Anti-Influenza Virus Activity and Chemical Components from the Parasitic Plant Cuscuta japonica Choisy on Dimocarpus longans Lour.
Abstract
1. Introduction
2. Results
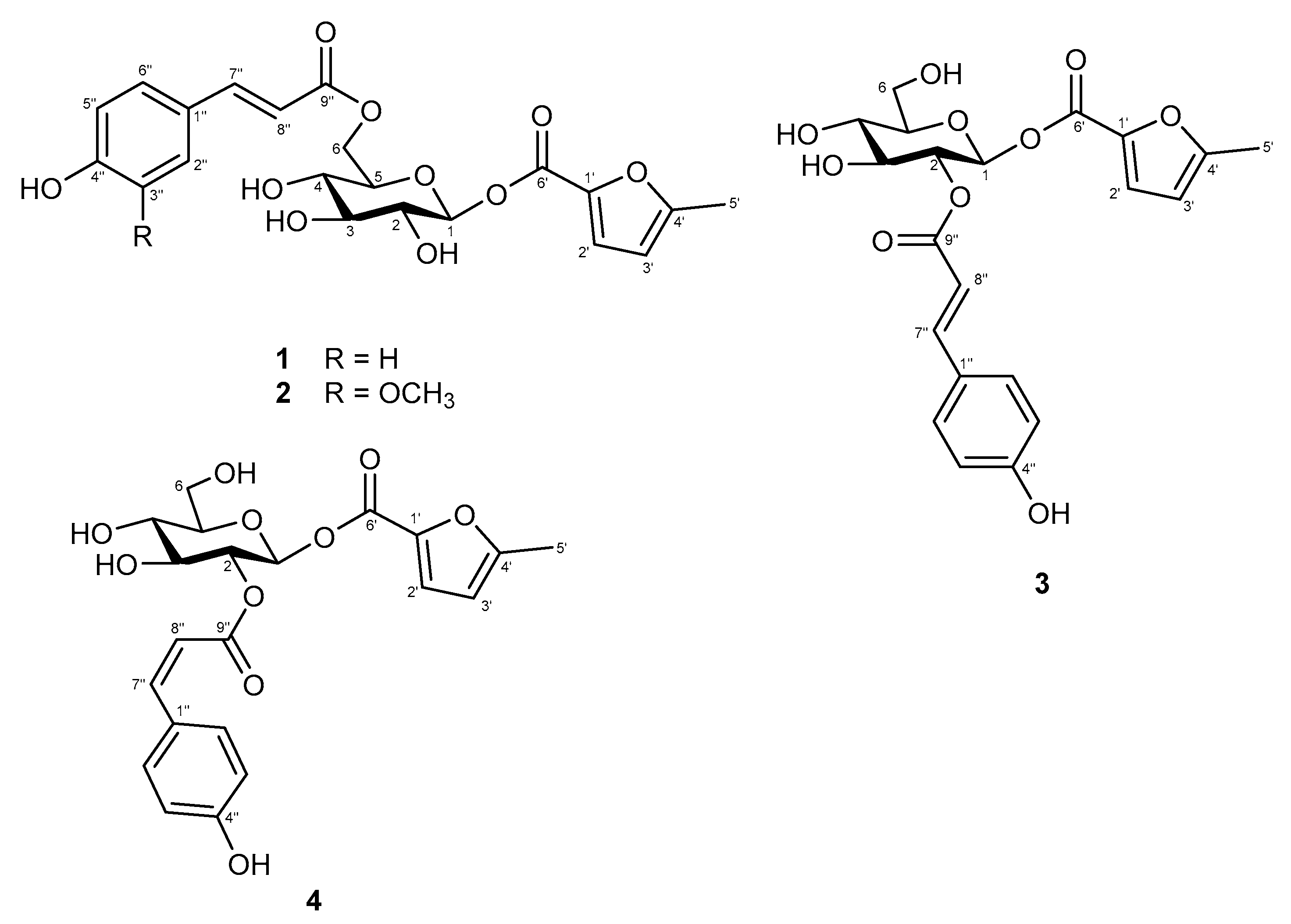
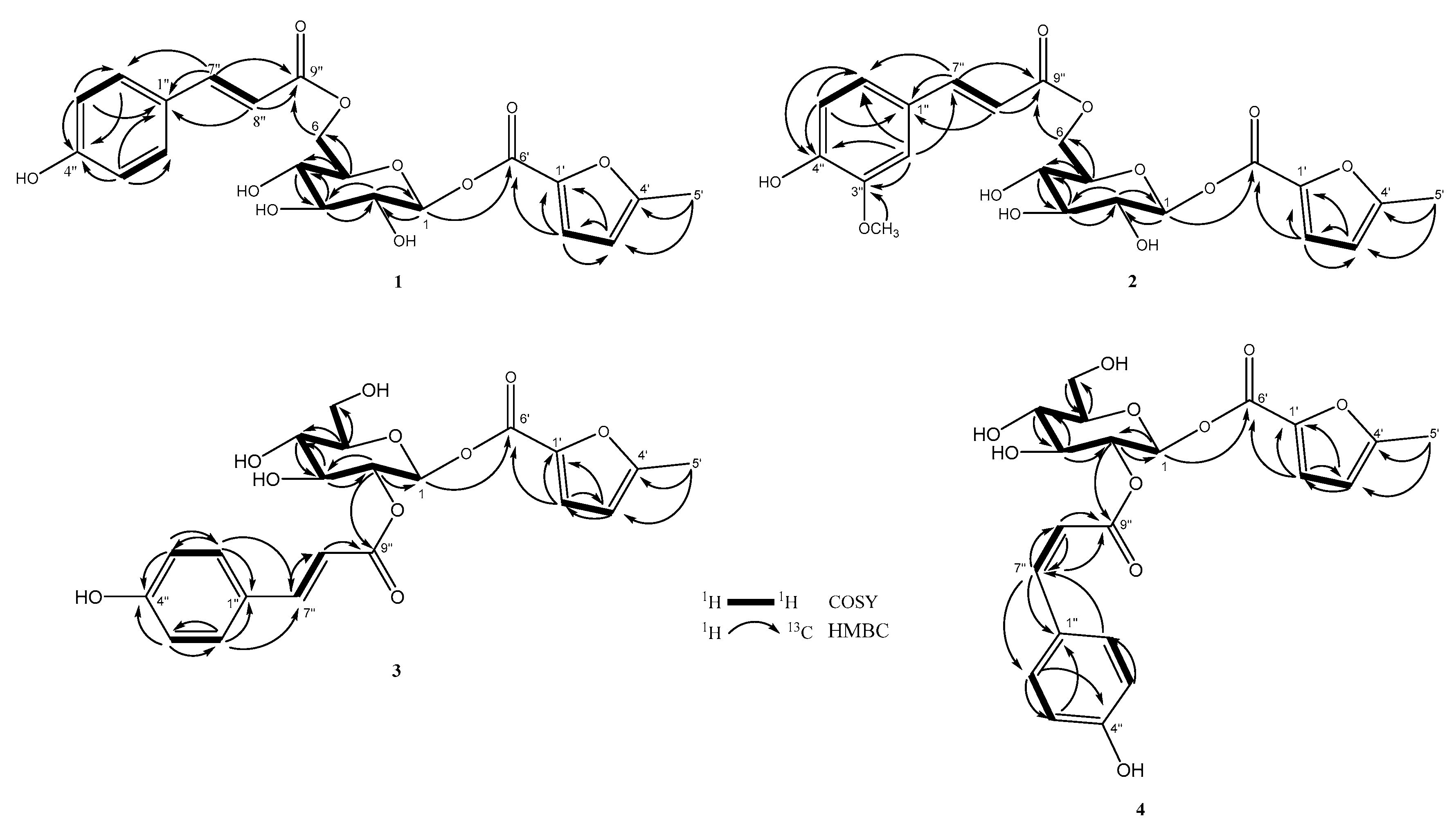
2.1. Structural Elucidation of New Compounds
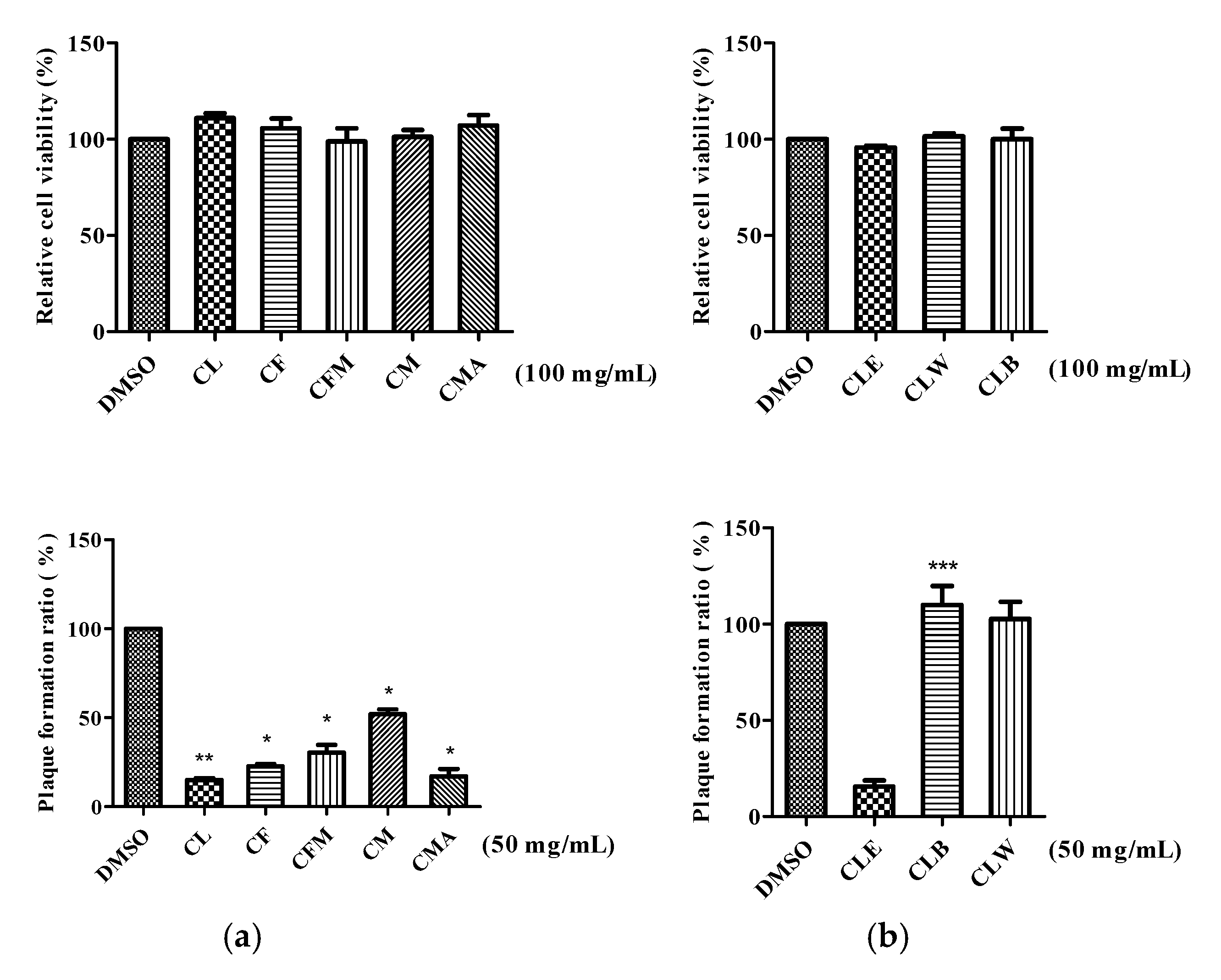
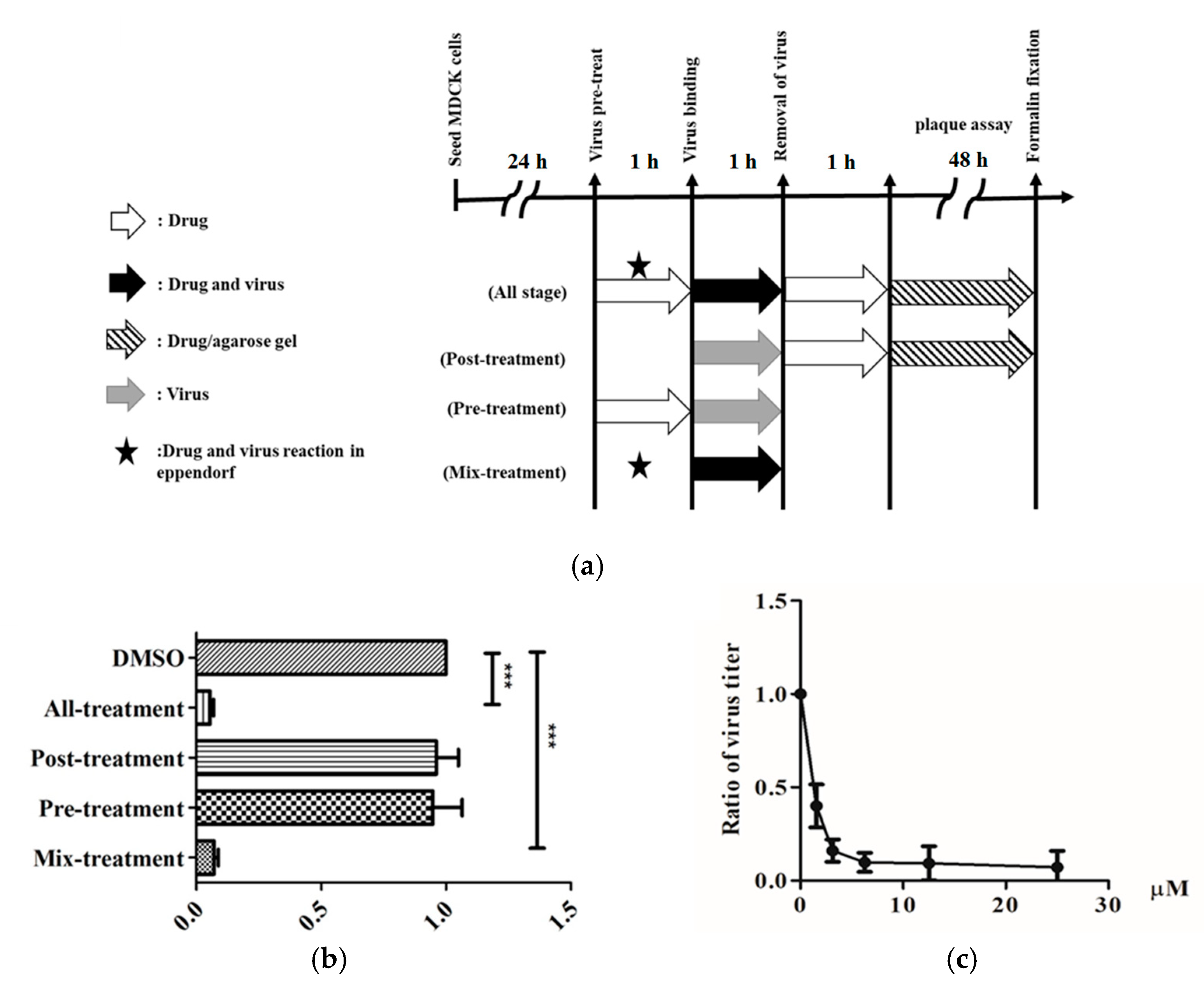
2.2. Antiviral Activity against Influenza A Virus (IVA)
3. Discussion
4. Materials and Methods
4.1. General
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Spectroscopic Data
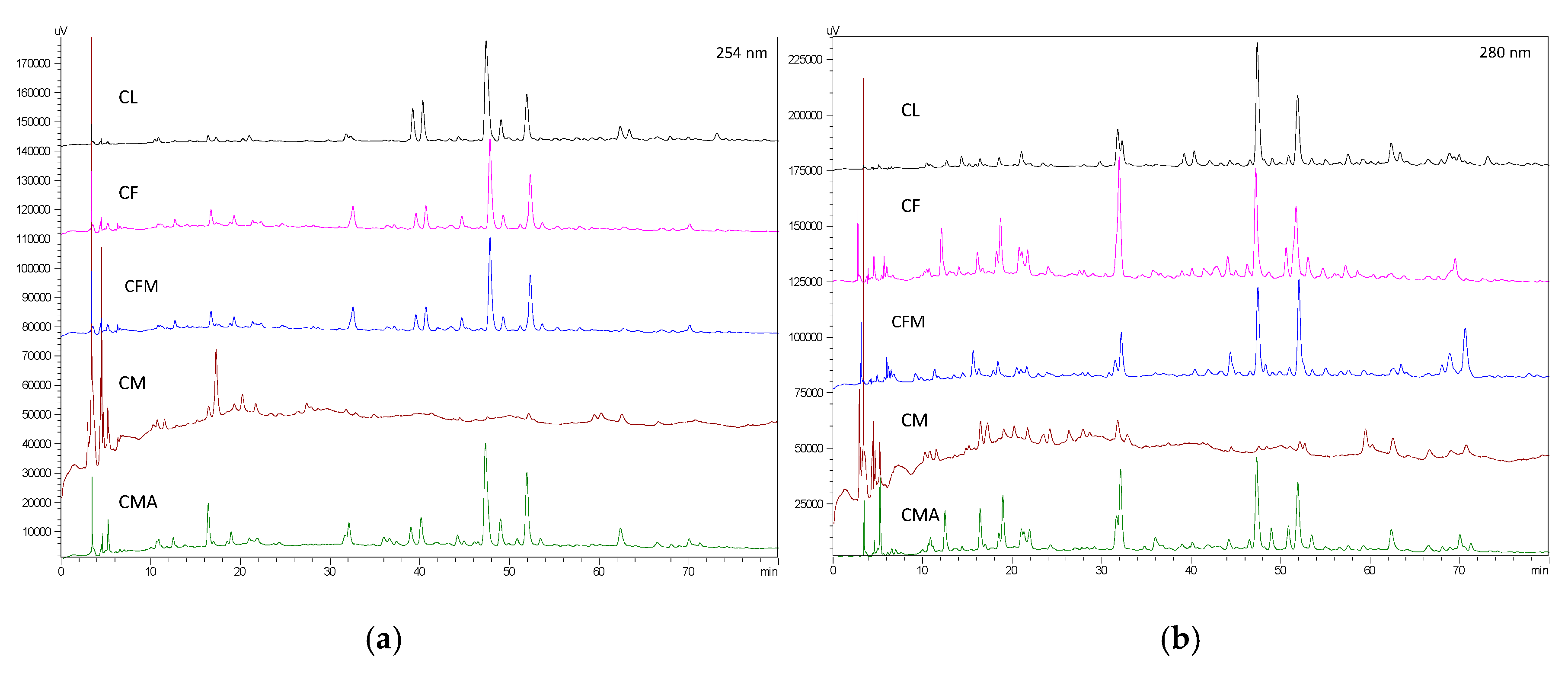
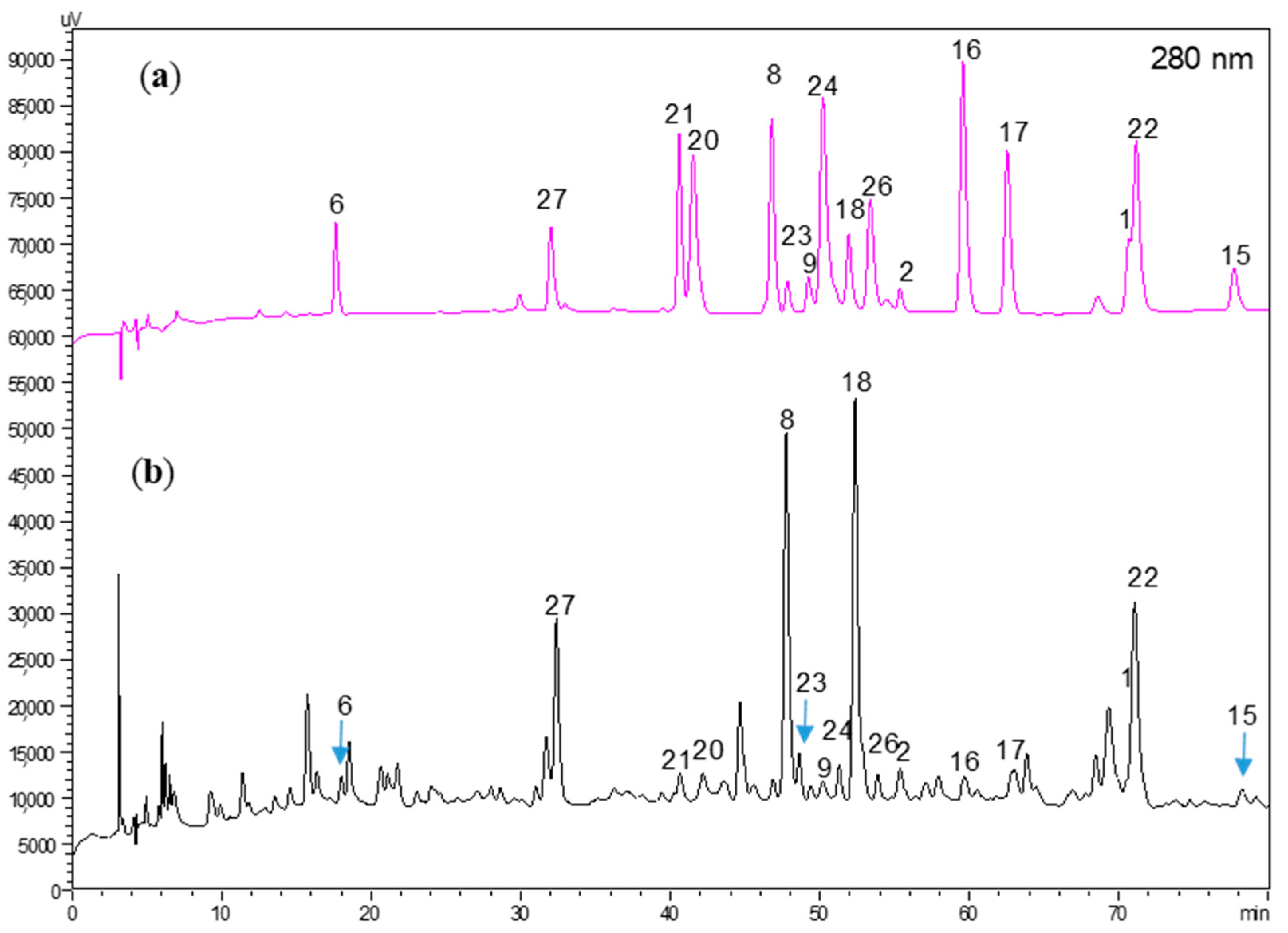
4.5. HPLC-DAD Analysis
4.6. Anti-Inflluenza Virus Assay
4.7. Time-Of-Addition Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, R.; Liu, T.; Liu, M.; Chen, F.; Liu, S.; Yang, J. Anti-influenza A virus activity of dendrobine and its mechanism of action. J. Agric. Food Chem. 2017, 65, 3665–3674. [Google Scholar] [CrossRef]
- Chen, X.; Liu, S.; Goraya, M.U.; Maarouf, M.; Huang, S.; Chen, Y. Host immune response to influenza A virus infection. Front. Immunol. 2018, 9, 320. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Galvin, H.D.; Haw, T.Y.; Nutsford, A.N.; Husain, M. Drug Resistance in Influenza A Virus: The Epidemiology and Management. Infect. Drug Resist. 2017, 10, 121–134. [Google Scholar] [CrossRef]
- Lee, J.Y.; Abundo, M.E.C.; Lee, C.W. Herbal medicines with antiviral activity against the influenza virus, a systematic review. Am. J. Chin. Med. 2018, 46, 1663–1700. [Google Scholar] [CrossRef] [PubMed]
