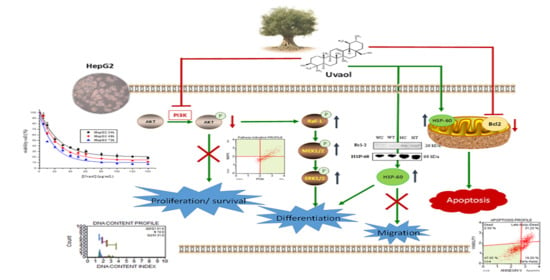
Antiproliferative and Pro-Apoptotic Effect of Uvaol in Human Hepatocarcinoma HepG2 Cells by Affecting G0/G1 Cell Cycle Arrest, ROS Production and AKT/PI3K Signaling Pathway
Abstract
1. Introduction
2. Results
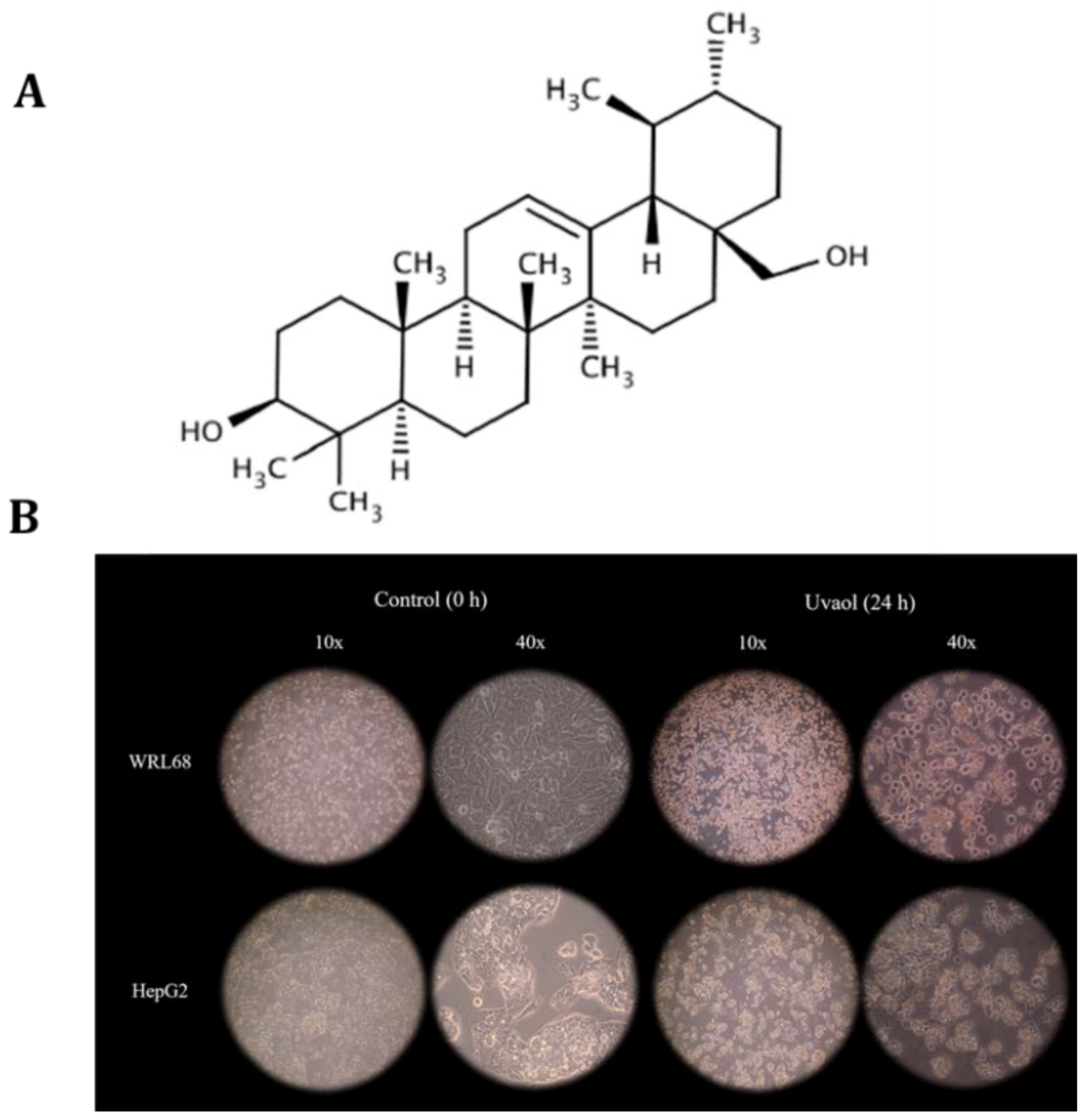
2.1. Uvaol Produces Morphological Changes in HepG2 Cells
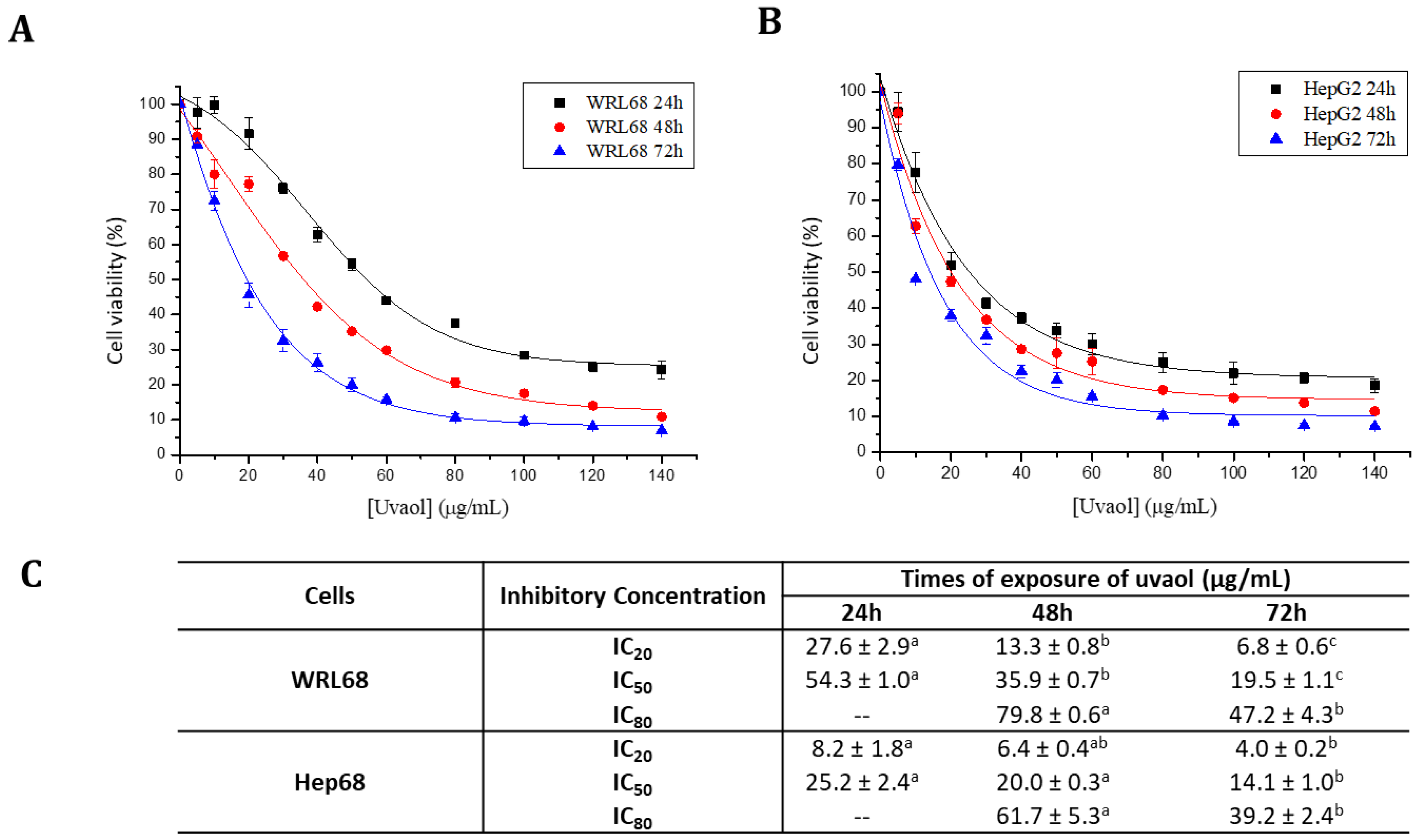
2.2. Uvaol Has a Selectivity Cytotoxic Effect on the HepG2 Cell Lines
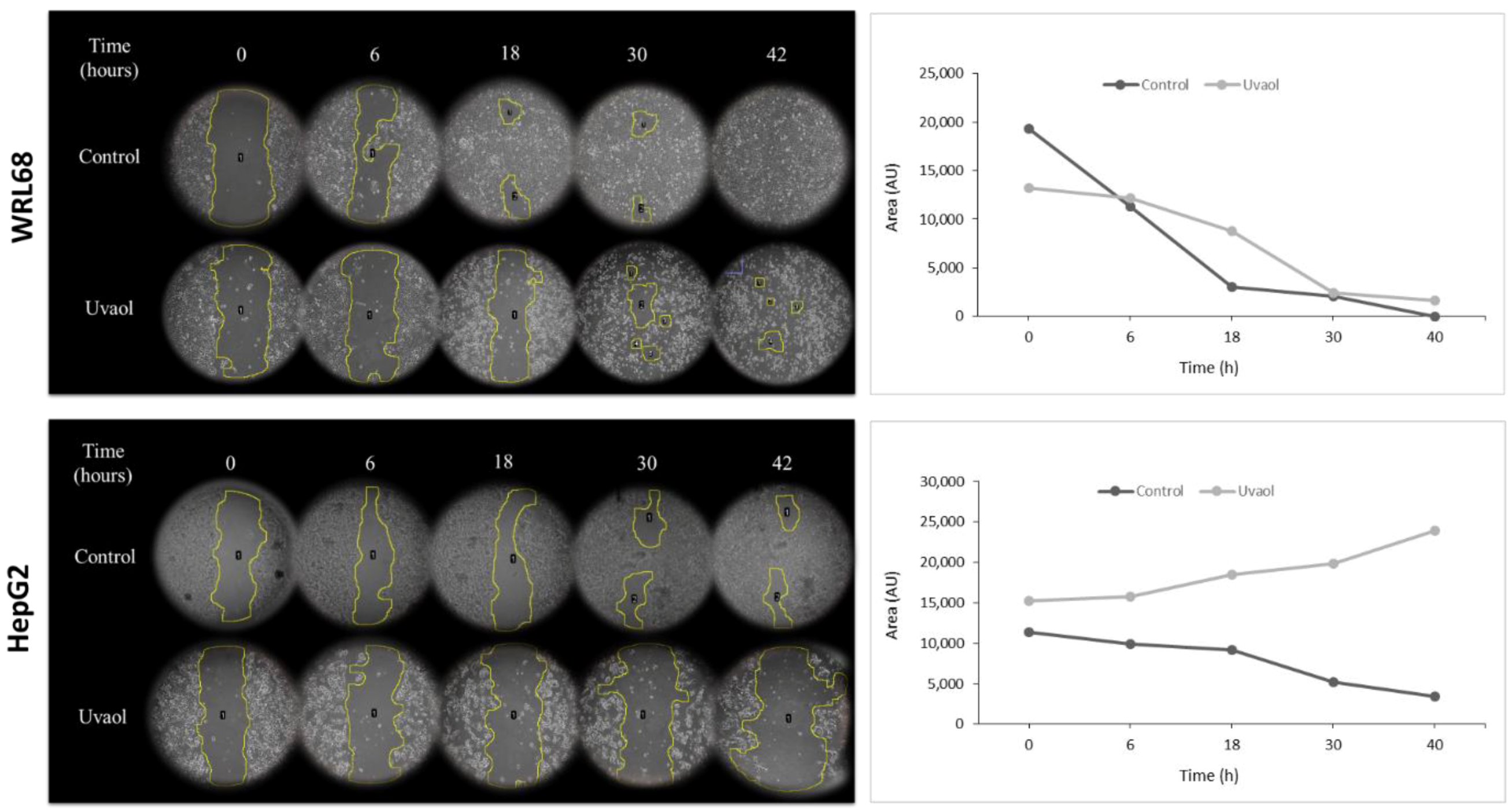
2.3. Anti-Migratory Activity of Uvaol in HepG2 Cells
2.4. Uvaol Induces G0/G1 Arrest in HepG2 Cells
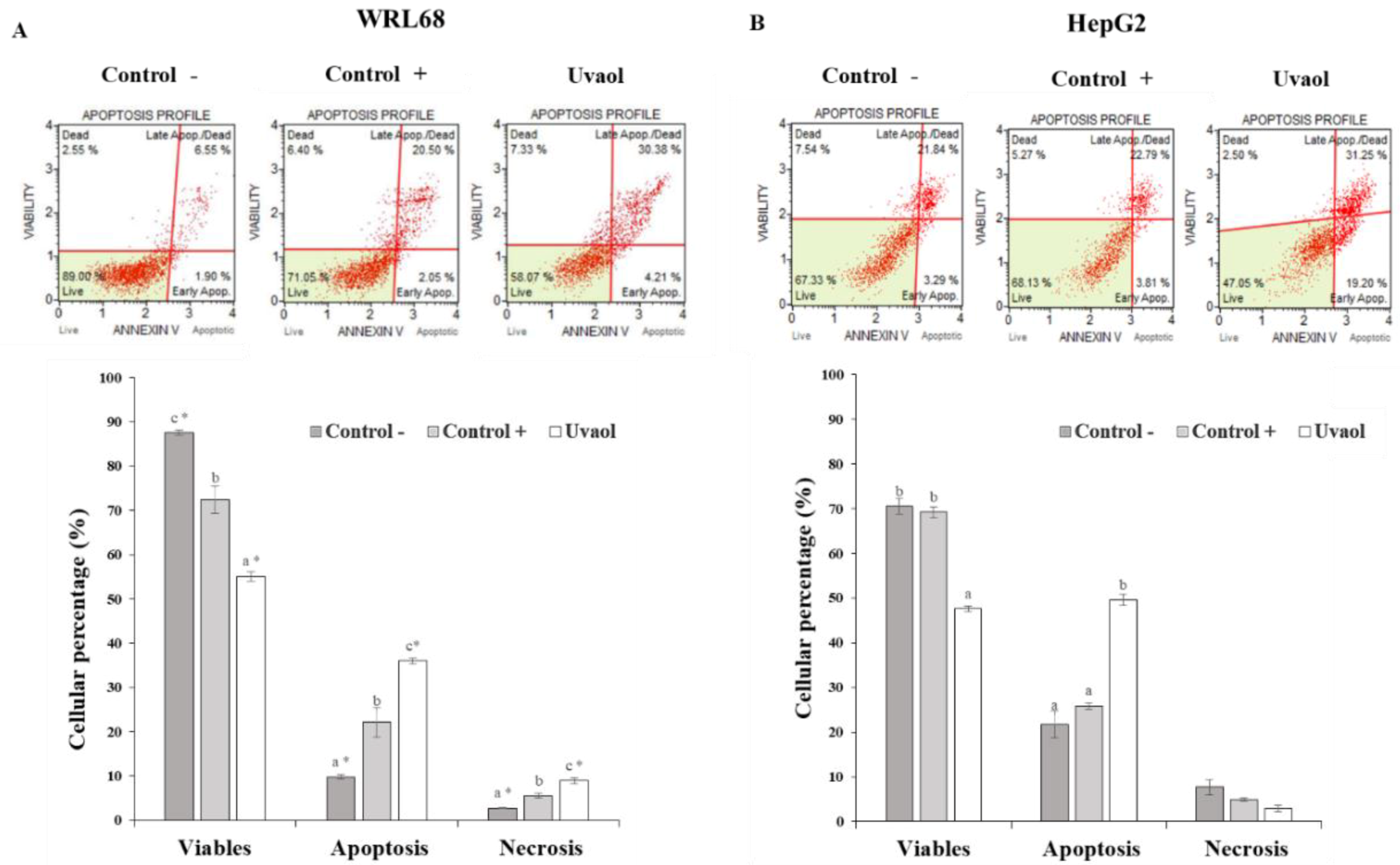
2.5. Apoptosis Is Enhanced in HepG2 Cells by Uvaol
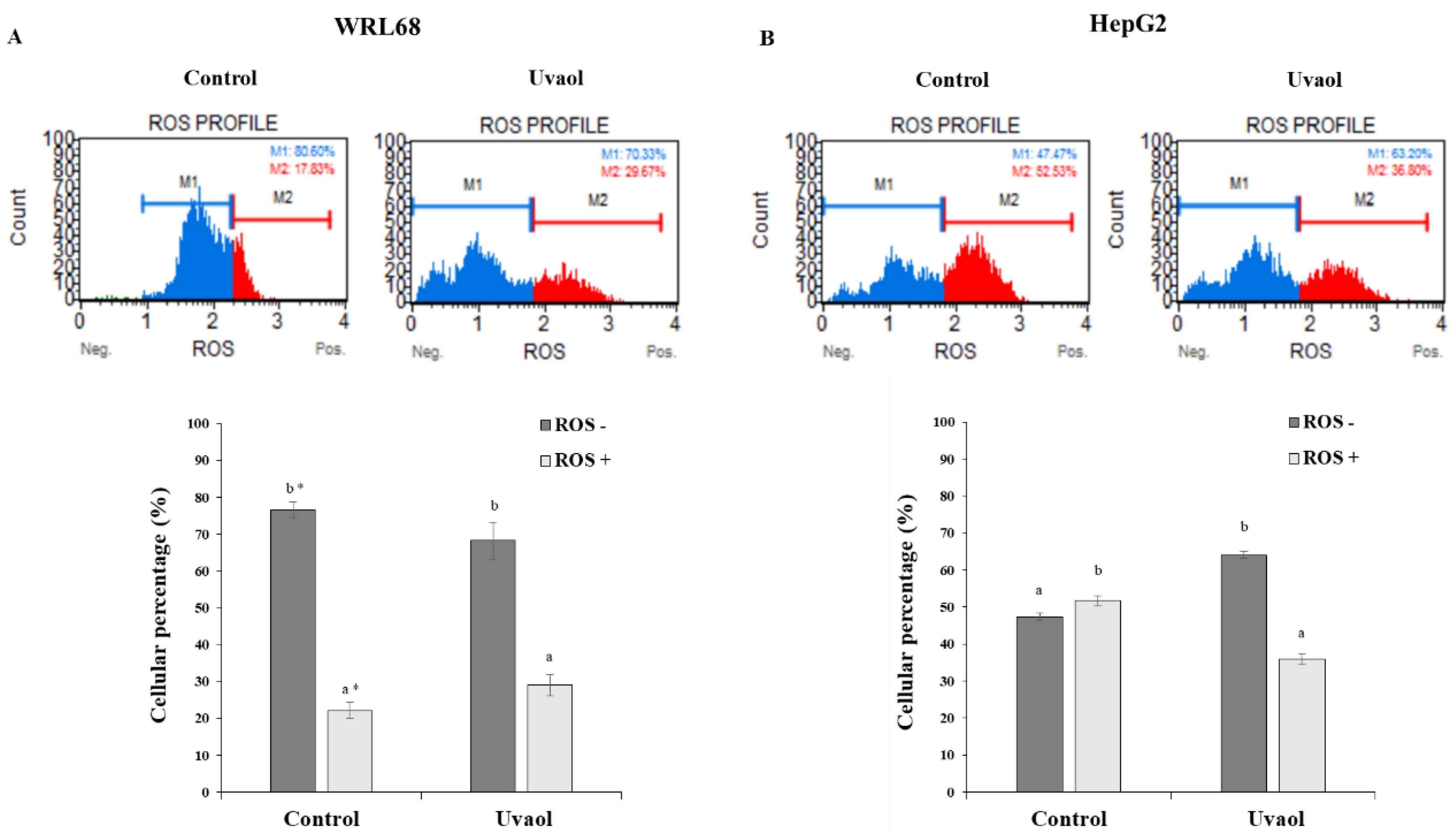
2.6. Uvaol Decreases ROS Production in HepG2 Cells
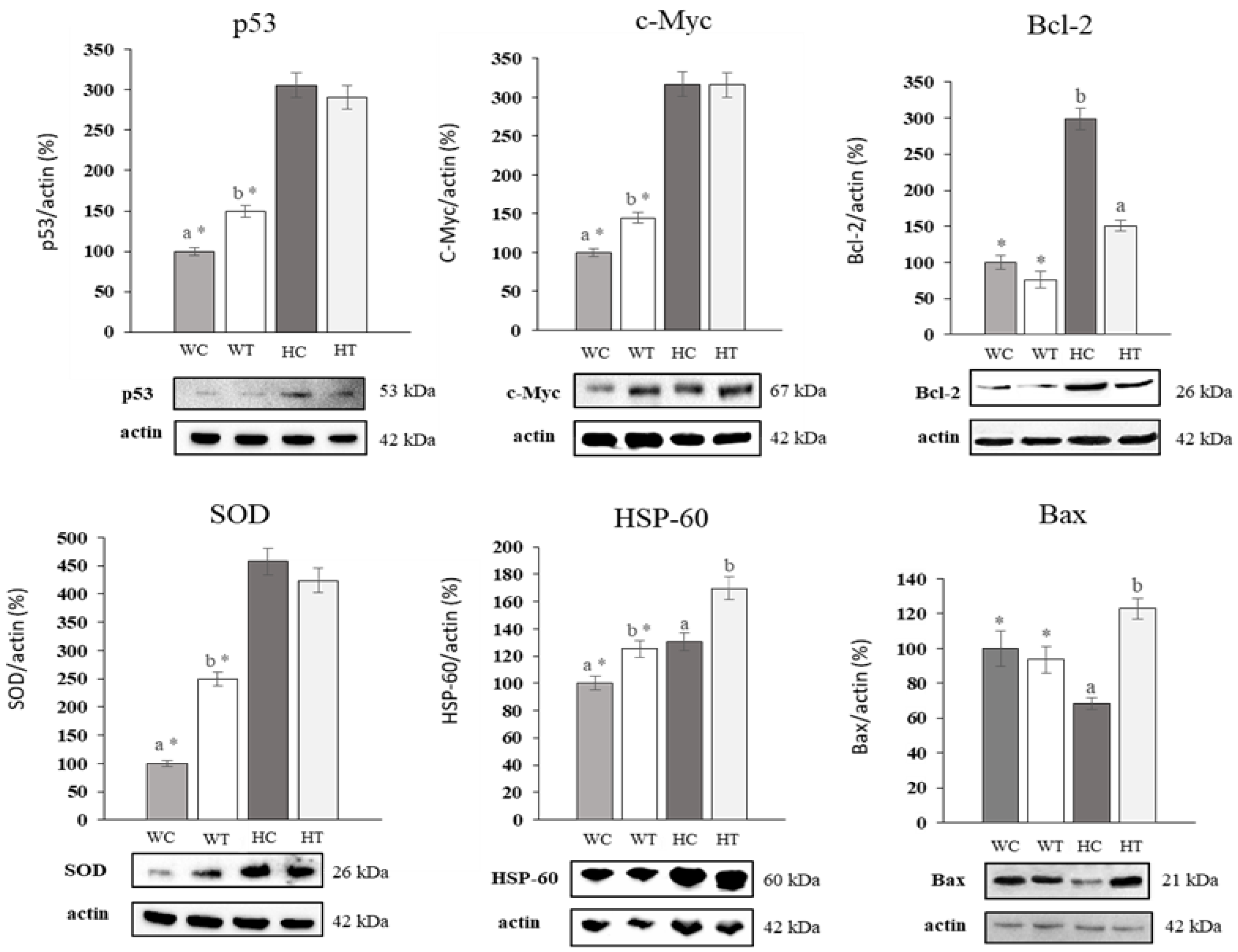
2.7. Uvaol Produces Up and Down-Regulation of Target Proteins in HepG2 Cells
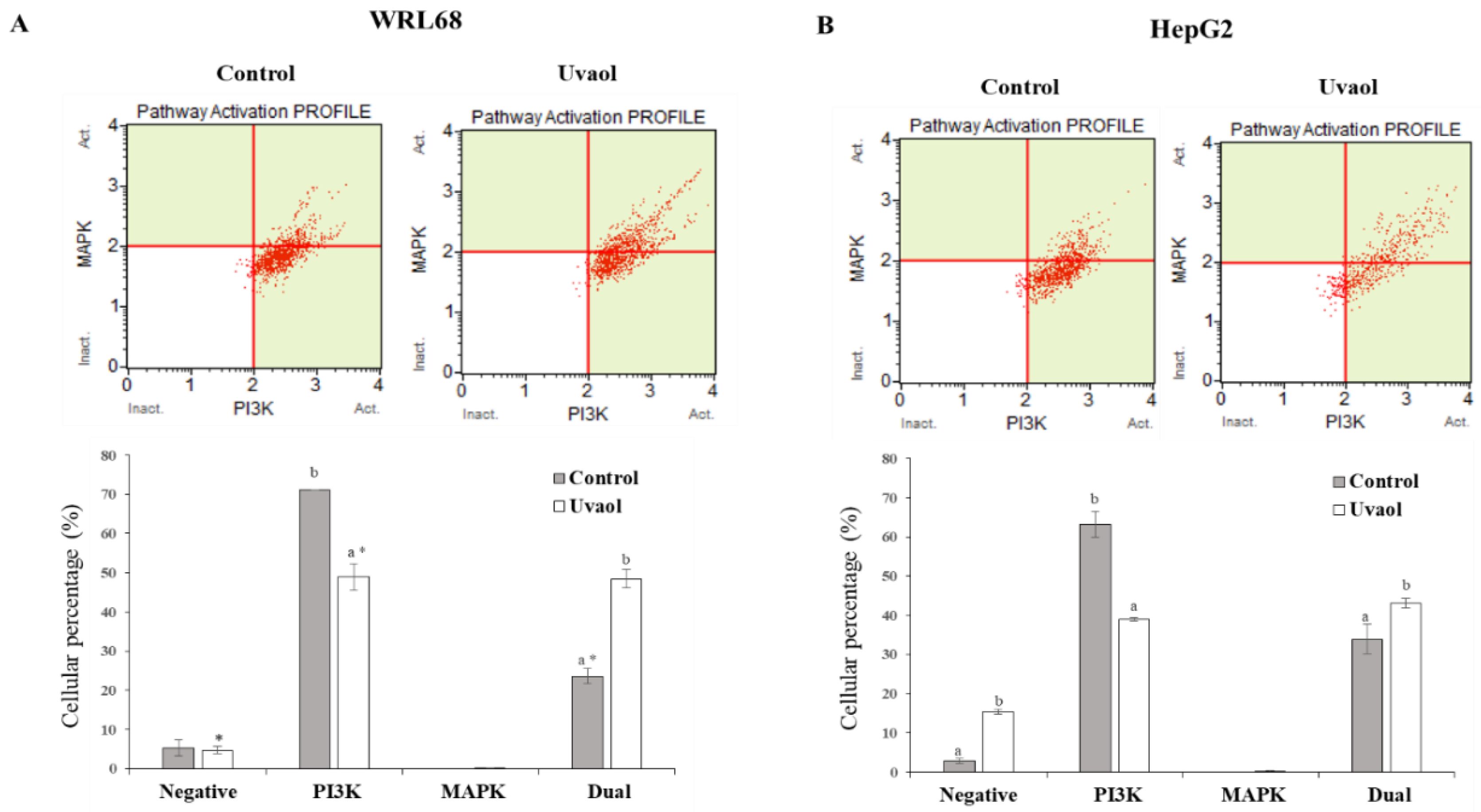
2.8. Uvaol Modulate Dual PI3K/MAPK Signaling Pathway in HepG2 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Uvaol Solution
4.3. Morphological Changes
4.4. MTT Assay
4.5. Scratch Assay
4.6. Cell Sorting by Flow Cytometry
4.6.1. Cell Cycle Assay
4.6.2. Apoptosis Assay
4.6.3. Measurement of ROS Production
4.7. Protein Extraction and Western Blot Analysis
4.8. PI3K/MAPK Dual Pathway Activation Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Chung, Y.H. Molecular targeted therapy for hepatocellular carcinoma: Current and future. World J. Gastroenterol. 2013, 19, 6144–6155. [Google Scholar] [CrossRef] [PubMed]
- Justman, Q.A. Looking beyond the stop sign: Cell-cycle checkpoints reconsidered. Cell Syst. 2017, 5, 438–440. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, J.D. The cell-cycle arrest and apoptotic functions of p53 in tumor initiation and progression. Csh. Perspect Med. 2016, 6, a026104. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A.; Newell, P.; Chiang, D.Y.; Friedman, S.L.; Llovet, J.M. Genomics and signaling pathways in hepatocellular carcinoma. Semin. Liver Dis. 2007, 27, 55–76. [Google Scholar] [CrossRef]
