Abstract
Calixarene-analogous metacyclophanes (CAMs) are a special class of cyclophanes that are cyclic polyaromatic hydrocarbons containing three or more aromatic rings linked by one or more methylene bridging groups. They can be considered to be analogues of calixarenes, since, in both types of molecules, the component aromatic rings are linked by methylene groups, which are meta to each other. Since the prototype or classical calix[4]arene consists of four benzene rings each linked by methylene bridges, which are also meta to each other, it can be considered to be an example of a functionalized [1.1.1.1]metacyclophane. A metacyclophane (MCP) that consists of three individual hydroxyl-group functionalized aromatic rings linked by methylene groups, e.g., a trihydroxy[1.1.1]MCP may therefore, by analogy, be termed in the broadest sense as a “calix[3]arene” or a “calix[3]arene-analogous metacyclophane”. Most of the CAMs reported have been synthesized by fragment coupling approaches. The design, synthesis and development of functionalized CAMs, MCPs, calixarenes and calixarene analogues has been an area of great activity in the past few decades, due their potential applications as molecular receptors, sensors and ligands for metal binding, and for theoretical studies, etc. In this review article, we focus mainly on the synthesis, structure and conformational properties of [1.1.1]CAMs, i.e., “calix[3]arenes” and their analogues, which contain three functionalized aromatic rings and which provide new scaffolds for further explorations in supramolecular and sensor chemistry.
1. Introduction
Cyclophanes are an important class of bridged aromatic hydrocarbons, consisting of one or more aromatic units linked by methylene group chains in such a way that the aromatic units form part of a macrocycle. A generic synthetic structure of cyclophanes in which two or more aromatic groups are linked via their ortho, meta or para positions by variably sized methylene groups is shown in Figure 1. These molecules have been extensively pursued and studied for a variety of different considerations [1,2]. Cyclophane chemistry can be considered to have been initiated by Pellegrin [3] who, in 1899, reported the synthesis of [2.2]metacyclophane (i.e., [2,2]MCP) 1 by Wurtz coupling of 1,3-bis(bromomethyl)benzene. The real development of the chemistry of cyclophanes, however, is well-acknowledged to have occurred after Cram and Steinberg’s synthesis of [2.2]paracyclophane 2 in 1951 [4].
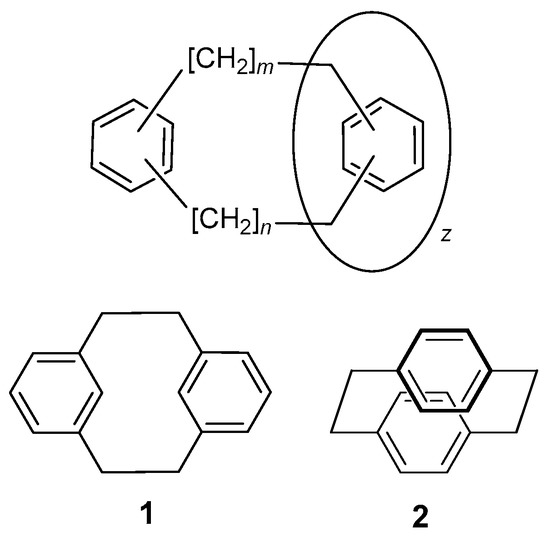
Figure 1.
Top: Generic cyclophane structure with the ortho-, meta- or para-substitution of the phenyl rings and where m, n and z are variable. Compound 1 is [2.2]metacyclophane and 2 is [2.2]paracyclophane, where m = n = 2 and z = 1.
On the other hand, calixarenes such as 3a–d (Figure 2), which can be considered to be examples of [1n]metacyclophanes (where n = 4, 5, 6 or 8, respectively), have also become an important class of compounds since Zincke and Zeigler’s first report in 1944 of a cyclic tetrameric compound (later confirmed to be 3a) from the base-induced reaction of para-substituted tert-butylphenol with formaldehyde [5]. The importance of this class of compounds derives from the fact that calixarenes have “basket”- or “cup”-like 3-D topologies, with the hydroxyl groups forming a narrower or “lower” rim due to the strong intermolecular hydrogen bonds. The opposite rim is termed the “upper” rim and is wider due to the steric repulsion of the para-substituents and the orientation of the aromatic rings although for the larger calix[n]arenes (n > 6); however, their shapes or conformations can vary or be more complex. As a result, these molecules can be selectively functionalized at either rim and they can serve as building blocks for a variety of applications, such as, for example, ionic or molecular receptors. Gutsche and coworkers pioneered and developed methodologies for synthesizing p-tert-butylcalix[4]arene 3a [6] (also commonly and inaccurately referred to simply as “calix[4]arene”, which should strictly be confined to the de-tert-butylated derivative of 3a), p-tert-butylcalix[5]arene 3b [7], p-tert-butylcalix[6]arene 3c [8], and p-tert-butylcalix[8]arene 3d [9] (recently, Haase reported a highly selective and high-yield synthesis for the production of calix[8]arenes [10]) as major products. Gutsche’s syntheses were achieved using p-tert-butylphenol and formaldehyde under different but reproducible reaction conditions, which have allowed these compounds, and derivatives thereof, to become easily accessible to be widely studied. Derivatives of 3a are well-known to be able to exist in four different major conformationally immobile conformers known as cone, partial-cone, 1,3-alternate, and 1,2-alternate conformers (Figure 3) and which can be selectively generated and isolated by introducing different functional groups onto the lower rim of 3a [11].
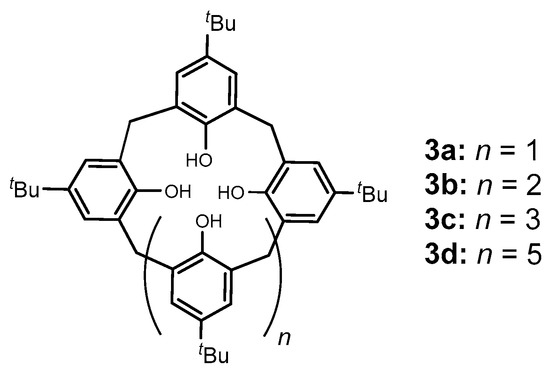
Figure 2.
Basic structural motifs of calix[n]arenes 3a–d.
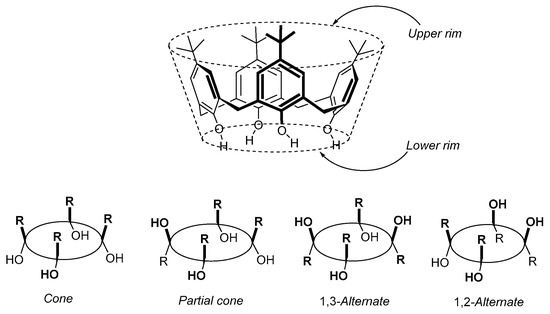
Figure 3.
General structure of p-substituted calix[4]arene (e.g., R = t-Bu) and schematic representations of its formal conformational isomers.
Many calixarene-based molecular receptors have been synthesized and studied, but calix[4]arenes and its derivatives with amide, ketone and ester functionalities at the lower rim, which have shown significant cation affinity [12,13], have received the most attention to date. In general, modifications of calixarenes have been via functionalization of their lower or upper rims. The spirodienone route [14] and Lhtoak’s mercuration [15] route, however, are notable exceptions for effecting the direct modifications to the basic calix[4]arene skeleton not specifically involving the functionalization of the upper and/or lower rims, or via convergent fragment-based approaches. Since there are many excellent reviews and monographs that have been published on calixarenes, and their more typical analogues, including homocalixarenes [16], homooxacalixarenes [17,18], azacalixarenes [19] and hexahomoazacalixarenes [20], and other types [21,22] the reader is referred to these so will not be further elaborated upon here. Instead, for this review, we have chosen to emphasize the MCP skeleton that has been extensively explored as a versatile and stable platform, or scaffold, for functionalization and study. In particular, we present a specific focus upon those functionalized MCPs, which in the broadest sense, can be considered to be directly analogous with calix[3]arenes in general. A review of the syntheses and properties of cyclophanes incorporating three aromatic units is presented below.
