The Effect of POSS Type on the Shape Memory Properties of Epoxy-Based Nanocomposites
Abstract
1. Introduction
2. Results and Discussion
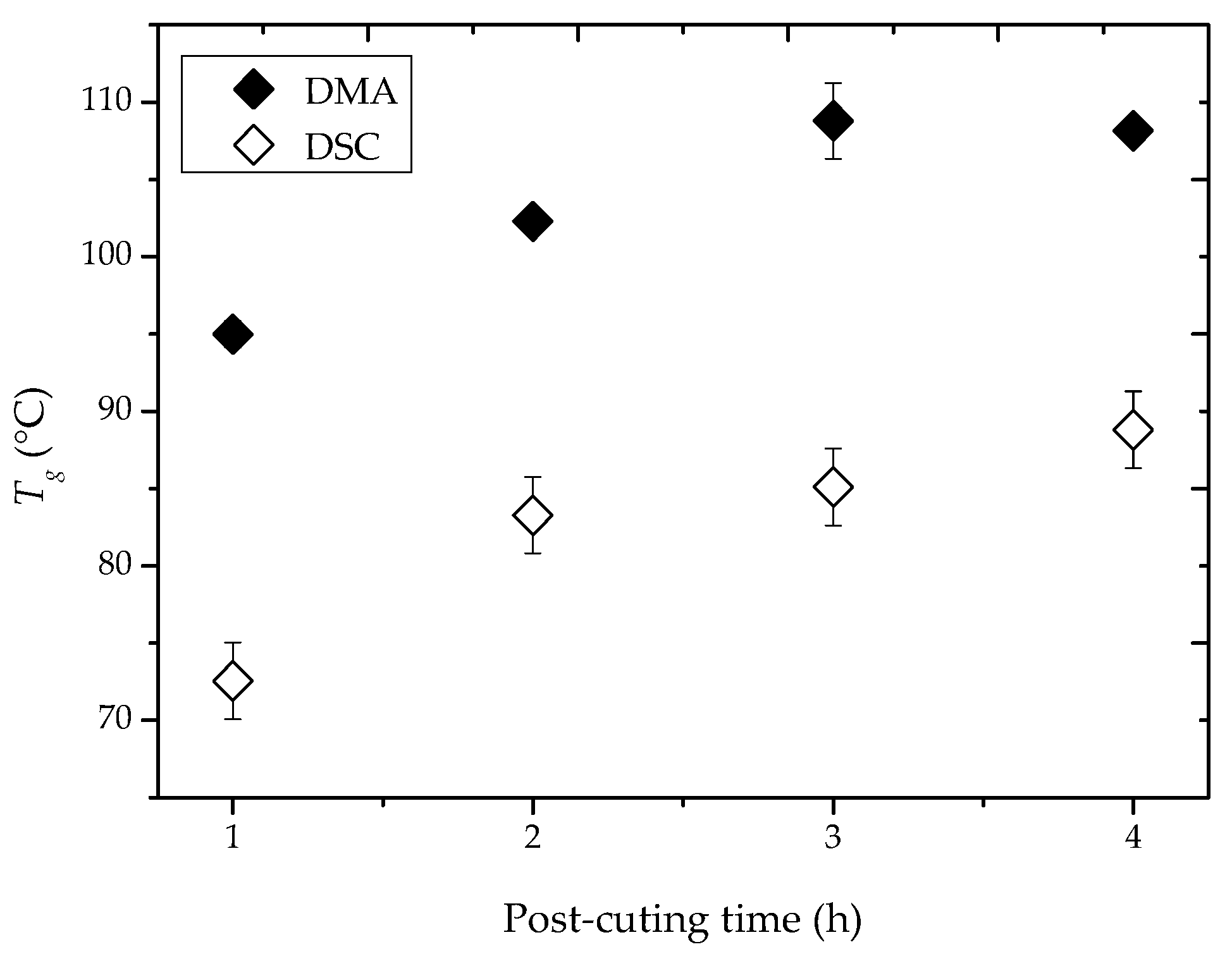
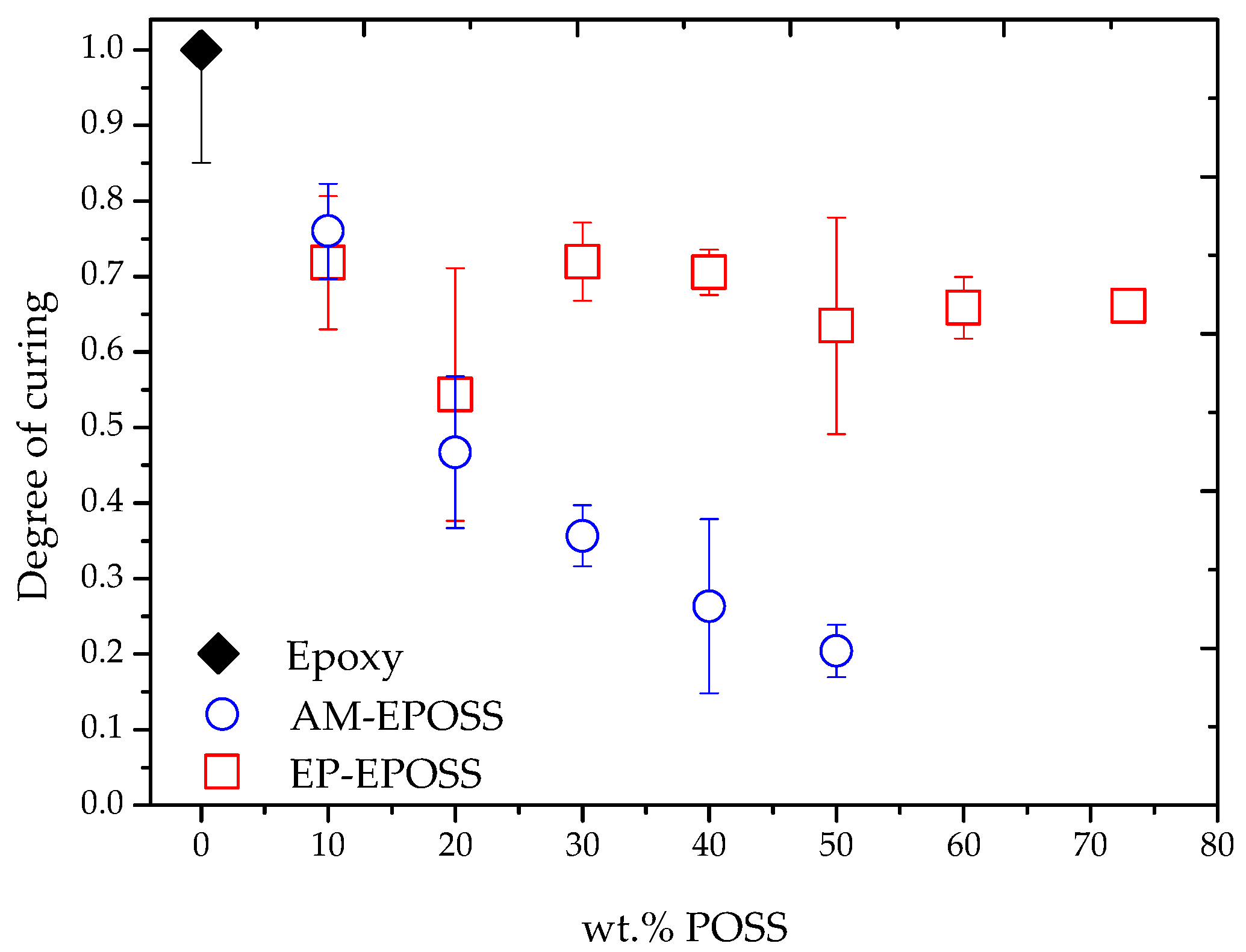
2.1. Curing and Post-Curing Conditions
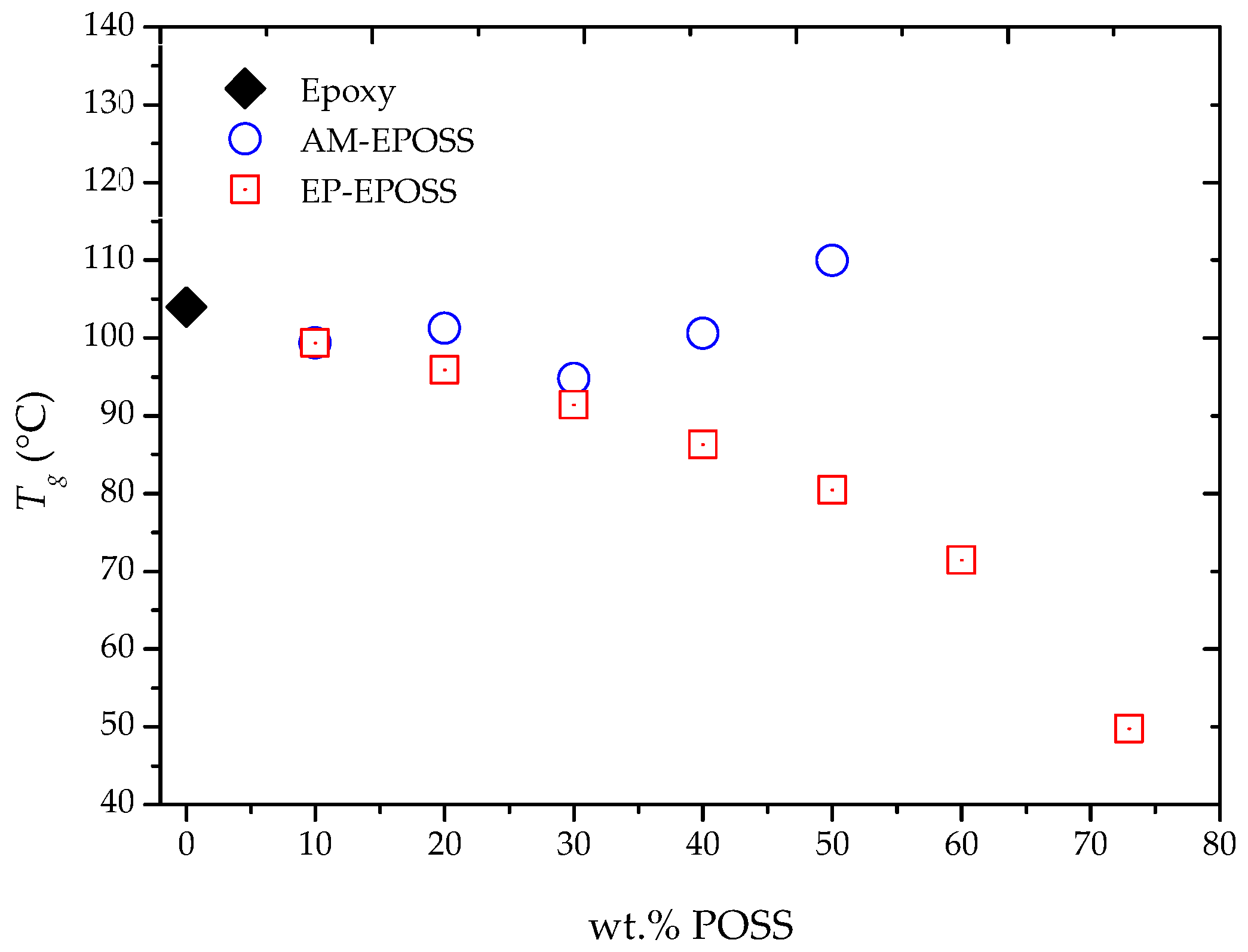
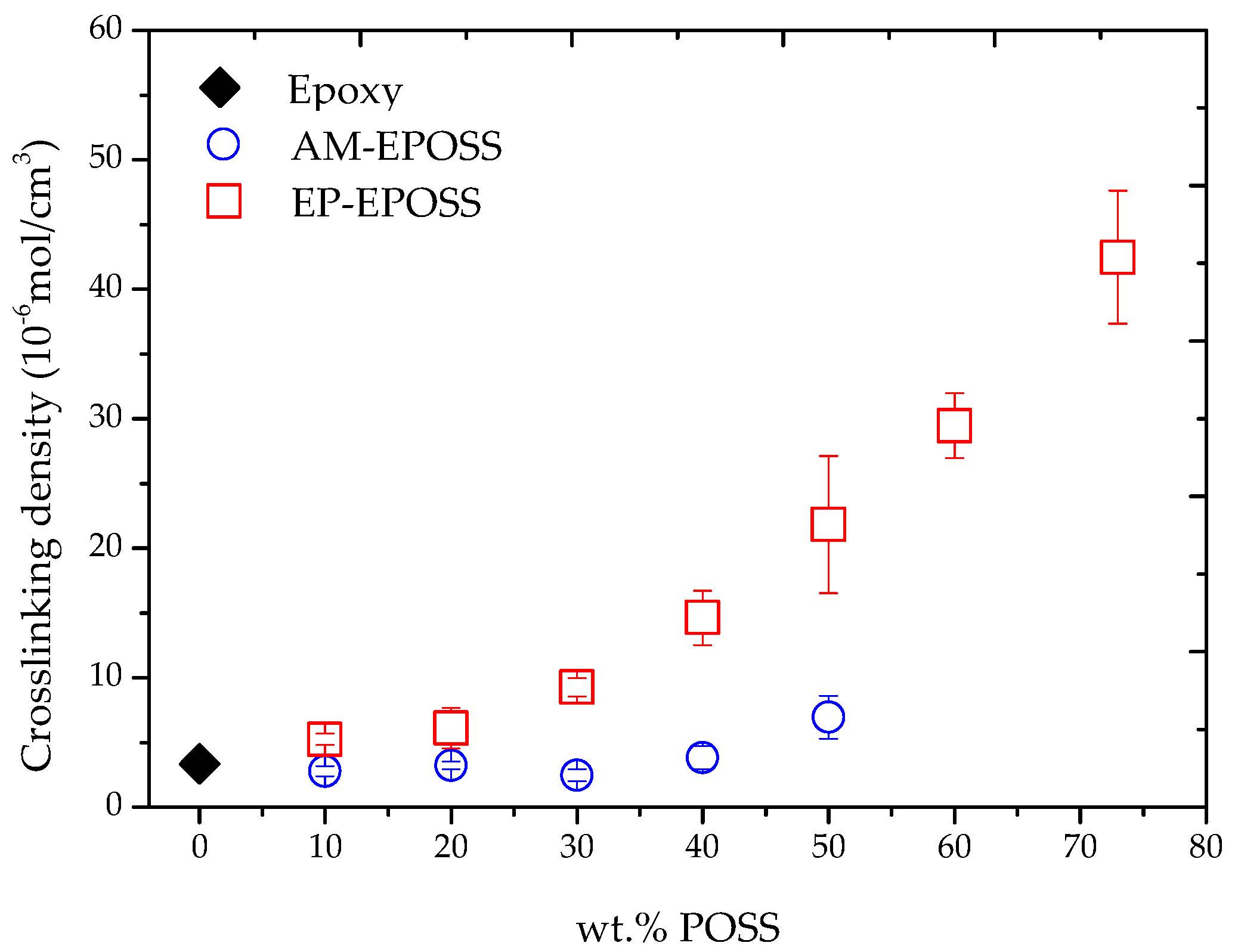
2.2. Mechanical and Thermomechanical Properties
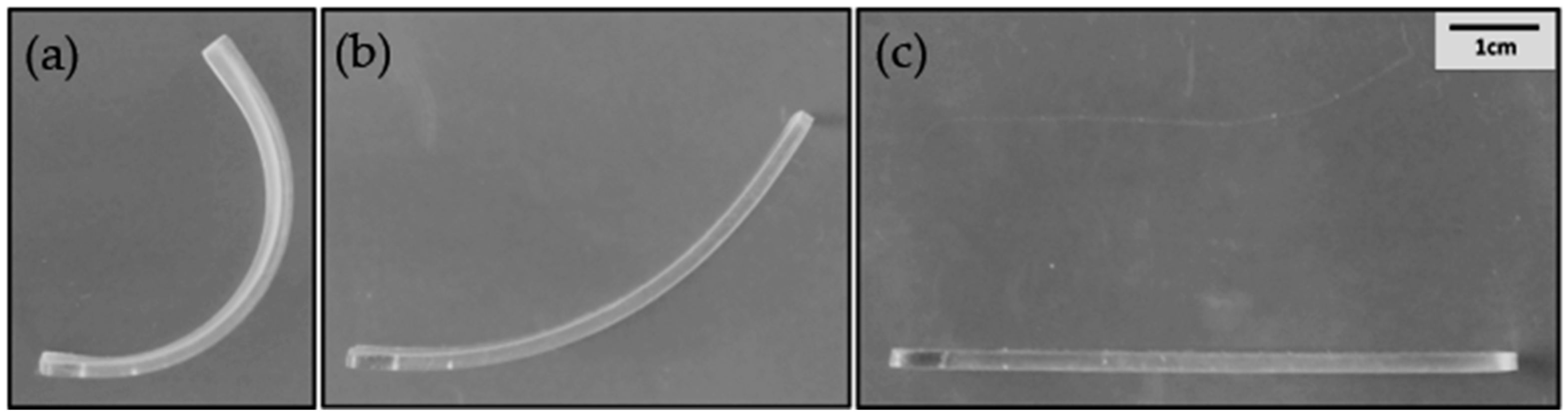
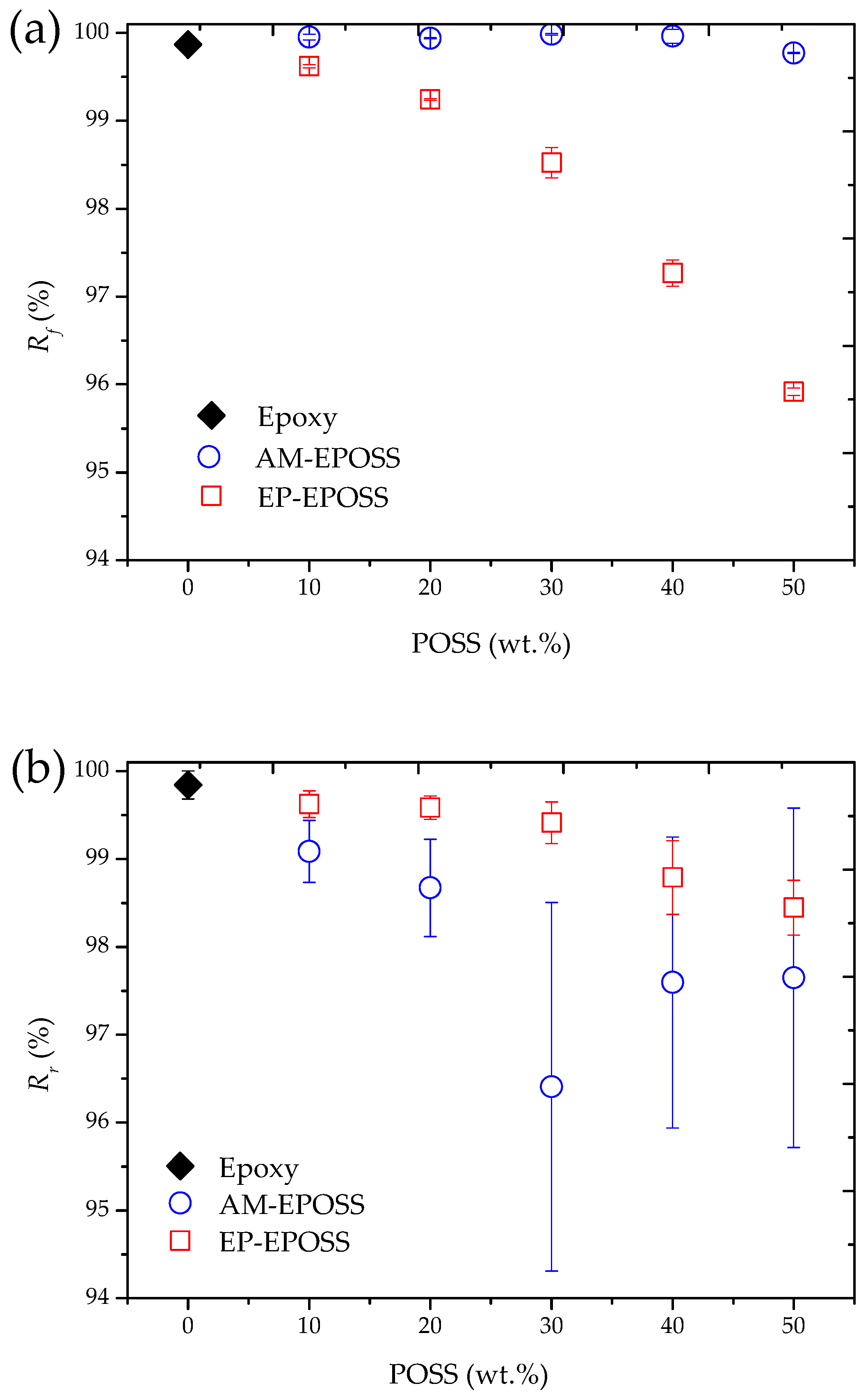
2.3. Shape Memory Properties
3. Materials and Methods
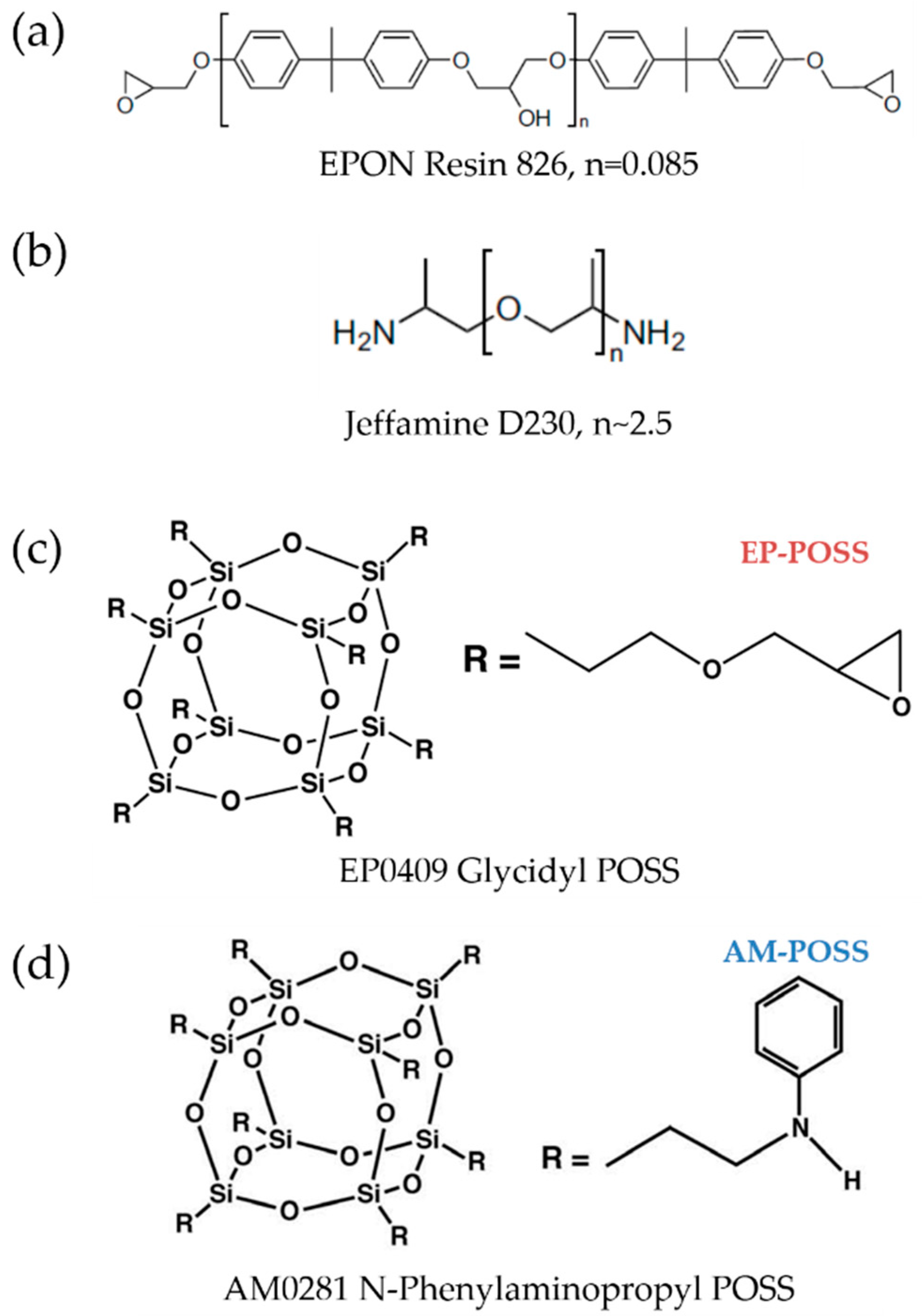
3.1. Materials
3.2. Epoxy and EPOSS Preparation
3.3. Characterization Methods
3.4. Shape Memory Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crisp, N.H.; Smith, K.L.; Hollingsworth, P.M. An integrated design methodology for the deployment of constellations of small satellites. Aeronaut. J. 2019, 123, 1193–1215. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H.; Liu, L.; Leng, J. Shape memory polymers and their composites in aerospace applications: A review. Smart Mater. Struct. 2014, 23, 23001. [Google Scholar] [CrossRef]
- Sun, J.; Guan, Q.; Liu, Y.; Leng, J. Morphing aircraft based on smart materials and structures: A state-of-the-art review. J. Intell. Mater. Syst. Struct. 2016, 27, 2289–2312. [Google Scholar] [CrossRef]
- Margoy, D.; Gouzman, I.; Grossman, E.; Bolker, A.; Eliaz, N.; Verker, R. Epoxy-based shape memory composite for space applications. Acta Astronaut. 2020. Available online: https://doi.org/10.1016/j.actaastro (accessed on 26 August 2020). [CrossRef]
- Tobushi, H.; Hayashi, S.; Sugimoto, Y.; Date, K. Two-way bending properties of shape memory composite with SMA and SMP. Materials 2009, 2, 1180–1192. [Google Scholar] [CrossRef]
- Zheng, X.; Zhou, S.; Li, X.; Weng, J. Shape memory properties of poly(d,l-lactide)/hydroxyapatite composites. Biomaterials 2006, 27, 4288–4295. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Leng, J. Recent developments in shape memory polymer nanocomposites: Actuation methods and mechanisms. Coord. Chem. Rev. 2016, 320, 38–52. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, T.; Yao, Y.; Zhang, F.; Liu, L.; Liu, Y.; Leng, J. Stimulus methods of multi-functional shape memory polymer nanocomposites: A review. Compos. Part A Appl. Sci. Manuf. 2017, 100, 20–30. [Google Scholar] [CrossRef]
