Phenolic Compounds in Poorly Represented Mediterranean Plants in Istria: Health Impacts and Food Authentication
Abstract
:1. Introduction
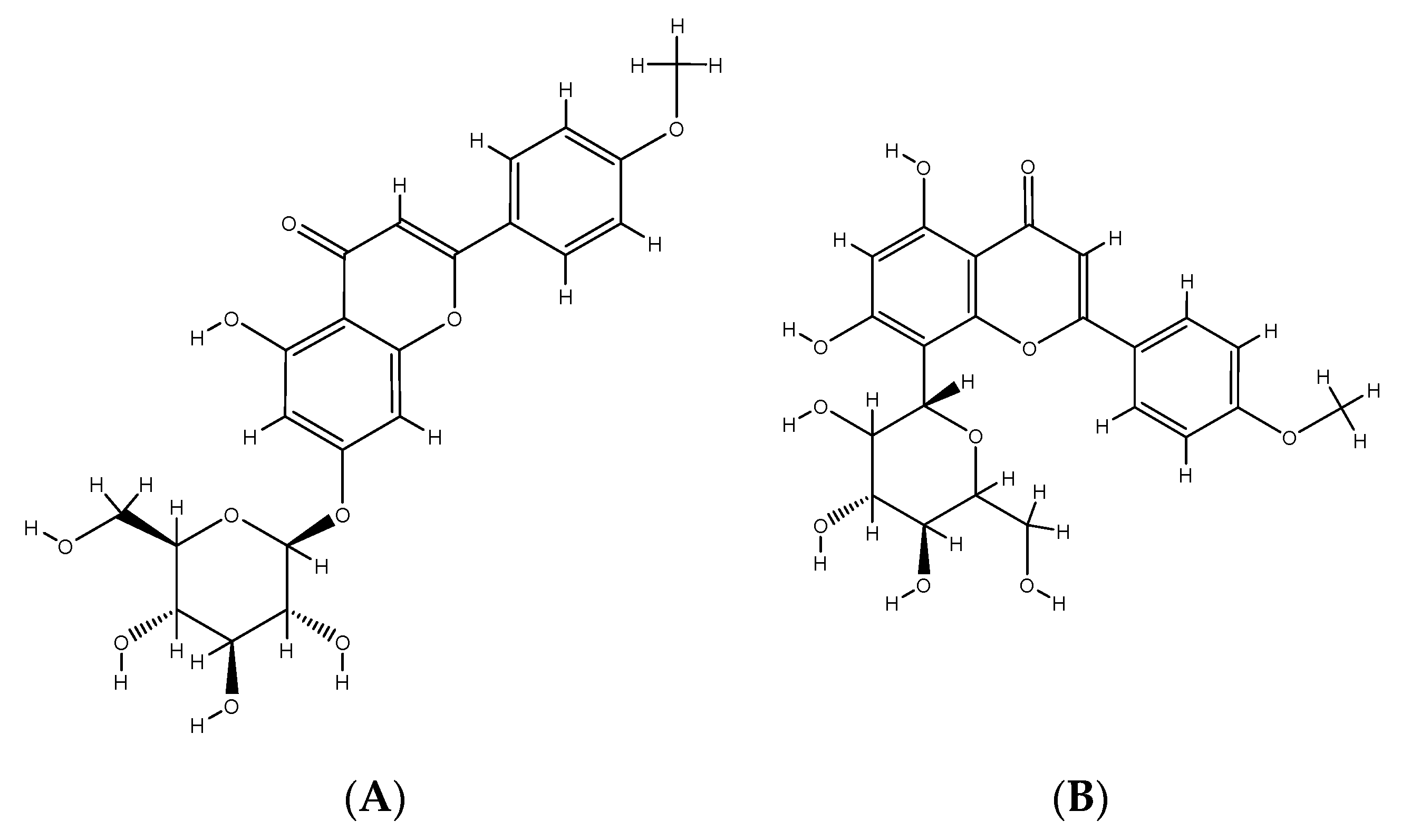
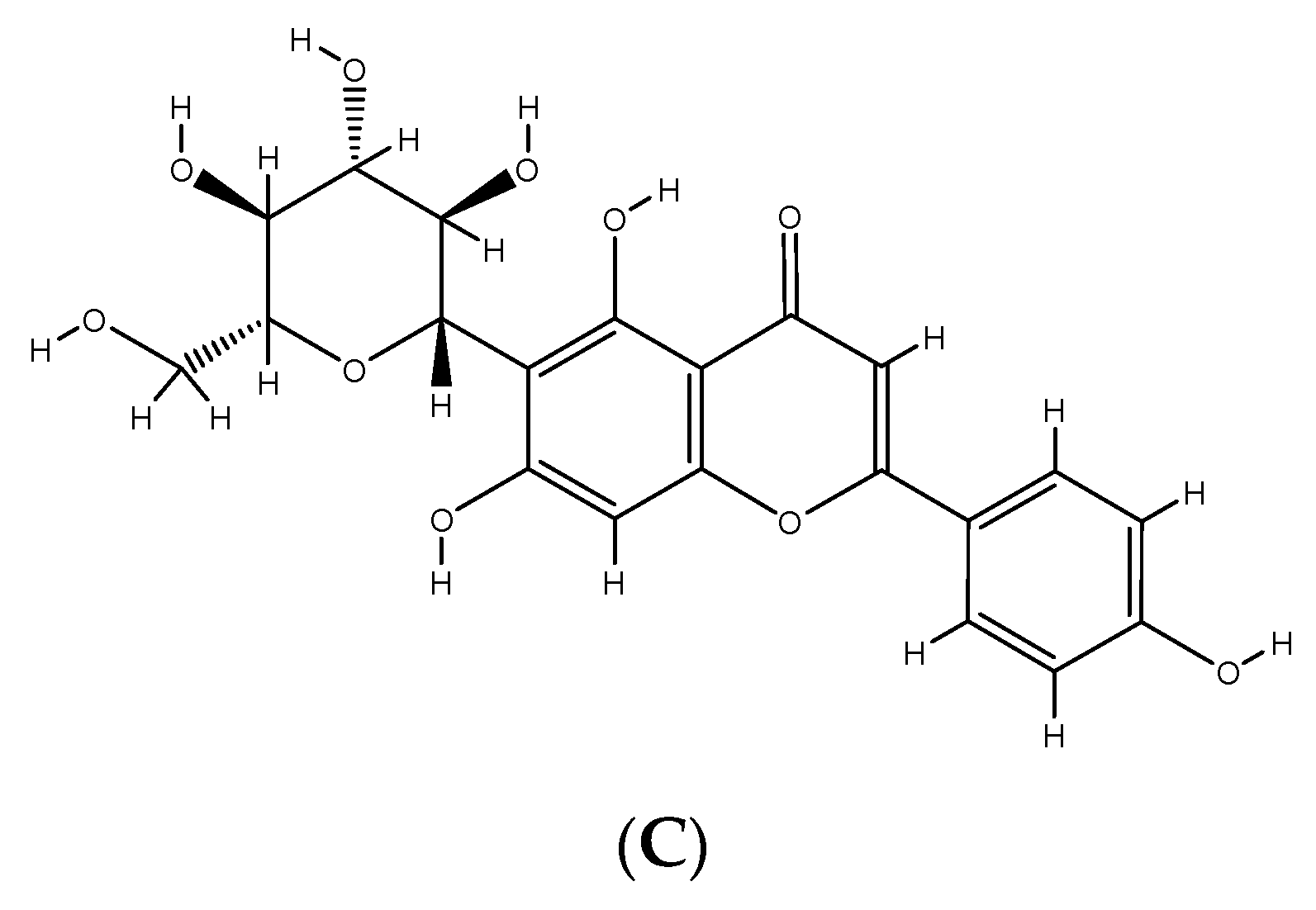
2. Phenolic Compounds from Different Plant Sources
3. Phenolic Compounds and Their Impact on Human Health
3.1. Pomegranate
3.2. Jujube
3.3. Strawberry Tree
3.4. Mediterranean Hackberry
3.5. Fig
3.6. Globe Artichoke
4. Food Authentication Technique
Phenolic Compounds Characterization for Determination of Authenticity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mertik, M. Building food market communities with the open source LOKeT project. J. Appl. Comput. Inf. Technol. 2014, 18, 2014. Available online: https://www.citrenz.ac.nz/jacit/JACIT1801/2014Mertik_LOKeTProject.pdf (accessed on 4 July 2020).
