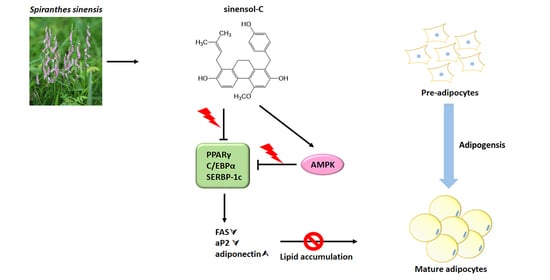
Sinensol-C Isolated from Spiranthes sinensis Inhibits Adipogenesis in 3T3-L1 Cells through the Regulation of Adipogenic Transcription Factors and AMPK Activation
Abstract
1. Introduction
2. Results
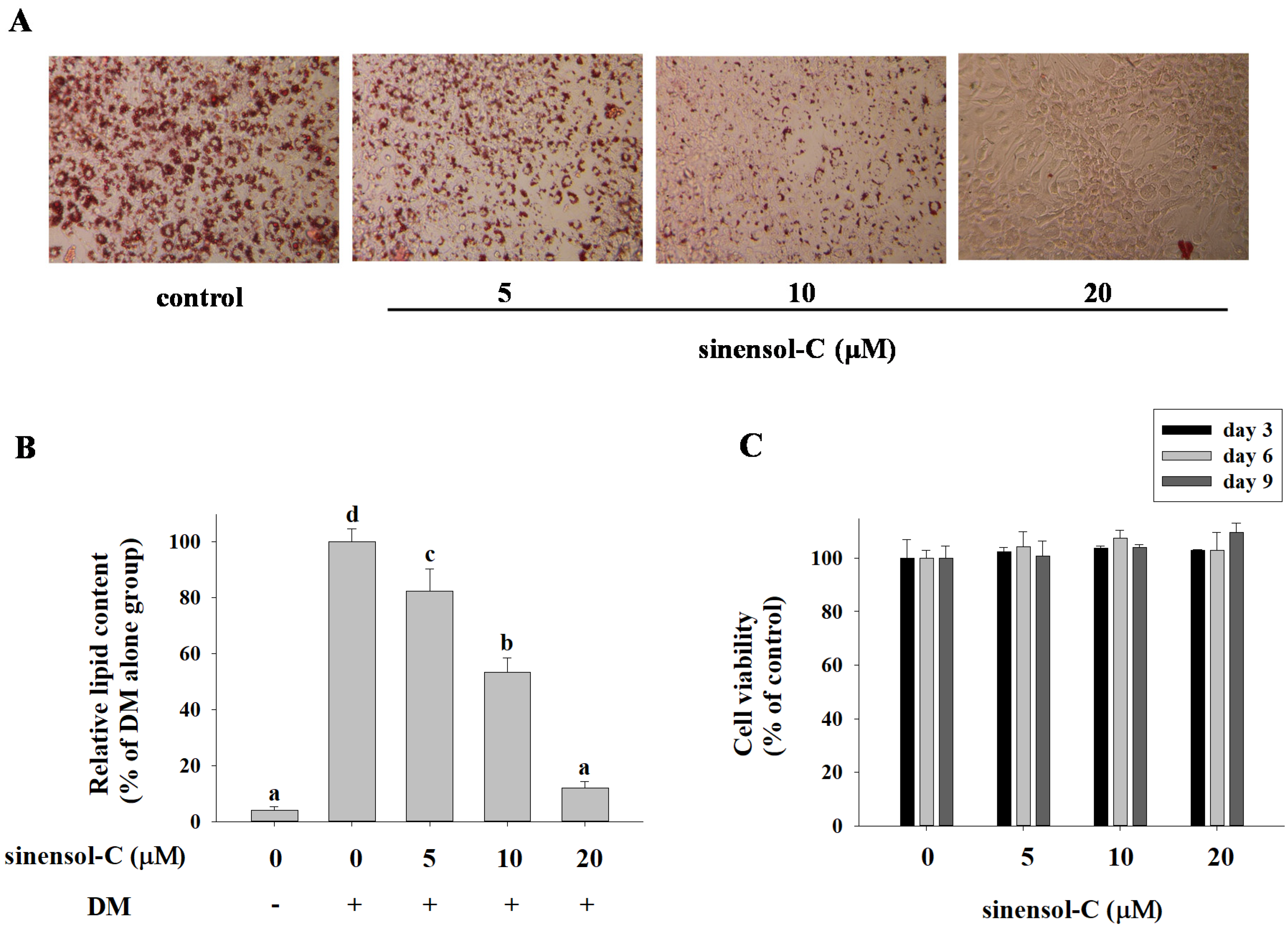
2.1. Effect of the Compounds Isolated from S. Sinensis on Adipocyte Differentiation in 3T3-L1 Cells
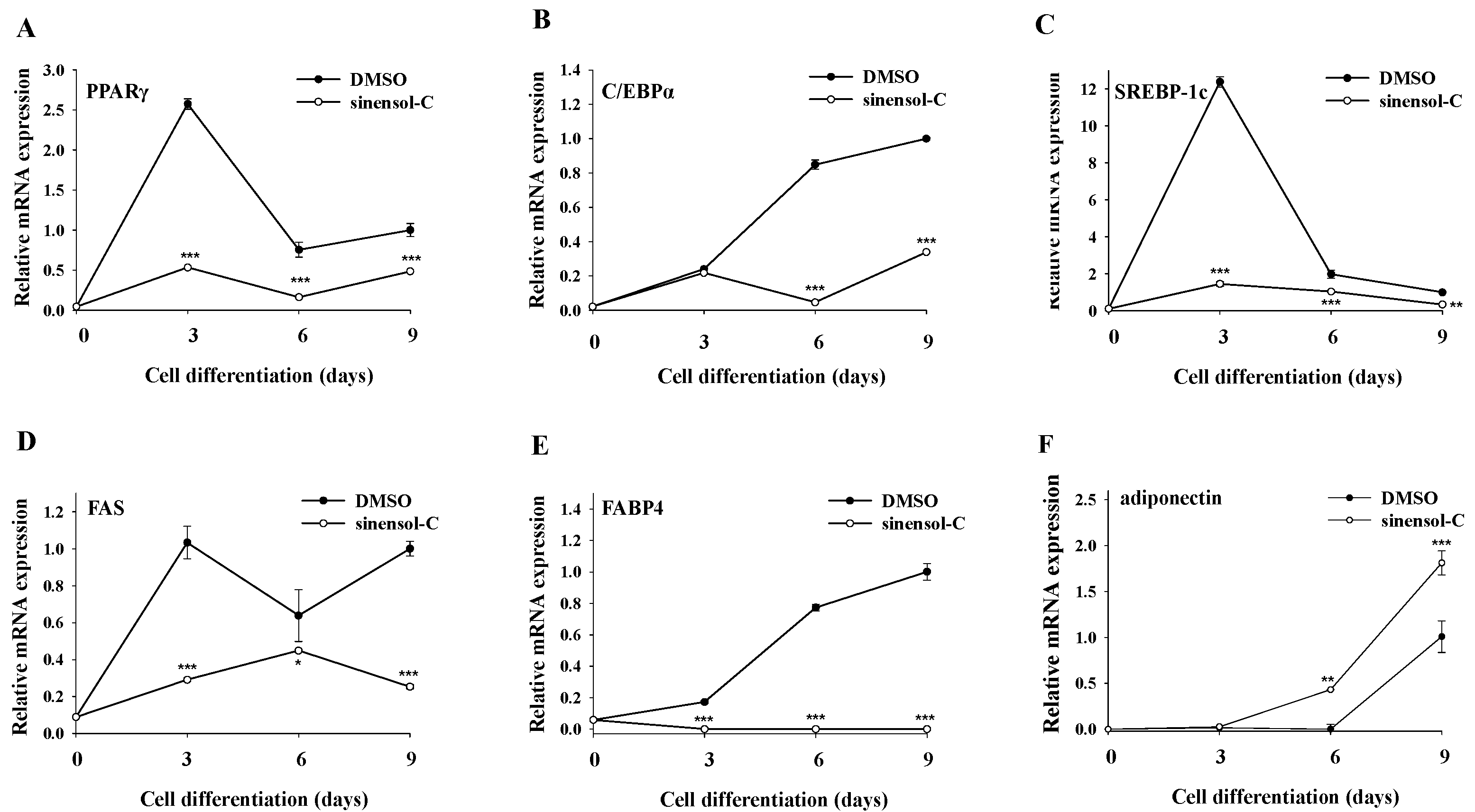
2.2. Effect of Sinensol-C on Adipogenesis-Related Gene Expression during the Entire Differentiation Period
2.3. Effect of Sinensol-C on the Expression of Adipogenesis-Related Protein in 3T3-L1 Adipocytes
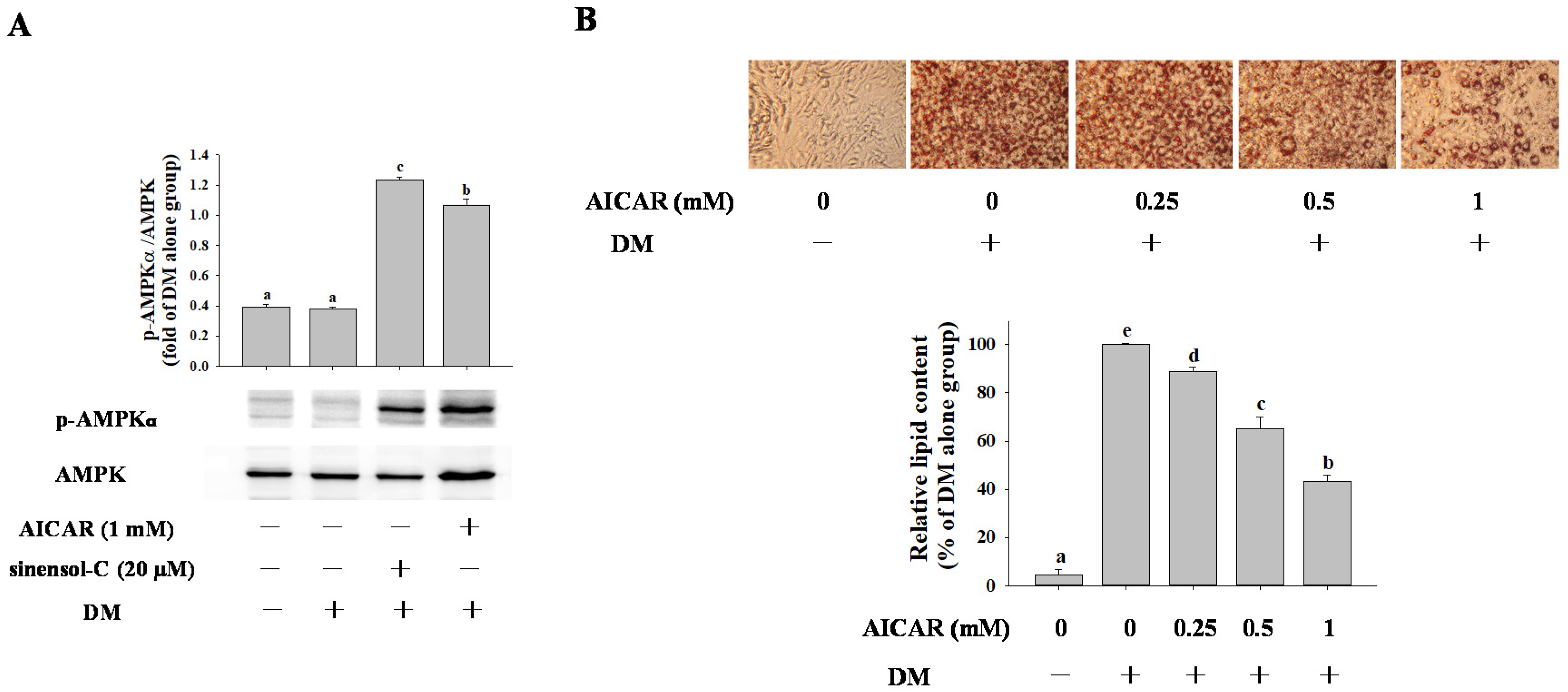
2.4. Effect of AMPK Activation on Adipocyte Differentiation in 3T3-L1 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material
4.3. Isolation and Identification of Sinensol-C from S. Sinensis
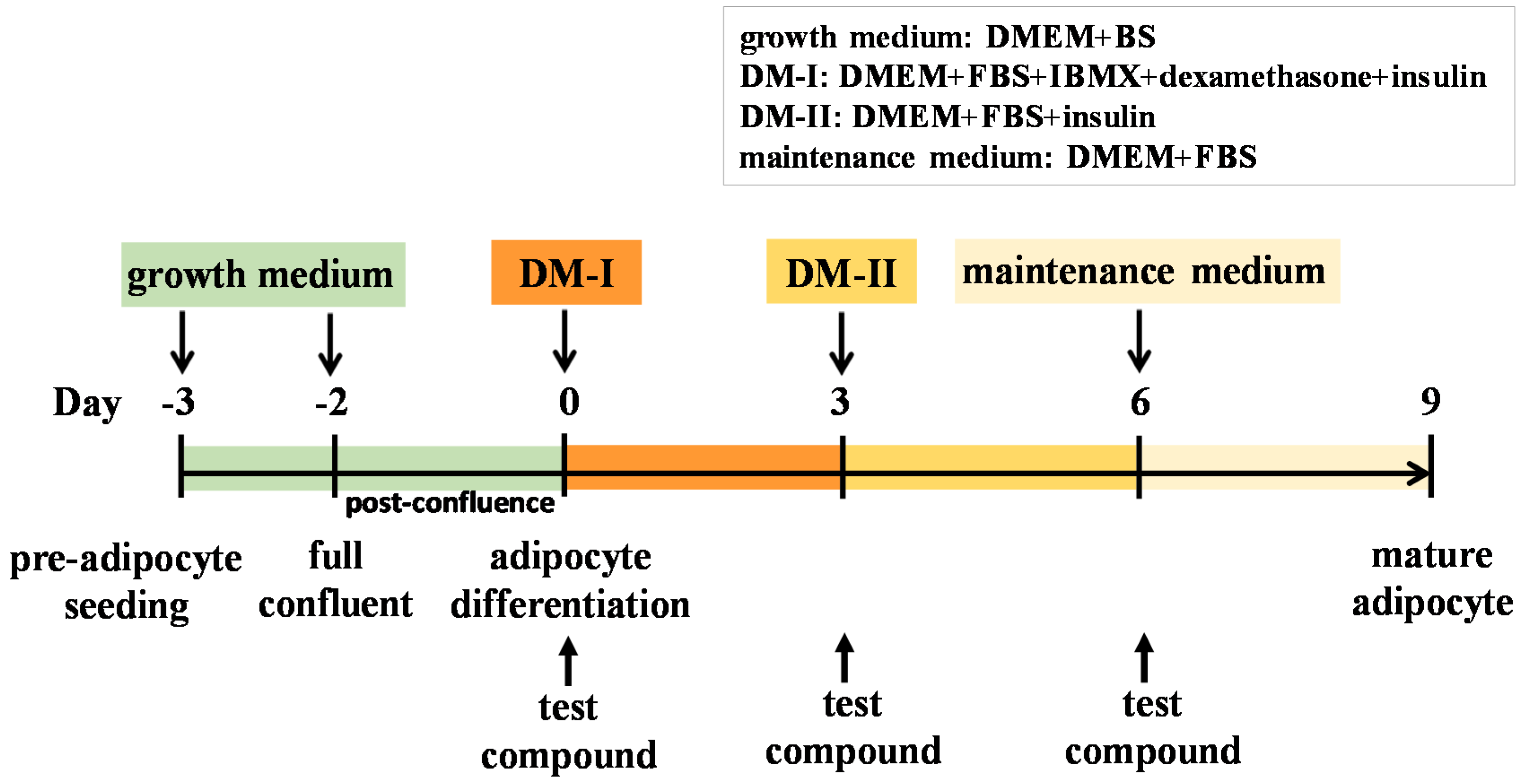
4.4. Cell Culture and Adipocyte Differentiation
4.5. Cell Viability
4.6. Oil Red O Staining
4.7. Western Blot Analysis
4.8. RNA Extraction and Q-PCR Analysis
4.9. Data Processing and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Surveswaran, S.; Kumar, P.; Sun, M. Spiranthes himalayensis (Orchidaceae, Orchidoideae) a new species from Asia. Phytokeys 2017, 89, 115–128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, L.H.; Tsay, J.S.; Chi, H.S. Embryological studies on Spiranthes sinensis (Pers.) Ames. Flora 2016, 224, 191–202. [Google Scholar] [CrossRef]
- Gutiérrez, R.M.P. Orchids: A review of uses in traditional medicine, its phytochemistry and pharmacology. J. Med. Plants Res. 2010, 4, 592–638. [Google Scholar] [CrossRef]
- Lin, Y.L.; Huang, R.L.; Don, M.J.; Kuo, Y.H. Dihydrophenanthrenes from Spiranthes sinensis. J. Nat. Prod. 2000, 63, 1608–1610. [Google Scholar] [CrossRef]
- Shie, P.H.; Huang, S.S.; Deng, J.S.; Huang, G.J. Spiranthes sinensis suppresses production of pro-inflammatory mediators by down-regulating the NF-κB signaling pathway and up-regulating HO-1/Nrf2 anti-oxidant protein. Am. J. Chin. Med. 2015, 43, 969–989. [Google Scholar] [CrossRef]