- Hager, M.D.; Bode, S.; Weber, C.; Schubert, U.S. Shape memory polymers: Past, present and future developments. Prog. Polym. Sci. 2015, 49–50, 3–33. [Google Scholar] [CrossRef]
- Liu, C.; Qin, H.; Mather, P.T. Review of progress in shape-memory polymers. J. Mater. Chem. 2007, 17, 1543–1558. [Google Scholar] [CrossRef]
- Lin, J.; Knoll, C.F.; Willey, C. Shape memory rigidizable inflatable (RI) structures for large space systems applications. Paper AIAA 2006-1896. In Proceedings of the 47th AIAA/ASME/ASCE/AHS/ASC Structures, Structural Dynamics, and Materials Conference, Newport, RI, USA, 1–4 May 2006. [Google Scholar]
- Liu, Y.; Leng, J. Applications of shape-memory polymers in aerospace. In Shape-Memory Polymers and Multifunctional Composites; Du, S., Leng, J., Eds.; CRC Press: Boca Raton, FL, USA, 2010; Chapter 8; pp. 233–264. [Google Scholar]
- Lendlein, A.; Kelch, S. Shape-memory polymers. Angew. Chem. Int. Ed. 2002, 41, 2034–2057. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, Y.; Huang, H.; Lu, J. Recent advances in shape–memory polymers: Structure, mechanism, functionality, modeling and applications. Prog. Polym. Sci. 2012, 37, 1720–1763. [Google Scholar] [CrossRef]
- Meng, H.; Li, G. A review of stimuli-responsive shape memory polymer composites. Polymer 2013, 54, 2199–2221. [Google Scholar] [CrossRef]
- Zhao, Q.; Jerry, H.; Xie, T. Recent progress in shape memory polymer: New behavior, enabling materials and mechanistic understanding. Prog. Polym. Sci. 2015, 49, 79–120. [Google Scholar] [CrossRef]
- Li, J.; Duan, Q.; Zhang, E.; Wang, J. Applications of shape memory polymers in kinetic buildings. Adv. Mater. Sci. Eng. 2018, 4, 1–13. [Google Scholar] [CrossRef]
- Raimondo, M.; Guadagno, L.; Speranza, V.; Bonnaud, L.; Dubois, P.; Lafdi, K. Multifunctional graphene/POSS epoxy resin tailored for aircraft lightning strike protection. Compos. Part B Eng. 2018, 140, 44–56. [Google Scholar] [CrossRef]
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Mishra, K.; Gidley, D.; Singh, R.P. Influence of self-assembled compliant domains on the polymer network and mechanical properties of POSS-epoxy nanocomposites under cryogenic conditions. Europ. Polym. J. 2019, 116, 283–290. [Google Scholar] [CrossRef]