- Ilbery, B.; Kneafsey, M.; Bamford, M. Protecting and promoting regional speciality food and drink products in the European Union. Outlook Agric. 2000, 29, 31–37. [Google Scholar] [CrossRef]
- Martinez, S.W. Policies supporting local food in the United States. Agriculture 2016, 6, 43. [Google Scholar] [CrossRef] [Green Version]
- Willis, D.B.; Carpio, C.E.; Boys, K.A. Supporting local food system development through food price premium donations: A policy proposal. J. Agric. Appl. Econ. 2016, 48, 192–217. [Google Scholar] [CrossRef] [Green Version]
- Izumi, B.T.; Rostant, O.S.; Moss, M.J.; Hamm, M.W. Results from the 2004 Michigan Farm-to-School Survey. J. Sch. Health 2006, 76, 169–174. [Google Scholar] [CrossRef]
- Vogt, R.A.; Kaiser, L.L. Still a Time to Act: A Review of Institutional Marketing of Regionally-Grown Food. Agric. Hum. Val. 2008, 25, 241–255. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.A. Pomegranate and its many functional components as related to human health: A review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654. [Google Scholar] [CrossRef]
- Jurenka, J. Therapeutic applications of pomegranate (Punica granatum L.): A review. Altern. Med. Rev. 2008, 13, 128. [Google Scholar]
- Preeti, T.S.; Tripathi, S. Ziziphus jujuba: A phytopharmacological review. Int. J. Res. Dev. Pharm. Life Sci. 2014, 3, 959–966. [Google Scholar]
- Ji, X.; Peng, Q.; Yuan, Y.; Shen, J.; Xie, X.; Wang, M. Isolation, structures and bioactivities of the polysaccharides from jujube fruit (Ziziphus jujuba Mill.): A review. Food Chem. 2017, 227, 349–357. [Google Scholar] [CrossRef]
- Bento, I.; Pereira, J.A. Arbutus unedo L. and its benefits on human health. J. Food Nutr. Res. 2011, 50, 73–85. [Google Scholar]
- Pawlowska, A.M.; De Leo, M.; Braca, A. Phenolics of Arbutus unedo L. (Ericaceae) fruits: Identification of anthocyanins and gallic acid derivatives. J. Agric. Food Chem. 2006, 54, 10234–10238. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, A. The Encyclopedia of Medicinal Plants; Dorling Kindersley: London, UK, 1996. [Google Scholar]
- Chopra, R.N.; Nayar, S.L.; Chopra, I.C.; Asolkar, L.; Kakkar, K. Glossary of Indian Medicinal Plants; Council of Scientific and Industrial Research (CSIR): New Delhi, India, 1986. [Google Scholar]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Foods 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Santos–Sanchez, F.N.; Salas–Coronado, R.; Villanueva–Canongo, C.; Hernandez–Carlos, B. Antioxidant compounds and their antioxidant mechanism. In Antioxidants; IntechOpen: London, UK, 2019. [Google Scholar]
- Cavazza, A.; Corradini, C.; Musci, M.; Salvadeo, P. High-performance liquid chromatographic phenolic compound fingerprint for authenticity assessment of honey. J. Sci. Food Agric. 2013, 93, 1169–1175. [Google Scholar] [CrossRef]
- Ferreres, F.; Gil-Izquierdo, A.; Vinholes, J.; Silva, S.T.; Valentão, P.; Andrade, P.B. Bauhinia forficata Link authenticity using flavonoids profile: Relation with their biological properties. Food Chem. 2012, 134, 894–904. [Google Scholar] [CrossRef]
- Malacarne, M.; Nardin, T.; Bertoldi, D.; Nicolini, G.; Larcher, R. Verifying the botanical authenticity of commercial tannins through sugars and simple phenols profiles. Food Chem. 2016, 206, 274–283. [Google Scholar] [CrossRef]
- Silva, B.M.; Andrade, P.B.; Valentão, P.; Mendes, G.C.; Seabra, R.M.; Ferreira, M.A. Phenolic profile in the evaluation of commercial quince jellies authenticity. Food Chem. 2000, 71, 281–285. [Google Scholar] [CrossRef]
- Dragovic-Uzelac, V.; Delonga, K.; Levaj, B.; Djakovic, S.; Pospisil, J. Phenolic profiles of raw apricots, pumpkins, and their purees in the evaluation of apricot nectar and jam authenticity. J. Agric. Food Chem. 2005, 53, 4836–4842. [Google Scholar] [CrossRef]
