, X.; Zhou, H.; Hao, Y.; Wang, M.-M. Fabrication of iron oxide@MOF-808 as a sorbent for magnetic solid phase extraction of benzoylurea insecticides in tea beverages and juice samples. J. Chromatogr. A 2020, 1615, 460766. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.; El-Shafie, A.S.; Zaher, N.; El-Azazy, M. Application of Pineapple Leaves as Adsorbents for Removal of Rose Bengal from Wastewater: Process Optimization Operating Face-Centered Central Composite Design (FCCCD). Molecules 2020, 25, 3752. [Google Scholar] [CrossRef]
- El-Azazy, M.; El-Shafie, A.S.; Ashraf, A.; Issa, A.A. Eco-Structured Biosorptive Removal of Basic Fuchsin Using Pistachio Nutshells: A Definitive Screening Design—Based Approach. Appl. Sci. 2019, 9, 4855. [Google Scholar] [CrossRef]
- El-Azazy, M.; El-Shafie, A.S.; El-Shafie, A.S.; Issa, A.A. Bio-Waste Aloe vera Leaves as an Efficient Adsorbent for Titan Yellow from Wastewater: Structuring of a Novel Adsorbent Using Plackett-Burman Factorial Design. Appl. Sci. 2019, 9, 4856. [Google Scholar] [CrossRef]
- Elazazy, M.; El-Shafie, A.S.; Issa, A.A.; Al-Sulaiti, M.; Al-Yafie, J.; Shomar, B.; Al-Saad, K. Potato Peels as an Adsorbent for Heavy Metals from Aqueous Solutions: Eco-Structuring of a Green Adsorbent Operating Plackett–Burman Design. J. Chem. 2019, 2019, 4926240. [Google Scholar] [CrossRef]
- Elazazy, M.; Kalla, R.N.; Issa, A.A.; Al-Sulaiti, M.; El-Shafie, A.S.; Shomar, B.; Al-Saad, K. Pomegranate peels as versatile adsorbents for water purification: Application of Box–Behnken design as a methodological optimization approach. Environ. Prog. Sustain. Energy 2019, 38, 13223. [Google Scholar] [CrossRef]
- Elazazy, M.S. Factorial Design and Machine Learning Strategies: Impacts on Pharmaceutical Analysis. In Spectroscopic Analyses—Developments and Applications; Sharmin, E., Zafar, F., Eds.; IntechOpen: London, UK, 2017. [Google Scholar]
- Elazazy, M.S. Determination of midodrine hydrochloride via Hantzsch condensation reaction: A factorial design based spectrophotometric approach. RSC Adv. 2015, 5, 48474–48483. [Google Scholar] [CrossRef]
- Al-Saad, K.; Elazazy, M.; Issa, A.A.; Al-Yafie, A.; El-Shafie, A.S.; Al-Sulaiti, M.; Shomar, B. Recycling of Date Pits Into a Green Adsorbent for Removal of Heavy Metals: A Fractional Factorial Design-Based Approach. Front. Chem. 2019, 7, 552. [Google Scholar] [CrossRef]
- Elazazy, M.S.; Ganesh, K.; Sivakumar, V.; Huessein, Y.H.A. Interaction of p-synephrine with p-chloranil: Experimental design and multiple response optimization. RSC Adv. 2016, 6, 64967–64976. [Google Scholar] [CrossRef]