- Koutb, F.; Abdel-Rahman, S.; Hassona, E.; Haggag, A. Association of C-myc and p53 gene expression and polymorphisms with hepatitis c (HCV) chronic infection, cirrhosis and hepatocellular carcinoma (HCC) stages in Egypt. Asian Pac. J. Cancer Prev. 2017, 18, 2049–2057. [Google Scholar] [CrossRef]
- Merle, P.; Trepo, C. Molecular mechanisms underlying hepatocellular carcinoma. Viruses Basel 2009, 1, 852–872. [Google Scholar] [CrossRef]
- Zhang, X.R.; Chen, Y.L.; Cai, G.S.; Li, X.; Wang, D. Carnosic acid induces apoptosis of hepatocellular carcinoma cells via ROS-mediated mitochondrial pathway. Chem. Biol. Interact. 2017, 277, 91–100. [Google Scholar] [CrossRef]
- Pilz, R.B.; Willis, R.C.; Boss, G.R. The influence of ribose 5-phosphate availability on purine synthesis of cultured human lymphoblasts and mitogen-stimulated lymphocytes. J. Biol. Chem. 1984, 259, 2927–2935. [Google Scholar]
- Kan, B.; London, I.M.; Levin, D.H. Role of reversing factor in the inhibition of protein synthesis initiation by oxidized glutathione. J. Biol. Chem. 1988, 263, 15652–15656. [Google Scholar] [PubMed]
- Lupiáñez, J.A.; Adroher, F.J.; Vargas, A.M.; Osuna, A. Differential behaviour of glucose 6-phosphate dehydrogenase in two morphological forms of Trypanosoma cruzi. Int. J. Biochem. 1987, 19, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Barroso, J.B.; García-Salguero, L.; Peragón, J.; de la Higuera, M.; Lupiáñez, J.A. The influence of dietary-protein on the kinetics of NADPH production systems in various tissues of rainbow-trout (Oncorhynchus mykiss). Aquaculture 1994, 124, 47–59. [Google Scholar] [CrossRef]
