Natural Compounds for Wood Protection against Fungi—A Review
Abstract
1. Introduction
2. Antifungal Substances of Plant Origin
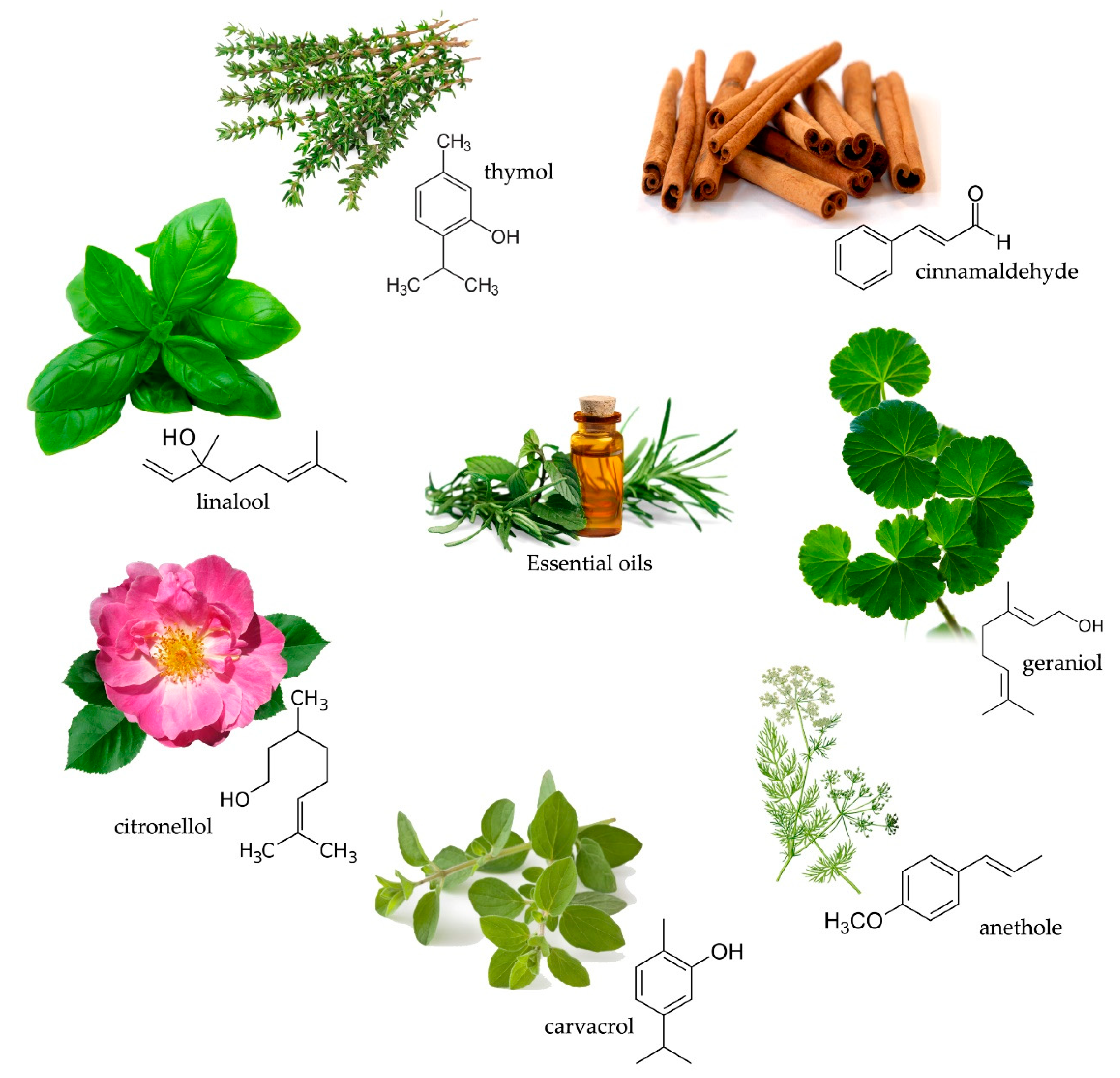
2.1. Essential Oils
Essential oils in Wood Protection
2.2. Tannins
2.2.1. Tannins in Wood Protection
2.2.2. Tannins in Combination with Other Substances
2.3. Wood Extractives
2.4. Other Plant Extracts
3. Antifungal Substances of Animal Origin
3.1. Propolis
3.1.1. Propolis in Wood Protection
3.1.2. Propolis Activity against Mould
3.1.3. Propolis Activity against Wood-Decaying Fungi
3.1.4. Propolis in Combination with Polymers
3.2. Chitin and Chitosan
Chitosan in Wood Protection
4. Conclusions
- -
- different biological compounds able to degrade pit membranes thus increasing their penetrability into wood tissue;
- -
- various natural polymers and cross-linkers to fix natural compounds inside the wood structure and prevent their leaching;
- -
- other substances such as antioxidants, biological control agents or chelators to enhance their antimicrobial activity and durability.