- George, W.; Yang, S.Z. Convolvulaceae. In Flora of Taiwan, 2nd ed.; Editorial Committee of the Flora of Taiwan, Ed.; Department of Botany, National Taiwan University: Taipei, Taiwan, 1998; Volume 4, pp. 344–347. [Google Scholar]
- Lin, M.K.; Lee, M.S.; Chang, W.T.; You, B.J. Extract and Method for Preparing the Same and Uses of Cuscuta sp. TWI382845B, 21 January 2013. [Google Scholar]
- Donnapee, S.; Li, J.; Yang, X.; Ge, A.H.; Donkor, P.O.; Gao, X.; Chang, Y. Cuscuta chinensis Lam.: A systematic review on ethnopharmacology, phytochemistry and pharmacology of an important traditional herbal medicine. J. Ethnopharmacol. 2014, 157, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.C.; Chang, W.T.; Lee, M.S.; Chiu, Y.J.; Chao, W.K.; Lin, Y.C.; Lin, M.K.; Peng, W.H. Antinociceptive and anti-inflammatory activities of Cuscuta chinensis seeds in mice. Am. J. Chin. Med. 2014, 42, 223–242. [Google Scholar] [CrossRef]
- Ye, M.; Yan, Y.; Guo, D.A. Characterization of phenolic compounds in the Chinese herbal drug Tu-Si-Zi by liquid chromatography coupled to electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 1469–1484. [Google Scholar] [CrossRef]
- Du, X.M.; Kohinata, K.; Kawasaki, T.; Guo, Y.T.; Miyahara, K. Components of the ether-insoluble resin glycoside like fraction from Cuscuta chinensis. Phytochem. 1998, 48, 843–850. [Google Scholar] [CrossRef]