2. Calixarene-Analogous MCPs Containing Three Aromatic Rings: Calix[3]arene Analogues
Many reports during the past few decades have dealt with the modification and studies of the properties of calix[n]arenes, particularly those in which n = 4,5,6 or 8, but not many have concerned calix[3]arenes containing only three aromatic rings, i.e., calix[3]arenes or [1.1.1]MCPs (or [1n]MCPs), with the exception of the hexahomoxacalix[3]arenes in which the bridging groups are one or more -CH2OCH2- groups [17,18]. [1.1.1]MCPs cyclophanes, which incorporate only three aromatic rings, have been a major focus of our group in recent years and provide useful molecular platforms, particularly for their synthesis and study of their conformational properties and molecular strain. In 1982, Moshfegh and co-workers reported the first synthesis of a series of functionalized [1.1.1]MCPs, i.e., p-halocalix[3]-arenes 4a–c [23] (Figure 4).
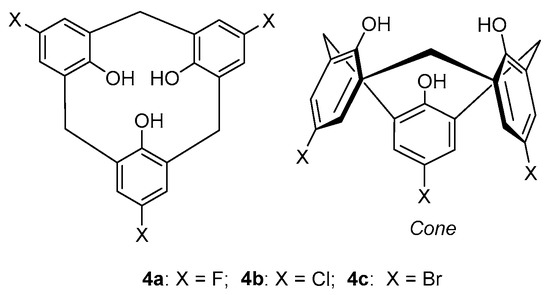
Figure 4.
General structures of the [1.1.1]metacyclophane (MCP) calixarene analogues 4a–c.
These compounds which contained intra-annular hydroxy groups and halogens on the para positions of one, two or all three of the phenyl groups and which adopted cone type conformations, were obtained in 69–90% yields by the cyclocondensation(s) of the corresponding precursor mono- or dihalo-2,2’-dihydroxydiphenyl-methane with 2,6-bis(hydroxymethyl)phenol, or with 4-halo-2,6-bis(hydroxymethyl)phenols [23]. The antimicrobial properties of these compounds which were named as “phloroglucide” analogues were reported but surprisingly, to date, no other studies have been reported with these particular compounds.
3. Homocalixarene-Analogous MCPs Containing Three Aromatic Rings: Homocalix[3]arene Analogues
Expanded macrocyclic calixarene-analogous metacyclophanes (CAMs) containing polymethylene bridging groups such as [3.1.1]MCP 7 which contains a single trimethylene (−(CH2)3−) and two methylene bridges can be regarded as an example of an unsymmetrical homocalix[3]arene analogous MCP. Thus, 7 which can be considered to be an example of a [3.1.1]dihomocalix[3]arene-analogous MCP was synthesized using the Nafion-H catalyzed cyclobenzylation of 5 and tert-butylphenol 6 [24,25]. The trimethoxy and trihydroxy derivatives of 7 were also reported. The room temperature 1H-NMR spectrum of dimethoxy 7 revealed two sets of doublets for the methylene protons which implied that it is in an asymmetric “2-partial-cone” conformation, in which the two aromatic rings linked by the trimethylene group are anti to each other as shown in Scheme 1. However, at 80 °C in CDBr3, coalescence of the methylene protons indicates that there is conformational ring flipping above this temperature. A mechanism was proposed in which two possible structures results from the alternating hydrogen bonding formed between one of the methoxy groups and the hydroxyl group. The triol however was fixed in a typical cone conformation with all three hydroxyl groups syn to one another.
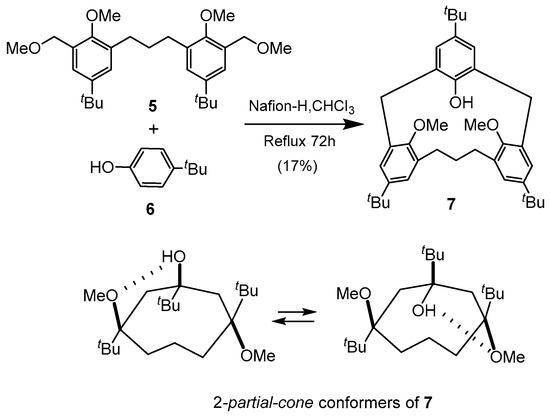
Scheme 1.
Synthesis of hydroxy[3.1.1]MCP calixarene analogue 7 and schematic representation of interconverting 2-partial-cone conformer structures.
Tashiro and coworkers reported the synthesis and conformational properties of the dihomocalix[3]arene containing one methylene and two dimethylene (−(CH2)2−) bridges, i.e., trimethoxy[2.2.1]MCP 8 and several of its derivatives. [26] The synthesis of 8, which can also be considered to be a dihomocalix[3]arene analogue, was found to be a 2:1 mixture of asymmetric and symmetrical atropisoisomers 8a and 8b, respectively (Scheme 2). The synthesis was accomplished via the corresponding dithia[3.3.1]MCP precursor, which was transformed by the oxidation and sulfone pyrolysis-extrusion methodology under vacuum pyrolysis at 450 °C [27,28]. Based upon their 1H-NMR spectra, and with the use of Pirkle’s reagent, these molecules were shown to exist in alternate conformations designated as 2,1-alternate 8a and 2,2-alternate 8b, which could undergo rapid interconversion.
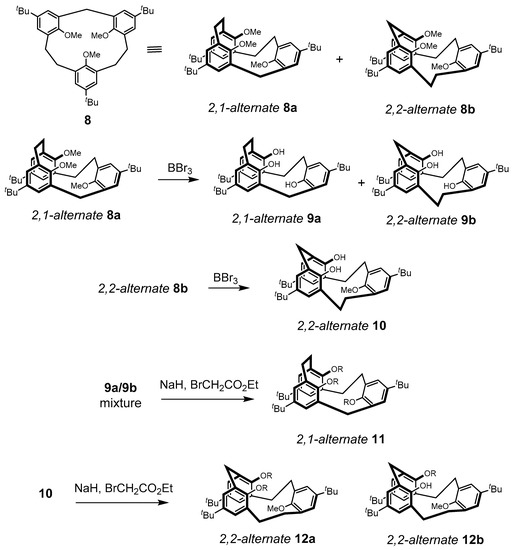
Scheme 2.
Conformers of [2.2.1]MCP dihomocalix[3]arene analogues 8–12 (R = CH2CO2Et).
Trihydroxy[2.2.1]MCPs 9a and 9b were obtained by the simple demethylation of 8a, but only dihydroxymethoxy[2.2.1]MCP 10 was obtained from the demethylation of 8b, the remaining methoxy group presumably being sterically shielded from the BBr3 attack. Treatment of the mixture of 9a and 9b with NaH/ethyl bromoacetate afforded only MCP 11. On the other hand, a 2:1 mixture of both MCPs 12a and 12b was obtained from similar treatment of 2,2-alternate 10. The single-crystal X-ray structures of both 8a and 8b further confirmed their structures. Tashiro’s group had previously reported the synthesis of trimethyl [2.2.2]MCPs [29]. These molecules, however, contained only intra-annular methyl groups and thus cannot be considered to be calix[3]arene-analogous MCPs, which, of course, typically contain hydroxyl or O-alkylated functional groups in the corresponding intra-annular positions.