Funding
Conflicts of Interest
References
- Brischke, C.; Alfredsen, G. Wood-water relationships and their role for wood susceptibility to fungal decay. Appl. Microbiol. Biotechnol. 2020, 104, 3781–3795. [Google Scholar] [CrossRef] [PubMed]
- Goodell, B.; Qian, Y.; Jellison, J. Fungal Decay of Wood: Soft Rot—Brown Rot—White Rot. In Development of Commercial Wood Preservatives; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2008; Volume 982, pp. 9–31. ISBN 978-0-8412-3951-7. [Google Scholar]
- Riley, R.; Salamov, A.A.; Brown, D.W.; Nagy, L.G.; Floudas, D.; Held, B.W.; Levasseur, A.; Lombard, V.; Morin, E.; Otillar, R.; et al. Extensive sampling of basidiomycete genomes demonstrates inadequacy of the white-rot/brown-rot paradigm for wood decay fungi. Proc. Natl. Acad. Sci. USA 2014, 111, 9923–9928. [Google Scholar] [CrossRef] [PubMed]
- Daniel, G. Microview of Wood under Degradation by Bacteria and Fungi. In Wood Deterioration and Preservation; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2003; Volume 845, pp. 34–72. ISBN 978-0-8412-3797-1. [Google Scholar]
- Zabel, R.A.; Morrell, J.J. Wood Microbiology: Decay and its Prevention; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Meyer, L.; Brischke, C. Fungal decay at different moisture levels of selected European-grown wood species. Int. Biodeter. Biodegr. 2015, 103, 23–29. [Google Scholar] [CrossRef]
- Edlund, M.-L.; Nilsson, T. Performance of Copper and Non-Copper Based Wood Preservatives in Terrestrial Microcosms. Holzforschung 1999, 53, 369–375. [Google Scholar] [CrossRef]
- Freeman, M.H.; McIntyre, C.R. Copper-based wood preservatives. For. Prod. J. 2008, 58, 6–27. [Google Scholar]
- Lesar, B.; Budija, F.; Kralj, P.; Petrič, M.; Humar, M. Leaching of boron from wood impregnated with preservative solutions based on boric acid and liquefied wood. Eur. J. Wood Wood Prod. 2012, 70, 365–367. [Google Scholar] [CrossRef]
- Edlich, R.F.; Winters, K.L.; Long, W.B., 3rd. Treated Wood Preservatives Linked to Aquatic Damage, Human Illness, and Death–A Societal Problem. JLT 2005, 15. [Google Scholar] [CrossRef]
- Singh, T.; Singh, A.P. A review on natural products as wood protectant. Wood Sci. Technol. 2012, 46, 851–870. [Google Scholar] [CrossRef]
- González-Laredo, R.F.; Rosales-Castro, M.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Moreno-Jiménez, M.R.; Karchesy, J.J. Wood preservation using natural products. Madera y Bosques 2015, 21, 63–76. [Google Scholar] [CrossRef]
- Humar, M.; Lesar, B. Efficacy of linseed-and tung-oil-treated wood against wood-decay fungi and water uptake. Int. Biodeter. Biodegr. 2013, 85, 223–227. [Google Scholar] [CrossRef]
- Patachia, S.; Croitoru, C. Biopolymers for wood preservation. In Biopolymers and Biotech Admixtures for Eco-Efficient Construction Materials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 305–332. [Google Scholar]
- Terziev, N.; Panov, D. Plant Oils as “Green” Substances for Wood Protection. Minimising the Environmental Impact of the Forest Products Industries; Springer: Berlin/Heidelberg, Germany, 2011; pp. 143–149. [Google Scholar]
- Teacă, C.A.; Roşu, D.; Mustaţă, F.; Rusu, T.; Roşu, L.; Roşca, I.; Varganici, C.-D. Natural Bio-Based Products for Wood Coating and Protection against Degradation: A Review. BioResources 2019, 14, 4873–4901. [Google Scholar]
- Susi, P.; Aktuganov, G.; Himanen, J.; Korpela, T. Biological control of wood decay against fungal infection. J. Environ. Manage. 2011, 92, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Mazid, M.; Khan, T.A.; Mohammad, F. Role of secondary metabolites in defense mechanisms of plants. Biol. Med. 2011, 3, 232–249. [Google Scholar]