- Amarasinghe, N.R.; Jayasinghe, U.; Hara, N.; Fujimoto, Y. Flacourside, a new 4-oxo-2-cyclopentenylmethyl glucoside from the fruit juice of Flacourtia indica. Food Chem. 2007, 102, 95–97. [Google Scholar] [CrossRef]
- Huang, H.C.; Chao, C.L.; Liaw, C.C.; Hwang, S.Y.; Kuo, Y.H.; Chang, T.C.; Chao, C.H.; Chen, C.J.; Kuo, Y.H. Hypoglycemic constituents isolated from Trapa natans L. pericarps. J. Agric. Food Chem. 2016, 64, 3794–3803. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.I.; Naheed, N.; Abbaskhan, A.; Ali, S. Hemiterpene glucosides and other constituents from Spiraea canescens. Phytochemistry 2009, 70, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Matsufuji, H.; Otsuki, T.; Takeda, T.; Chino, M.; Takeda, M. Identification of reaction products of acylated anthocyanins from red radish with peroxyl radicals. J. Agric. Food Chem. 2003, 51, 3157–3161. [Google Scholar] [CrossRef] [PubMed]
- Feistel, F.; Paetz, C.; Lorenz, S.; Beran, F.; Kunert, G.; Schneider, B. Idesia polycarpa (Salicaceae) leaf constituents and their toxic effect on Cerura vinula and Lymantria dispar (Lepidoptera) larvae. Phytochemistry 2017, 143, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mangelinckx, S.; Ma, L.; Wang, Z.; Li, W.; De Kimpe, N. Caffeoylquinic acid derivatives isolated from the aerial parts of Gynura divaricata and their yeast α-glucosidase and PTP1B inhibitory activity. Fitoterapia 2014, 99, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.X.; Chen, Y.J.; Zhao, J.; Song, Q.Y.; Gao, K. Chemical constituents from the aerial parts of Triosteum pinnatifidum. Chem. Nat. Compd. 2013, 49, 95–96. [Google Scholar] [CrossRef]