- Liang, C.P.; Chang, C.H.; Liang, C.C.; Hung, K.Y.; Hsieh, C.W. In vitro antioxidant activities, free radical scavenging capacity, and tyrosinase inhibitory of flavonoid compounds and ferulic acid from Spiranthes sinensis (Pers.) Ames. Molecules 2014, 19, 4681–4694. [Google Scholar] [CrossRef]
- Matu, E.N.; van Staden, J. Antibacterial and anti-inflammatory activities of some plants used for medicinal purposes in Kenya. J. Ethnopharmacol. 2003, 87, 35–41. [Google Scholar] [CrossRef]
- Peng, J.; Xu, Q.; Xu, Y.; Qi, Y.; Han, X.; Xu, L. A new anticancer dihydroflavanoid from the root of Spiranthes australis (R. Brown) Lindl. Nat. Prod. Res. 2007, 21, 641–645. [Google Scholar] [CrossRef]
- Patra, S.; Nithya, S.; Srinithya, B.; Meenakshi, S. Review of medicinal plants for anti-obesity activity. Transl. Biomed. 2015, 6, 1–22. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, W.; Wang, D.; Ma, X. Chinese medicine in the battle against obesity and metabolic diseases. Front. Physiol. 2018, 9, 850. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, M.; Yao, H.; Liu, Y.; Gao, R. Herbal medicine for the treatment of obesity: An overview of scientific evidence from 2007 to 2017. Evid. Based Complement. Alternat. Med. 2017, 2017, 8943059. [Google Scholar] [CrossRef]
- Vermaak, I.; Viljoen, A.M.; Hamman, J.H. Natural products in anti-obesity therapy. Nat. Prod. Rep. 2011, 28, 1493–1533. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, Y.; Ji, L.; Hirano, H.; Ueda, M.; Nagashima, K.; Kikuchi, T. Studies on the Constituents of Orchidaceous Plants. IX.: Constituents of Spiranthes sinensis (PERS.) AMES var. amoena (M. BIEBERSON) HARA. (2). Structures of Spirantheosol, Spiranthoquinone, Spiranthol-C, and Spirasineol-B, New Isopentenyldihydrophenanthrenes. Chem. Pharm. Bull. 1990, 38, 629–635. [Google Scholar] [CrossRef]
- Li, C.Y.; Liu, J.; Su, X.H.; Yuan, Z.P.; Zhong, Y.J.; Li, Y.F.; Liang, B. New dimeric phenanthrene and flavone from Spiranthes sinensis. J. Asian Nat. Prod. Res. 2013, 15, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.Y.; Han, X.; Xu, L.N.; Qi, Y.; Xu, Y.W.; Xu, Q.W. Two new prenylated coumarins from Spiranthes sinensis (Pers.) Ames. J. Asian Nat. Prod. Res. 2008, 10, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, Y.; Ueda, M.; Kikuchi, T. Studies on the constituents of Orchidaceous plants. VIII. Constituents of Spiranthes sinensis (Pers.) Ames var. amoena (M. Bieberson) Hara. (1). Isolation and structure elucidation of spiranthol-A, spiranthol-B, and spirasineol-A, new isopentenyldihydrophenanthrenes. Chem. Pharm. Bull. 1989, 37, 3195–3199. [Google Scholar] [CrossRef]
- Lin, Y.L.; Wang, W.Y.; Kuo, Y.H.; Liu, Y.H. Homocyclotirucallane and two dihydrophenanthrenes from Spiranthes sinensis. Chem. Pharm. Bull. 2001, 49, 1098–1101. [Google Scholar] [CrossRef]
- Lean, M.E.J.; Astrup, A.; Roberts, S.B. Making progress on the global crisis of obesity and weight management. Brit. Med. J. 2018, 361, 1–7. [Google Scholar] [CrossRef]
- Hand, G.A.; Blair, S.N. Energy flux and its role in obesity and metabolic disease. Eur. Endocrinol. 2014, 10, 131–135. [Google Scholar] [CrossRef]
- Sullivan, P.W.; Ghushchyan, V.H.; Ben-Joseph, R. The impact of obesity on diabetes, hyperlipidemia and hypertension in the United States. Qual. Life Res. 2008, 17, 1063–1071. [Google Scholar] [CrossRef]
- Rayner, J.J.; Neubauer, S.; Rider, O.J. The paradox of obesity cardiomyopathy and the potential for weight loss as a therapy. Obes. Rev. 2015, 16, 679–690. [Google Scholar] [CrossRef] [PubMed]
- McArdle, M.A.; Finucane, O.M.; Connaughton, R.M.; McMorrow, A.M.; Roche, H.M. Mechanisms of obesity-induced inflammation and insulin resistance: Insights into the emerging role of nutritional strategies. Front. Endocrinol. 2013, 4, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.G.; Park, C.Y. Anti-obesity drugs: A review about their effects and safety. Diabetes Metab. J. 2012, 36, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [CrossRef] [PubMed]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.; et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef]
- Dagon, Y.; Avraham, Y.; Berry, E.M. AMPK activation regulates apoptosis, adipogenesis, and lipolysis by eIF2alpha in adipocytes. Biochem. Biophys. Res. Commun. 2006, 340, 43–47. [Google Scholar] [CrossRef]