- Adamczyk, B.; Simon, J.; Kitunen, V.; Adamczyk, S.; Smolander, A. Tannins and Their Complex Interaction with Different Organic Nitrogen Compounds and Enzymes: Old Paradigms versus Recent Advances. ChemistryOpen 2017, 6, 610–614. [Google Scholar] [CrossRef]
- Vasconsuelo, A.; Boland, R. Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Sci. 2007, 172, 861–875. [Google Scholar] [CrossRef]
- Bhagat, S.; Birah, A.; Kumar, R.; Yadav, M.S.; Chattopadhyay, C. Plant disease management: Prospects of pesticides of plant origin. In Advances in Plant Biopesticides; Springer: Berlin/Heidelberg, Germany, 2014; pp. 119–129. [Google Scholar]
- Masango, P. Cleaner production of essential oils by steam distillation. J. Clean. Prod. 2005, 13, 833–839. [Google Scholar] [CrossRef]
- Edris, A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid. Based Complement. Altern. Med. 2016, 2016. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P.; Domagalska, B.W.; Młynarczyk, A. Essential oils and herbal extracts as antimicrobial agents in cosmetic emulsion. Indian J. Microbiol. 2013, 53, 232–237. [Google Scholar] [CrossRef]
- Prakash, B.; Kedia, A.; Mishra, P.K.; Dubey, N.K. Plant essential oils as food preservatives to control moulds, mycotoxin contamination and oxidative deterioration of agri-food commodities–Potentials and challenges. Food Control 2015, 47, 381–391. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential oils: Sources of antimicrobials and food preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef] [PubMed]
- Voda, K.; Boh, B.; Vrtačnik, M.; Pohleven, F. Effect of the antifungal activity of oxygenated aromatic essential oil compounds on the white-rot Trametes versicolor and the brown-rot Coniophora puteana. Int. Biodeter. Biodegr. 2003, 51, 51–59. [Google Scholar] [CrossRef]
- Hussain, A.; Shrivastav, A.; Jain, S.K. Antifungal Activity of Essential Oils against Local Wood Degrading Cellulolytic Filamentous Fungi. Adv. Biores. 2013, 4, 161–167. [Google Scholar]
- Bahmani, M.; Schmidt, O. Plant essential oils for environment-friendly protection of wood objects against fungi. Maderas-Cienc. Tecnol. 2018, 20, 325–332. [Google Scholar] [CrossRef]
- Kartal, S.N.; Hwang, W.-J.; Imamura, Y.; Sekine, Y. Effect of essential oil compounds and plant extracts on decay and termite resistance of wood. Holz als Roh-und Werkstoff 2006, 64, 455. [Google Scholar] [CrossRef]
- Pánek, M.; Reinprecht, L.; Hulla, M. Ten essential oils for beech wood protection-Efficacy against wood-destroying fungi and moulds, and effect on wood discoloration. BioResources 2014, 9, 5588–5603. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, Z.; Huang, Q.; Zhang, D. Antifungal activity of several essential oils and major components against wood-rot fungi. Ind. Crops Prod. 2017, 108, 278–285. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, T.; Mi, N.; Wang, Y.; Li, G.; Wang, L.; Xie, Y. Antifungal activity of monoterpenes against wood white-rot fungi. Int. Biodeter. Biodegr. 2016, 106, 157–160. [Google Scholar] [CrossRef]
- Voda, K.; Boh, B.; Vrtačnik, M. A quantitative structure–antifungal activity relationship study of oxygenated aromatic essential oil compounds using data structuring and PLS regression analysis. J. Mol. Model. 2004, 10, 76–84. [Google Scholar] [CrossRef]
- Chittenden, C.; Singh, T. Antifungal activity of essential oils against wood degrading fungi and their applications as wood preservatives. Int. Wood Prod. J. 2011, 2, 44–48. [Google Scholar] [CrossRef]
- Cheng, S.-S.; Liu, J.-Y.; Chang, E.-H.; Chang, S.-T. Antifungal activity of cinnamaldehyde and eugenol congeners against wood-rot fungi. Bioresour. Technol. 2008, 99, 5145–5149. [Google Scholar] [CrossRef] [PubMed]