- Marova, I.; Parilova, K.; Friedl, Z.; Obruca, S.; Duronova, K. Analysis of phenolic compounds in lager beers of different origin: A contribution to potential determination of the authenticity of Czech beer. Chromatographia 2011, 73, 83. [Google Scholar] [CrossRef]
- Aung, M.M.; Chang, Y.S. Traceability in a food supply chain: Safety and quality perspectives. Food Control. 2014, 39, 172–184. [Google Scholar] [CrossRef]
- Esslinger, S.; Riedl, J.; Fauhl-Hassek, C. Potential and limitations of non-targeted fingerprinting for authentication of food in official control. Food Res. Internat. 2014, 60, 189–204. [Google Scholar] [CrossRef]
- Danezis, G.P.; Tsagkaris, A.S.; Camin, F.; Brusic, V.; Georgiou, C.A. Food authentication: Techniques, trends & emerging approaches. Trends Anal. Chem. 2016, 85, 123–132. [Google Scholar] [CrossRef]
- Primrose, S.; Woolfe, M.; Rollinson, S. Food forensics: Methods for determining the authenticity of foodstuffs. Trends Food Sci. Technol. 2010, 21, 582–590. [Google Scholar] [CrossRef]
- Bai, L.; Zhang, H.; Liu, Q.; Zhao, Y.; Cui, X.; Guo, S.; Zhang, L.; Ho, C.T.; Bai, N. Chemical characterization of the main bioactive constituents from fruits of Ziziphus jujuba. Food Funct. 2016, 7, 2870–2877. [Google Scholar] [CrossRef] [PubMed]
- Miklavčič Višnjevec, A.; Ota, A.; Skrt, M.; Butinar, B.; Smole Možina, S.; Gunde Cimerman, N.; Nečemer, M.; Baruca Arbeiter, A.; Hladnik, M.; Krapac, M.; et al. Genetic, biochemical, nutritional and antimicrobial characteristics of pomegranate (Punica granatum L.) grown in Istria. Food Technol. Biotechnol. 2017, 55, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Miklavčič Višnjevec, A.; Baruca Arbeiter, A.; Hladnik, M.; Ota, A.; Skrt, M.; Butinar, B.; Nečemer, M.; Krapac, M.; Ban, D.; Bučar-Miklavčič, M.; et al. The first an integrated characterization of jujube (Ziziphus jujube Mill.) grown in the North Adriatic region. Food Technol. Biotechnol. 2019, 57, 17. [Google Scholar] [CrossRef] [PubMed]
- Spitaler, R.; Gurschler, S.; Ellmerer, E.; Schubert, B.; Sgarbossa, M.; Zidorn, C. Flavonoids from Celtis australis (Cannabaceae). Biochem. Syst. Ecol. 2009, 37, 120–121. [Google Scholar] [CrossRef]
- Zehrmann, N.; Zidorn, C.; Ganzera, M. Analysis of rare flavonoid C-glycosides in Celtis australis L. by micellar electrokinetic chromatography. J. Pharm. Biomed. Anal. 2010, 51, 1165–1168. [Google Scholar] [CrossRef]
- Sommavilla, V.; Haidacher-Gasser, D.; Sgarbossa, M.; Zidorn, C. Seasonal variation in phenolics in leaves of Celtis australis (Cannabaceae). Biochem. Syst. Ecol. 2012, 41, 110–114. [Google Scholar] [CrossRef]
- Veberic, R.; Colaric, M.; Stampar, F. Phenolic acids and flavonoids of fig fruit (Ficus carica L.) in the northern Mediterranean region. Food Chem. 2008, 106, 153–157. [Google Scholar] [CrossRef]
- Fratianni, F.; Tucci, M.; De Palma, M.; Pepe, R.; Nazzaro, F. Polyphenolic composition in different parts of some cultivars of globe artichoke (Cynara cardunculus L. var. scolymus (L.) Fiori). Food Chem. 2007, 104, 1282–1286. [Google Scholar] [CrossRef]
- Gumienna, M.; Szwengiel, A.; Górna, B. Bioactive components of pomegranate fruit and their transformation by fermentation processes. Eur. Food Res. Technol. 2005, 242, 631–640. [Google Scholar] [CrossRef] [Green Version]
- Pareek, S. Nutritional composition of jujube fruit. Emir. J. Food Agric. 2013, 25, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Alarcão-E-Silva, M.L.C.M.M.; Leitão, A.E.B.; Azinheira, H.G.; Leitão, M.C.A. The Arbutus berry: Studies on its color and chemical characteristics at two mature stages. J. Food Compos. Anal. 2001, 14, 27–35. [Google Scholar] [CrossRef]
- Vidrih, R.; Hribar, J.; Prgomet, Ž.; Poklar Ulrih, N. The physico-chemical properties of strawberry tree (Arbutus unedo L.) fruits. Croat. J. Food Sci. Technol. 2013, 5, 29–33. [Google Scholar]