- Sozio, M.S.; Lu, C.; Zeng, Y.; Liangpunsakul, S.; Crabb, D.W. Activated AMPK inhibits PPAR-alpha and PPAR-gamma transcriptional activity in hepatoma cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, 739–747. [Google Scholar] [CrossRef]
- Tandon, P.; Wafer, R.; Minchin, J.E.N. Adipose morphology and metabolic disease. J. Exp. Biol. 2018, 221, 1–11. [Google Scholar] [CrossRef]
- Ejaz, A.; Wu, D.; Kwan, P.; Meydani, M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J. Nutr. 2009, 139, 919–925. [Google Scholar] [CrossRef]
- Sakuma, S.; Sumida, M.; Endoh, Y.; Kurita, A.; Yamaguchi, A.; Watanabe, T.; Kohda, T.; Tsukiyama, Y.; Fujimoto, Y. Curcumin inhibits adipogenesis induced by benzyl butyl phthalate in 3T3-L1 cells. Toxicol. Appl. Pharmacol. 2017, 329, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Y.; Chen, C.W.; Chen, L.K.; Chou, H.Y.; Chang, C.L.; Juan, C.C. Curcumin attenuates adipogenesis by inducing preadipocyte apoptosis and inhibiting adipocyte differentiation. Nutrients 2019, 11, 2307. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, A.; Vasas, A.; Hohmann, J. Natural phenanthrenes and their biological activity. Phytochemistry 2008, 69, 1084–1110. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Walkey, C.J.; Puigserver, P.; Spiegelman, B.M. Transcriptional regulation of adipogenesis. Genes Dev. 2000, 14, 1293–1307. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R.; Barrick, C.; Kim, K.A.; Lindner, J.; Blondeau, B.; Fujimoto, Y.; Shiota, M.; Kesterson, R.A.; Kahn, B.B.; Magnuson, M.A. Deletion of PPARgamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2005, 102, 6207–6212. [Google Scholar] [CrossRef]
- Morrison, R.F.; Farmer, S.R. Hormonal signaling and transcriptional control of adipocyte differentiation. J. Nutr. 2000, 130, 3116s–3121s. [Google Scholar] [CrossRef]
- Lowe, C.E.; O’Rahilly, S.; Rochford, J.J. Adipogenesis at a glance. J. Cell Sci. 2011, 124, 2681–2686. [Google Scholar] [CrossRef]
- Kim, J.B.; Spiegelman, B.M. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996, 10, 1096–1107. [Google Scholar] [CrossRef]