- Barroso, J.B.; Peragón, J.; García-Salguero, L.; de la Higuera, M.; Lupiáñez, J.A. Carbohydrate deprivation reduces NADPH-production in fish liver but not in adipose tissue. Int. J. Biochem. Cell Biol. 2001, 33, 785–796. [Google Scholar] [CrossRef]
- Peragón, J.; Barroso, J.B.; García-Salguero, L.; Aranda, F.; de la Higuera, M.; Lupiáñez, J.A. Selective changes in the protein-turnover rates and nature of growth induced in trout liver by long-term starvation followed by re-feeding. Mol. Cell Biochem. 1999, 201, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Peragón, J.; Barroso, J.B.; García-Salguero, L.; de la Higuera, M.; Lupiáñez, J.A. Dietary alterations in protein, carbohydrates and fat increase liver protein-turnover rate and decrease overall growth rate in the rainbow trout (Oncorhynchus mykiss). Mol. Cell Biochem. 2000, 209, 97–104. [Google Scholar] [CrossRef]
- Rufino-Palomares, E.; Reyes-Zurita, F.J.; Fuentes-Almagro, C.A.; de la Higuera, M.; Lupiáñez, J.A.; Peragón, J. Proteomics in the liver of gilthead sea bream (Sparus aurata) to elucidate the cellular response induced by the intake of maslinic acid. Proteomics 2011, 11, 3312–3325. [Google Scholar] [CrossRef]
- Rufino-Palomares, E.E.; Pérez-Jiménez, A.; Reyes-Zurita, F.J.; García-Salguero, L.; Mokhtari, K.; Herrera-Merchán, A.; Medina, P.P.; Peragón, J.; Lupiáñez, J.A. Anti-cancer and anti-angiogenic properties of various natural pentacyclic tri-terpenoids and some of their chemical derivatives. Curr. Org. Chem. 2015, 19, 919–947. [Google Scholar] [CrossRef]
- Mokhtari, K.; Rufino-Palomares, E.E.; Pérez-Jiménez, A.; Reyes-Zurita, F.J.; Figuera, C.; García-Salguero, L.; Medina, P.P.; Peragón, J.; Lupiáñez, J.A. Maslinic acid, a triterpene from olive, affects the antioxidant and mitochondrial status of B16F10 melanoma cells grown under stressful conditions. Evid. Based Compl. Alt. 2015, 2015, 272457. [Google Scholar] [CrossRef]
