4. Chemical Characterization
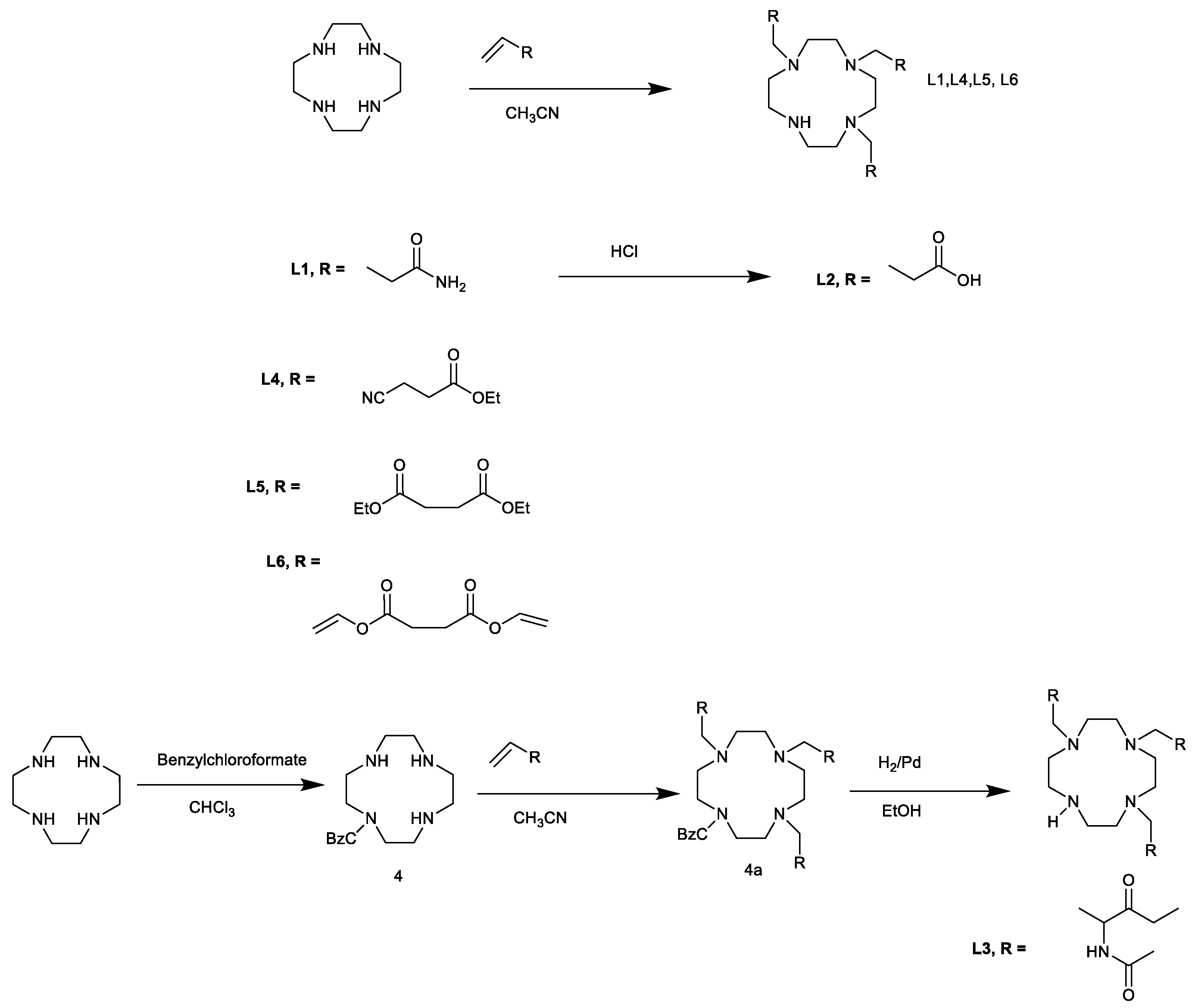
4.1. L1
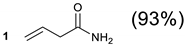
Acrylamide (8.9 g, 100 mmol) and cyclen (4.31 g, 25 mmol) were dissolved in 125 mL methanol. The solution was heated under reflux for 24 h. The methanol was removed under reduced pressure and the residue stirred with Et2O to give a white paste. The Et2O was then decanted and methanol added. After stirring for a few minutes, the product was filtered on a Buchner funnel, washed with ethanol (4 × 10 mL), then Et2O. The product was dried in vacuo at 50 °C overnight to give 3.9 as a white solid. Yield: 9.0 g (93%). m.p. 170–172 °C. Rf = 0.57. 1H-NMR (300 MHz, D2O, δ): 2.29 (6H, t, 3J = 6.7 Hz, NCH2CH2CONH2), 2.42 (4H, br s, ring CH2), 2.55 (12H, br s, ring CH2), 2.62 (6H, t, 3J = 6.8 Hz, NCH2CH2CONH2); 13C-NMR (75 MHz, D2O, δ); 32.36 (CH2CONH2), 45.15 (CH2), 50.01 (CH2), 50.42 (CH2,), 50.59 (CH2), 51.16 (CH2), 51.33 (CH2), 178.62 (C = O), 179.11 (C = O).
Anal. Calcd (found) for C17H35N7O3*1.0 H2O*0.4 C2H5OH: C, 50.7 (50.5); H, 9.4 (9.1); N, 23.3 (23.5). IR (νmax/(cm-1), KBr): 3349 (NH), 3154 (NH), 2969 (CH), 2837, 1673 (C = O), 1424.
HRMS (+ES): found [M + H] + 386.2878. C17H36N7O3 requires 386.2874.
4.2. L2
L1 (0.3 g, 0.73 M) was dissolved in 10 mL HCl (12 M) and refluxed vigorously for 2 h. The reaction was switched off and allowed to cool overnight. The white solid, which developed was filtered off and re-dissolved in H2O. After the H2O was removed by lyophilisation, the white solid was recrystallised from aqueous methanol. The product was then dried in a vacuum oven at 50 °C for 12 h to give 3.10 as a white solid. Yield: 0.34 g (91.5%). m.p. 180–183 °C. 1H-NMR (300 MHz, D2O, δ): 2.63 (6H, t, 3J = 6.6 Hz, CH2CH2COOH), 3.01 (22 H, br s, CH2 NCH2CH2); 13C-NMR (75 MHz, D2O, δ); 28.83 (CH2), 49.07 (CH2), 176.13 (C = O).
Anal. Calcd (found) for C17H32N4O6*1.8 H2O*2.5 HCl: C, 40.3 (40.7); H, 7.5 (7.1); N, 11.1 (10.9).
IR (νmax/(cm−1), KBr): 3076 (OH), 1753 (C = O), 1411, 798, 653.
HRMS (+ES): found [M + H] + 389.2395. C17H33N4O6 requires 389.2395.
4.3. 4
A solution of benzyl chloroformate (2.7 g, 15.9 mmol) in CHCl3 (20 mL, dry) was added slowly over 1.5 h to a cooled solution of cyclen (2.5 g, 14.5 mmol) in chloroform (50 mL). The reaction was allowed to reach ambient temperature and then stirred for 12 h. Some of the organic solvent was removed under reduced pressure and the reaction stirred vigorously after the addition of 5% NaOH (75 mL). The organic layer was separated and the aqueous layer re-extracted with CHCl3 (2 × 25 mL). The organic extracts were combined, dried over MgSO4 and evaporated in vacuo. The resultant oil was purified on a silica gel column with 1% MeOH in CHCl3 containing 0.1% iPrNH2 to yield an oil which after trituration with acetonitrile provided 10 as a white, crystalline solid (2.6 g, 53%). A small amount of di-Z-cyclen eluted from the column first. Rf mono-Z-cyclen = 0.51 (SiO2, 5% MeOH in CHCl3. m.p. 146–152 °C; 1H-NMR (300 MHz, CD3OD, δ); 3.13 (16H, m, ring CH2), 5.15 (2H, s, OCH2), 7.36 (5H, m, Ph); 13C-NMR (75 MHz, CD3OD, δ); 47.87 (ring CH2), 48.44 (ring CH2), 49.58 (ring CH2), 50.51 (ring CH2), 68.56 (OCH2Ph), 128.89 (3-Ph), 128.93 (4-Ph), 129.08 (2-Ph), 129.37 (1-Ph), 157.52 (C = O): IR (νmax/(cm−1), KBr): 3391 (NH), 3183 (NH), 2959 (CH), 2837, 2352, 1673 (C = O), 1455, 1012, 776, 728 (arom), 558, 456.
4.4. 4a
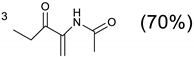
The 10 (0.25 g, 0.82 mmol) was dissolved in a 3:1 mixture of CH3Cl and CH3CN (20 mL). Methyl 2-acetamidoacrylate (0.37 g, 2.61 mmol) was then added. Triflic acid (50 µL, 0.7 eq.) was promptly added with an automatic pipette. This resulted in a clear solution, which was then stirred under argon for 24 h. After the solvent was removed in vacuo, the residue was partitioned between 1 M ammonia solution (10 mL) and chloroform (20 mL). After the aqueous layer was washed with another 10 mL chloroform, the organic extracts were pooled and evaporated to yield a viscous, pale yellow oil. The resulting oil was dissolved in a small amount of CHCl3 and Et2O was added to induce crystallisation. A colourless, crystalline solid was obtained. Yield: 0.42 g (70.4%). Rf = 0.62 (SiO2, 3% iPrNH2 in CH2Cl2); m.p. 209 °C (with decomposition); 1H-NMR (300 MHz, CDCl3, δ): 1.98 (6H, s, CH3), 2.06 (3H, s, CH3), 2.72–3.25 (20H, m, ring CH2), 3.69 (9H, s, CH3), 3.78 (2H, s br, NCH2CO), 4.55 (m, 3H, CH), 5.80 (2H, s, OCH2Ph), 7.24 (5H, m, Ph), 7.68 (br s, 3H, NH); 13C-NMR (75 MHz, CDCl3, δ); 22.86 (CH3), 23.24 (CH3), 47.71 (CH2), 48.65 (CH), 52.49 (CH3), 68.56 (OCH2Ph), 127.98 (Ph), 128.69 (Ph), 130.95 (Ph), 170.19 (C = O), 172.19 (C = O).