The efficient syntheses of larger macrocyclic MCPs in which the aromatic groups are linked by varying larger numbers of −CH2− groups can also be effectively accomplished using two other non-sulfur extrusion processes, namely the low-valent zinc McMurry coupling reaction, or the TosMIC (p-tolylsulfonylmethyl isocyanide) [30,31] methodology that was used to great advantage by Vogtle’s group, for the synthesis of [3n]MCPs [32]. The synthesis, conformations and properties of several types of the larger homocalix[3]arene-analogue MCPs [33,34,35,36,37,38], using TosMIC has been reported by our group. For example, the synthesis of the symmetrical all-trimethylene-bridged hexahomocalix[3]arene analogue MCP, namely tri-tert-butyl-trimethoxy[3.3.3]MCP 13a, was achieved via the TosMIC-NaH mediated cyclotrimerization reaction of 2,6-bis(bromomethyl)-4-tert-butylanisole 14, followed by acid hydrolysis, to form trione 15 along with dione 16 in 22% and 10% yields, respectively (Scheme 3) [33], although better yields of 15 (68%) could be obtained using the 1 + 1 coupling reaction of bis-TosMIC adduct 17 with 14, instead, as shown in Scheme 3. Wolff–Kishner reduction of 15 produced the desired tri-tert-butyl-trimethoxy[3.3.3]MCP 13a in 86% yield. The demethylation of 13a with BBr3 in dichloromethane gave the tri-tert-butyl-trihydroxy[3.3.3]MCP 13b in 89% yield. The O-Alkylation of 13b could be achieved in high yields with several alkyl halides (RX: R = Et, Pr, and n-Bu), in the presence of Cs2CO3 under reflux conditions in acetone, to predominantly yield the partial-cone conformers of derivatives 13c–g. The partial-cone conformers of 13c–e were formed exclusively, but, in the cases of 13f and 13g, the partial-cone:cone ratio fell from 95:5 to 67:33. On the other hand, when NaH was used instead as the base to form 13f and 13g also in high yields (>95%), the cone conformers were now exclusively formed. The authors rationalized their results by a mechanism in which ring-inversion of one of the rings could occur at different rates, leading to the formation of the partial-cone isomers with the ethyl, propyl and butyl derivatives. However, with the corresponding larger ethoxycarbonylmethoxy and N,N-diethylaminocarbonylmethoxy derivatives, a “metal-templating” effect with Cs2+ ion and more strongly with the Na+ ion, prevents complete ring inversion. Similar metal-templating effects had been noted previously in the case of O-alkylation reactions with calix[4]arenes. “Doubly-bridged” derivatives of 13b [34] in which two of the hydroxy groups were capped using 3,5-bis(bromomethyl)toluene, or 1-tert-butyl-3,5-bis(bromomethyl)-benzene, Cs2CO3 and acetone under reflux conditions in acetone, was deduced to also be in a partial-cone conformation.
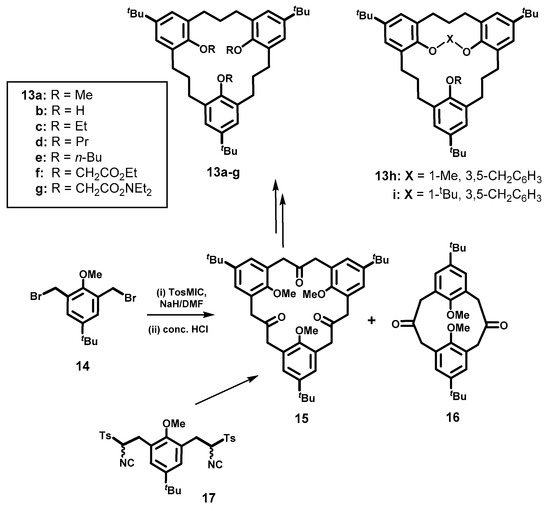
Scheme 3.
Hexahomocalix[3]arene analogue MCPs 13a–g and capped 13h–i.
In a similar manner as with the synthesis of 13a, the tetrahomocalix[3]arene-analogue MCP, tri-tert-butyl-trimethoxy-[3.3.1]MCP 20a was synthesized by a NaH/DMF-mediated cyclocondensation of 17 and 18 followed by Wolff–Kishner reduction of the diketone intermediate 19 (Figure 5). The inherent chirality of the resulting C1-symmetrical 20e was confirmed by its room temperature 1H-NMR spectrum with added Pirkle’s chiral shift reagent, which caused a splitting of the OMe groups and AB patterns corresponding to the methylene protons indicating a 2-partial-cone conformation.
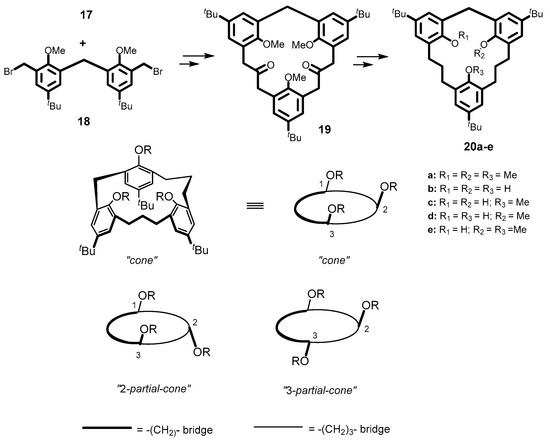
Figure 5.
Synthesis and conformers of tetrahomocalix[3]arene analogue MCPs 20a–e.
The demethylation of 20a using tetramethylsilyl iodide (TMSI) in acetonitrile produced the corresponding trihydroxy[3.3.1]MCP, 20b, as expected, but when BBr3 in dichloromethane was used, the partial demethylation of 20a resulted, forming only the dihydroxy[3.3.1]MCP, 20c. Upon further treatment with TMSI/MeCN, however, 20c afforded the trihydroxy[3.3.1]MCP 20b [38]. Its 1H-NMR spectrum (in CDCl3) exhibits the signals for the hydroxyl groups at ~ δ 3.33 and 6.25 ppm, which is evidence for the formation of intramolecular hydrogen bonding and a cone conformation [38]. In contrast, both the 1H-NMR spectrum and a single crystal analysis confirmed the 2-partial-cone conformation of macrocycle 20c. Its 1H-NMR spectrum shows two sets of doublets at δ 3.61 and 4.36 ppm (J = 13.3 Hz) for the ArCH2Ar methylene protons and a single peak at δ 1.72 ppm for the methoxy protons consistent with a 2-partial-cone conformation. To date, no further studies on either the computational or supramolecular properties of these compounds have been reported.
4. Calixbenzofuran-Analogous MCPs: Calix[3]benzofuran Analogues
The demethylation of [3.3.1]MCP-dione 19 in acetonitrile with in-situ-generated TMSI produced a mixture of benzofuran ring-containing products, the symmetrical 22a and unsymmetrical 23a and a new spirobisdihydro-furan 24 in 24, 45 and 5% yields, respectively (Figure 6) [39]. It is presumed that the trihydroxy-diketo intermediate 21 is first formed, and then undergoes intramolecular TMSI-mediated cyclizations to form the observed products [39,40,41]. Sawada and co-workers had previously reported that the treatment of tetramethoxy [2.1.2.1]MCPs with TMSI formed hemisphere-shaped calixarene analogues containing a dihydrobenzofuran ring [42,43]. The CDCl3 1H-NMR spectrum of the symmetrical 22a shows the hydroxyl signal at δ 6.54 ppm, indicating intramolecular hydrogen bonding between the hydroxyl and the oxygen of one of the benzofuran rings. The single-crystal X-ray structure of 22a confirmed the intramolecular hydrogen bond as predicted from the 1H-NMR spectroscopic data, with a distance of 2.182 Å between the hydroxyl proton and one of the benzofuran oxygens. The unsymmetrical regioisomer 23a is fixed in an asymmetrical hemispherically shaped cone conformation and the spirobisdihydrofuran 24 is presumed to have been formed by a nucleophilic attack on one of the formed benzofurans in 22a.
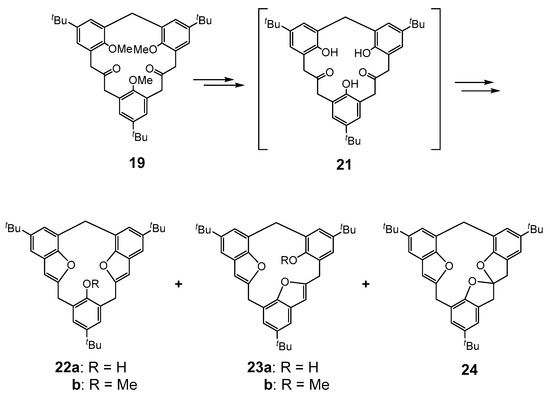
Figure 6.
Calix[3]dibenzofuran analogue MCPs 22–24.
The O-methylation of 23a with MeI/K2CO3 in acetone resulted in the folding of the arene ring into the macrocycle to form the non-symmetrical partial-cone methoxy[1.1.1]MCP 23b, which is evident from the 1H signals observed for the bridge methylene hydrogen atoms. The methoxy group of 23b is shifted to the high field as a singlet at δ 1.97 ppm due to its shielding by the benzofuran rings; these 1H signals correspond to an unsymmetrical partial-cone structure as depicted from density functional theory (DFT)-optimized energy structures. Its 1H-NMR spectra with added Pirkle’s reagent revealed the racemic mixture of P- and M-enantiomers (Figure 7). Single-crystal X-ray analysis of 23b revealed that the macrocyclic skeleton adopts a highly asymmetric hemisphere-shaped cone-type conformation in which the methoxy group is pointed upwards and is exo to the two benzofuran rings, predicted from the 1H-NMR spectra. These molecules, therefore, by adopting curvature in their planar structures that have no symmetry planes in their three-dimensional representations, fit Szumna’s expanded definition of inherent chirality [44,45]. Others, including Böhmer [46,47] and Mandolini [48], had previously proposed and studied inherent chirality in calixarenes.