- Laikhtman, A.; Gouzman, I.; Verker, R.; Grossman, E.; Pippin, H.G. Atomic oxygen and UV irradiation effects on fluorosilicone rubber: Comparison of RF plasma and in-flight exposure. High Perf. Polym. 2008, 20, 447–460. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Wang, M.; Shen, Z. An experimental study on improving the atomic oxygen resistance of epoxy resin/silica nanocomposites. Polym. Eng. Sci. 2007, 47, 1156–1162. [Google Scholar] [CrossRef]
- Grossman, E.; Gouzman, I. Space environment effects on polymers in low earth orbit. Nucl. Instrum. Meth. Phys. Res. Sect. B 2003, 208, 48–57. [Google Scholar] [CrossRef]
- Packirisamy, S.; Schwam, D.; Litt, M.H. Atomic oxygen resistant coatings for low earth orbit space structures. J. Mater. Sci. 1995, 30, 308–320. [Google Scholar] [CrossRef]
- Choi, C.; Kim, Y.; Sathish Kumar, S.K.; Kim, C.-G. Enhanced resistance to atomic oxygen of OG POSS/epoxy nanocomposites. Compos. Struct. 2018, 202, 959–966. [Google Scholar] [CrossRef]
- Rivera Lopez, M.Y.; Lambas, J.M.; Stacey, J.P.; Gamage, S.; Suliga, A.; Viquerat, A.; Scarpa, F.; Hamerton, I. Development of cycloaliphatic epoxy-POSS nanocomposite matrices with enhanced resistance to atomic oxygen. Molecules 2020, 25, 1483. [Google Scholar] [CrossRef] [PubMed]
- Verker, R.; Grossman, E.; Eliaz, N. Erosion of POSS-polyimide films under hypervelocity impact and atomic oxygen: The role of mechanical properties at elevated temperatures. Acta Mater. 2009, 57, 1112–1119. [Google Scholar] [CrossRef]
- Verker, R.; Grossman, E.; Gouzman, I.; Eliaz, N. POSS-polyimide nanocomposite films: Simulated hypervelocity space debris and atomic oxygen effects. High. Perform. Polym. 2008, 20, 475–491. [Google Scholar] [CrossRef]
- Gouzman, I.; Grossman, E.; Verker, R.; Atar, N.; Bolker, A.; Eliaz, N. Advances in polyimide-based materials for space applications. Adv. Mater. 2019, 31, 1807738. [Google Scholar] [CrossRef]
- Wu, J.; Mather, P.T. POSS polymers: Physical properties and biomaterials applications. Polym. Rev. 2009, 49, 25–63. [Google Scholar] [CrossRef]
- Lichtenhan, J.D.; Pielichowski, K.; Blanco, I. POSS-Based Polymers. Polymers 2019, 11, 1727. [Google Scholar] [CrossRef]
- Dodiuk, H.; Kenig, S.; Blinsky, I.; Dotan, A.; Buchman, A. Nanotailoring of epoxy adhesives by polyhedral-oligomeric-sil-sesquioxanes (POSS). Int. J. Adhes. Adhes. 2005, 25, 211–218. [Google Scholar] [CrossRef]
- Blanco, I. The Rediscovery of POSS: A Molecule Rather than a Filler. Polymers 2018, 10, 904. [Google Scholar] [CrossRef] [PubMed]