- Ayaz, F.A.; Kucukislamoglu, M.; Reunanen, M. Sugar, non-volatile and phenolic acids composition of strawberry tree (Arbutus unedo L. var. ellipsoidea) fruits. J. Food Compos. Anal. 2000, 13, 171–177. [Google Scholar] [CrossRef]
- Demır, F.; Doğan, H.; Özcan, M.; Haciseferoğullari, H. Nutritional and physical properties of hackberry (Celtis australis L.). J. Food Eng. 2002, 54, 241–247. [Google Scholar] [CrossRef]
- Ota, A.; Miklavčič Višnjevec, A.; Vidrih, R.; Prgomet, Ž.; Nečemer, M.; Hribar, J.; Gunde Cimerman, N.; Smole Možina, S.; Bučar-Miklavčič, M.; Poklar Ulrih, N. Nutritional, antioxidative, and antimicrobial analysis of the Mediterranean hackberry (Celtis australis L.). Food Sci. Nutr. 2017, 5, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Slavin, J.L. Figs: Past, present, and future. Nutr. Today 2006, 41, 180–184. [Google Scholar] [CrossRef]
- Solomon, A.; Golubowicz, S.; Yablowicz, Z.; Grossman, S.; Bergman, M.; Gttlieb, H.E.; Altman, A.; Kerem, Z.; Flaishman, M.A. Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.). J. Agric. Food Chem. 2006, 54, 7717–7723. [Google Scholar] [CrossRef] [PubMed]
- Croteau, R.; Kutchan, T.M.; Lewis, N.G. Natural products (secondary metabolites). Biochem. Mol. Biol. Plants 2000, 24, 1250–1319. [Google Scholar]
- Herrmann, K. Occurrence and content of hydroxycinnamic and hydroxybenzoic acid compounds in foods. Crit. Rev. Food Sci. Nutr. 1989, 28, 315–347. [Google Scholar] [CrossRef] [PubMed]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, R.H.; El-Said, M.A.A.; Khalifa, A.M.S.; Göransson, U.; Bohlin, L.; Borg-Karlson, A.K.; Verpoorte, R. Biosynthesis, Natural Sources, Dietary Intake, Pharmacokinetic Properties, and Biological Activities of Hydroxycinnamic Acids. J. Agric. Food Chem. 2012, 50, 10877–10895. [Google Scholar] [CrossRef]
- Niemetz, R.; Gross, G.G. Enzymology of gallotannin and ellagitannin biosynthesis. Phytochemistry 2005, 66, 2001–2011. [Google Scholar] [CrossRef]
- Veberic, R.; Trobec, M.; Herbinger, K.; Hofer, M.; Grill, D.; Stampar, F. Phenolic compounds in some apple (Malus domestica Borkh) cultivars of organic and integrated production. J. Sci. Food Agric. 2005, 85, 1687–1694. [Google Scholar] [CrossRef]
- Steinmann, D.; Ganzera, M. Recent advances on HPLC/MS in medicinal plant analysis. J. Pharm. Biomed. Anal. 2011, 55, 744–757. [Google Scholar] [CrossRef]
- Integrated Taxonomic Information System Report. Available online: http://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=27074 (accessed on 20 September 2018).
- Stover, E.D.; Mercure, E.W. The pomegranate: A new look at the fruit of paradise. Hort. Sci. 2007, 42, 1088–1092. [Google Scholar] [CrossRef] [Green Version]
- Smaoui, S.; Hlima, H.B.; Mtibaa, A.C.; Fourati, M.; Sellem, I.; Elhadef, K.; Mellouli, L. Pomegranate peel as phenolic compounds source: Advanced analytical strategies and practical use in meat products. Meat Sci. 2019, 158, 107914. [Google Scholar] [CrossRef]
- Ismail, T.; Sestili, P.; Akhtar, S. Pomegranate peel and fruit extracts: A review of potential anti-inflammatory and anti-infective effects. J. Ethnopharmacol. 2012, 143, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Saleem, M.; Krueger, C.G.; Reed, J.D.; Mukhtar, H. Anthocyanin-and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-κB pathways and inhibits skin tumorigenesis in CD-1 mice. Int. J. Cancer 2005, 113, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Negi, P.S.; Jayaprakasha, G.K.; Jena, B.S. Antioxidant and antimutagenic activities of pomegranate peel extracts. Food Chem. 2003, 80, 393–397. [Google Scholar] [CrossRef]
- Cerdá, B.; Cerón, J.J.; Tomás-Barberán, F.A.; Espín, J.C. Repeated oral administration of high doses of the pomegranate ellagitannin punicalagin to rats for 37 days is not toxic. J. Agric. Food Chem. 2003, 51, 3493–3501. [Google Scholar] [CrossRef]