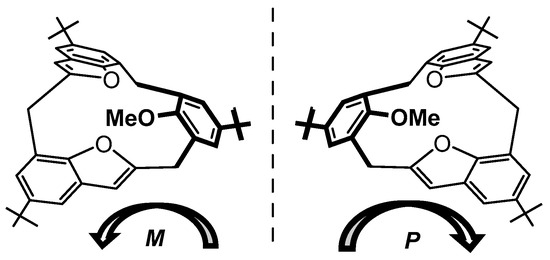
Figure 7.
Schematic diagram of M-23b (left) and P-23b (right). Figure adapted from [39].
The DFT calculations conducted on these molecules using Gaussian-09 [49] showed that the energy-minimized structures of the hemisphere-shaped cone conformers were in agreement with the observed single-crystal X-ray structures. The DFT gas phase calculations using B3LYP/6-31G(d) showed that of the regioisomers 22a and 23a, the latter of which was energetically more favored by 3.791 kJ mole−1. The DFT calculations of the corresponding conformations of methoxy derivatives 22b and 23b showed that the latter was also similarly more favored by 2.358 kJ mole−1, with the methoxy groups being favored to be in exo rather than endo orientations to the benzofuran rings, as shown in Figure 8.
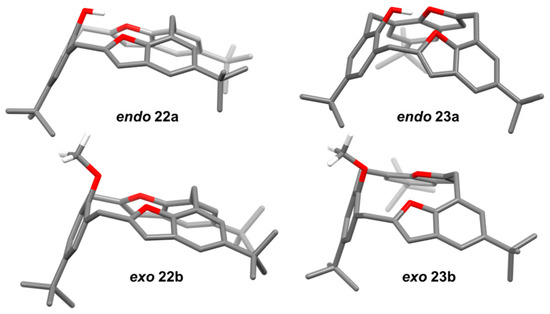
Figure 8.
Geometry-optimized structures of endo 22a and endo 23a; exo 22b and exo 23b. All hydrogens except methoxy and phenolic hydrogens are omitted for clarity. Figure adapted and re-calculated from [39].
A series of derivatives 25b–e of calix[3]benzofuran 25a were synthesized using typical electrophilic aromatic substitution reactions to investigate the influence of the substituents on the conformations of the calix[3]benzofurans. The 1H-NMR spectra of 25a–e reveal that they adopt diverse conformations in solution and in some cases undergo very fast conformational changes relative to the NMR time scale. For example, 25a freely interconverts between cone and saddle conformers (Figure 9) in solution, but the tribromocalix[3]benzofuran 25b, adopts a rigid cone type hemisphere-shaped symmetrical structure in the solid state. The triformyl derivative 25c showed fast cone-saddle interconversion in solution, but, when the three formyl groups were reduced to form the corresponding trihydroxymethyl derivative 25d, the molecule adopted a fixed-cone conformation as with 25b. The acylation of 25a to form 25e once again led to the formation of a slowly-interconverting mixture of cone-saddle conformers (Table 1) [40].
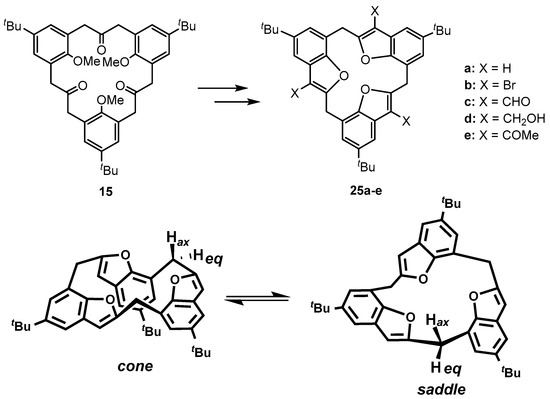
Figure 9.
Calix[3]benzofuran analogue MCPs, 25a–e and cone and saddle conformers of 25a [40].

Table 1.
Influence of substituents on the conformations of benzofurans 25a–e [40].
Solvent and gas-phase DFT determinations with calix[3]benzofuran MCPs 25a–e [40] reveal that both the saddle and corresponding cone conformers have lower ground-state energies in the solvent system than in the gas phase (Figure 10).
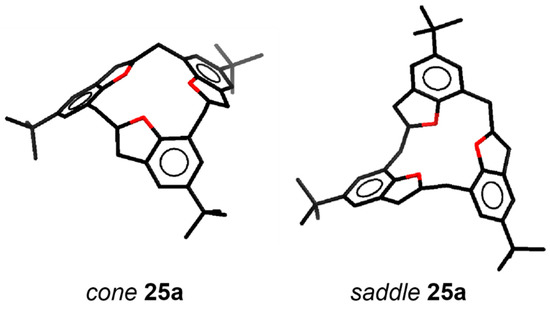
Figure 10.
Geometry-optimized structures of the cone and saddle conformers of 25a. Similar structures were obtained for the cone and saddle conformers of 25b–e and are not shown.
The calculations also show that, among the calix[3]benzofurans, the saddle conformers are energetically more favored than their corresponding cone conformers in both the solvent and gas-phase. The energy differences are in the following order: 23a > 25e > 25d > 25c > 25a > 23b > 22b. Thus, by introducing the different groups at the furan moieties, their saddle conformers become energetically more favored, roughly according to the increasing size of the groups (i.e., COMe > CH2OH > CHO), except for 25b. In the case of 25b, there may be two factors influencing the stability of the cone conformers: bromine is electronegative in nature and also has greater electron density due to multiple lone-pairs of electrons. A single-crystal structure of 25b, however, revealed it to be in a cone conformation in the solid state, but it should be noted that it co-crystallized with a well-defined chloroform molecule and with disordered solvent methanol molecules.
The cycloaddition reaction of 17 and 26, followed by acidic work-up afforded [3.3.3]MCP 27a (Figure 11) which, in turn, when treated with TMSI, generated in situ from chlorotrimethylsilane/sodium iodide in CH3CN, generated, instead of the expected trihydroxy 27b, two calix[3]benzofuran[3.1.1]MCP-analogues 28a and 29 in 52% and 7% yields, respectively [41]. The regioisomer of 28a, namely the unsymmetrical 29, however, could not be isolated, although it was detected in the 1H NMR spectra of the crude reaction mixture. The 1H-NMR spectrum of 28a exhibits a single peak at δ = 4.09 ppm for the ArCH2Ar methylene bridge protons and the trimethylene protons appeared as a broad multiplet at δ 2.01 and 2.98 ppm. The position of the hydroxyl group at δ 6.34 ppm is consistent with the existence of intramolecular hydrogen bonding between the hydroxyl group and the oxygen of one of the benzofuran rings. The formation of 30, which contains the spirobisdihydrofuran moiety, is analogous to the spirobishydrofuran 24 described previously [41].
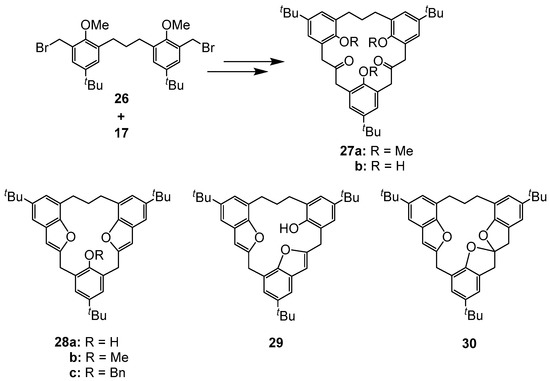
Figure 11.
Structures of calix[3]benzofuran analogue MCPs 27–30.
When 28a was treated with MeI in the presence of K2CO3 in acetone methoxy[3.1.1]MCP 28b was produced as its cone-conformer. This is evident from the 1H signals observed for the bridge methylene hydrogen atoms that are split and appear as a pair of doublets at δ = 3.79 and 4.07 ppm. Previously, we had noted that the O-methylation of 23a under the same conditions resulted instead in the inversion of one of the benzofuran rings, affording the non-symmetrical partial-cone 23b (Figure 6). Thus, the size of the linking methylene chains can have significant effects on the resulting conformers, which are formed [41]. The O-benzylation of 28a, produced the cone-benzyloxy[3.1.1]MCP 28c with no isomerization being observed. This is evident from the 1H signals for the bridge methylene hydrogen atoms that are split and appear as a pair of doublets at δ = 3.77 and 4.45 ppm [41]. In principle, these molecules could adopt three possible conformations, which are schematically represented in Figure 12.