- Asgharinezhad, A.A.; Ebrahimzadeh, H.; Mirbabaei, F.; Mollazadeh, N.; Shekari, N. Dispersive micro-solid-phase extraction of benzodiazepines from biological fluids based on polyaniline/magnetic nanoparticles composite. Anal. Chim. Acta 2014, 844, 80–89. [Google Scholar] [CrossRef]
- Abolhasani, J.; Khanmiri, R.H.; Ghorbani-Kalhor, E.; Hassanpour, A.; Asgharinezhad, A.A.; Shekari, N.; Fathi, A. An Fe3O4@SiO2@polypyrrole magnetic nanocomposite for the extraction and preconcentration of Cd(ii) and Ni(ii). Anal. Methods 2015, 7, 313–320. [Google Scholar] [CrossRef]
- Chen, X.; Lam, K.F.; Yeung, K.L. Selective removal of chromium from different aqueous systems using magnetic MCM-41 nanosorbents. Chem. Eng. J. 2011, 172, 728–734. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations Revisited, Rebutted. J. Am. Stat. Assoc. 1982, 77, 209–210. [Google Scholar] [CrossRef]
- Anderson, T.W.; Darling, D.A. A test of goodness of fit. J. Am. Stat. Assoc. 1954, 49, 765–769. [Google Scholar] [CrossRef]
- Hasniyati, M.; Zuhailawati, H.; Sivakumar, R.; Dhindaw, B. Optimization of multiple responses using overlaid contour plot and steepest methods analysis on hydroxyapatite coated magnesium via cold spray deposition. Surf. Coatings Technol. 2015, 280, 250–255. [Google Scholar] [CrossRef]
- Derringer, G.; Suich, R. Simultaneous Optimization of Several Response Variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Brinker, J.C.; Scherer, G.W. Sol-gel Science: The physics and chemistry of sol-gel processing; Academic Press: New York, NY, USA, 1990. [Google Scholar]
- Minitab®18 Statistical Software; Version 18; Software for Statistical Analysis: State College, PA, USA, 2018; Available online: https://www.minitab.com/en-us/.
- Issa, A.A.; Al-Saad, K.A.; Luyt, A.S. Magnetic solid phase extraction for chromatographic separation of carbamates. J. Am. Ceram. Soc. 2017, 101, 2038–2049. [Google Scholar] [CrossRef]
Sample Availability: Not available. |



| Assessed Variables | Variable Code | Level | |
|---|---|---|---|
| Low (−) | High (+) | ||
| Concentration of TEOS ([TEOS], M) | A | 0.01 | 0.50 |
| Concentration of Ammonia (Ammonia, M) | B | 2.0 | 3.2 |
| Dose of Fe3O4 (Dose, mg/25 mL) | C | 10.0 | 50.0 |
| Addition Mode | D | One time | Gradually |
| Responses | Highest magnetic properties Smallest PS (monodispersed) Narrowest PSD (uniform distribution) | ||
| Run# | 24 FFD in Coded Units with Actual Domains * | Details of the Synthetic Procedure for Each Experiment ** | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Block | A, M | B, M | C, mg/25 mL | D | Ammonia (mL) | Water (mL) | Dose of Fe3O4 (mL/25 mL) | Used TEOS (1 M) | Ethanol (mL) | |
| 01 | 1 | 0 (0.255) | 0 (2.6) | 0 (30) | −1 (One time) | 5.4 | 1.04 | 3 | 6.375 | 9.185 |
| 02 | 1 | +1 (0.500) | +1 (3.2) | +1 (50) | −1 (One time) | 6.7 | 0 | 5 | 12.5 | 0.8 |