Anal. Calcd (found) for C34H53N7O11: C, 55.50 (55.10); H, 7.26 (7.61); N, 13.32 (13.06). HRMS (+ES): found [M + H] + 736.3871. C34H53N7O11 requires 736.3876.
4.5. L3
The 10a (0.40 g, 0.54 mM) was stirred in a suspension of 10% palladium on carbon (50 mg) in ethanol (20 mL). A slow flowrate of H2 was bubbled through the suspension in a fume cupboard. After 12 h, the reaction mixture was centrifuged to remove the catalyst. The supernatant was filtered through a syringe filter (0.2 µM, polypropylene) and the solvent removed under reduced pressure to give a white solid: [M + H]+ = 603). This solid was then heated in 12 M HCl (20 mL) at 60 °C for 3 h. After the acid was removed under reduced pressure, the residue was dissolved in EtOH (20 mL) and treated with activated carbon (0.25 g). The suspension was filtered through a bed of celite on a Buchner funnel and the filtrate was evaporated under reduced pressure. The resulting residue was recrystallised from aqueous EtOH to give 3.31 as a hygroscopic, white solid. Yield: 0.204g (86%). 1H-NMR (300 MHz, D2O, δ): 1.84 (4H, s, CH2), 2.57–3.10 (18H, m, ring CH2), 4.60–4.70 (9H, br s, 3NH3+); 13C-NMR (75 MHz, D2O, δ); 47.2, 48.6 (CH2), 176.61 (C = O).
MS (ES+): 434.
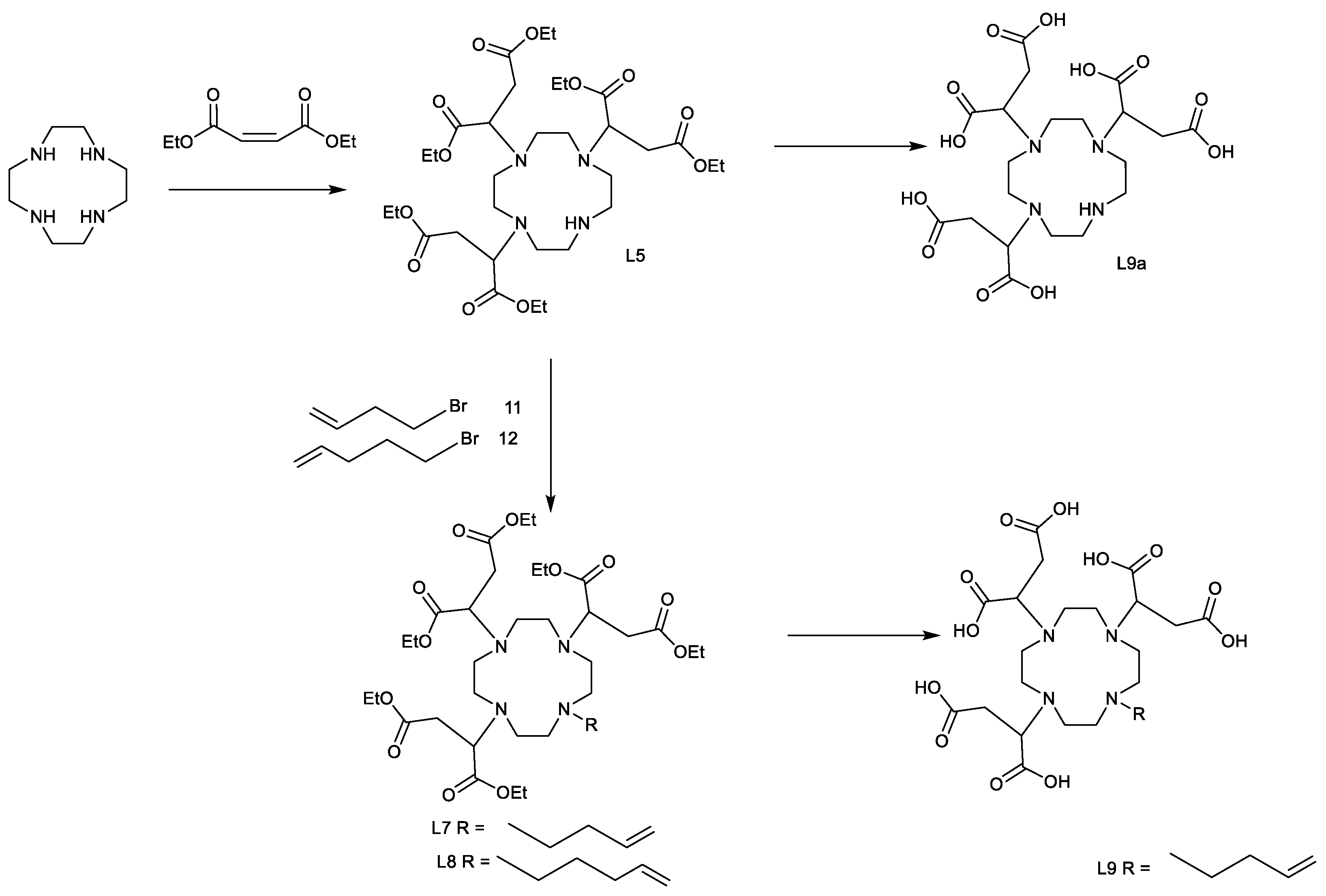
4.6. L5
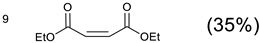
The 1,4,7,10-tetraazacyclododecane (3 g, 0.01734 mol) and potassium carbonate (7.8 g, 0.05652 mol) were dissolved in anhydrous acetonitrile (100 mL). To this a solution of diethyl maleate (126) (42 g, 0.2439 mol) in anhydrous acetonitrile (100 mL) was added dropwise over 2 h. The reaction was heated to 80 °C and stirred for 72 h. The reaction was cooled to room temperature and the solids were removed. The solvent was removed using reduced pressure to yield a crude oil. The crude extract was chromatographed on silica (Si–60) (350 g), and the product was eluted with DCM/EtOH (95:5).
Yield = 0.25 g, 35.58%.
1H-NMR (300 MHz, CDCl3, δ): 1.21 (18H, t, 3J = 5.01 Hz), 1.80–2.41 (22H, m CH2), 3.54 (1H, q, 3J = 5.35 Hz CH), 3.77 (4H, q, 3J = 7.03 Hz CH2), 4.08 (12H, q, 3J = 7.01 CH2).
13C-NMR (75 MHz, CDCl3, δ): 14.18 (CH3), 35.58 (CH2), 46.46 (CH2), 47.44 (CH2), 50.18 (CH2), 50.40 (CH2), 59.58 (C = O), 60.52 (CH2), 170.93 (C = O), 171.46 (C = O).
FTIR (thin-film, KBr disk): 2983, 1736, 1464, 1368, 1302, 1256, 1170.
HRMS: Theoretical C32H56N4O12 688.3945 Found 711.3792 [M + Na + H].
CHN Theoretical C 55.80 H 8.19 N 8.13 Found C 55.23 H 8.35 N 8.27.
4.7. L6
Cyclen (390 mg, 2.27 mmol) was dissolved in dry MeCN (35 mL), to which anhydrous potassium carbonate (K2CO3) (1040 mg, 7.51 mmol, 3.3 eq.) was added, heated to 80 °C under an argon atmosphere, and stirred. Added to this mixture, over the course of 1 h, was a solution of diallyl maleate (2.39 mL, 2230 mg, 11.35 mmol, 5 eq.) in dry MeCN (35 mL). The reaction mixture was heated to reflux at 80 °C for 50 h under an argon atmosphere. The organic phase was separated using silica gel chromatography and eluted with a gradient of 1% to 5% EtOH in DCM eluent.
Yield 580mg 33%.
Rf = 0.80.
1H-NMR (300 MHz, CDCl3, δ): 2.57 (2H, m, CH2), 2.73 (2H, m, CH2), 2.89 (2H, m, CH2), 3.09 (2H, m, CH2), 3.84 (1H, m, CH), 4.03 (2H, m, CH2) 4.59 (2H, m, CH2), 5.19 (2H, m, CH2) 5.22 (2H, m, CH2), 5.30 (1H, m, CH) 5.34 (1H, m, CH), 5.86 (1H, m, CH).
13C-NMR (75 MHz, CDCl3): 34.84 (CH2), 47.36 (CH), 47.89 (CH), 50.09 (CH2), 51.80 (CH2), 57.12 (CH2), 61.65 (CH2), 65.79 (CH2), 66.01 (CH2), 66.16 (CH2), 118.80 (CH2), 119.45 (CH2), 119.74 (CH2), 131.53 (CH), 131.77 (CH), 170.72 (C = O), 170.80 (C = O), 170.86 (C = O).
HRMS theoretical C33H58N4O12 714.1965 found 714.3542.
CHN Theoretical C 56.12 H 8.20 N 8.11 Found C 57.07 H 8.35 N 8.14.