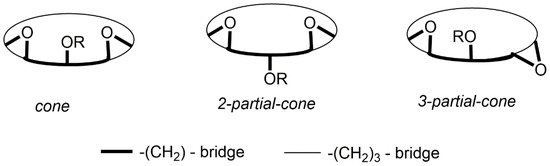
Figure 12.
Schematic representations of the three basic possible conformers of 28 and 29.
Gas-phase DFT computational analysis of the three basic types of conformers of compounds 27a–30 was undertaken using the geometry-optimized structures of each of these conformers [41]. The calculated optimized-energy differences (kJ mol–1) for 27a–30 showed that, of the various conformational isomers of 27a–30, the cone-shaped structures are the most favored energetically, in the following order: cone > 2-partial-cone > 1,3-alternate. For example, the cone conformer of 28a is −13.4 and −23.9 kJ mol−1 more stable than its corresponding 2-partial-cone and 1,3-alternate conformers, respectively. The findings were also consistent with the experimental evidence that the phenolic hydrogen forms weak intramolecular hydrogen bonding with an oxygen atom of one of the benzofuran rings. The distances between the hydroxyl hydrogen and the benzofuran oxygens are, respectively, 2.014 Å and 3.374 Å, 2.698 Å and 4.253 Å, and 2.010 Å and 3.493 Å for the cone, 2-partial-cone and 1,3-alternate conformers of 28a. For the corresponding conformers of 29, the respective distances are 1.836 Å vs. 3.277 Å, 2.605 Å vs. 3.065 Å, and 3.660 Å vs. 3.836 Å.
5. Homooxacalixarene-Analogue MCPs Containing Three Aromatic Rings: “Oxacalix[3]arene” Analogues
Hexahomotrioxacalix[3]arene 31 (Figure 13; R = H), commonly also called “oxacalix[3]arene”, was first reported in 1962 by Hultzsch who isolated it in less than 1% yield from the reaction of formaldehyde with p-tert-butylphenol [50], and the evolution of the chemistry of this and related analogues have been well reviewed recently [17,18]. Hexahomotrioxacalix[3]arene 31 can be considered to be an example of a [3.3.3]MCP in which the phenolic units are linked by −CH2OCH2− bridges. In 1983, Dhawan and Gutsche described detailed reproducible syntheses of 31 [51], which can be conducted in relatively large-scale. Hampton and co-workers also have described an alternative smaller-scale acid-catalyzed procedure which has been used for the synthesis of several different “wide-rim” para-substituted analogues of 31 [52]. Shinkai’s group and others have extensively studied 31 as a platform to generate several versatile hosts [53,54,55,56] since, in comparison with the basic calix[4]arene structure, 31 offers some potential advantages: (1) its intra-annular cavity consists of a 18-membered ring, whereas that of calix[4]arene is a 16-membered ring; (2) the rate of ring inversion for derivatives of 31 should be much faster than that for calix[4]arenes because of the flexibility of the dimethyleneoxa linkages; (3) conformational isomerism is much more simplified, as there are only two types of formal conformations possible, namely cone and partial-cone (Figure 13), in contrast to the four possible formal conformations in calix[4]arenes; (4) the ethereal ring oxygens may act cooperatively with the phenolic oxygens upon binding with metal ions; and (5) its cone and partial-cone conformers can have C3v and/or Cs symmetry, which is particularly useful for the design of receptors for biologically relevant RNH3+ammonium ions.
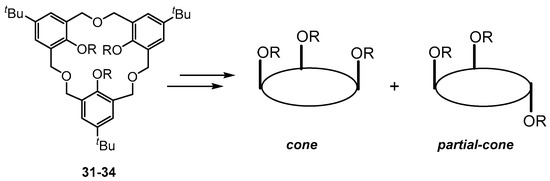
Figure 13.
Structures of hexahomotrioxacalix[3]arene 31 (R = H) and schematic representations of the cone and partial-cone conformers of its tri-O-functionalized derivatives 32 (R = alkyl); 33 (R= ethoxycarbonylmethoxy) and 34 (R = N,N-diethyl O-methylacetamido).
In 1993, Shinkai and coworkers reported that treatment of 31 with four alkyl halides in DMF in the presence of NaH, K2CO3, Cs2CO3, t-BuOK or K afforded the corresponding O-methoxy, O-ethoxy, O-n-propyloxy and O-n-butyloxy derivatives that can adopt cone and/or partial-cone conformations (Figure 13) in varying yields ratios. They showed that a metal templating and solvent effect could lead to preferential thermodynamic and/or a kinetic preference for the partial-cone conformers of the corresponding tri-O-alkylated derivatives. In particular, tri-O-n-butylated 32 (R – n-Bu) was formed exclusively with the use of NaH in DMF but also in a 99:1% ratio over the cone conformer when Cs2CO3 in DMF was used [53]. Shinkai [54,55,56] also reported the synthesis of the tris-O-ethoxycarbonylmethoxy derivative 33 (R = CH2CO2Et) by the reaction of excess ethyl bromoacetate with 31 in acetone under reflux conditions with K2CO3 or Cs2CO3, and that an alkali-metal template effect led exclusively to the formation of the partial-cone-33 conformer. With NaH or t-BuOK in THF, however, a mixture of cone and partial-cone products was formed, with the cone-33 conformer being formed in only a 20–22% yield. Shinkai was also able to show both a metal templating and solvent effect for the efficient formation of the tris-O-amido derivatives 34 (R = CH2CONEt2) from the reactions of 31 with diethylchloroacetamide [55]. With NaH in THF, cone-34 was formed exclusively, but, with K2CO3 or Cs2CO3 in acetone, partial-cone-34 was exclusively formed. With NaH in DMF, a lower overall yield (29 and 26%, respectively) of the cone and partial-cone-34 was obtained. However, with t-BuOK in THF a mixture of cone and partial-cone-34 products was formed in 60 and 38% overall yield.
Recently, 35 the C3-symmetric N-pyridyl O-methylacetamido functionalized derivative of 31 (Figure 14) was reported to be able to selectively and cooperatively bind Ag+ and n-butylammonium ions and be controlled by the metal ion [57]. The geometries of the molecular structures were optimized in the gas phase using the PBE0 functional theory with the LANL2DZ basis set. The calculated binding or interaction energies (IE) for cone-35 ⊃n-BuNH3+, cone-35 ⊃t-BuNH3+, cone-35 ⊃Ag+, and nBuNH3+ ⊂ [cone-35 ⊃Ag+] are −298.8 kJ mol−1, −268.3 kJ mol−1, −457.1 kJ mol−1, and –525.8 kJ·mol−1, respectively, and were in agreement with the trend for the complexation data determined by the 1H-NMR spectroscopic titration experiments with the corresponding perchlorate salts [57]. A conceptualization of the complexation of nBuNH3+ by the receptor cone-35 is shown in Figure 15.
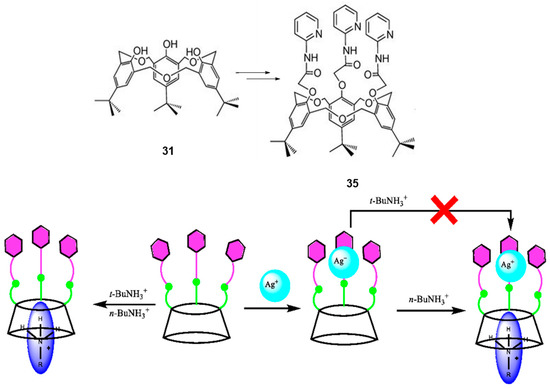
Figure 14.
A schematic representation showing the cooperative binding mode of 35 (shown in a cone conformation) with and without Ag+ complexation and selective binding with n-BuNH3+ and t-BuNH3+ cations. The green circles represent the CONH− and the hexagons represent the pyridyl groups. (Figure take from [57]).