| 03 | 1 | +1 (0.500) | −1 (2.0) | −1 (10) | −1 (One time) | 4.2 | 2 | 1 | 12.5 | 5.3 |
| 04 | 1 | −1 (0.010) | −1 (2.0) | +1 (50) | −1 (One time) | 4.2 | 2 | 5 | 0.25 | 13.55 |
| 05 | 1 | −1 (0.010) | −1 (2.0) | −1 (10) | +1 (Gradually) | 4.2 | 2 | 1 | 0.25 | 17.55 |
| 06 | 1 | 0 (0.255) | 0 (2.6) | 0 (30) | +1 (Gradually) | 5.4 | 1.04 | 3 | 6.375 | 9.185 |
| 07 | 1 | +1 (0.500) | +1 (3.2) | −1 (10) | +1 (Gradually) | 6.7 | 0 | 1 | 12.5 | 4.8 |
| 08 | 1 | −1 (0.010) | +1 (3.2) | +1 (50) | +1 (Gradually) | 6.7 | 0 | 5 | 0.25 | 13.05 |
| 09 | 1 | −1 (0.010) | +1 (3.2) | −1 (10) | −1(One time) | 6.7 | 0 | 1 | 0.25 | 17.05 |
| 10 | 1 | +1 (0.500) | −1 (2.0) | +1 (50) | +1 (Gradually) | 4.2 | 2 | 5 | 12.5 | 1.3 |
| 11 | 2 | −1 (0.010) | +1 (3.2) | −1 (10) | +1 (Gradually) | 6.7 | 0 | 1 | 0.25 | 17.05 |
| 12 | 2 | +1 (0.500) | −1 (2.0) | +1 (50) | −1 (One time) | 4.2 | 2 | 5 | 12.5 | 1.3 |
| 13 | 2 | +1 (0.500) | −1 (2.0) | −1 (10) | +1 (Gradually) | 4.2 | 2 | 1 | 12.5 | 5.3 |
| 14 | 2 | 0 (0.255) | 0 (2.6) | 0 (30) | +1 (Gradually) | 5.4 | 1.04 | 3 | 6.375 | 9.185 |
| 15 | 2 | −1 (0.010) | −1 (2.0) | +1 (50) | +1 (Gradually) | 4.2 | 2 | 5 | 0.25 | 13.55 |
| 16 | 2 | +1 (0.500) | +1 (3.2) | +1 (50) | +1 (Gradually) | 6.7 | 0 | 5 | 12.5 | 0.8 |
| 17 | 2 | −1 (0.010) | +1 (3.2) | +1 (50) | −1 (One time) | 6.7 | 0 | 5 | 0.25 | 13.05 |
| 18 | 2 | 0 (0.255) | 0 (2.6) | 0 (30) | −1 (One time) | 5.4 | 1.04 | 3 | 6.375 | 9.185 |
| 19 | 2 | −1 (0.010) | −1 (2.0) | −1 (10) | −1 (One time) | 4.2 | 2 | 1 | 0.25 | 17.55 |
| 20 | 2 | +1 (0.500) | +1 (3.2) | −1 (10) | −1 (One time) | 6.7 | 0 | 1 | 12.5 | 4.8 |
| Response | Transformation | R2 | Adjusted R2 | Predicted R2 |
|---|---|---|---|---|
| Magnetic Properties | Box–Cox transformation (λ = 0); Forward selection of terms (α to enter = 0.25). | 95.15% | 92.91% | 88.15% |
| PS | Box–Cox transformation (λ = 0); Backward elimination of terms (α to remove = 0.10) | 97.08% | 94.46% | 88.00% |
| PSD | Box–Cox transformation (λ = 0); Backward elimination of terms (α to remove = 0.10) | 97.22% | 95.59% | 91.58% |
| Adsorbents | TEOS (mL) | Ammonia (mL) | Fe3O4 (mg/mL) | Water (mL) | Addition Mode |
|---|---|---|---|---|---|
| TMS-mNP55 | 0.6 | 66.70 | 50 mL (10 mg/mL) | 0 mL | One time |
| TMS-mNP100 | 0.6 | 41.66 | 20 mL | One time |
| Carbamate Pesticides (Stock Solution 40 ppm) | Rt (min.) | Control | Extracted (ppm) | ||
|---|---|---|---|---|---|
| ppm | % | TMS-mNP55 | TMS-mNP100 | ||
| Aldicarb | 9.091 | 35.90 | 89.75 | 0 | 0 |
| Dioxacarb | 14.08 | 19.77 | 49.42 | 0.182 | 0.263 |
| Methiocarb sulfoxide | 15.73 | 28.98 | 72.44 | 2.406 | 2.651 |
| Propoxur | 16.52 | 34.80 | 86.99 | 0.604 | 0.899 |
| Promecarb | 17.42 | 34.82 | 87.06 | 1.706 | 2.587 |