4.8. L7
L5 (0.64 g, 0.00093 mol) was dissolved in anhydrous acetonitrile (20 mL) to which potassium carbonate (1.00 g, 0.0072 mol) had been added. The reaction was cooled in an ice/NaCl/IMS bath. To this 4-bromobutene (78) (2.66 g, 0.0197 mol, 2.00 mL) was added and the reaction was allowed to warm up to room temperature overnight. The reaction was then stirred for 78 h at room temperature. The solids within the reaction mixture were removed using a centrifuge, and the solvent was decanted. The solvent was then removed using reduced pressure, followed by 24 h on high vacuum to yield a clear, light-yellow oil. The crude product was then chromatographed on silica eluted with DCM and EtOH (95:5%).
Yield 0.54 g, 0.00072, 77.92%.
1H-NMR (300 MHz, CDCl3, δ): 1.18 (18H, q, 3J = 5.7 Hz CH3), 2.09 (2H, d, 3J = 6.98 Hz CH2), 2.3–2.8 (24H, m, CH2), 3.75 (1H, t, 3J = 6.0 Hz CH), 4.11 (12H, m CH2), 4.93 (2H, q, 3J = 17.4 Hz CH), 5.71 (1H, t–t, 3J = 6.75 Hz CH).
13C-NMR (75 MHz, CDCl3): 14.12 (CH3), 14.38 (CH3), 29.64 (CH2), 35.52 (CH2), 49.87 (CH2), 50.60 (CH2), 51.18 (CH2), 53.55 (CH2), 53.92 (CH2), 54.66 (CH2), 59.57 (CH), 59.84 (CH), 59.92 (CH), 60.44 (CH2), 60.84 (CH2), 115.31 (CH2), 137.09 (CH), 171.39 (C = O), 171.89 (C = O).
HRMS: calculated 743.4437 [M + H], found 743.4445 [M + H].
FTIR (thin-film CDCl3 NaCl): 2982, 2253, 1723, 1461, 1371, 1300, 1260.
4.9. L8
sDO3A-(ethyl) (4.06 g, 0.0059 mol) was dissolved in anhydrous acetonitrile (40 mL) to which potassium carbonate (4.1 g, 0.03 mol) was added. The reaction was cooled to −18 °C, then 5-bromopent-1-ene (134) (2.65 g, 0.0177 mol) was added, followed by stirring for a period of 48 h while being kept at room temperature. The solid was removed using a centrifuge, and the solvent layer was decanted. The solvent was then removed using reduced pressure to yield a dark-yellow oil. The crude product was then chromatographed on silica eluted with DCM and EtOH (95:5%).
Yield, 2.77 g, 62%.
1H-NMR (300 MHz, CDCl3, δ):1.18 (18H, q, 3J = 5.6 Hz CH3), 2.10 (2H, d, 3J = 6.70 Hz CH2), 2.3-28 (26H, m, CH2), 3.76 (1H, t, 3J = 6.0 Hz CH), 4.11 (12H, m, CH2), 4.94 (2H, q, 17.5Hz, CH), 5.72 (1H, t–t, 3J = 6.75 Hz, CH).
13C-NMR (75 MHz, CDCl3): 14.12 (CH3), 14.40 (CH3), 29.71 (CH2), 30.21 (CH2), 35.50 (CH2), 49.90 (CH2), 50.58 (CH2), 51.19 (CH2), 53.55 (CH2), 53.92 (CH2), 54.67 (CH2), 59.60 (CH), 59.82 (CH), 59.90 (CH), 60.46 (CH2), 60.82 (CH2), 115.30 (CH2), 137.11 (CH), 171.41 (C = O), 171.91 (C = O).
FTIR (thin-film NaCl): 2981, 2940, 2832, 1728. 1448, 1372, 1297, 1258, 1175, 1117.
HRMS: predicted 757.4593 [M + H], found 757.4590 [M + H].
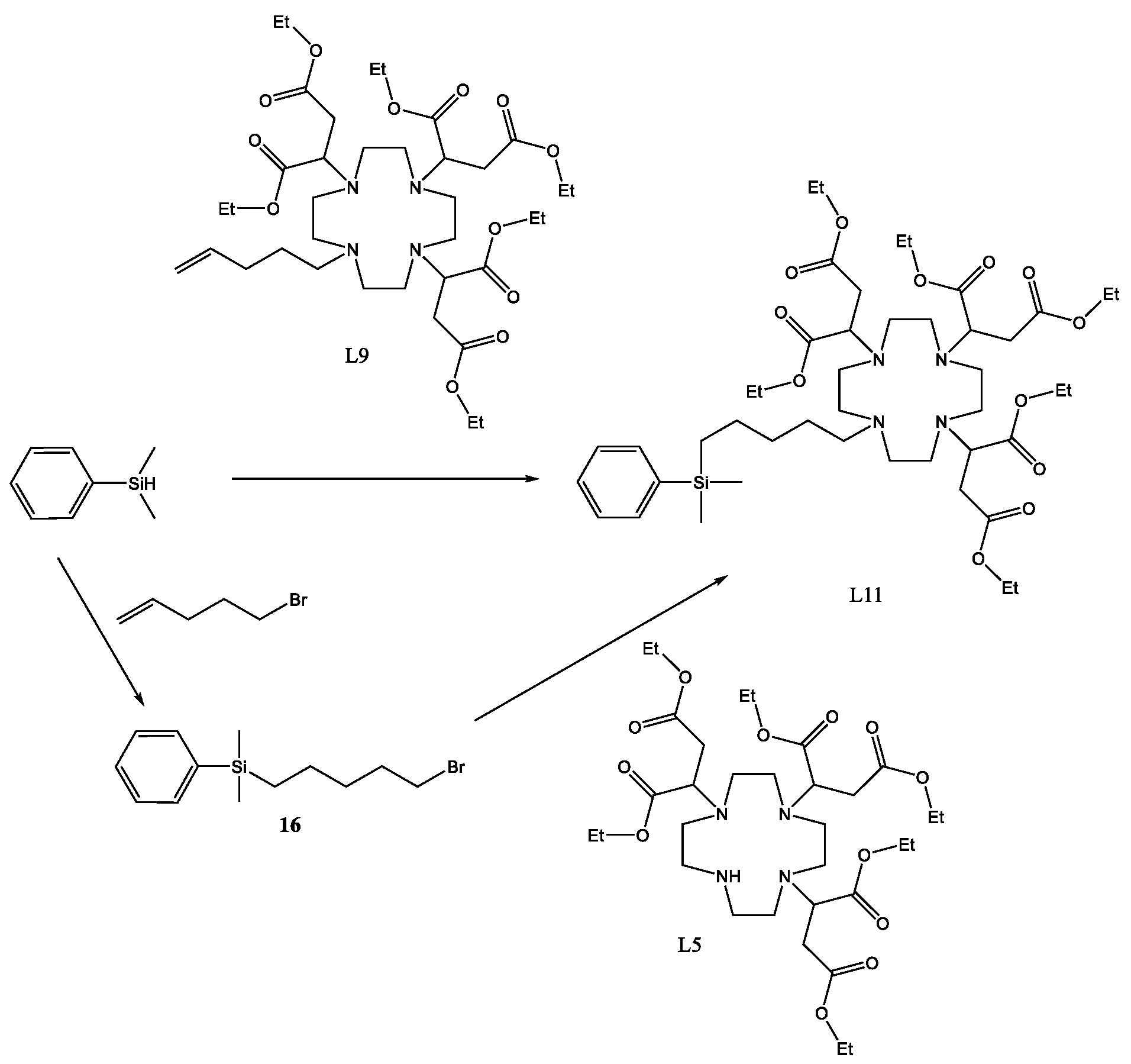
4.10. L9
L7 (0.25 g, 0.0003355 mol) was dissolved in formic acid (1.4 M, 15 mL), and heated to 60 °C for 48 h. The temperature was increased to 70 °C for 24 h. The solvent was removed using lyophilisation; the solid residue was taken up into water (20 mL) and lyophilised.
Yield 0.21 g, 91%.
1H-NMR (300 MHz, CDCl3, δ):1.26 (12H, t, 3J = 6.01 Hz CH3), 2.05 (2H, q, 3J = 8.61 Hz CH2), 2.54–2.99 (24H, m, CH2), 3.77 (1H, q, 3J = 3.87 Hz CH), 4.12 (1H, 3J = 4.98 Hz CH), 5.02 (2H, q, 3J = 8.43 Hz, CH), 5.66 (1H, q, 3J = 10.53 Hz, CH).
13C-NMR (75 MHz, CDCl3): 14.09 (CH3), 35.33 (CH2), 44.82 (CH2), 48.11 (CH2), 49.89 (CH2), 61.10 (CH2), 63.16 (CH2), 116.87 (CH2), 134.85 (CH), 170.79 (C = O), 170.81 (C = 0), 170.92 (C = O).
LCMS(postive): 687.81 [M + H] [C32H54N4O12]−.
FTIR (CDCl3–NaCl): 2983, 2858, 2254, 1729, 1609, 1465, 1372, 1299, 1262, 1180.
4.11. L9a
L5 (0.25 g, 0.00036 mol) was dissolved in ethanol (1 mL), and was added to a solution of formic acid (2.5 mL, 3.05 g, 0.06587 mol) and water (2 mL). The reaction was heated to 90 °C for 24 h, and then cooled to room temperature. The solvents and formic acid were removed using lyophilisation. The lyophilisation was repeated three times by dissolving the crude material in water (10 mL).
1H-NMR (300 MHz, D2O, δ): 1.31 (4H, m, CH2), 2.16–2.35 (15H, m, CH2), 2.52 (6H,m, CH2), 2.54 (1H, m, CH), 3.42 (2H, m, CH) 4.15 (1H, m, CH).