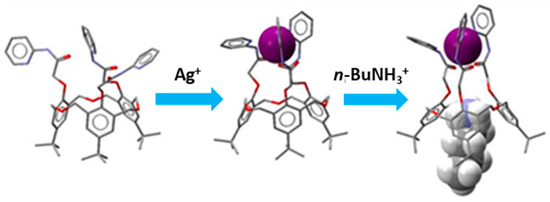
Figure 15.
Geometry-optimized (PBE0/LANL2DZ) structures of cone-35 and its complex with Ag+ and nBuNH3+. Left: The free cone-35, Middle: 1:1 cone-35 ⊃Ag+ complex, and right: nBuNH3+ ⊂ [cone-35 ⊃Ag+] complex. Figure taken from reference [57].
Chirality is also possible with unsymmetrically substituted hexahomooxacalix[3]arenes. Araki et al. had previously reported the “pseudo C2-symmetric inherent chirality” of the di-O-benzylated derivative 36a and the di-O-benzylated-mono-O-methoxy derivative (Figure 16) 36b of oxacalix[3]arene 31, by O-alkylation at the lower rim of 31 and that these molecules were useful for the recognition of α-amino acid derivatives [58].
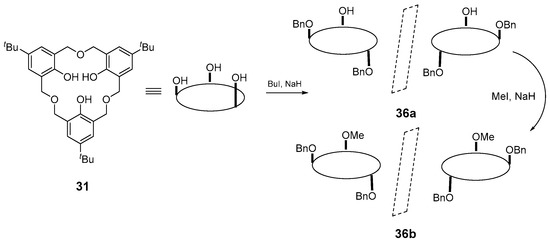
Figure 16.
Synthesis of chiral oxacalixarene MCP analogues 36a–b showing the chiral atropisomers. Figure adapted from reference [58].
In a very recent contribution, Marcos and co-workers reported their latest results with cation and anion ditopic receptors based upon trisubstituted derivatives of 31 with lower-rim O-tert-butylurea, phenylthiourea or phenylurea functionalities, which are connected to the hexahomotrioxacalix[3]arene scaffold via butyl spacers. [59] Both cone and partial-cone conformers were obtained with the phenylurea but only partial-cone products, as determined by solution NMR studies and their binding properties towards several relevant anions with different geometries were assessed by proton NMR titrations. The cone conformation of the trisphenylurea derivative was also confirmed by single-crystal X-ray crystallography and also proved to be the best an anion receptor, with the highest affinity being shown toward the trigonal planar acetate and benzoate anions (log Kassoc = 4.12 and 4.00, respectively) amongst the spherical, linear and tetrahedral anions tested. It also proved to be an effective ditopic receptor for several biogenic amine hydrochlorides monoamine neurotransmitters and trace amine hydrochlorides, in different solvents.
Jabin’s group [60] compared the binding of a series of ammonium ions with two previously reported tris-O-amido derivatives, 34 [54] and the tris-mediated cryptand 37 previously reported by Yamato and coworkers [61] in 10% yields, and later in an improved 50% yield by Jabin and coworkers [62]. Both of these receptors are locked in cone-conformations (Figure 17) and 1H-NMR studies with both formed endo-complexes with seventeen different primary ammonium ion guests, including biomolecules, even in protic media.
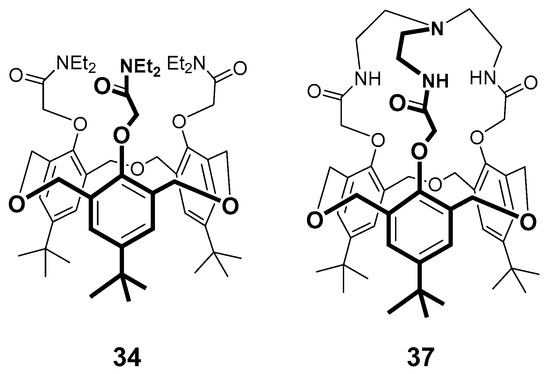
Figure 17.
Structures of hexahomotrioxacalix[3]arene-analogue-MCPs 34 and 37.
The ammonium ions of each of these guests inserted deeply into the polyaromatic cavity of the host receptor molecules. The NH3+ moieties were situated very close to the 18-atom lower-rim macrocyclic plane, forming H-bonds with the three oxygen atoms of the macrocycle. The overall topological complementarity between primary ammonium ions and the two cavity-based receptors resulted in the previously unreported complexation specificity for the primary ammonium ions over the secondary, tertiary and quaternary ones that were tested in this study. The authors were able to take advantage of this and demonstrated selective liquid–liquid extraction of primary ammonium salts from water solutions and for the selective recognition of lysine-containing peptides, with obvious potential for peptide sensing. Interestingly, also, 34 showed better binding than 37 presumably since the capping reduced the flexibility for the polyaromatic cavity to accommodate the guests.
To date, there is only one report of a 2-naphthol ring-analogue of 31, namely oxacalix[3]naphthalene 38, which has its naphthol units linked by -CH2OCH2- bridges and can be considered to be an example of a [3.3.3]metanaphthalenophane analogue of the more common [3.3.3]homooxacalixarenes described above. This compound that was reported in 2001 by our group, was formed in 25% yield along with its unsymmetrical regioisomer in lower yield, via a fragment-based approach in which the linear precursor underwent cyclo-condensation under acidic conditions [63] similar to those described Hampton [51]. Only a limited study of its complexation properties with metal ions was conducted; however, a 1H NMR study of its complexation properties with C60 fullerene was reported; additionally, it formed a solid state 2:1 supramolecular complex in its cone conformation with C60 (Figure 18) [64] in contrast to the 1:1 complexes observed for C60 with 31 and several derivatives thereof reported earlier by Tsubaki et al. [65].
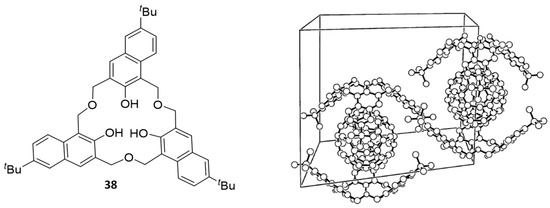
Figure 18.
Hexahomotrioxacalix[3]naphthalene 38 and the single-crystal X-ray structure of its 2:1 complex with fullerene-C60. Figure adapted from Reference [64].
6. Conclusions
In this review article, we have focused mainly upon the synthesis, structure and conformational properties of calixarene-analogous metacyclophanes, which contain three aromatic rings. These molecules can be considered to be analogues of the relatively less-known “calix[3]arenes”. This group of molecules also encompasses homocalix[3]arenes and homooxacalix[3]arenes and can be considered as homocalix[3]arene- and homooxacalix[3]arene-analogous metacyclophanes. To date, the latter, including their supramolecular properties, have been the most extensively studied. This review highlights the origins, synthesis, structural aspects including their inherent chirality, and some of the different modifications that are possible with these molecules as a group that can lead to diverse application possibilities. We have mostly considered and cited the more recent literature that has been published and, where available, the more recently published reviews. The supramolecular properties of the new synthetic homocalix[3]arene-analogous metacyclophanes, which the Yamato group has mainly been focused on, however, have been untapped and will be subjected to further investigation.
Funding
This research received no external funding.