- Zieleniak, A.; Wojcik, M.; Wozniak, L.A. Structure and physiological functions of the human peroxisome proliferator-activated receptor gamma. Arch. Immunol. Ther. Exp. 2008, 56, 331–345. [Google Scholar] [CrossRef]
- Lowell, B.B. PPARgamma: An essential regulator of adipogenesis and modulator of fat cell function. Cell 1999, 99, 239–242. [Google Scholar] [CrossRef]
- Ma, X.; Wang, D.; Zhao, W.; Xu, L. Deciphering the Roles of PPARgamma in adipocytes via dynamic change of transcription complex. Front. Endocrinol. 2018, 9, 473. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, M.; Matsuda, M.; Maeda, N.; Funahashi, T.; Matsuzawa, Y.; Makishima, M.; Shimomura, I. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes 2003, 52, 1655–1663. [Google Scholar] [CrossRef]
- Havel, P.J. Control of energy homeostasis and insulin action by adipocyte hormones: Leptin, acylation stimulating protein, and adiponectin. Curr. Opin. Lipidol. 2002, 13, 51–59. [Google Scholar] [CrossRef]
- Bauche, I.B.; El Mkadem, S.A.; Pottier, A.M.; Senou, M.; Many, M.C.; Rezsohazy, R.; Penicaud, L.; Maeda, N.; Funahashi, T.; Brichard, S.M. Overexpression of adiponectin targeted to adipose tissue in transgenic mice: Impaired adipocyte differentiation. Endocrinology 2007, 148, 1539–1549. [Google Scholar] [CrossRef]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef]
- Lindsay, R.S.; Funahashi, T.; Hanson, R.L.; Matsuzawa, Y.; Tanaka, S.; Tataranni, P.A.; Knowler, W.C.; Krakoff, J. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet 2002, 360, 57–58. [Google Scholar] [CrossRef]
- Wu, X.; Motoshima, H.; Mahadev, K.; Stalker, T.J.; Scalia, R.; Goldstein, B.J. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes 2003, 52, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Yoshida, H. Beneficial effects of adiponectin on glucose and lipid metabolism and atherosclerotic progression: Mechanisms and perspectives. Int. J. Mol Sci. 2019, 20, 1190. [Google Scholar] [CrossRef]