- Brunsvold, A.L.; Minton, T.K.; Gouzman, I.; Grossman, E.; Gonzalez, R. An investigation of the resistance of polyhedral oligomeric silsesquioxane polyimide to atomic-oxygen attack. High. Perform. Polym. 2004, 16, 303–318. [Google Scholar] [CrossRef]
- Pistor, V.; Ornaghi, F.G.; Ornaghi Jr, H.L.; Zattera, A.J. Degradation kinetic of epoxy nanocomposites containing different percentage of epoxycyclohexyl—POSS. Polym. Compos. 2012, 33, 1224–1232. [Google Scholar] [CrossRef]
- Strachota, A.; Kroutilová, I.; Kovářová, J.; Matějka, L. Epoxy networks reinforced with polyhedral oligomeric silsesquioxanes (POSS). Thermomechanical properties. Macromolecules 2004, 37, 9457–9464. [Google Scholar] [CrossRef]
- Pellice, S.A.; Fasce, D.P.; Williams, R.J.J. Properties of epoxy networks derived from the reaction of diglycidyl ether of bisphenol A with polyhedral oligomeric silsesquioxanes bearing OH-functionalized organic substituents. J. Polym. Sci. Part. B Polym. Phys. 2003, 41, 1451–1461. [Google Scholar] [CrossRef]
- Xiao, F.; Sun, Y.; Xiu, Y.; Wong, C.P. Preparation, thermal and mechanical properties of POSS epoxy hybrid composites. J. Appl. Polym. Sci. 2007, 104, 2113–2121. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, Y.; Liu, S. Polyhedral oligomeric silsesquioxane (POSS)-modified thermoplastic and thermosetting nanocomposites: A review. Polym. Polym. Compos. 2008, 16, 483–500. [Google Scholar] [CrossRef]
- Jones, I.K.; Zhou, Y.X.; Jeelani, S.; Mabry, J.M. Effect of polyhedral-oligomeric-sil-sesquioxanes on thermal and mechanical behavior of SC15 epoxy. eXPRESS Polym. Lett. 2008, 2, 494–501. [Google Scholar] [CrossRef]
- Ramírez, C.; Rico, M.; Torres, A.; Barral, L.; López, J.; Montero, B. Epoxy/POSS organic–inorganic hybrids ATR-FTIR and DSC studies. Eur. Polym. J. 2008, 44, 3035–3045. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, Y.; Deng, Y.; Yang, C.; Chen, J.; Dai, L. Morphology and thermal properties of organic–inorganic hybrid material involving monofunctional-anhydride POSS and epoxy resin. Mater. Chem. Phys. 2011, 125, 174–183. [Google Scholar] [CrossRef]
- Matějka, L.; Murias, P.; Pleštil, J. Effect of POSS on thermomechanical properties of epoxy–POSS nanocomposites. Eur. Polym. J. 2012, 48, 260–274. [Google Scholar] [CrossRef]