| Carbofuran | 18.01 | 42.27 | 105.7 | 0.631 | 1.165 |
| Dioxacarb | 19.35 | 73.26 | 183.2 | 8.948 | 13.52 |
| 3-Hydroxycarbofuran | 19.63 | 73.94 | 184.8 | 0.181 | 0.296 |
| Methiocarb | 20.14 | 46.78 | 116.9 | 12.28 | 23.61 |
| Rt (min.) | Organophosphorus Pesticide | Stock | Control | % | TMS-mNP55 | TMS-mNP100 |
|---|---|---|---|---|---|---|
| Conc. (ppm) | Conc. (ppm) | ppm | ppm | |||
| 14.29 | Mevinphos | 10 | 4.065 | 40.65 | 0 | 0 |
| 17.32 | Sulfotep | 5 | 3.955 | 79.10 | 0.294 | 0.468 |
| 17.80 | Demeton-S | 20 | 16.96 | 84.79 | 0.072 | 0.152 |
| 18.28 | Dioxothion | 60 | 52.24 | 87.06 | 15.67 | 15.90 |
| 18.70 | Disulfoton | 10 | 7.095 | 70.95 | 0.535 | 0.875 |
| 19.37 | Dichlofenthion | 10 | 7.484 | 74.84 | 0.851 | 1.276 |
| 20.51 | Fenthion | 10 | 8.974 | 89.74 | 0.830 | 1.278 |
| 20.80 | Trichloronat | 10 | 7.739 | 77.39 | 0.857 | 1.153 |
| 21.10 | Clofenvinfos | 5 | 5.318 | 106.4 | 1.520 | 1.851 |
| 21.33 | Crotoxyphos | 20 | 20.99 | 105.0 | 0 | 0.027 |
| 22.22 | Prothiofos | 10 | 9.641 | 96.41 | 0.796 | 1.174 |
| 23.20 | Ethion | 10 | 9.623 | 96.23 | 1.129 | 1.442 |
| 23.61 | Famphur | 20 | 25.98 | 129.9 | 0 | 0 |
| 24.75 | Phosmet | 20 | 21.29 | 106.5 | 0 | 0 |
| 25.48 | Leptophos | 10 | 10.58 | 105.8 | 0.254 | 0.435 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Saad, K.; Issa, A.A.; Idoudi, S.; Shomar, B.; Al-Ghouti, M.A.; Al-Hashimi, N.; El-Azazy, M. Smart Synthesis of Trimethyl Ethoxysilane (TMS) Functionalized Core–Shell Magnetic Nanosorbents Fe3O4@SiO2: Process Optimization and Application for Extraction of Pesticides. Molecules 2020, 25, 4827. https://doi.org/10.3390/molecules25204827
Al-Saad K, Issa AA, Idoudi S, Shomar B, Al-Ghouti MA, Al-Hashimi N, El-Azazy M. Smart Synthesis of Trimethyl Ethoxysilane (TMS) Functionalized Core–Shell Magnetic Nanosorbents Fe3O4@SiO2: Process Optimization and Application for Extraction of Pesticides. Molecules. 2020; 25(20):4827. https://doi.org/10.3390/molecules25204827
Chicago/Turabian StyleAl-Saad, Khalid, Ahmed A. Issa, Sourour Idoudi, Basem Shomar, Mohammad A. Al-Ghouti, Nessreen Al-Hashimi, and Marwa El-Azazy. 2020. "Smart Synthesis of Trimethyl Ethoxysilane (TMS) Functionalized Core–Shell Magnetic Nanosorbents Fe3O4@SiO2: Process Optimization and Application for Extraction of Pesticides" Molecules 25, no. 20: 4827. https://doi.org/10.3390/molecules25204827
APA StyleAl-Saad, K., Issa, A. A., Idoudi, S., Shomar, B., Al-Ghouti, M. A., Al-Hashimi, N., & El-Azazy, M. (2020). Smart Synthesis of Trimethyl Ethoxysilane (TMS) Functionalized Core–Shell Magnetic Nanosorbents Fe3O4@SiO2: Process Optimization and Application for Extraction of Pesticides. Molecules, 25(20), 4827. https://doi.org/10.3390/molecules25204827