13C-NMR (CD3OD): 13.960 (CH3), 43.235 (CH2), 58.122 (CH2), 134.816 (CH), 169.232 (C = O), 175.253 (C = O), 175.944 (C = O), 176.478 (C = O).
HRMS Theoretical C37H64N4O12 756.4582 Found 779.2096 [M + Na].
CHN Theoretical C 58.71 H 8.52 N 7.40 C 58.55 H 8.43 N 7.34.
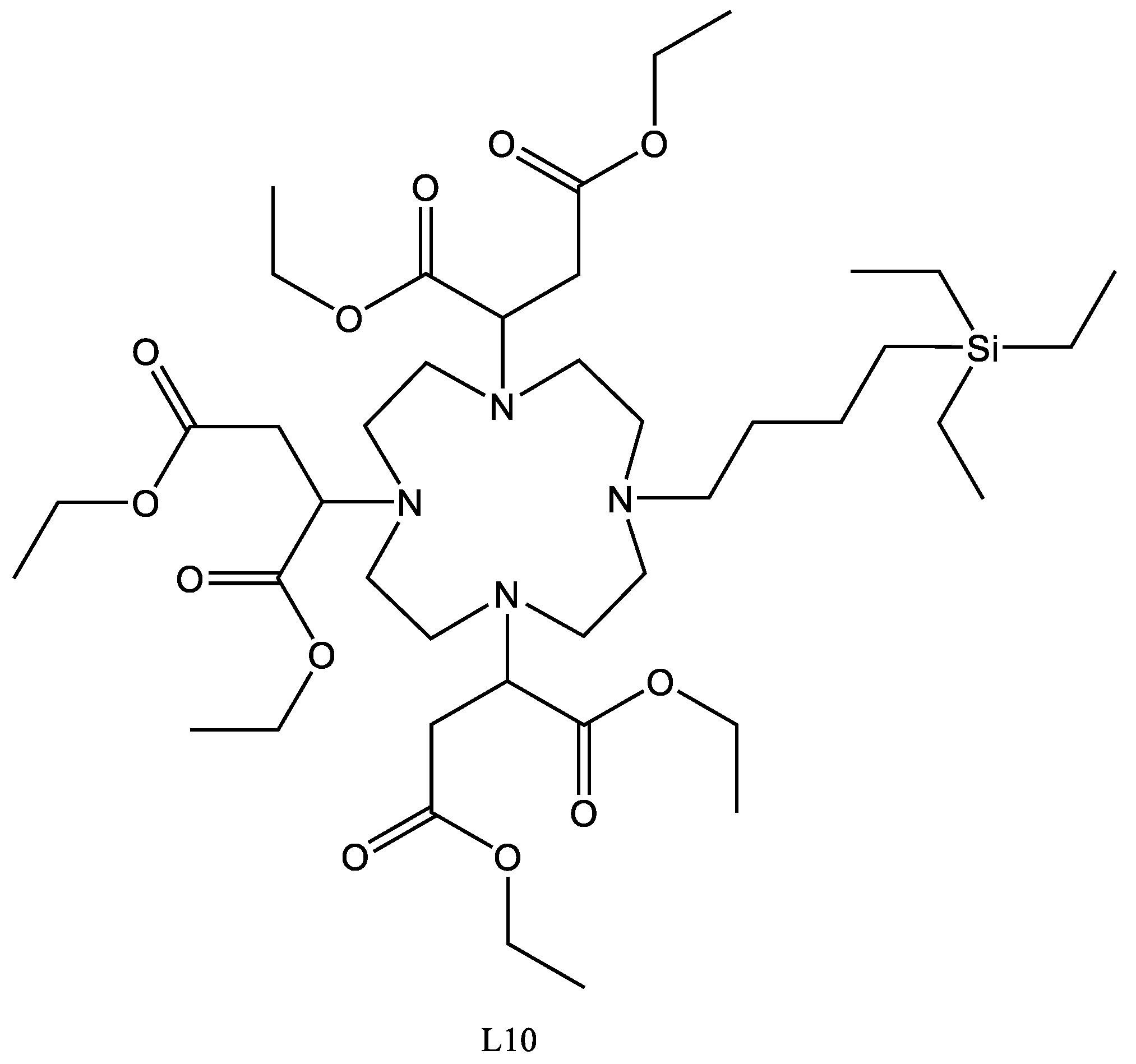
4.12. L10
L5 (250 mg, 0.000336 mol) was dissolved in toluene (1 mL), to which triethylsilane (58 mg, 0.0005 mol, 0.08 mL) was added. To the stirred reaction mixture, Speier’s catalyst (20 µL) was added and the reaction was heated to 80 °C for 24 h. The reaction was cooled and passed through an activated charcoal plug. The solvent was then removed using reduced pressure, yielding a clear gum.
Yield 126mg 40%.
1H-NMR (300 MHz, CDCl3, δ):0.53 (6H, q, 3J = 7.86 Hz, CH3), 0.98 (9H, t, 3J = 5.54 Hz CH3), 1.09 (2H, d, 3J = 6.03 Hz CH2), 1.21 (9H, t, 3J = 7.14 Hz), 1.67 (1H, s, CH), 2.28–2.83 (22H, m CH2), 3.73 (2H, m CH), 4.09 (2H, q, 3J = 4.57 Hz).
13C-NMR (75 MHz, CDCl3): 0.93 (CH3), 5.75 (CH2), 6.51 (CH2), 14.02 (CH3), 14.35 (CH3), 21.35 (CH2), 22.57 (CH2), 26.59 (CH2), 29.13(CH2), 29.78 (CH2), 35.22 (CH2), 35.40 (CH2), 49.98 (CH2), 50.65 (CH2), 51.05 (CH2), 51.19 (CH2), 53.68 (CH2), 53.98 (CH2), 55.40 (CH2), 59.59 (CH), 60.43 (CH3), 171.39 (C = O), 171.44 (C = O), 171.86 (C = O).
29Si NMR (CDCl3): 19.57, −21.49
FTIR (thin-film NaCl): 3027, 2920, 2874, 1734, 1604, 1495, 1461, 1379, 1177.
HRMS Theoretical C42H78N4O12Si 858.5482 Found 858.5865.
CHN Theoretical C 58.71 H 9.15 N 6.52 Found C 59.22 H 9.54 N 6.76.
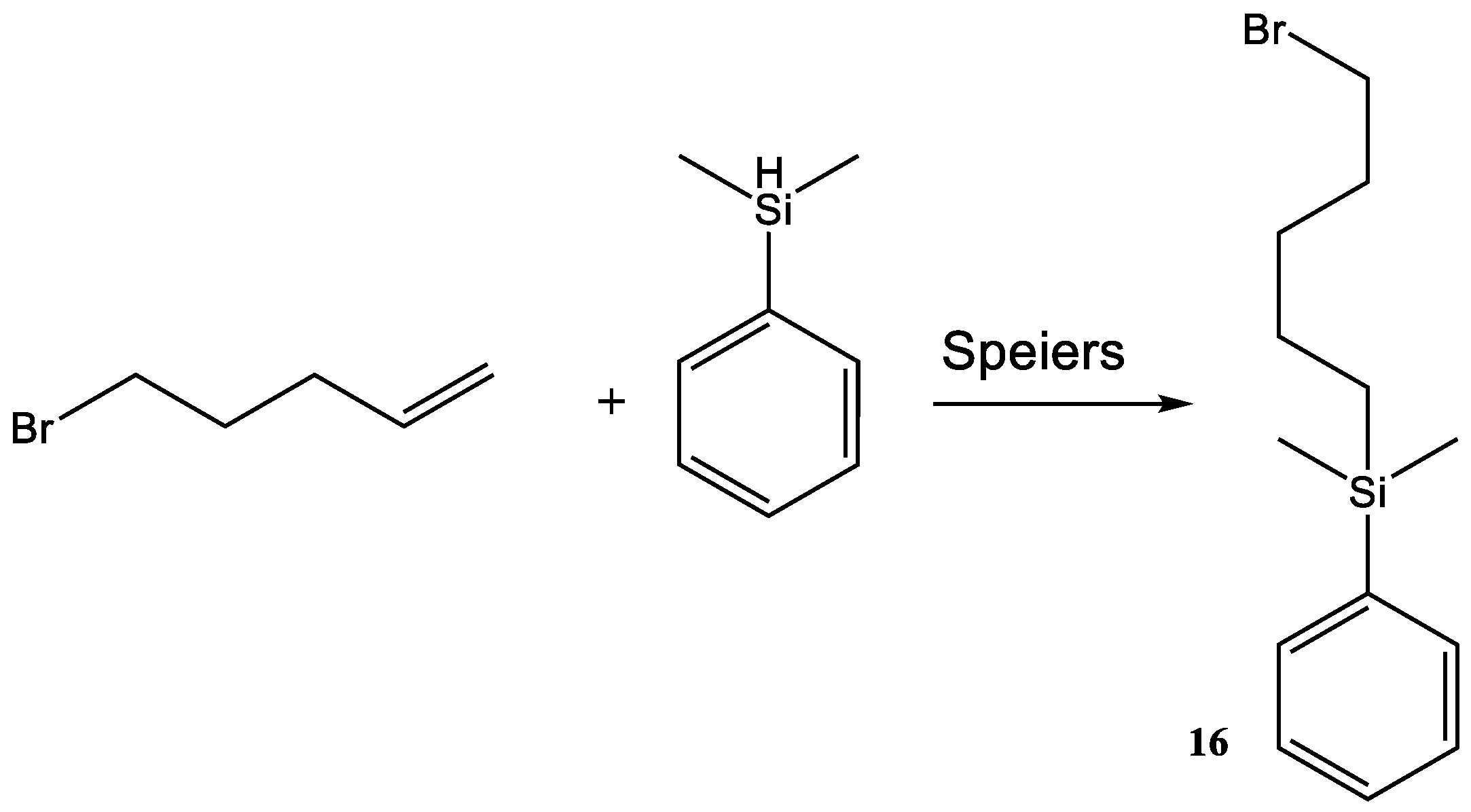
4.13. 16
Dimethylphenyl silane (1.0 mL, 876 mg, 0.007338 mol) was added to 5-bromopent-1-ene (134) (1.4 eq, 1.04 mL, 1.31 g, 0.008806 mol) along with Speier’s catalyst (100 µL). The reaction mixture was sealed under argon and heated to 60 °C for 34 h. The cooled reaction was added to DCM (150 mL) and activated charcoal was added; the reaction was then refluxed for 30 min. The solids were removed using filtration and the solvent was removed by reduced pressure. The excess 5-bromopent-1-ene was removed using reduced pressure (50 mbar) at 40 °C; the product was then placed under high vacuum for 34 h. The resulting oil was centrifuged to remove trace amounts of activated charcoal, yielding an oil.