- Zahin, M.; Aqil, F.; Ahmad, I. Broad spectrum antimutagenic activity of antioxidant active fraction of Punica granatum L. peel extracts. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2010, 703, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Haleem, S.; Akhtar, M.; Zia-ul-Haq, M.; Akbar, J. Efficiency of pomegranate peel extracts in stabilization of sunflower oil under accelerated conditions. Food Res. Int. 2008, 41, 194–200. [Google Scholar] [CrossRef]
- Singh, R.P.; Chidambara Murthy, K.N.; Jayaprakasha, G.K. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J. Agric. Food Chem. 2002, 50, 81–86. [Google Scholar] [CrossRef]
- Panichayupakaranant, P.; Tewtrakul, S.; Yuenyongsawad, S. Antibacterial, anti-inflammatory and anti-allergic activities of standardised pomegranate rind extract. Food Chem. 2010, 123, 400–403. [Google Scholar] [CrossRef]
- Lee, S.I.; Kim, B.S.; Kim, K.S.; Lee, S.; Shin, K.S.; Lim, J.S. Immune-suppressive activity of punicalagin via inhibition of NFAT activation. Biochem. Biophys. Res. Commun. 2008, 371, 799–803. [Google Scholar] [CrossRef]
- Romier, B.; Van De Walle, J.; During, A.; Larondelle, Y.; Schneider, Y.J. Modulation of signalling nuclear factor-κB activation pathway by polyphenols in human intestinal Caco-2 cells. Br. J. Nutr. 2008, 100, 542–551. [Google Scholar] [CrossRef] [Green Version]
- Chevion, M. A site-specific mechanism for free radical induced biological damage: The essential role of redox-active transition metals. Free Radic. Biol. Med. 1988, 5, 27–37. [Google Scholar] [CrossRef]
- Sies, H.E.L.M.U.T. Physiological society symposium: Impaired endothelial and smooth muscle cell function in oxidative stress. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Fallarero, A.; Peña, B.R.; Medina, M.E.; Gra, B.; Rivera, F.; Gutierrez, Y.; Vuorela, P.M. Studies on the toxicity of Punica granatum L.(Punicaceae) whole fruit extracts. J. Ethnopharmacol. 2003, 89, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhao, Z. Origin, distribution, germplasm, and growing of Chinese dates. In Chinese Dates: A Traditional Functional Food; CRC Press: New York, NY, USA, 2016; pp. 23–31. [Google Scholar]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Feng, W.; Cao, J.; Cao, D.; Jiang, W. Antioxidant activity and total phenolic contents in peel and pulp of Chinese jujube (Ziziphus jujuba Mill) fruits. J. Food Biochem. 2009, 33, 613–629. [Google Scholar] [CrossRef]
- Sun, Y.F.; Liang, Z.S.; Shan, C.J.; Viernstein, H.; Unger, F. Comprehensive evaluation of natural antioxidants and antioxidant potentials in Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex HF Chou fruits based on geographical origin by TOPSIS method. Food Chem. 2011, 124, 1612–1619. [Google Scholar] [CrossRef]
- Gao, Q.H.; Wu, C.S.; Yu, J.G.; Wang, M.; Ma, Y.J.; Li, C.L. Textural characteristic, antioxidant activity, sugar, organic acid, and phenolic profiles of 10 promising jujube (Ziziphus jujuba Mill.) selections. J. Food Sci. 2012, 77, C1218–C1225. [Google Scholar] [CrossRef]
- Choi, S.H.; Ahn, J.B.; Kim, H.J.; Im, N.K.; Kozukue, N.; Levin, C.E.; Friedman, M. Changes in free amino acid, protein, and flavonoid content in jujube (Ziziphus jujube) fruit during eight stages of growth and antioxidative and cancer cell inhibitory effects by extracts. J. Agric. Food Chem. 2012, 60, 10245–10255. [Google Scholar] [CrossRef]
- Guo, S.; Duan, J.A.; Tang, Y.; Qian, D.; Zhu, Z.; Qian, Y.; Shang, E.; Su, S. UHPLC-TOFMS coupled with chemometric method as a powerful technique for rapid exploring of differentiating components between two Ziziphus species. J. Sep. Sci. 2011, 34, 659–666. [Google Scholar] [CrossRef]