- Pistor, V.; Soares, B.G.; Mauler, R.S. Influence of the polyhedral oligomeric silsesquioxane n-phenylaminopropyl-POSS in the thermal stability and the glass transition temperature of epoxy resin. Polímeros 2013, 23, 331–338. [Google Scholar] [CrossRef]
- Pistor, V.; Soares, B.G.; Mauler, R.S. Influence of different concentrations of n-phenylaminopropyl–POSS on the thermodynamic fragility of the cured epoxy resin. Polymer 2013, 54, 2292–2298. [Google Scholar] [CrossRef]
- Longhi, M.; Pistor, V.; Pandolphi Zini, L.; Jonko Birriel, E.; Raquel Kunst, S.; Zattera, A.J. Influence of the functionality of polyhedral oligomeric silsesquioxane-POSS containing glycidyl groups on the dispersion and interaction with epoxy nanocomposites. Polym. Compos. 2016, 38, 229–236. [Google Scholar] [CrossRef]
- Longhi, M.; Pistor, V.; Zini, L.P.; Kunst, S.R.; Zattera, A.J. Influence of functionality of polyhedral oligomeric silsesquioxane (POSS) dispersed in epoxy resin for application in hybrid coating. Mater. Sci. Forum 2017, 899, 278–282. [Google Scholar] [CrossRef]
- Mishra, K.; Pandey, G.; Singh, R.P. Enhancing the mechanical properties of an epoxy resin using polyhedral oligomeric silsesquioxane (POSS) as nano-reinforcement. Polym. Test. 2017, 62, 210–218. [Google Scholar] [CrossRef]
- Mishra, K.; Singh, R.P. Quantitative evaluation of the effect of dispersion techniques on the mechanical properties of polyhedral oligomeric silsesquioxane (POSS)-epoxy nanocomposites. Polym. Compos. 2018, 39, 2445–2453. [Google Scholar] [CrossRef]
- Mishra, K.; Babu, L.K.; Dhakal, D.; Lamichhane, P.; Vaidyanathan, R.K. The effect of solvent on the mechanical properties of polyhedral oligomeric silsesquioxane (POSS)–epoxy nanocomposites. SN Appl. Sci. 2019, 1, 898. [Google Scholar] [CrossRef]
- Xie, T.; Rousseau, I.A. Facile tailoring of thermal transition temperatures of epoxy shape memory polymers. Polymer 2009, 50, 1852–1856. [Google Scholar] [CrossRef]
- Cicala, G.; Blanco, I.; Latteri, A.; Ognibene, G.; Agatino Bottino, F.; Fragalà, M.E. PES/POSS soluble veils as advanced modifiers for multifunctional fiber reinforced composites. Polymers 2017, 9, 281. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Nie, K.; Zheng, S. Thermomechanical, surface and shape memory properties of thermosetting blends of epoxy with Poly (ethylene oxide): An impact of POSS microdomain formation. Mater. Chem. Phys. 2020, 240, 122183. [Google Scholar] [CrossRef]