Yield 1.23 g, 59.0%.
1H-NMR (300 MHz, CDCl3, δ):0.03 (6H, s, CH3), 0.52 (2H, t, 3J = 6.2 Hz CH2), 1.13 (4H, m, CH2), 1.54 (2H, t, 3J = 6.8 Hz, CH2), 3.03 (2H, t, 3J = 6.9 Hz, CH2), 7.07 (3H, t, 3J = 3.8 Hz, CH), 7.26 (2H, q, 3J = 3.5 Hz, CH).
13C-NMR (75 MHz, CDCl3):−1.15 (CH3), 15.49 (CH2), 23.01 (CH2), 31.80 (CH2), 32.27 (CH2), 33.52 (CH2), 127.60 (CH), 129.27 (CH), 133.35 (CH), 139.02 (C).
29Si NMR (400 MHz CDCl3): −2.65 (Si-R).
FTIR (thin-film NaCl plate): 3068, 2961, 2935, 1642, 1579, 1475, 1252, 1119.
LC–MS (positive): 206.15 [M − Br + H].
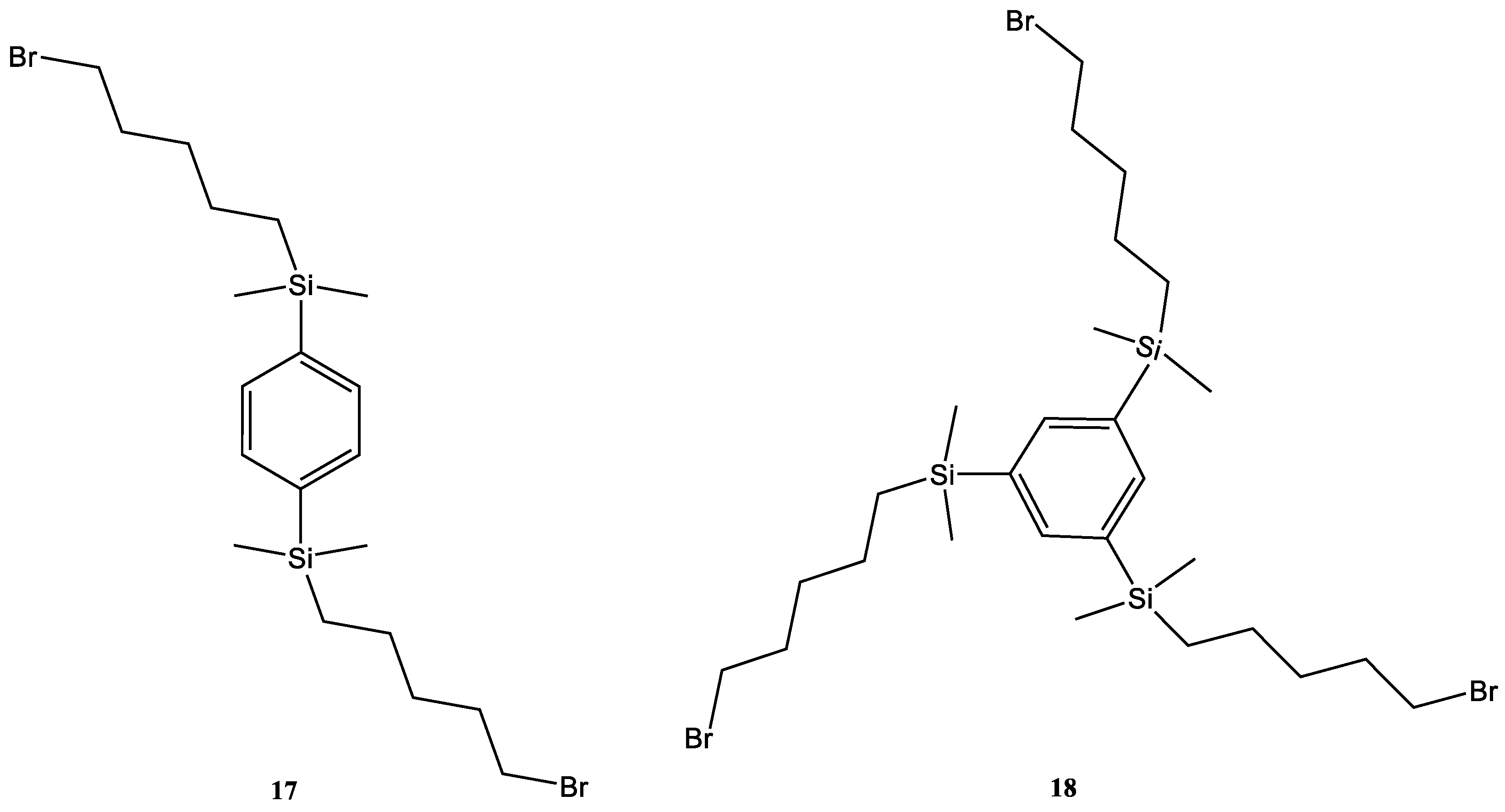
4.14. 17
The 1,4-Bis(dimethylsilyl)benzene (1 mL, 875 mg. 0.0045 mol) and 5-bromo-pent-1-ene (2.8 eq, 0.0126 mol, 1.877 g, 1.50 mL) were combined in a sealed tube, to which Speier’s catalyst (100 µL) was added. The reaction was sealed under argon and heated to 60 °C for 48 h. After this step, the reaction was added to DCM (100 mL) and activated charcoal was added; a further stage of reflux for 30 min followed. The solids were filtered out and the solvent removed using reduced pressure, yielding a clear oil. The oil was then placed under high vacuum for 48 h.
Yield 1.37 g, 62.3%.
1H-NMR (300 MHz, CDCl3, δ):0.01 (12H, s, CH3), 0.10 (4H, d, 3J = 5.7 Hz CH2), 0.49 (4H, t, 3J = 6.8 Hz CH2), 1.18 (8H, m, CH2), 1.53 (4H, p, 3J = 7.3 Hz CH2), 3.03 (4H, t, 3J = 6.9 Hz CH2), 7.22 (4H, s CH).
13C-NMR (75 MHz, CDCl3): −3.09 (CH3), 15.45 (CH2), 23.03 (CH2), 30.67 (CH2), 31.87 (CH2), 33.58 (CH2), 132.22 (CH), 139.66 (C).
29Si NMR (400 MHz CDCl3): −2.66. (Si-R).
FTIR (thin-film NaCl plate): 2955, 2926, 1459, 1438, 1379, 1250, 1135, 1056.
LC–MS (positive) 508.1[M + NH4].
4.15. 18
The 1,3,5-tri(dimethylsilyl)benzene (1.00 g, 0.00401 mol), was added to 5-bromopent-1-ene (4 eq, 2.39 g, 0.016 mol, 1.90 mL) along with Speier’s catalyst (100 µL). The reaction was sealed under argon and heated to 60 °C for 48 h. The cooled reaction was added to DCM (100 mL) alongside activated charcoal, and refluxed for 30 min. The solids were removed using filtration and the solvent removed, yielding a clear oil. The oil was then placed under high vacuum for 48 h.
Yield 1.51 g, 54.1%.
1H-NMR (300 MHz, CDCl3, δ):0.02 (18H, s CH3), 0.49 (6H, t, 3J = 6.9 Hz CH2), 1.14 (H12, m, CH2), 1.54 (6H, t, 3J = 6.8 Hz, CH2), 3.08 (6H, t, 3J = 6.8 Hz CH2), 7.35 (3H, s, CH).
13C-NMR (75 MHz, CDCl3): −3.08 (CH3), 15.46 (CH2), 23.05 (CH2), 31.79 (CH2), 32.39 (CH2), 33.66 (CH2), 137.02 (CH), 139.11 (Cq).
29Si NMR (400 MHz CDCl3): −2.59 (Si-R).
FTIR (thin-film NaCl plate): 2957, 2927, 1711, 1460, 1439, 1411, 1367, 1252, 1222, 1138, 1070.