- Nigro, E.; Scudiero, O.; Monaco, M.L.; Palmieri, A.; Mazzarella, G.; Costagliola, C.; Bianco, A.; Daniele, A. New insight into adiponectin role in obesity and obesity-related diseases. Biomed. Res. Int. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhou, X.; Huang, N.; Li, H.; Tian, J.; Li, T.; Yao, K.; Nyachoti, C.M.; Kim, S.W.; Yin, Y. AMPK regulation of glucose, lipid and protein metabolism: Mechanisms and nutritional significance. Curr. Protein Pept. Sci. 2017, 18, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.C.; Zierath, J.R. AMP-activated protein kinase signaling in metabolic regulation. J. Clin. Investig. 2006, 116, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.E.; Martins, L.; Muckett, P.J.; Khadayate, S.; Bornot, A.; Clausen, M.; Admyre, T.; Bjursell, M.; Fiadeiro, R.; Wilson, L.; et al. AMPK activation protects against diet induced obesity through Ucp1-independent thermogenesis in subcutaneous white adipose tissue. Nat. Metab. 2019, 1, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.; Arraiz, N.; Aguirre, M.; Velasco, M.; Bermudez, V. AMPK as target for intervention in childhood and adolescent obesity. J. Obes. 2011, 2011, 252817. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, T.; Sawada, K.; Yamamoto, N.; Ashida, H. 4-Hydroxyderricin and xanthoangelol from Ashitaba (Angelica keiskei) suppress differentiation of preadiopocytes to adipocytes via AMPK and MAPK pathways. Mol. Nutr. Food Res. 2013, 57, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.H.; Jang, J.; Chung, S.I.; Yoon, Y. AMPK and SREBP-1c mediate the anti-adipogenic effect of beta-hydroxyisovalerylshikonin. Int. J. Mol. Med. 2016, 37, 816–824. [Google Scholar] [CrossRef]
- Ahmad, B.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Molecular mechanisms of adipogenesis: The anti-adipogenic role of AMP-activated protein kinase. Front. Mol. Biosci. 2020, 7, 76. [Google Scholar] [CrossRef]
- Kelly, M.; Ruderman, N.B.; Tomas, E. AMP-activated protein kinase and its regulation by adiponectin and interleukin-6. Scand. J. Food Nutr. 2006, 50, 85–91. [Google Scholar] [CrossRef]