- Chang, P.; Xu, S.; Zhao, B.; Zheng, S. A design of shape memory networks of poly(ε-caprolactone)s via POSS-POSS interactions. Polym. Adv. Technol. 2018, 30, 713–725. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, G.; Shen, X.; Yan, X.; Nie, J. Poly(ε-caprolactone)-based shape memory polymers crosslinked by polyhedral oligomeric silsesquioxane. RSC Adv. 2016, 6, 90212–90219. [Google Scholar] [CrossRef]
- Gu, S.Y.; Gao, X.F. Improved shape memory performance of star-shaped POSS-polylactide based polyurethanes (POSS-PLAUs). RSC Adv. 2015, 5, 90209–90216. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, S.; Li, L.; Zheng, S. Physically cross-linked networks of POSS-capped poly (acrylate amide) s: Synthesis, morphologies, and shape memory behaviour. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 587–600. [Google Scholar] [CrossRef]
- Chatterjee, T.; Naskar, K. Thermo-sensitive shape memory polymer nanocomposite based on polyhedral oligomeric silsesquioxane (POSS) filled polyolefins. Polym. Plast. Technol. Eng. 2019, 58, 630–640. [Google Scholar] [CrossRef]
- Kazemi, F.; Sadeghi, G.M.M.; Kazemi, H.R. Synthesis and evaluation of the effect of structural parameters on recovery rate of shape memory polyurethane-POSS nanocomposites. Eur. Polym. J. 2019, 114, 446–451. [Google Scholar] [CrossRef]
- Lascano, D.; Quiles-Carrillo, L.; Torres-Giner, S.; Boronat, T.; Montanes, N. Optimization of the curing and post-curing conditions for the manufacturing of partially bio-based epoxy resins with improved toughness. Polymers 2019, 11, 1354. [Google Scholar] [CrossRef]
- Bandzierz, K.; Reuvekamp, L.; Dryzek, J.; Dierkes, W.; Blume, A.; Bielinski, D. Influence of network structure on glass transition temperature of elastomers. Materials 2016, 9, 607. [Google Scholar] [CrossRef]
- Blanco, I.; Abate, L.; Bottino, F.A. Mono substituted octaphenyl POSSs: The effects of substituents on thermal properties and solubility. Thermochim. Acta 2017, 655, 117–123. [Google Scholar] [CrossRef]
- Celikbag, Y.; Robinson, T.J.; Via, B.K.; Adhikari, S.; Auad, M.L. Pyrolysis oil substituted epoxy resin: Improved ratio optimization and crosslinking efficiency. J. Appl. Polym. Sci. 2015, 132, 42239. [Google Scholar] [CrossRef]
- Wang, X.; Gillham, J.K. Competitive primary amine/epoxy and secondary amine/epoxy reactions: Effect on the isothermal time-to-vitrify. J. Appl. Polym. Sci. 1991, 43, 2267–2277. [Google Scholar] [CrossRef]