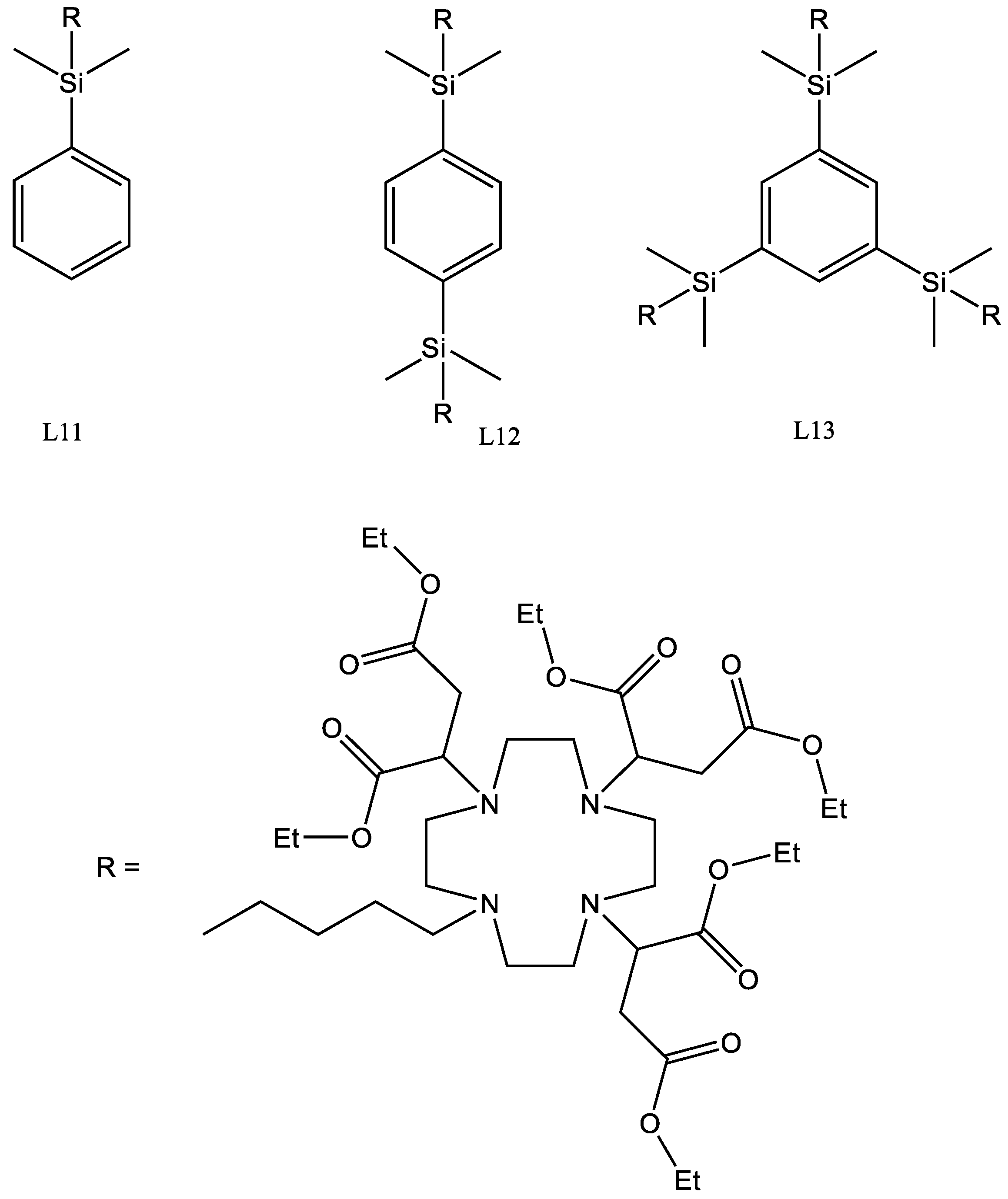
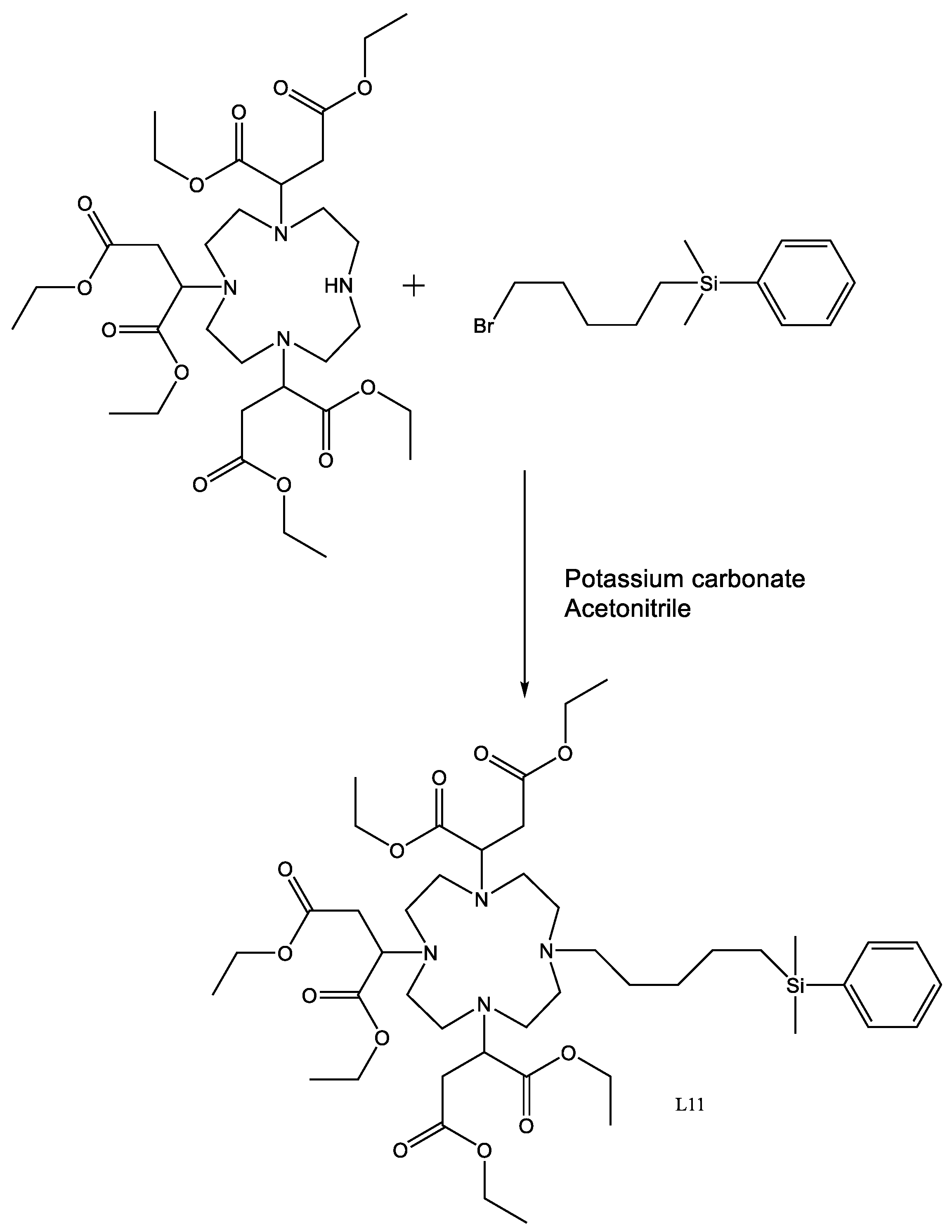
4.16. L11
L5 (1.15 g, 0.00152 mol) was dissolved in anhydrous acetonitrile (10 mL) to which potassium carbonate (0.90 g, 0.00666 mol) was added along with (5-bromopentyl) dimethyl(phenyl)silane (450 mg, 0.00158 mol). The reaction was sealed under argon and heated to 60 °C for 48 h. The reaction was cooled and the solids removed using a centrifuge. The solvent was then removed using reduced pressure to yield a yellow oil. The crude product was then chromatographed on silica, eluted with a gradient of 100% DCM to 95% DCM + 5% EtOH.
Yield 550 mg, 40.5%.
1H-NMR (300 MHz, CDCl3, δ):0.20 (6H, s CH3), 0.69 (2H, t, 3J = 6.4 Hz CH2), 1.16 (2H, d, 3J = 3.5 Hz CH2), 1.19 (6H, t, 3J = 2.7 Hz CH3), 1.24 (18H, t, 3J = 6.9 Hz CH3), 2.20 (2H, s, CH2), 2.43–2.65 (22H, m, Ch2), 3.74 (4H, m CH2), 4.10 (12H, q, 3J = 7.0 Hz CH2), 7.29 (3H, t, 3J = 3.3 Hz CH), 7.64 (2H, dd, 2.5 Hz CH).
13C-NMR (75 MHz, CDCl3):−3.17 (CH3), 14.09 (CH3), 14.30 (CH3), 15.65 (CH2), 18.28 (CH / CH3), 23.77 (CH2), 26.43 (CH2), 31.48 (CH2), 35.31 (CH2), 49.81 (CH2), 50.51 (CH2), 51.06 (CH2), 53.94 (CH2), 55.26 (CH2), 58.09 (CH), 59.89 (CH2), 60.38 (CH2), 127.56 (CH), 128.61 (CH), 133.39 (CH), 139.43 (C), 171.28 (C = O), 171.76 (C = O).
29Si NMR (400 MHz CDCl3): −2.73. (Si-R).
FTIR: 2922, 2361, 1724, 1634, 1645, 1284, 1252, 1180, 1110, 1088.
HRMS: Theoretical C48H76N4O12Si calculated 892.5201, found 893.5286 [M + H].
CHN Theoretical C 60.51 H 8.56 N 6.27 Found C 61.14 H 8.44 N 6.32.