- Yuan, X.X.; Wang, B.Y.; Yang, L.; Zhang, Y.L. The role of ROS-induced autophagy in hepatocellular carcinoma. Clin. Res. Hepatol. Gas 2018, 42, 306–312. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J.; Krause, K. H, Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Kulik, L.; El-Serag, H.B. Epidemiology and management of hepatocellular carcinoma. Gastroenterology 2019, 156, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Villanueva, A.; Lachenmayer, A.; Finn, R.S. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat. Rev. Clin. Oncol. 2015, 12, 408–424. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.D.; Ma, Q.Q.; Ye, L.; Piao, G.C. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Escrich, E.; Ramírez-Tortosa, M.C.; Sánchez-Rovira, P.; Colomer, R.; Solanas, M.; Gaforio, J.J. Olive oil in cancer prevention and progression. Nutr. Rev. 2006, 64, S40–S52. [Google Scholar] [CrossRef]
- López-García, E.; Rodríguez-Artalejo, F.; Li, T.Y.; Fung, T.T.; Li, S.S.; Willett, W.C.; Rimm, E.B.; Hu, F.B. The mediterranean-style dietary pattern and mortality among men and women with cardiovascular disease. Am. J. Clin. Nutr. 2014, 99, 172–180. [Google Scholar] [CrossRef]
- Yubero-Serrano, E.M.; López-Moreno, J.; Gómez-Delgado, F.; López-Miranda, J. Extra virgin olive oil: More than a healthy fat. Eur. J. Clin. Nutr. 2019, 72, 8–17. [Google Scholar] [CrossRef]
- Preedy, V.R.; Watson, R.R. Olives and Olive Oil in Health and Disease Prevention; Academic Press: San Diego, CA, USA, 2010; p. 1520. [Google Scholar]
- Sánchez-Quesada, C.; López-Biedma, A.; Gaforio, J.J. The differential localization of a methyl group confers a different anti-breast cancer activity to two triterpenes present in olives. Food Funct. 2015, 6, 249–256. [Google Scholar] [CrossRef]
- Sánchez-Quesada, C.; López-Biedma, A.; Warleta, F.; Campos, M.; Beltrán, G.; Gaforio, J.J. Bioactive properties of the main triterpenes found in olives, virgin olive oil, and leaves of Olea europaea. J. Agric. Food Chem. 2013, 61, 12173–12182. [Google Scholar] [CrossRef]