- Choi, S.H.; Ahn, J.B.; Kozukue, N.; Levin, C.E.; Friedman, M. Distribution of free amino acids, flavonoids, total phenolics, and antioxidative activities of jujube (Ziziphus jujuba) fruits and seeds harvested from plants grown in Korea. J. Agric. Food Chem. 2011, 59, 6594–6604. [Google Scholar] [CrossRef]
- Wang, B.N.; Liu, H.F.; Zheng, J.B.; Fan, M.T.; Cao, W. Distribution of phenolic acids in different tissues of jujube and their antioxidant activity. J. Agric. Food Chem. 2011, 59, 1288–1292. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.H.; Wu, C.S.; Wang, M. The jujube (Ziziphus jujuba Mill.) fruit: A review of current knowledge of fruit composition and health benefits. J. Agric. Food Chem. 2013, 61, 3351–3363. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G.; Faleiro, M.L.; Guerreiro, A.C.; Antunes, M.D. Arbutus unedo L.: Chemical and biological properties. Molecules 2014, 19, 15799–15823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guimarães, R.; Barros, L.; Dueñas, M.; Carvalho, A.M.; Queiroz, M.J.R.; Santos-Buelga, C.; Ferreira, I.C. Characterisation of phenolic compounds in wild fruits from Northeastern Portugal. Food Chem. 2013, 141, 3721–3730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendes, L.; de Freitas, V.; Baptista, P.; Carvalho, M. Comparative antihemolytic and radical scavenging activities of strawberry tree (Arbutus unedo L.) leaf and fruit. Food Chem. Toxicol. 2011, 49, 2285–2291. [Google Scholar] [CrossRef]
- Fortalezas, S.; Tavares, L.; Pimpão, R.; Tyagi, M.; Pontes, V.; Alves, P.M.; McDougall, G.; Stewart, D.; Ferreira, R.B.; Santos, C.N. Antioxidant properties and neuroprotective capacity of strawberry tree fruit (Arbutus unedo). Nutrients 2010, 2, 214–229. [Google Scholar] [CrossRef]
- Pallauf, K.; Rivas-Gonzalo, J.C.; Del Castillo, M.D.; Cano, M.P.; de Pascual-Teresa, S. Characterization of the antioxidant composition of strawberry tree (Arbutus unedo L.) fruits. J. Food Compos. Anal. 2008, 21, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Sytsma, K.J.; Morawetz, J.; Pires, J.C.; Nepokroeff, M.; Conti, E.; Zjhra, M.; Hall, J.C.; Chase, M.W. Urticalean rosids: Circumscription, rosid ancestry, and phylogenetics based on rbcL, trnL-F, and ndhF sequences. Am. J. Bot. 2002, 89, 1531–1546. [Google Scholar] [CrossRef] [Green Version]
- Baytop. Turkish Plants Names Dictionary, 578th ed.; High Language and History Association: Ankara, Turkey, 1994. [Google Scholar]
- Baldini, M.; Ban, D.; Bandelj, D.; Arbeiter, A.B.; Bučar-Miklavčič, M.; Butinar, B.; Cigić, B.; Ferenac, M.; Godena, S.; Cimerman, N.G.; et al. Pomegranate (Punica granatum L.), Jujube (Ziziphus jujuba Mill.), Almond (Prunus dulcis (Mill.) D.A. Webb), Strawberry Tree (Arbutus unedo L.) and Common Hackberry (Celtis australis L.) in Istria; Annales: Koper, Slovenia, 2015; pp. 13–88. [Google Scholar]
- Vrhovnik, I. The possibility of growing figs in Slovenia. SAD Rev. Za Sadjarstvo Vinograd. Vinar. 2007, 18, 3–5. [Google Scholar]
- Caliskan, O. Mediterranean figs (Ficus carica L.) functional food properties. In The Mediterranean Diet; Academic Press: San Diego, CA, USA, 2015; pp. 629–637. [Google Scholar]
- Barolo, M.I.; Mostacero, N.R.; López, S.N. Ficus carica L.(Moraceae): An ancient source of food and health. Food Chem. 2014, 164, 119–127. [Google Scholar] [CrossRef]
- Dueñas, M.; Pérez-Alonso, J.J.; Santos-Buelga, C.; Escribano-Bailón, T. Anthocyanin composition in fig (Ficus carica L.). J. Food Compos. Anal. 2008, 21, 107–115. [Google Scholar] [CrossRef]
- Bianco, V.V. Present situation and future potential of artichoke in the Mediterranean basin. Acta Hortic. 2005, 681, 39–55. [Google Scholar] [CrossRef]
- Gebhardt, R.; Fausel, M. Antioxidant and hepatoprotective effects of artichoke extracts and constituents in cultured rat hepatocytes. Toxicol. Vitr. 1997, 11, 669–672. [Google Scholar] [CrossRef]