- Reinprecht, L.; Pop, D.-M.; Vidholdová, Z.; Timar, M.C. Anti-decay potential of five essential oils against the wood-decaying fungi Serpula lacrymans and Trametes versicolor. Acta Fac. Xylol. Zvolen Res Publica Slovaca 2019, 61, 63–72. [Google Scholar]
- Jones, D.; Howard, N.; Suttie, E. The potential of propolis and other naturally occurring products for preventing biological decay. In Proceedings of the 42nd Annual Meeting of the International Research Group on Wood Protection, Queenstown, New Zealand, 8–12 May 2011; IRG Secretariat: Stockholm, Sweden, 2011. [Google Scholar]
- Yang, V.W.; Clausen, C.A. Antifungal effect of essential oils on southern yellow pine. Int. Biodeter. Biodegr. 2007, 59, 302–306. [Google Scholar] [CrossRef][Green Version]
- Salem, M.Z.M.; Zidan, Y.E.; Mansour, M.M.A.; El Hadidi, N.M.N.; Elgat, W.A.A. Antifungal activities of two essential oils used in the treatment of three commercial woods deteriorated by five common mold fungi. Int. Biodeter. Biodegr. 2016, 106, 88–96. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Chen, P.-F.; Chang, S.-T. Antifungal activities of essential oils and their constituents from indigenous cinnamon (Cinnamomum osmophloeum) leaves against wood decay fungi. Bioresource Technol. 2005, 96, 813–818. [Google Scholar] [CrossRef]
- Su, Y.-C.; Hsu, K.-P.; Wang, E.I.-C.; Ho, C.-L. The composition, anti-mildew and anti-wood-decay fungal activities of the leaf and fruit oils of Juniperus formosana from Taiwan. Nat. Prod. Commun. 2013, 8, 1934578X1300800936. [Google Scholar] [CrossRef]
- Su, Y.-C.; Ho, C.-L.; Wang, E.I.-C.; Chang, S.-T. Antifungal activities and chemical compositions of essential oils from leaves of four eucalypts. Taiwan J. For. Sci. 2006, 21, 49–61. [Google Scholar]
- Cheng, S.-S.; Wu, C.-L.; Chang, H.-T.; Kao, Y.-T.; Chang, S.-T. Antitermitic and antifungal activities of essential oil of Calocedrus formosana leaf and its composition. J. Chem. Ecol. 2004, 30, 1957–1967. [Google Scholar] [CrossRef]
- Mohareb, A.S.; Badawy, M.E.; Abdelgaleil, S.A. Antifungal activity of essential oils isolated from Egyptian plants against wood decay fungi. J. Wood Sci. 2013, 59, 499–505. [Google Scholar] [CrossRef]
- Rawat, K.; Sahoo, U.K.; Hegde, N.; Kumar, A. Effectiveness of neem (Azadirachta indica A. Juss) oil against decay fungi. Sci. Technol. J 2018, 5, 48–51. [Google Scholar] [CrossRef]
- Cai, L.; Lim, H.; Nicholas, D.D.; Kim, Y. Bio-based Preservative using Methyl-β-cyclodextrin-Essential Oil Complexes for Wood Protection. Int. J. Biol. Macromol. 2020, 147, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, A. Tannins: Prospectives and actual industrial applications. Biomolecules 2019, 9, 344. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Kumar, V.; Kaur, J.; Tanwar, B.; Goyal, A.; Sharma, R.; Gat, Y.; Kumar, A. Health effects, sources, utilization and safety of tannins: A critical review. Toxin Rev. 2019, 1–13. [Google Scholar] [CrossRef]
- Hernes, P.J.; Hedges, J.I. Tannin signatures of barks, needles, leaves, cones, and wood at the molecular level11Associate editor: C. Arnosti. Geochim. Cosmochim. Acta 2004, 68, 1293–1307. [Google Scholar] [CrossRef]
- China, C.R.; Hilonga, A.; Nyandoro, S.S.; Schroepfer, M.; Kanth, S.V.; Meyer, M.; Njau, K.N. Suitability of selected vegetable tannins traditionally used in leather making in Tanzania. J. Clean. Prod. 2020, 251, 119687. [Google Scholar] [CrossRef]
- Raji, P.; Samrot, A.V.; Bhavya, K.S.; Sharan, M.; Priya, S.; Paulraj, P. Greener Approach for Leather Tanning Using Less Chrome with Plant Tannins and Tannins Mediated Nanoparticles. J. Clust. Sci. 2019, 30, 1533–1543. [Google Scholar] [CrossRef]