- Martín, R.; Ibeas, E.; Carvalho-Tavares, J.; Hernández, M.; Ruiz-Gutiérrez, V.; Nieto, M.L. Natural triterpenic diols promote apoptosis in astrocytoma cells through ROS-mediated mitochondrial depolarization and JNK activation. PLoS ONE 2009, 4, e5975. [Google Scholar] [CrossRef]
- Allouche, Y.; Warleta, F.; Campos, M.; Sánchez-Quesada, C.; Uceda, M.; Beltrán, G.; Gaforio, J.J. Antioxidant, antiproliferative, and pro-apoptotic capacities of pentacyclic triterpenes found in the skin of olives on MCF-7 human breast cancer cells and their effects on DNA damage. J. Agric. Food Chem. 2011, 59, 121–130. [Google Scholar] [CrossRef]
- Grandhi, M.S.; Kim, A.K.; Ronnekleiv-Kelly, S.M.; Kamel, I.R.; Ghasebeh, M.A.; Pawlik, T.M. Hepatocellular carcinoma: From diagnosis to treatment. Surg. Oncol. 2016, 25, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Peragón, J.; Rufino-Palomares, E.E.; Muñoz-Espada, I.; Reyes-Zurita, F.J.; Lupiáñez, J.A. A new HPLC-MS method for measuring maslinic acid and oleanolic acid in HT29 and HepG2 human cancer cells. Int. J. Mol. Sci. 2015, 16, 21681–21694. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Zhang, W.; Gao, K.; Lu, Y.Y.; Tang, H.F.; Sun, X.L. Oleanane-type saponins from Anemone taipaiensis and their cytotoxic activities. Fitoterapia 2013, 89, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Y.; Huang, H.Y.; Wu, Y.L. Anticancer and apoptotic activities of oleanolic acid are mediated through cell cycle arrest and disruption of mitochondrial membrane potential in HepG2 human hepatocellular carcinoma cells. Mol. Med. Rep. 2015, 12, 5012–5018. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Bretones, G.; Delgado, M.D.; León, J. Myc and cell cycle control. Biochim. Biophys. Acta 2015, 1849, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Frenzel, A.; Grespi, F.; Chmelewskij, W.; Villunger, A. Bcl2 Family proteins in carcinogenesis and the treatment of cancer. Apoptosis 2009, 14, 584–596. [Google Scholar] [CrossRef]