- Zhou, L.; Deepa, S.S.; Etzler, J.C.; Ryu, J.; Mao, X.; Fang, Q.; Liu, D.D.; Torres, J.M.; Jia, W.; Lechleiter, J.D.; et al. Adiponectin activates AMP-activated protein kinase in muscle cells via APPL1/LKB1-dependent and phospholipase C/Ca2+/Ca2+/calmodulin-dependent protein kinase kinase-dependent pathways. J. Biol. Chem. 2009, 284, 22426–22435. [Google Scholar] [CrossRef]
- Lihn, A.S.; Jessen, N.; Pedersen, S.B.; Lund, S.; Richelsen, B. AICAR stimulates adiponectin and inhibits cytokines in adipose tissue. Biochem. Biophys. Res. Commun. 2004, 316, 853–858. [Google Scholar] [CrossRef]
- Habinowski, S.A.; Witters, L.A. The effects of AICAR on adipocyte differentiation of 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2001, 286, 852–856. [Google Scholar] [CrossRef]
- Qi, Y.; Shang, J.Y.; Ma, L.J.; Sun, B.B.; Hu, X.G.; Liu, B.; Zhang, G.J. Inhibition of AMPK expression in skeletal muscle by systemic inflammation in COPD rats. Respir. Res. 2014, 15, 156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giri, S.; Rattan, R.; Haq, E.; Khan, M.; Yasmin, R.; Won, J.S.; Key, L.; Singh, A.K.; Singh, I. AICAR inhibits adipocyte differentiation in 3T3L1 and restores metabolic alterations in diet-induced obesity mice model. Nutr. Metab. 2006, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kang, R.; Bae, S.; Yoon, Y. AICAR, an activator of AMPK, inhibits adipogenesis via the WNT/beta-catenin pathway in 3T3-L1 adipocytes. Int. J. Mol. Med. 2011, 28, 65–71. [Google Scholar] [CrossRef]
- Li, Y.; Wang, P.; Zhuang, Y.; Lin, H.; Li, Y.; Liu, L.; Meng, Q.; Cui, T.; Liu, J.; Li, Z. Activation of AMPK by berberine promotes adiponectin multimerization in 3T3-L1 adipocytes. FEBS lett. 2011, 585, 1735–1740. [Google Scholar] [CrossRef]
- Fisch, M.H.; Flick, B.H.; Arditti, J. Structure and antifungal activity of hircinol, loroglossol and orchinol. Phytochemistry 1973, 12, 437–441. [Google Scholar] [CrossRef]
- Takenouchi, T.; Takayama, Y.; Takezawa, T. Co-treatment with dexamethasone and octanoate induces adipogenesis in 3T3-L1 cells. Cell Biol. Int. 2004, 28, 209–216. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
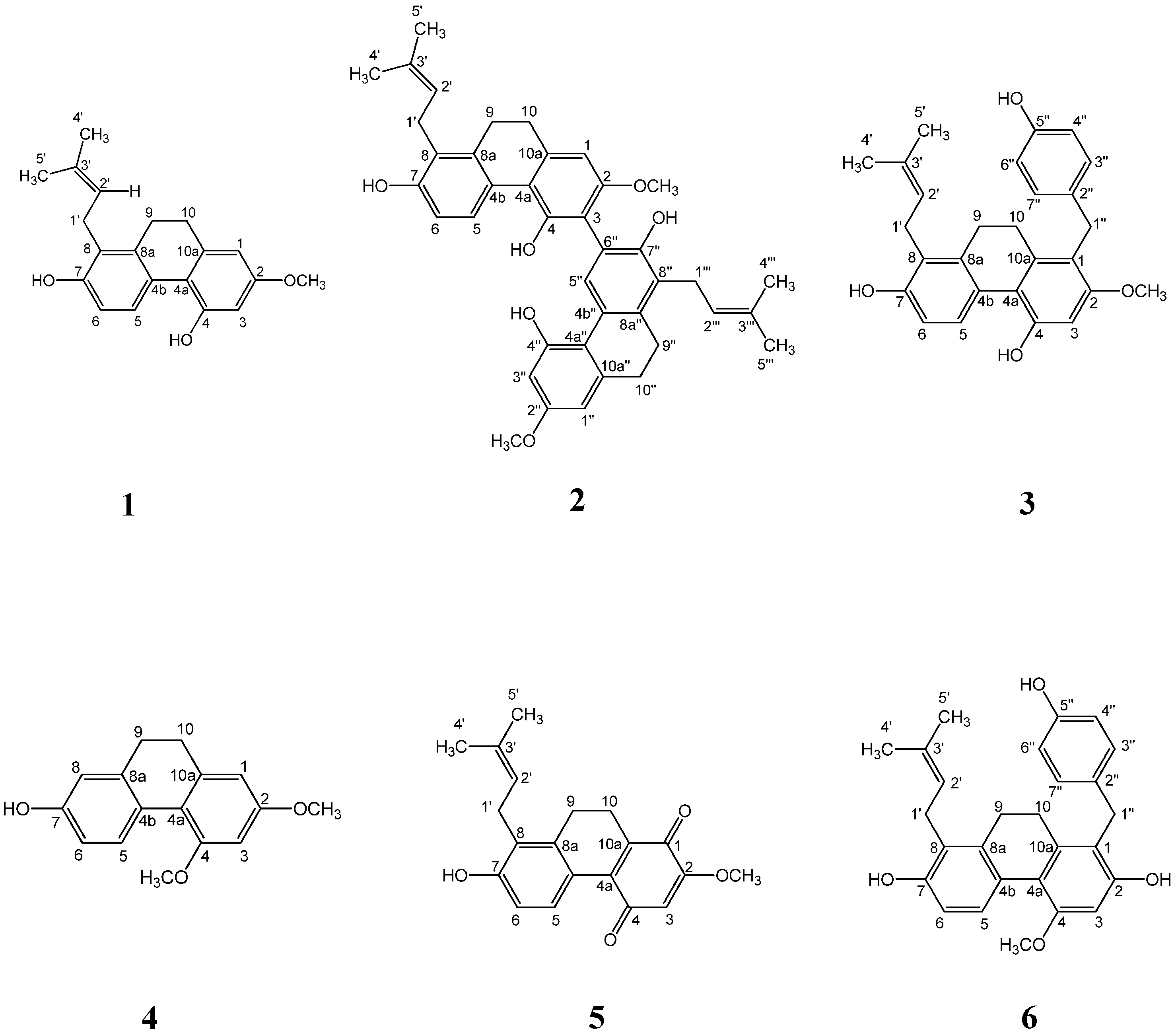



| Compound | IC50 (μM) a |
|---|---|
| spiranthol-A (1) | >60 |
| spiranthesol (2) | 38.93 ± 0.83 |