- Wang, A.X.; Zhang, Q.; Jia, Z.J. Phenylpropanosids, lignans and other constituents from Cremanthodium ellisii. Pharmazie 2005, 36, 889–892. [Google Scholar] [CrossRef]
- Wang, G.K.; Bin Lin, B.; Rao, R.; Zhu, K.; Qin, X.Y.; Xie, G.Y.; Qin, M. A new lignan with anti-HBV activity from the roots of Bombax ceiba. Nat. Prod. Res. 2013, 27, 1348–1352. [Google Scholar] [CrossRef]
- Chen, J.J.; Fang, H.Y.; Duh, C.Y.; Chen, I.S. New indolopyridoquinazoline, benzo[c]phenanthridines and cytotoxic constituents from Zanthoxylum integrifoliolum. Planta Medica 2005, 71, 470–475. [Google Scholar] [CrossRef]
- Achanta, S.; Liautard, V.; Paugh, R.; Organ, M.G. The development of a general strategy for the synthesis of tyramine-based natural products by using continuous flow techniques. Chem. Eur. J. 2010, 16, 12797–12800. [Google Scholar] [CrossRef]
- Hsieh, T.J.; Chang, F.R.; Chia, Y.C.; Chen, C.Y.; Chiu, H.F.; Wu, Y.C. Cytotoxic constituents of the fruits of Cananga odorata. J. Nat. Prod. 2001, 64, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Goda, Y.; Shibuya, M.; Sankawa, U. Inhibitors of the arachidonate cascade from Allium chinense and their effect on in vitro platelet aggregation. Chem. Pharm. Bull. 1987, 35, 2668–2674. [Google Scholar] [CrossRef] [PubMed]
- Munoz, O.; Piovano, M.; Garbarino, J.; Hellwing, V.; Breitmaier, E. Tropane alkaloids from Schizanthus litoralis. Phytochemistry 1996, 43, 709–713. [Google Scholar] [CrossRef]