- Wang, J.J.; Han, H.; Chen, X.F.; Yi, Y.H.; Sun, H.X. Cytotoxic and apoptosis-inducing activity of triterpene glycosides from Holothuria scabra and Cucumaria frondosa against HepG2 cells. Mar. Drugs 2014, 12, 4274–4290. [Google Scholar] [CrossRef]
- Parra, A.; Martín-Fonseca, S.; Rivas, F.; Reyes-Zurita, F.J.; Medina-O’Donnell, M.; Rufino-Palomares, E.E.; Martínez, A.; García-Granados, A.; Lupiáñez, J.A.; Albericio, F. Solid-phase library synthesis of bi-functional derivatives of oleanolic and maslinic acids and their cytotoxicity on three cancer cell lines. Acs Comb. Sci. 2014, 16, 428–447. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, X.C.; Chang, H.L.; Huang, X.J.; Guo, X.; Du, X.H.; Tian, S.Y.; Wang, L.X.; Lyv, Y.H.; Yuan, P.; et al. Hsp60 exerts a tumor suppressor function by inducing cell differentiation and inhibiting invasion in hepatocellular carcinoma. Oncotarget 2016, 7, 68976–68989. [Google Scholar] [CrossRef]
- Kauffmann-Zeh, A.; Rodríguez-Viciana, P.; Ulrich, E.; Gilbert, C.; Coffer, P.; Downward, J.; Evan, G. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature 1997, 385, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, L.; You, Q.D.; Nie, F.F.; Gu, H.Y.; Zhao, L.; Wang, X.T.; Guo, Q.L. Differential Apoptotic Induction of gambogic acid, a novel anticancer natural product, on hepatoma cells and normal hepatocytes. Cancer Lett. 2007, 256, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Duronio, V.; Scheid, M.P.; Ettinger, S. Downstream signalling events regulated by phosphatidylinositol 3-kinase activity. Cell Signal. 1998, 10, 233–239. [Google Scholar] [CrossRef]
- Vivanco, I.; Sawyers, C.L. The phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nat. Rev. Cancer 2002, 2, 489–501. [Google Scholar] [CrossRef]