Acknowledgments
M.M.I. and T.Y. thank the OTEC at Saga University for financial support. This work was performed under the Cooperative Research Program of “Network Joint Research Center for Materials and Devices (Institute for Materials Chemistry and Engineering, Kyushu University)”. S.R. would like to thank A. Alodhayb and the Deanship of Scientific Research, King Saud University, Saudi Arabia, ongoing support.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Gleiter, R.; Hopf, H. Modern Cyclophane Chemistry; Wiley-VCH: Weinheim, Germany, 2004; ISBN 978-3-527-30713-5. [Google Scholar]
- Ghasemabadi, P.G.; Yao, T.; Bodwell, G.J. Cyclophanes containing large polycyclic aromatic hydrocarbons. Chem. Soc. Rev. 2015, 44, 6494–6518. [Google Scholar] [CrossRef]
- Pellegrin, M.M. Contribution à l’étude de la réaction de Fittig. Recl. Trav. Chim. Pays-Bas 1899, 18, 457–465. [Google Scholar] [CrossRef]
- Cram, D.J.; Steinberg, H. Macro rings. I. Preparation and spectra of the paracyclophanes. J. Am. Chem. Soc. 1951, 73, 5691–5704. [Google Scholar] [CrossRef]
- Zincke, A.; Ziegler, E. Zur kenntnis des härtungsprozesses von phenol-formaldehyd-harzen, X. mitteilung. Ber. Dtsch. Chem. Ges. 1944, 77, 264–272. [Google Scholar] [CrossRef]
- Gutsche, C.D.; Iqbal, M. p-tert-Butylcalix[4]arene. Org. Synth. 1990, 68, 234. [Google Scholar] [CrossRef]
- Stewart, D.R.; Gutsche, C.D. The one-step synthesis of p-tert-butylcalix[5]arene. Org. Prep. Proc. Intl. 1993, 25, 137–139. [Google Scholar] [CrossRef]
- Gutsche, C.D.; Dhawan, B.; Leonis, M.; Stewart, D.R. p-tert-Butylcalix[6]arene. Org. Synth. 1990, 68, 238. [Google Scholar] [CrossRef]
- Munch, J.H.; Gutsche, C.D. p-tert-Butylcalix[8]arene. Org. Synth. 1990, 68, 243. [Google Scholar] [CrossRef]
- Haase, C.H.W. Path to industrial production of calix[8 and 4]arenes. J. Org. Chem. 2020, 85, 603–611. [Google Scholar] [CrossRef]
- Neri, P.; Sessler, J.; Wang, M.-X. Calixarenes and Beyond; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-31867-7. [Google Scholar]
- Agrawal, Y.K.; Kunji, S.; Menon, S.K. Analytical applications of calixarenes. Rev. Anal. Chem. 1998, 17, 69–139. [Google Scholar] [CrossRef]
- Van Loon, J.D.; Verboom, W.; Reinhoudt, D.N. Selective functionalization and conformational properties of calix[4]arenes, a review. Org. Prep. Proced. Int. 1992, 24, 437–462. [Google Scholar] [CrossRef]
- Gaeta, C.; Talotta, C.; Neri, P. The calixarene p-bromodienone route: From a chemical curiosity to an useful synthetic tool. J. Incl. Phenom. Macrocycl. Chem. 2014, 79, 23–46. [Google Scholar] [CrossRef]
- Tlusty, M.; Eigner, V.; Babor, M.; Kohouta, M.; Lhotak, P. Synthesis of upper rim-double-bridged calix[4]arenes bearing seven membered rings and related compounds. RSC Adv. 2019, 9, 22017–22030. [Google Scholar] [CrossRef]
- Nakamura, Y.; Fujii, T.; Inokuma, S.; Nishimura, J. Homocalixarenes. In Calixarenes 2001; Asfari, Z., Böhmer, V., Harrowfield, J., Vicens, J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 219–234. [Google Scholar] [CrossRef]
- Cottet, K.; Marcos, P.M.; Cragg, P.J. Fifty years of oxacalix[3]arenes: A review. Beilstein J. Org. Chem. 2012, 8, 201–226. [Google Scholar] [CrossRef] [PubMed]
- Masci, B. Homooxa- and homoaza-calixarenes. In Calixarenes 2001; Asfari, Z., Böhmer, V., Harrowfield, J., Vicens, J., Saadioui, M., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 235–249. [Google Scholar] [CrossRef]
- Wang, D.-X.; Wang, M.-X. Azacalixaromatics. In Calixarenes and Beyond; Neri, P., Sessler, J., Wang, M.-X., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 363–397. [Google Scholar] [CrossRef]
- Kaewtong, C.; Pulpoka, P. Azacalix[3]arenes: Chemistry and recent developments in functionalization for specific anion and cation recognition. J. Incl. Phenom. Macrocycl. Chem. 2009, 65, 129–136. [Google Scholar] [CrossRef]
- Chen, C.-F.; Han, Y. Triptycene-derived macrocyclic arenes: From calixarenes to helicarenes. Acc. Chem. Res. 2018, 51, 2093–2106. [Google Scholar] [CrossRef]
- Kohnke, F.H. Calixpyrroles: From anion ligands to potential anticancer drugs. Eur. J. Org. Chem. 2020, 2020, 4261–4272. [Google Scholar] [CrossRef]
- Moshfegh, A.A.; Beladi, E.; Radnia, L.; Hosseini, A.S.; Tofigh, S.; Hakimelahi, G.H. The synthesis of 5,11,17-trihalotetracyclo [13.3.1.13,7.19,13]henicosa-1 (19),3,5,7,9,11,13, 15,17-nonaene-19,20,21-triols and 5,11,17-trihalo-19,20,21-trihydroxytetracyclo [13.3.1.13,7. 19,13]henicosa-1(19),3,5,7(20),9,11,13(21), 15,17-nonaene-8,14-dione. Cyclo-derivatives of phloroglucide analogues. Helv. Chim. Acta 1982, 65, 1264–1270. [Google Scholar] [CrossRef]
- Yamato, T.L.; Doamekpor, K.; Tsuzuki, H.; Tashiro, M. A novel calixarene-analogous macrocyclic metacyclophane ‘molecular pendulum’. Chem. Lett. 1995, 24, 89–90. [Google Scholar] [CrossRef]
- Yamato, T.L.; Doamekpor, K.; Tsuzuki, H. Synthesis and conformational studies of novel calixarene-analogous macrocyclic [3.1.1]metacyclophanes. Liebigs Ann. Recl. 1997, 1997, 1537–1544. [Google Scholar] [CrossRef]
- Tsuge, A.; Sawada, T.; Mataka, S.; Nishiyama, N.; Sakashita, H.; Tashiro, M. Preparation and structural properties of hydroxy- and alkoxy-[2.2.1]metacyclophanes. J. Chem. Soc. Perkin Trans. 1 1992, 1489–1494. [Google Scholar] [CrossRef]
- Laufenberg, S.; Feuerbacher, N.; Pischel, I.; Börsch, O.; Nieger, M.; Vögtle, F. “New biphenylenophanes and biphenylophanes–1,8-dimethylbiphenylene by continuous vacuum pyrolysis. Liebigs Ann. Chem. 1997, 1901–1906. [Google Scholar] [CrossRef]
- Vögtle, F. Cyclophane Chemistry: Synthetics, Structures and Reactions; John Wiley & Sons: Chichester, UK, 1993; ISBN 10: 0471931993. [Google Scholar]
- Tashiro, M.; Watanabe, T.; Tsuge, A.; Sawada, T.; Mataka, S. Metacyclophanes and related compounds. 24. Preparation and reaction of trimethyl[2.2.2]- and tetramethyl[2.2.2.2]metacyclophane. J. Org. Chem. 1989, 54, 2632–2638. [Google Scholar] [CrossRef]
- Possel, O.; van Leusen, A.M. Tosylmethyl isocyanide employed in a novel synthesis of ketones. A new masked formaldehyde reagent. Tetrahedron Lett. 1977, 18, 4229–4231. [Google Scholar] [CrossRef]
- van Leusen, A.M.; Boerna, G.J.; Helholdt, R.B.; Siderius, H.; Strating, J. Chemistry of sulfonylmethylisocyanides. Simple synthetic approaches to a new versatile chemical building block. Tetrahedron Lett. 1972, 13, 2367–2368. [Google Scholar] [CrossRef]
- Breitenbach, J.; Vögtle, F. Macrocyclizations with TosMIC-Yielding [3n]metacyclophanes. Synthesis 1992, 41–43. [Google Scholar] [CrossRef]
- Yamato, T.; Doamekpor, L.K.; Koizumi, K.; Kishi, K.; Haraguchi, M.; Tashiro, M. Synthesis and conformational studies of calixarene-analogous trihydroxy[3.3.3]metacyclophanes and their O-alkylated derivatives. Liebigs Ann. 1995, 7, 1259–1267. [Google Scholar] [CrossRef]
- Yamato, T.; Zhang, F. The synthesis and probable conformation of m-xylenyl capped homocalix[3]arenes derived from a trihydroxy[3.3.3]metacyclophane. J. Chem. Res. 1999, 23, 34–35. [Google Scholar] [CrossRef]