- Zheng, N.; Fang, G.; Cao, Z.; Zhao, Q.; Xie, T. High strain epoxy shape memory polymer. Polym. Chem. 2015, 6, 3046–3053. [Google Scholar] [CrossRef]
- Joshi, V.; Srividhya, M.; Dubey, M.; Ghosh, A.K.; Saxena, A. Effect of functionalization on dispersion of POSS-silicone rubber nanocomposites. J. Appl. Polym. Sci. 2013, 130, 92–99. [Google Scholar] [CrossRef]
- Catalán-Toledo, J.; Nenen, A.; Vallejos, G.A.; Oyarzun-Ampuero, F.; Shibue, T.; Nishide, H.; Moreno-Villoslada, I. A simple and green methodology to assemble poly (4-vinylpyridine) and a sulfonated azo-dye for obtaining stable polymeric nanoparticles. Polymer 2018, 158, 289–296. [Google Scholar] [CrossRef]
- Moreno-Villoslada, I.; González, F.; Rivera, L.; Hess, S.; Rivas, B.L.; Shibue, T.; Nishide, H. Aromatic− aromatic interaction between 2,3,5-Triphenyl-2H-tetrazolium chloride and poly (sodium 4-styrenesulfonate). J. Phys. Chem. B 2007, 111, 6146–6150. [Google Scholar] [CrossRef]
- Edwards, S.F.; Takano, H.; Terentjev, E.M. Dynamic mechanical response of polymer networks. J. Chem. Phys. 2000, 113, 5531–5538. [Google Scholar] [CrossRef]
- Kourki, H.; Mortezaei, M.; Navid Famili, M.H. Filler networking in the highly nanofilled systems. J. Thermoplast. Compos. Mater. 2016, 29, 1047–1063. [Google Scholar] [CrossRef]
- Lee, A.; Lichtenhan, J.D. Viscoelastic responses of polyhedral oligosilsesquioxane reinforced epoxy systems. Macromolecules 1998, 31, 4970–4974. [Google Scholar] [CrossRef]
- Pan, R.; Shanks, R.; Yang, Q.; Luo, H. Critical role of tetrasilanolphenyl–POSS moieties in competing mechanism of rigid cages and soft segments and its effect on the glass transition temperature of epoxy hybrids. Comp. Mater. Sci. 2018, 152, 78–84. [Google Scholar] [CrossRef]
- Pistor, V.; Soares, B.G.; Mauler, R.S. Influence of different percentages of N-phenylaminopropyl-poss on the degradation kinetic of epoxy resin. Polym. Compos. 2012, 33, 1437–1444. [Google Scholar] [CrossRef]
- Sombatsompop, N.; Wood, A.K. Measurement of thermal conductivity of polymers using an improved Lee’s Disc apparatus. Polym. Test. 1997, 16, 203–223. [Google Scholar] [CrossRef]
- Hexion Corporation, EPON™ Resin 826-Technical Data Sheet, 2005. Available online: https://www.hexion.com/CustomServices/PDFDownloader.aspx?type=tds&pid=f0b7842c-5814-6fe3-ae8a-ff0300fcd525 (accessed on 13 September 2020).