- Moelling, K.; Schad, K.; Bosse, M.; Zimmermann, S.; Schweneker, M. Regulation of Raf-Akt cross-talk. J. Biol. Chem. 2002, 277, 31099–31106. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.L.; Lin, P.Y.; Lin, H.C.; Chen, Y.L. The apoptotic effect of ursolic acid on SK-Hep-1 cells is regulated by the PI3K/Akt, p38 and JNK MAPK signaling pathways. Molecules 2016, 21, 460. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Du, X.Y.; Zhang, Y.; Wang, R. Platycodin D, a triterpenoid saponin from Platycodon grandiflorum, induces G2/M arrest and apoptosis in human hepatoma HepG2 cells by modulating the PI3K/Akt pathway. Tumor Biol. 2014, 35, 1267–1274. [Google Scholar] [CrossRef]
- Tang, C.; Lu, Y.H.; Xie, J.H.; Wang, F.; Zou, J.N.; Yang, J.S.; Xing, Y.Y.; Xi, T. Downregulation of survivin and activation of caspase-3 through the PI3K/Akt pathway in ursolic acid-induced HepG2 cell apoptosis. AntiCancer Drugs 2009, 20, 249–258. [Google Scholar] [CrossRef]
- Pérez-Jiménez, A.; Rufino-Palomares, E.E.; Fernández-Gallego, N.; Ortuño-Costela, M.C.; Reyes-Zurita, F.J.; Peragón, J.; García-Salguero, L.; Mokhtari, K.; Medina, P.P.; Lupiáñez, J.A. Target Molecules in 3T3-L1 adipocytes differentiation are regulated by maslinic acid, a natural triterpene from Olea europaea. Phytomedicine 2016, 23, 1301–1311. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compound of uvaol are available from the authors. |

| Protein | Primary Antibody | Secondary Antibody |
|---|---|---|
| p53 | Rabbit anti-p53 antibody (1:500) | Anti-mouse IgG antibody (1:5000) |
| SOD | Rabbit anti-SOD antibody (1:250) | Anti-rabbit IgG antibody (1:5000) |
| c-Myc | Goat anti-c-Myc antibody (1:500) | Anti-goat IgG antibody (1:5000) |
| HSP-60 | Mouse anti-HSP-60 antibody (1:250) | Anti-mouse IgG antibody (1:5000) |
| Bcl-2 | Rabbit anti-Bcl-2 antibody (1:500) | Anti-rabbit IgG antibody (1:5000) |