- Jimenez-Escrig, A.; Dragsted, L.O.; Daneshvar, B.; Pulido, R.; Saura-Calixto, F. In vitro antioxidant activities of edible artichoke (Cynara scolymus L.) and effect on biomarkers of antioxidants in rats. J. Agric. Food Chem. 2003, 51, 5540–5545. [Google Scholar] [CrossRef] [Green Version]
- Pandino, G.; Lombardo, S.; Mauromicale, G.; Williamson, G. Profile of polyphenols and phenolic acids in bracts and receptacles of globe artichoke (Cynara cardunculus var. scolymus) germplasm. J. Food Compos. Anal. 2011, 24, 148–153. [Google Scholar] [CrossRef]
- Brat, P.; Georgé, S.; Bellamy, A.; Chaffaut, L.D.; Scalbert, A.; Mennen, L.; Arnault, N.; Amiot, M.J. Daily polyphenol intake in France from fruit and vegetables. J. Nutr. 2006, 136, 2368–2373. [Google Scholar] [CrossRef] [Green Version]
- Georgiou, C.A.; Danezis, G.P. Elemental and isotopic mass spectrometry. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; Volume 68, pp. 131–243. [Google Scholar] [CrossRef]
- de Villiers, A.; Venter, P.; Pasch, H. Recent advances and trends in the liquid-chromatography–mass spectrometry analysis of flavonoids. J. Chromatogr. A 2016, 1430, 16–78. [Google Scholar] [CrossRef]
- Makita, C.; Chimuka, L.; Steenkamp, P.; Cukrowska, E.; Madala, E. Comparative analyses of flavonoid content in Moringa oleifera and Moringa ovalifolia with the aid of UHPLC-qTOF-MS fingerprinting. S. Afr. J. Bot. 2016, 105, 116–122. [Google Scholar] [CrossRef]
- Taylor, M.J.; Keenan, G.A.; Reid, K.B.; Fernández, D.U. The utility of ultra-performance liquid chromatography/electrospray ionisation time-of-flight mass spectrometry for multi-residue determination of pesticides in strawberry. Rapid Commun. Mass Spectrom. An Int. J. Devoted Rapid Dissem. Minute Res. Mass Spectrom. 2008, 22, 2731–2746. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Ganzera, M.; Sturm, S. Recent advances on HPLC/MS in medicinal plant analysis—An update covering 2011–2016. J. Pharm. Biomed. Anal. 2018, 147, 211–233. [Google Scholar] [CrossRef] [PubMed]
- Fügel, R.; Carle, R.; Schieber, A. Quality and authenticity control of fruit purées, fruit preparations and jams—A review. Trends Food Sci. Technol. 2005, 16, 433–441. [Google Scholar] [CrossRef]
- Obón, J.M.; Díaz-García, M.C.; Castellar, M.R. Red fruit juice quality and authenticity control by HPLC. J. Food Compos. Anal. 2011, 24, 760–771. [Google Scholar] [CrossRef]
- Wen, L.; Wrolstad, R.E. Phenolic composition of authentic pineapple juice. J. Food Sci. 2002, 67, 155–161. [Google Scholar] [CrossRef]
- Dragovic-Uzelac, V.; Pospišil, J.; Levaj, B.; Delonga, K. The study of phenolic profiles of raw apricots and apples and their purees by HPLC for the evaluation of apricot nectars and jams authenticity. Food Chem. 2005, 91, 373–383. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miklavčič Višnjevec, A.; Schwarzkopf, M. Phenolic Compounds in Poorly Represented Mediterranean Plants in Istria: Health Impacts and Food Authentication. Molecules 2020, 25, 3645. https://doi.org/10.3390/molecules25163645
Miklavčič Višnjevec A, Schwarzkopf M. Phenolic Compounds in Poorly Represented Mediterranean Plants in Istria: Health Impacts and Food Authentication. Molecules. 2020; 25(16):3645. https://doi.org/10.3390/molecules25163645
Chicago/Turabian StyleMiklavčič Višnjevec, Ana, and Matthew Schwarzkopf. 2020. "Phenolic Compounds in Poorly Represented Mediterranean Plants in Istria: Health Impacts and Food Authentication" Molecules 25, no. 16: 3645. https://doi.org/10.3390/molecules25163645
APA StyleMiklavčič Višnjevec, A., & Schwarzkopf, M. (2020). Phenolic Compounds in Poorly Represented Mediterranean Plants in Istria: Health Impacts and Food Authentication. Molecules, 25(16), 3645. https://doi.org/10.3390/molecules25163645