- Liao, J.; Yuan, C.; Di, Y.; He, H.; Hu, X. A new indole alkaloid from the fruits of Capparis masaikai. Asian J. Chem. 2014, 26, 4504–4506. [Google Scholar] [CrossRef]
- Tian, S.; Yang, Y.; Liu, K.; Xiong, Z.; Xu, L.; Zhao, L. Antimicrobial metabolites from a novel halophilic actinomycete Nocardiopsis terrae YIM 90022. Nat. Prod. Res. 2013, 28, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L.; Zhang, L.J.; Chen, R.Y.; Kuo, L.M.Y.; Huang, J.P.; Huang, H.C.; Lee, K.H.; Wu, Y.C.; Kuo, Y.H. Antioxidant and anti-inflammatory phenylpropanoid derivatives from Calamus quiquesetinervius. J. Nat. Prod. 2010, 73, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.L.; Huang, H.C.; Lin, H.C.; Chang, T.C.; Chang, W.L. Sesquiterpenes from Baizhu stimulate glucose uptake by activating AMPK and PI3K. Am. J. Chin. Med. 2016, 44, 963–979. [Google Scholar] [CrossRef]
- Lin, Y.L.; Wang, W.Y.; Kuo, Y.H.; Chen, C.F. Nonsteroidal constituents from Solanum Incanum L. J. Chin. Chem. Soc. 2000, 47, 247–251. [Google Scholar] [CrossRef]
- Asres, K.; Mascagni, P.; O’Neill, M.J.; Phillipson, D. Isoflavonoids from Bolusanthus speciosus (Bolus) Harms Leguminosae. Zeitschrift für Naturforschung C 1985, 40, 617–620. [Google Scholar] [CrossRef]
- Von Massow, F.; Smith, M.A.R. Indirect 13C–1H coupling in asymmetrically trisubstituted benzenes: A carbon-13 nuclear magnetic resonance study. J. Chem. Soc. Perkin Trans. 2 1976, 2, 977. [Google Scholar] [CrossRef]