| Bax | Mouse anti-Bax antibody (1:500) | Anti-mouse IgG antibody (1:5000) |
| Actin | Mouse anti-actin antibody (1:1.000) | Anti-mouse IgG antibody (1:5000) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonel-Pérez, G.C.; Pérez-Jiménez, A.; Gris-Cárdenas, I.; Parra-Pérez, A.M.; Lupiáñez, J.A.; Reyes-Zurita, F.J.; Siles, E.; Csuk, R.; Peragón, J.; Rufino-Palomares, E.E. Antiproliferative and Pro-Apoptotic Effect of Uvaol in Human Hepatocarcinoma HepG2 Cells by Affecting G0/G1 Cell Cycle Arrest, ROS Production and AKT/PI3K Signaling Pathway. Molecules 2020, 25, 4254. https://doi.org/10.3390/molecules25184254
Bonel-Pérez GC, Pérez-Jiménez A, Gris-Cárdenas I, Parra-Pérez AM, Lupiáñez JA, Reyes-Zurita FJ, Siles E, Csuk R, Peragón J, Rufino-Palomares EE. Antiproliferative and Pro-Apoptotic Effect of Uvaol in Human Hepatocarcinoma HepG2 Cells by Affecting G0/G1 Cell Cycle Arrest, ROS Production and AKT/PI3K Signaling Pathway. Molecules. 2020; 25(18):4254. https://doi.org/10.3390/molecules25184254
Chicago/Turabian StyleBonel-Pérez, Gloria C., Amalia Pérez-Jiménez, Isabel Gris-Cárdenas, Alberto M. Parra-Pérez, José Antonio Lupiáñez, Fernando J. Reyes-Zurita, Eva Siles, René Csuk, Juan Peragón, and Eva E. Rufino-Palomares. 2020. "Antiproliferative and Pro-Apoptotic Effect of Uvaol in Human Hepatocarcinoma HepG2 Cells by Affecting G0/G1 Cell Cycle Arrest, ROS Production and AKT/PI3K Signaling Pathway" Molecules 25, no. 18: 4254. https://doi.org/10.3390/molecules25184254
APA StyleBonel-Pérez, G. C., Pérez-Jiménez, A., Gris-Cárdenas, I., Parra-Pérez, A. M., Lupiáñez, J. A., Reyes-Zurita, F. J., Siles, E., Csuk, R., Peragón, J., & Rufino-Palomares, E. E. (2020). Antiproliferative and Pro-Apoptotic Effect of Uvaol in Human Hepatocarcinoma HepG2 Cells by Affecting G0/G1 Cell Cycle Arrest, ROS Production and AKT/PI3K Signaling Pathway. Molecules, 25(18), 4254. https://doi.org/10.3390/molecules25184254