| spirasineol-A (3) | 31.45 ± 2.48 |
| orchinol (4) | >60 |
| spiranthoquinone (5) | >60 |
| sinensol-C (6) | 12.67 ± 0.69 |
| curcumin b | 33.66 ± 0.95 |
| Genes | Sequence |
|---|---|
| PPAR-γ | F: 5′-CAA GAA TAC CAA AGT GCG ATC AA-3′ |
| R: 5′-GAG CTG GGT CTT TTC AGA ATA ATA AG-3′ | |
| C/EBPα | F: 5′-AGC AAC GAG TAC CGG GTA CG-3′ |
| R: 5′-TGT TTG GCT TTA TCT CGG CTC-3′ | |
| SERBP-1c | F: 5′-GAT CAA AGA GGA GCC AGT GC-3′ |
| R: 5′-TAG ATG GTG GCT GCT GAG TG-3′ | |
| FAS | F: 5’-CCC TTG ATG AAG AGG GAT CA-3’ |
| R: 5′-ACT CCA CAG GTG GGG AAC AAG-3′ | |
| FABP4 | F: 5′-AGT GAA AAC TTC GAT GAT TAC ATG AA-3′ |
| R: 5′-GCC TGC CAC TTT CCT TGT G-3′ | |
| adiponectin | F: 5′-TCC TGG AGA GAA GGG AGA GAA AG-3′ |
| R: 5′-TCA GCT CCT GTC ATT CCA ACA T-3′ | |
| 18S rRNA | F: 5′-CGC CGC TAG AGG TGA AAT TCT-3′ |
| R:5′-CAT TCT TGG CAA ATG CTT TCG-3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shie, P.-H.; Yang, C.-P.; Huang, G.-J.; Wang, S.-Y.; Kuo, Y.-H. Sinensol-C Isolated from Spiranthes sinensis Inhibits Adipogenesis in 3T3-L1 Cells through the Regulation of Adipogenic Transcription Factors and AMPK Activation. Molecules 2020, 25, 4204. https://doi.org/10.3390/molecules25184204
Shie P-H, Yang C-P, Huang G-J, Wang S-Y, Kuo Y-H. Sinensol-C Isolated from Spiranthes sinensis Inhibits Adipogenesis in 3T3-L1 Cells through the Regulation of Adipogenic Transcription Factors and AMPK Activation. Molecules. 2020; 25(18):4204. https://doi.org/10.3390/molecules25184204
Chicago/Turabian StyleShie, Pei-Hsin, Chung-Ping Yang, Guan-Jhong Huang, Sheng-Yang Wang, and Yueh-Hsiung Kuo. 2020. "Sinensol-C Isolated from Spiranthes sinensis Inhibits Adipogenesis in 3T3-L1 Cells through the Regulation of Adipogenic Transcription Factors and AMPK Activation" Molecules 25, no. 18: 4204. https://doi.org/10.3390/molecules25184204
APA StyleShie, P.-H., Yang, C.-P., Huang, G.-J., Wang, S.-Y., & Kuo, Y.-H. (2020). Sinensol-C Isolated from Spiranthes sinensis Inhibits Adipogenesis in 3T3-L1 Cells through the Regulation of Adipogenic Transcription Factors and AMPK Activation. Molecules, 25(18), 4204. https://doi.org/10.3390/molecules25184204