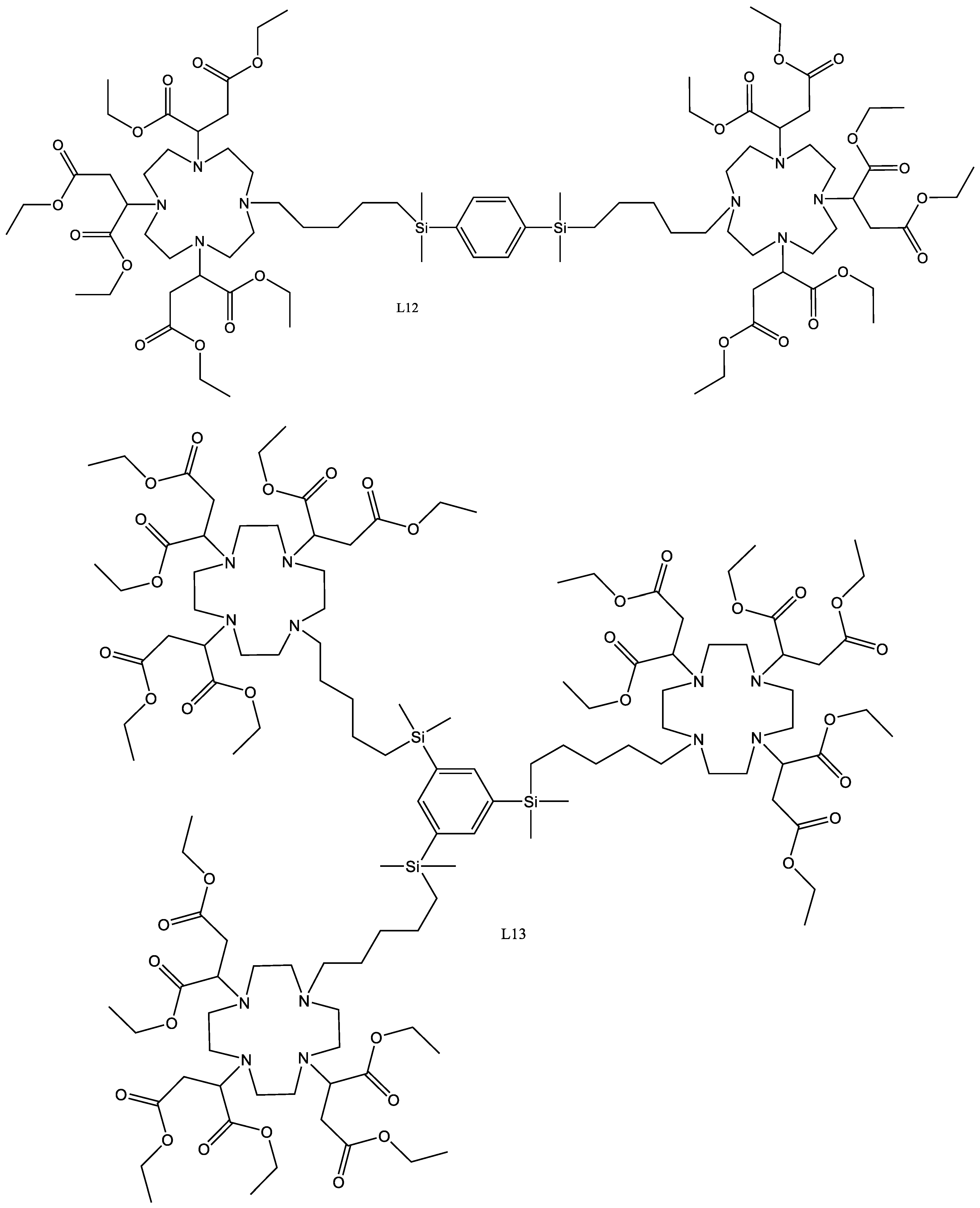
4.17. L12
L5 (590 mg, 0.0008576 mol) was dissolved in acetonitrile (10 mL) to which potassium carbonate (1.00 g, 0.007407 mol) was added. To the reaction mixture 1,4-bis((5-bromopentyl)dimethylsilyl)benzene (211 mg, 0.0004288 mol) was added and the reaction was sealed under argon and heated to 60 °C for 48 h. The solids were removed using centrifugation and the solvent was extracted by reduced pressure. The crude product was then taken up into DCM (100 mL) to which activated charcoal was added, and then refluxed for 30 min. The solids were removed by filtration and the solvent by reduced pressure to yield a clear oil. The product was then chromatographed on silica eluted with a gradient of DCM (100%) to DCM (95%)/EtOH (5%), yielding the product as an oil.
Yield 303.7 mg, 41.5%.
1H-NMR (300 MHz, CDCl3, δ):0.22 (12H, s, CH3), 0.72 (4H, s, CH2), 1.21 (8H, m CH2), 1.26 (36H, m CH3), 1.9 (4H, s, CH2), 2.23–2.75 (48H, m, CH2), 3.71 (6H, m, CH2), 4.07 (24H, m CH2), 7.49 (H4, s, CH).
13C-NMR (75 MHz, CDCl3):−3.20.
(CH3), 13.09 (CH3), 14.80 (CH3), 16.25 (CH2), 18.48 (CH / CH3), 24.07 (CH2), 26.43 (CH2), 31.48 (CH2), 35.31 (CH2), 49.81 (CH2), 50.51 (CH2), 51.06 (CH2), 53.94 (CH2), 55.26 (CH2), 58.09 (CH), 59.89 (CH2), 60.38 (CH2), 127.56 (CH), 128.61 (CH), 133.39 (CH), 138.44 (Cq), 171.22 (C = O), 174.06 (C = O).
29Si NMR (400 MHz CDCl3): −2.85 (Si-R).
FTIR (thin-film NaCl plates): 2983, 2930, 1724, 1465, 1447, 1371, 1302, 1260, 1170, 1031, 909.
HRMS: Theoretical C84H146N8O24Si2 1707.0921 Found 1707.7698.
CHN Theoretical C 59.06 H 8.61 N 6.56 Found C 58.86 H 8.91 N 6.23.
4.18. L13
The 1,3,5-bis((5-bromopentyl)dimethylsilyl)benzene (674.5 mg, 0.00109 mol), was dissolved in acetonitrile (10 mL) to which L5 (2.25 g, 0.00327 mol) was added, along with potassium carbonate (2.20 g, 0.016 mol). The reaction was heated to 60 °C for 48 h under argon, after which the solids were removed using centrifugation and the solvent was removed using reduced pressure. The crude oil was then taken up into DCM (100 mL) to which activated charcoal was added. The reaction was refluxed for 30 min, and the solids were removed using filtration. The solvent was then removed using reduced pressure to yield the crude product. The oil was chromatographed on silica eluted with a gradient of DCM (100%) to DCM (95%) EtOH (5%) leaving a clear oil.
Yield: 1.18 g, 43.0%.
1H-NMR (300 MHz, CDCl3, δ):0.33 (18H, s, CH3), 0.72 (6H, m, CH2), 1.37 (72H, m CH3), 2.36–2.71 (72H, m, CH2), 3.88 (9H, m, CH2), 4.12 (36H, m CH2), 7.69 (3H, s, CH).
13C-NMR (75 MHz, CDCl3):−3.13 (CH3), 13.98 (CH3), 14.25 (CH3), 15.68 (CH2), 18.19 (CH3), 23.90 (CH2), 25.96 (CH2), 28.12 (CH2), 31.66 (CH2), 35.52 (CH2), 35.17 (CH2), 35.31 (CH2), 46.58 (CH2), 47.83 (CH2), 51.81 (CH2), 51.06 (CH2), 53.70 (CH2), 57.92 (CH2), 58.54 (CH), 59.88 (CH), 60.33 (CH2), 60.37 (CH2), 61.50 (CH2), 137.23 (CH), 139.05 (Cq), 171.43 (C = O), 178.82 (C = O).
FTIR (flush sample CDCl3, NaCl plate): 2981, 2932, 2854, 2360, 2258, 1729, 1448, 1372, 1299, 1254, 1175, 1030.
29Si NMR (400 MHz CDCl3): −2.83 (Si-R).
MALDI: Theoretical C123H216N12O36Si3 2521.4 Found 2521.7.
CHN Theoretical C 58.55 H 8.63 N 6.66 Found C 59.86 H 8.71 N 6.73.
4.19. L14
L11 (0.55 g, 0.0006162 mol) was dissolved in 1M LiOH (5 mL) and heated to 100 °C for 24 h. The solution was cooled to room temperature, then was injected into the head of the ion-exchange column containing Bio-Rad AG1 A4I (OH− form), bed volume 500 cm3, eluting with ultra-pure water at a rate of 5 mL/min. After collection of 500 mL of eluent, the eluent was changed to formic acid (1 M), while the fraction was collected in 5 mL units and analysed using negative-ion LC–MS. The solvent was removed by lyophilisation to yield a white solid, which was then dissolved in water (10 mL) and the lyophilisation repeated to produce a white solid.
Yield 143 mg, 32%.
1H-NMR (300 MHz, D2O δ): 0.01 (6H, s, CH3), 0.53 (2H, s, CH2), 1.09 (4H, s, CH2), 1.43 (2H, s, CH2), 2.11–2.79 (24H, m, CH2), 3.83 (3H, m, CH), 7.17 (3H, m CH), 7.39 (2H, m, CH).
13C-NMR (75 MHz, D2O):−3.21 (CH3), 23.77 (CH2), 28.03 (CH2), 33.52(CH2), 36.36 (CH2), 48.61 (CH2), 50.67 (CH2), 51.30(CH2), 54.04 (CH2), 55.16 (CH2), 57.89 (CH), 59.89 (CH2), 60.38 (CH2), 129.03 (CH), 131.52 (CH), 135.11 (CH), 137.21 (Cq). 174.68 (C = O), 179.86 (C = O).
29Si NMR (400 MHz D2O): −2.80 (Si-R).
FTIR (KBr disk): 3012, 2880, 2583, 1726, 1512, 1485, 1376, 1327, 1296.