- Chung, C.P.; Hsia, S.M.; Lee, M.Y.; Chen, H.J.; Cheng, F.; Chan, L.C.; Kuo, Y.H.; Lin, Y.L.; Chiang, W. Gastroprotective activities of Adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) on the growth of the stomach cancer AGS cell line and indomethacin-induced gastric ulcers. J. Agric. Food Chem. 2011, 59, 6025–6033. [Google Scholar] [CrossRef] [PubMed]
- Fraser, B.H.; Perlmutter, P.; Wijesundera, C. Practical syntheses of triacylglycerol regioisomers containing long-chain polyunsaturated fatty acids. J. Am. Oil Chem. Soc. 2006, 84, 11–21. [Google Scholar] [CrossRef]
- Li, Y.H.; Lai, C.Y.; Su, M.C.; Cheng, J.C.; Chang, Y.S. Antiviral activity of Portulaca oleracea L. against influenza A viruses. J. Ethnopharmacol. 2019, 241, 112013. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1, 8, 10, 11, 16, 17, 22, 26–30 are available from the authors. |

| Position | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| δH, J in Hz | ||||
| 1 | 5.05 (d, 7.5) | 5.05 (d, 7.5) | 5.34 (d, 8.0) | 5.25 (d, 8.0) |
| 2 | 3.46 (dd, 9.0, 7.5) | 3.48 (dd, 8.5, 7.5) | 5.07 (dd, 9.0, 8.0) | 5.03 (dd, 9.0, 8.0) |
| 3 | 3.48 (t, 9.0) | 3.50 (t, 8.5) | 3.71 (t, 9.0) | 3.64 (t, 9.0) |
| 4 | 3.37 (t, 9.0) | 3.39 (t, 8.5) | 3.51 (br d, 9.0) | 3.47 (t, 9.0) |
| 5 | 3.77 (ddd, 9.0, 7.5, 2.0) | 3.78 (ddd, 8.5, 7.5, 2.0) | 3.57 (m) a | 3.53 (m) a |
| 6 | 4.23 (dd, 12.0, 7.5) 4.55 (dd, 12.0, 2.0) | 4.25 (dd, 12.0, 7.5) 4.57 (dd, 12.0, 2.0) | 3.74 (dd, 12.0, 5.5) 3.96 (dd, 12.0, 2.0) | 3.71 (dd, 12.0, 5.0) 3.90 (br d, 12.0) |