- Yamato, T.; Kohno, K.; Tsuchihashi, K. Synthesis, structures and ion selectivity of homocalix[3]arene thioketals derived from homocalix[3]arene ketones. J. Incl. Phenom. Macrocycl. Chem. 2002, 43, 137–144. [Google Scholar] [CrossRef]
- Yamato, T.; Haraguchi, M.; Nishikawa, J.; Ide, S. Synthesis, conformational studies and inclusion properties of O-benzylated calixarene analogues of trihydroxy[3.3.3]metacyclophanes. J. Chem. Soc. Perkin Trans. 1 1998, 609–614. [Google Scholar] [CrossRef]
- Yamato, T. Synthesis, conformations and inclusion properties of homocalix[3]arenes. J. Incl. Phenom. Macrocycl. Chem. 1998, 32, 195–207. [Google Scholar] [CrossRef]
- Islam, M.M.; Tomiyasu, H.; Thuery, P.; Matsumoto, T.; Tanaka, J.; Elsegood, M.R.J.; Redshaw, C.; Yamato, T. Synthesis and conformational studies of calixarene analogue chiral [3.3.1]metacyclophane. J. Mol. Struct. 2015, 1098, 47–54. [Google Scholar] [CrossRef]
- Islam, M.M.; Tomiyasu, H.; Matsumoto, T.; Tanaka, J.; Rahman, S.; Georghiou, P.E.; Redshaw, C.; Yamato, T. Synthesis and conformational studies of chiral macrocyclic [1.1.1]metacyclophanes containing benzofuran rings. Org. Biomol. Chem. 2015, 13, 9055–9064. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Akther, T.; Ikejiri, Y.; Matsumoto, T.; Tanaka, J.; Rahman, S.; Georghiou, P.E.; Hughes, D.L.; Redshaw, C.; Yamato, T. Synthesis, structural properties, electrophilic substitution reactions and DFT computational studies of calix[3]benzofurans. RSC Adv. 2016, 6, 50808–50817. [Google Scholar] [CrossRef]
- Islam, M.M.; Wang, C.-Z.; Feng, X.; Rahman, S.; Georghiou, P.E.; Alodhayb, A.; Yamato, T. Synthesis, structures and DFT computational studies of [3.1.1]metacyclophanes containing benzofuran rings. ChemistrySelect 2018, 3, 13542–13547. [Google Scholar] [CrossRef]
- Sawada, T.; Hongo, T.; Matsuo, N.; Konishi, M.; Kawaguchi, T.; Ihara, H. Hemisphere-shaped calixarenes and their analogs: Synthesis, structure, and chiral recognition ability. Tetrahedron 2011, 67, 4716–4722. [Google Scholar] [CrossRef]
- Sawada, T.; Nishiyama, Y.; Tabuchi, W.; Ishikawa, M.; Tsutsumi, E.; Kuwahara, Y.; Shosenji, H. Novel calixarene hemisphere synthesized via pinacol rearrangement of [2.1.2.1]metacyclophane. Org. Lett. 2006, 8, 1995–1997. [Google Scholar] [CrossRef]
- Szumna, A. Inherently chiral concave molecules-from synthesis to applications. Chem. Soc. Rev. 2010, 39, 4274–4285. [Google Scholar] [CrossRef]
- Wierzbicki, M.; Jędrzejewska, H.; Szumna, A. Chiral calixarenes and resorcinarenes. In Calixarenes and Beyond; Neri, P., Sessler, J., Wang, M.-X., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 13–42. ISBN 978-3-319-31867-7. [Google Scholar] [CrossRef]
- Wolff, A.; Böhmer, V.; Fogt, W.; Ugozzoli, F.; Andreetti, G.D. Dissymmetric calix[4]arenes with C2 and C4 symmetry. J. Org. Chem. 1990, 55, 5665–5667. [Google Scholar] [CrossRef]
- Pickard, S.T.; Pirkle, W.H.; Tabatabai, M.; Vogt, W.; Böhmer, V. Dissymmetric calix[4]arenes: Optical resolution of some conformationally fixed derivatives. Chirality 1993, 5, 310–314. [Google Scholar] [CrossRef]
- Mandolini, L.; Ungaro, R. Calixarenes in Action; Imperial College Press: London, UK, 2000. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Hultzsch, K. Ring condensates in alkyl phenol resins. Kunststoffe 1962, 52, 19–24. [Google Scholar]
- Dhawan, B.; Gutsche, C.D. Calixarenes. 10. Oxacalixarenes. J. Org. Chem. 1983, 48, 1536–1539. [Google Scholar] [CrossRef]
- Hampton, P.D.; Bencze, Z.; Tong, W.; Daitch, C.E. A new synthesis of oxacalix[3]arene macrocycles and alkali-metal-binding studies. J. Org. Chem. 1994, 59, 4838–4843. [Google Scholar] [CrossRef]
- Araki, K.; Inada, K.; Otsuka, H.; Shinkai, S. Conformational isomerism in and binding properties to alkali-metals and an ammonium salt of O-alkylated homooxacalix[3]arenes. Tetrahedron 1993, 49, 9465–9478. [Google Scholar] [CrossRef]
- Araki, K.; Hashimoto, N.; Otsuka, H.; Shinkai, S. Synthesis and ion selectivity of conformers derived from hexahomotrioxacalix[3]arene. J. Org. Chem. 1993, 58, 5958–5963. [Google Scholar] [CrossRef]
- Matsumoto, H.; Nishio, S.; Takeshita, M.; Shinkai, S. Syntheses and ion selectivities of tri-amide derivatives of hexahomotrioxacalix[3]arene. Remarkably large metal template effect on the ratio of cone vs. partial-cone conformers. Tetrahedron 1995, 51, 4647–4654. [Google Scholar] [CrossRef]
- Takeshita, M.; Inokuchi, F.; Shinkai, S. C3-Symmetrically-capped homotrioxacalix[3]arene. A preorganized host molecule for inclusion of primary ammonium ions. Tetrahedron Lett. 1995, 36, 3341–3344. [Google Scholar] [CrossRef]
- Jiang, X.-K.; Ikejiri, Y.; Wu, C.; Rahman, S.; Georghiou, P.E.; Zeng, X.; Elsegood, M.R.J.; Redshaw, C.; Teat, S.J.; Yamato, T. A hexahomotrioxacalix[3]arene-based ditopic receptor for alkylammonium ions controlled by Ag+ ions. Molecules 2018, 23, 467. [Google Scholar] [CrossRef]
- Araki, K.; Inada, K.; Shinkai, S. Chiral recognition of α-amino acid derivatives with a homooxacalix[3]arene: Construction of a pseudo-C2-symmetrical compound from a C3-symmetrical macrocycle. Angew. Chem. Int. Ed. Engl. 1996, 35, 72–74. [Google Scholar] [CrossRef]
- Teixeira, F.A.; Ascenso, J.R.; Cragg, P.J.; Hickey, N.; Geremia, S.; Marcos, P.M. Recognition of anions, monoamine neurotransmitter and trace amine hydrochlorides by ureido-hexahomotrioxacalix[3]arene ditopic receptors. Eur. J. Org. Chem. 2020, 1930–1940. [Google Scholar] [CrossRef]
- Lambert, S.; Bartik, K.; Jabin, I. Specific binding of primary ammonium ions and lysine-containing peptides in protic solvents by hexahomotrioxacalix[3]arenes. J. Org. Chem. 2020, 85, 10062–10071. [Google Scholar] [CrossRef]
- Jiang, X.-K.; Deng, M.; Mu, L.; Zeng, X.; Zhang, J.X.; Yamato, T. Synthesis and crystal structure of a novel hexahomotrioxacalix[3]cryptand. Asian J. Chem. 2013, 25, 515–517. [Google Scholar] [CrossRef]
- Zahim, S.; Ajami, D.; Laurent, P.; Valkenier, H.; Reinaud, O.; Luhmer, M.; Jabin, I. Synthesis and binding properties of a tren-capped hexahomotrioxacalix[3]arene. ChemPhysChem 2019, 21, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Ashram, M.; Mizyed, S.; Georghiou, P.E. Synthesis of hexahomotrioxacalix[3]naphthalenes and a study of their alkali-metal cation binding properties. J. Org. Chem. 2001, 66, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Mizyed, S.; Ashram, M.; Miller, D.O.; Georghiou, P.E. Supramolecular complexation of [60]fullerene with hexahomotrioxacalix[3]naphthalenes: A new class of naphthalene-based calixarenes. J. Chem. Soc. Perkin Trans. 2 2001, 1916–1919. [Google Scholar] [CrossRef]
- Tsubaki, K.; Tanaka, K.; Kinoshita, T.; Fuji, K. Complexation of C60 with hexahomooxacalix[3]arenes and supramolecular structures of complexes in the solid state. Chem. Commun. 1998, 895–896. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).