- Huntsman Corporation, The JEFFAMINE Polyetheramines-Huntsman, 2017. Available online: https://pdf4pro.com/view/the-jeffamine-polyetheramines-huntsman-24de09.html (accessed on 13 September 2020).
- Hybrid Plastics, AM7C81.02 (70 wt% AM0281 in 30 wt% PGMEA), 2018. Available online: https://hybridplastics.com/product/am0281-n-phenylaminopropyl-poss-cage-mixture (accessed on 13 September 2020).
- Hybrid Plastics, EP0409-Glycidyl POSS Cage Mixture, 2018. Available online: https://hybridplastics.com/product/ep0409-glycidyl-poss-cage-mixture/ (accessed on 13 September 2020).
- Lee, W.I.; Loos, A.C.; Springer, G.S. Heat of reaction, degree of cure, and viscosity of Hercules 3501-6 resin. J. Compos. Mater. 1982, 16, 510–520. [Google Scholar] [CrossRef]
- Iijima, T.; Yoshioka, N.; Tomoi, M. Effect of cross-link density on modification of epoxy resins with reactive acrylic elastomers. Eur. Polym. J. 1992, 28, 573–581. [Google Scholar] [CrossRef]
- Ruiz, Q.; Pourchet, S.; Placet, V.; Plasseraud, L.; Boni, G. New eco-friendly synthesized thermosets from isoeugenol-based epoxy resins. Polymers 2020, 12, 229. [Google Scholar] [CrossRef]
- Rousseau, I.A.; Xie, T. Shape memory epoxy: Composition, structure, properties and shape memory performances. J. Mater. Chem. 2010, 20, 3431–3441. [Google Scholar] [CrossRef]
- Li, F.; Qi, L.; Yang, J.; Xu, M.; Luo, X.; Ma, D. Polyurethane/conducting carbon black composites: Structure, electric conductivity, strain recovery behavior, and their relationships. J. Appl. Polym. Sci. 2000, 75, 68–77. [Google Scholar] [CrossRef]
Sample Availability: Samples are not available from the authors. |




| Sample Designation | wt.% Resin (Epoxide, mol) | wt.% Crosslinker Agent (Amine, mol) | wt.% POSS (Epoxide/Amine, mol) |
|---|---|---|---|
| Pristine epoxy (Epoxy) [51] | 75 (1) | 25 (1) | 0 (0) |
| 10 AM | 70 (1) | 20 (0.86) | 10 (0.14) |
| 20 AM | 65 (1) | 15 (0.70) | 20 (0.30) |
| 30 AM | 60 (1) | 10 (0.52) | 30 (0.48) |
| 40 AM | 55 (1) | 5 (0.28) | 40 (0.72) |
| 50 AM | 50 (1) | 0 (0) | 50 (1) |
| 10 EP | 65 (0.86) | 25 (1) | 10 (0.14) |
| 20 EP | 55 (0.72) | 25 (1) | 20 (0.28) |
| 30 EP | 45 (0.58) | 25 (1) | 30 (0.42) |
| 40 EP | 34 (0.44) | 26 (1) | 40 (0.56) |
| 50 EP | 24 (0.30) | 26 (1) | 50 (0.70) |
| 60 EP | 14 (0.18) | 26 (1) | 60 (0.82) |
| 73 EP | 0 (0) | 27 (1) | 73 (1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bram, A.I.; Gouzman, I.; Bolker, A.; Eliaz, N.; Verker, R. The Effect of POSS Type on the Shape Memory Properties of Epoxy-Based Nanocomposites. Molecules 2020, 25, 4203. https://doi.org/10.3390/molecules25184203
Bram AI, Gouzman I, Bolker A, Eliaz N, Verker R. The Effect of POSS Type on the Shape Memory Properties of Epoxy-Based Nanocomposites. Molecules. 2020; 25(18):4203. https://doi.org/10.3390/molecules25184203
Chicago/Turabian StyleBram, Avraham I., Irina Gouzman, Asaf Bolker, Noam Eliaz, and Ronen Verker. 2020. "The Effect of POSS Type on the Shape Memory Properties of Epoxy-Based Nanocomposites" Molecules 25, no. 18: 4203. https://doi.org/10.3390/molecules25184203
APA StyleBram, A. I., Gouzman, I., Bolker, A., Eliaz, N., & Verker, R. (2020). The Effect of POSS Type on the Shape Memory Properties of Epoxy-Based Nanocomposites. Molecules, 25(18), 4203. https://doi.org/10.3390/molecules25184203