| 2′ | 5.72 (d, 1.0) | 5.74 (d, 2.0) | 5.68 (d, 2.0) | 5.66 (br s) |
| 3′ | 6.09 (d, 1.0) | 6.08 (d, 2.0) | 5.98 (br s) | 5.95 (br s) |
| 4′ | - | - | - | - |
| 5′ | 2.20 (s) | 2.15 (s) | 2.16 (s) | 2.18 (s) |
| 2″ | 7.56 (d, 8.5) | 7.24 (d, 2.0) | 7.43 (d, 8.5) | 7.63 (d, 8.5) |
| 3″ | 6.79 (d, 8.5) | - | 6.79 (d, 8.5) | 6.73 (d, 8.5) |
| 4″ | - | - | - | |
| 5″ | 6.79 (d, 8.5) | 6.81 (d, 8.5) | 6.79 (d, 8.5) | 6.73 (d, 8.5) |
| 6″ | 7.56 (d, 8.5) | 7.09 (dd, 8.5, 2.0) | 7.43 (d, 8.5) | 7.73 (d, 8.5) |
| 7″ | 7.60 (d, 16.0) | 7.62 (d, 16.0) | 7.67 (d, 16.0) | 6.90 (d, 12.5) |
| 8″ | 6.37 (d, 16.0) | 6.42 (d, 16.0) | 6.36 (d, 16.0) | 5.80 (d, 12.5) |
| OCH3 | - | 3.92 s | - | - |
| Position | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| δc | ||||
| 1 | 100.9 | 100.8 | 98.9 | 98.9 |
| 2 | 74.5 | 74.4 | 74.4 | 74.4 |
| 3 | 77.8 | 77.8 | 75.9 | 76.0 |
| 4 | 71.7 | 71.7 | 71.1 | 71.2 |
| 5 | 76.4 | 76.1 | 78.9 | 78.9 |
| 6 | 64.7 | 64.7 | 62.2 | 62.2 |
| 1′ | 167.5 | 167.3 | 167.3 | 167.3 |
| 2′ | 92.0 | 92.1 | 91.9 | 91.9 |
| 3′ | 101.6 | 101.6 | 101.4 | 101.4 |
| 4′ | 164.9 | 164.8 | 165.1 | 165.1 |
| 5′ | 19.8 | 19.9 | 19.8 | 19.8 |
| 6′ | 171.4 | 171.3 | 171.0 | 171.5 |
| 1″ | 127.3 | 127.7 | 127.1 | 131.4 |
| 2″ | 131.5 | 111.9 | 131.4 | 133.8 |
| 3″ | 116.9 | 149.6 | 117.0 | 116.2 |
| 4″ | 161.5 | 151.0 | 161.5 | 162.3 |
| 5″ | 116.9 | 116.6 | 117.0 | 116.2 |
| 6″ | 131.5 | 124.6 | 131.4 | 133.8 |
| 7″ | 147.1 | 147.4 | 147.5 | 147.5 |