HRMS: Theoretical C33H52N4O12Si 724.3487 Found 747.5834 M + Na.
CHN Theoretical C 54.68 H 7.23 N 7.73 Found C 55.86 H 7.71 N 7.13.
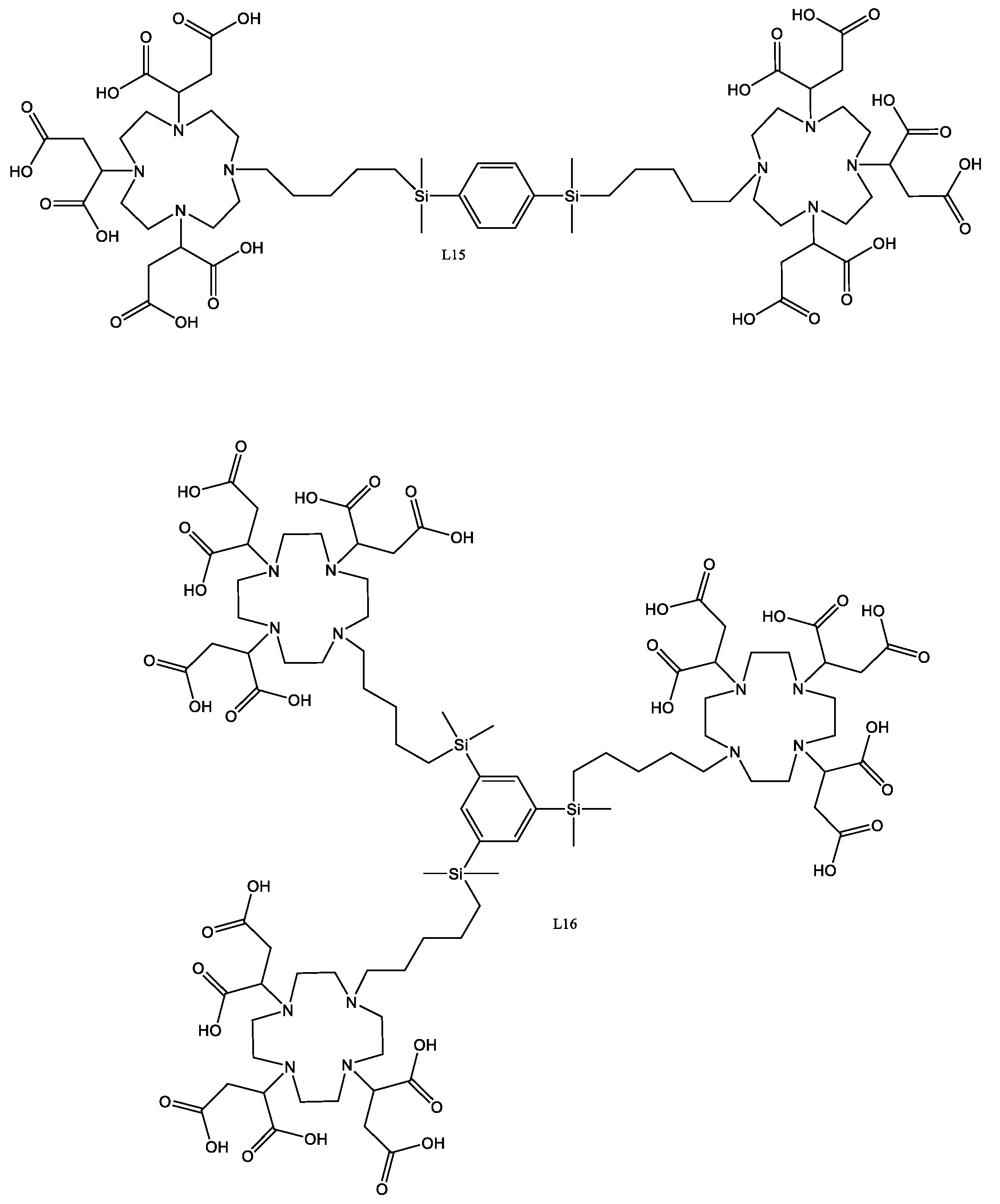
4.20. L15
L12 (1.72 g, 0.001 mol) was dissolved in LiOH (5 mL) and heated to 100 °C for 24 h. The solution was cooled to room temperature, then was injected into the head of the ion-exchange column containing Bio-Rad AG1 A4I (OH− form), bed volume 500 cm3, eluting with ultra-pure water at a rate of 5 mL/min. After collection of 500 mL of eluent, the eluent was changed to formic acid (1M), while the fraction was collected in 5 mL units and analysed using negative-ion LC–MS. The solvent was removed from the product-containing fractions by lyophilisation to yield a white solid, which was then dissolved in water (10 mL) and the lyophilisation repeated to give a white solid.
Yield, 629.1 mg, 45.9%.
1H-NMR (300 MHz, D2O δ): 0.02 (12H, s, CH3), 0.89 (4H, s CH2), 1.06 (4H, s, CH2), 1.09 (4H, s, CH2), 2.47–3.89 (50H44, m, CH2), 7.62 (4H, m, CH).
13C-NMR (75 MHz, D2O):− 3.19(CH3), 23.77 (CH2), 28.03 (CH2), 33.52(CH2), 36.36 (CH2), 48.61 (CH2), 50.67 (CH2), 51.30(CH2), 54.04 (CH2), 55.16 (CH2), 57.89 (CH), 59.89 (CH2), 60.38 (CH2), 129.03 (CH), 131.52 (CH), 135.11 (CH), 137.21 (Cq). 174.68 (C = O), 179.86 (C = O).
29Si NMR (400 MHz D2O): −2.82 (Si-R).
FTIR: 3218, 3052, 2953, 2576, 1927, 1725, 1631, 1580, 1467, 1392, 1248, 1187, 1135, 1086.
HRMS: Theoretical C60H98N8O24Si2 1370.6282 Found 1371.7254 M + H.
CHN Theoretical C 52.54 H 7.20 N 8.17 Found C 52.34 H 7.41 N 8.13.
4.21. L16
L13 (2.00 g, 0.000788 mol) was dissolved in LiOH (25 mL) and heated to 100 °C for 48 h, forming a colloidal solution. The solution was filtered through a 5 µm glass-fibre filter pad, then the solution was neutralised using formic acid (1M). The solution was lyophilised to form a white solid, which was then taken up into LiOH (1M) (50 mL) forming a clear solution. The solution was injected into the head of the ion-exchange column containing Bio-Rad AG1 A4I (OH− form), bed volume 500 cm3, eluting with ultra-pure water at a rate of 5 mL/min. After collection of 500 mL of eluent, the eluent was changed to formic acid (1M), while the fraction was collected in 5 mL units and analysed using negative-ion LC–MS The solvent was removed by lyophilisation to yield a white solid, which was then dissolved in water (10 mL) and lyophilisation was repeated.
Yield 283.2 mg, 17.65%.
1H-NMR (300 MHz, D2O δ): −0.05 (H18, s), 0.48 (H6, s), 0.87 (H6, s), 1.15 (H6, s), 1.60 (H6, s), 2.35–3.03 (H72, m), 3.93 (H9, m), 7.46 (H3, s).
13C-NMR (75 MHz, D2O):− 3.28 (CH3), 23.77 (CH2), 28.03 (CH2), 33.52(CH2), 36.36 (CH2), 48.61 (CH2), 50.67 (CH2), 51.30(CH2), 54.04 (CH2), 55.16 (CH2), 57.89 (CH), 59.89 (CH2), 60.38 (CH2), 129.03 (CH), 131.52 (CH), 135.11 (CH), 137.21 (Cq). 174.68 (C = O), 179.86 (C = O).
29Si NMR (400 MHz D2O): −2.83 (Si-R).
FTIR (KBr disk): 3402, 3132, 2975, 2858, 1734, 1639, 1597, 1384, 1251, 1179.
MALDI: Theoretical C87H144N12O36Si3 2016.9 Found 2018.2.
CHN Theoretical C 51.77 H 7.19 N 8.33 Found C 52.84 H 7.51 N 8.15.
4.22. GdL14
Dimethyl(phenyl)silane-pentyl-sDO3A (11.3 mg, 0.0000157 mol) was dissolved in water (10 mL) to which Gd2O3 (2.8 mg, 0.00000785 mol) was added. The pH of the solution was adjusted from pH 5 to pH 8–8.5 by addition of LiOH (1M) solution. The reaction was then heated to 80 °C for 48 h. The solution was cooled and the solids removed using a centrifuge, then the solution was decanted and passed through a 0.5 µm filter. The solution was lyophilised, producing a white powder.
Yield 10.1 mg, 73.1%.
FTIR (KBr disk): 3415, 2954, 2932, 2867, 2359, 1587, 1392, 1317, 1252, 1182, 1153, 1115, 1084.
CHN Theoretical C 41.49, H 5.81, N 5.61 found C 41.15 H 5.34, N 6.05.
4.23. EuL14
(pentane-sDO3A-) dimethyl(phenyl)silane (26.7 mg, 0.000031 mol) was dissolved in water (20 mL) to which Eu2O3 (5.5 mg, 0.0000155 mol) was added. The pH was adjusted from pH 5 to pH 8.5 using LiOH and the reaction was heated to 80 °C for 48 h. The solution was centrifuged and then passed through a 0.5 µm filter, after which it was lyophilised, producing a white powder.
Yield 22.5 mg, 83%.
FTIR (KBr disk): 3417, 2954, 2932, 2867, 2359, 1588, 1393, 1317, 1253, 1184, 1152, 1115, 1083.
CHN Theoretical C 40.89, H 5.79, N 5.66 found C 41.38 H 5.84, N 6.12.