| 8″ | 114.9 | 115.1 | 114.7 | 115.9 |
| 9″ | 169.1 | 169.1 | 168.2 | 168.2 |
| OCH3 | - | 56.7 | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.-C.; Liaw, C.-C.; Lin, M.-K.; Chen, C.-J.; Chao, C.-L.; Chao, C.-H.; Kuo, Y.-H.; Chiu, Y.-P.; Peng, Y.-S.; Huang, H.-C. Anti-Influenza Virus Activity and Chemical Components from the Parasitic Plant Cuscuta japonica Choisy on Dimocarpus longans Lour. Molecules 2020, 25, 4427. https://doi.org/10.3390/molecules25194427
Cheng J-C, Liaw C-C, Lin M-K, Chen C-J, Chao C-L, Chao C-H, Kuo Y-H, Chiu Y-P, Peng Y-S, Huang H-C. Anti-Influenza Virus Activity and Chemical Components from the Parasitic Plant Cuscuta japonica Choisy on Dimocarpus longans Lour. Molecules. 2020; 25(19):4427. https://doi.org/10.3390/molecules25194427
Chicago/Turabian StyleCheng, Ju-Chien, Chia-Ching Liaw, Ming-Kuem Lin, Chao-Jung Chen, Chien-Liang Chao, Chih-Hua Chao, Yueh-Hsiung Kuo, Yen-Po Chiu, Yu-Shin Peng, and Hui-Chi Huang. 2020. "Anti-Influenza Virus Activity and Chemical Components from the Parasitic Plant Cuscuta japonica Choisy on Dimocarpus longans Lour." Molecules 25, no. 19: 4427. https://doi.org/10.3390/molecules25194427
APA StyleCheng, J.-C., Liaw, C.-C., Lin, M.-K., Chen, C.-J., Chao, C.-L., Chao, C.-H., Kuo, Y.-H., Chiu, Y.-P., Peng, Y.-S., & Huang, H.-C. (2020). Anti-Influenza Virus Activity and Chemical Components from the Parasitic Plant Cuscuta japonica Choisy on Dimocarpus longans Lour. Molecules, 25(19), 4427. https://doi.org/10.3390/molecules25194427