- Picariello, L.; Gambuti, A.; Picariello, B.; Moio, L. Evolution of pigments, tannins and acetaldehyde during forced oxidation of red wine: Effect of tannins addition. LWT 2017, 77, 370–375. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological Application of Tannin-Based Extracts. Molecules 2020, 25, 614. [Google Scholar] [CrossRef]
- Sánchez-Martín, J.; Beltrán-Heredia, J.; Solera-Hernández, C. Surface water and wastewater treatment using a new tannin-based coagulant. Pilot Plant Trials. J. Environ. Manag. 2010, 91, 2051–2058. [Google Scholar] [CrossRef]
- Basso, M.C.; Pizzi, A.; Al-Marzouki, F.; Abdalla, S. Horticultural/hydroponics and floral natural foams from tannins. Ind. Crops Prod. 2016, 87, 177–181. [Google Scholar] [CrossRef]
- Tondi, G.; Petutschnigg, A. Tannin-Based Foams: The Innovative Material for Insulation Purposes. In Handbook of Composites from Renewable Materials, Structure and Chemistry; Wiley: Hoboken, NJ, USA, 2016; Volume 1, p. 93. [Google Scholar]
- Lei, H.; Pizzi, A.; Du, G. Environmentally friendly mixed tannin/lignin wood resins. J. Appl. Polym. Sci. 2008, 107, 203–209. [Google Scholar] [CrossRef]
- Yazaki, Y.; Collins, P.J. Wood adhesives based on tannin extracts from barks of some pine and spruce species. Holz als Roh-und Werkstoff 1994, 52, 307–310. [Google Scholar] [CrossRef]
- Missio, A.L.; Mattos, B.D.; Ferreira, D.d.F.; Magalhães, W.L.E.; Bertuol, D.A.; Gatto, D.A.; Petutschnigg, A.; Tondi, G. Nanocellulose-tannin films: From trees to sustainable active packaging. J. Clean. Prod. 2018, 184, 143–151. [Google Scholar] [CrossRef]
- Zhao, B.; Han, W.; Zhang, W.; Shi, B. Corrosion inhibition performance of tannins for mild steel in hydrochloric acid solution. Res. Chem. Intermediat. 2018, 44, 407–423. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, J.; Chen, N.G.; Shi, Z.; Qiu, J.; He, C.; Chen, M. Recent advances in anticancer activities and drug delivery systems of tannins. Med. Res. Rev. 2017, 37, 665–701. [Google Scholar] [CrossRef]
- Teodor, E.D.; Ungureanu, O.; Gatea, F.; Radu, G.L. The Potential of Flavonoids and Tannins from Medicinal Plants as Anticancer Agents. Anti-Cancer Agent. ME 2020. [Google Scholar] [CrossRef]
- Vilhelmova-Ilieva, N.; Galabov, A.S.; Mileva, M. Tannins as Antiviral Agents. In Tannins-Structural Properties, Biological Properties and Current Knowledge; IntechOpen: London, UK, 2019. [Google Scholar]
- Wang, H.; Chen, Y.; Zhang, W. A single-molecule atomic force microscopy study reveals the antiviral mechanism of tannin and its derivatives. Nanoscale 2019, 11, 16368–16376. [Google Scholar] [CrossRef]
- Li, M.; Feng, L.; Jiang, W.-D.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Zhou, X.-Q. Condensed tannins decreased the growth performance and impaired intestinal immune function in on-growing grass carp (Ctenopharyngodon idella). Br. J. Nutr. 2020, 123, 737–755. [Google Scholar] [CrossRef]
- Girard, M.; Bee, G. Invited review: Tannins as a potential alternative to antibiotics to prevent coliform diarrhea in weaned pigs. Animal 2020, 14, 95–107. [Google Scholar] [CrossRef]
- Akiyama, H.; Fujii, K.; Yamasaki, O.; Oono, T.; Iwatsuki, K. Antibacterial action of several tannins against Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 48, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Funatogawa, K.; Hayashi, S.; Shimomura, H.; Yoshida, T.; Hatano, T.; Ito, H.; Hirai, Y. Antibacterial activity of hydrolyzable tannins derived from medicinal plants against Helicobacter pylori. Microbiol. Immunol. 2004, 48, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Doss, A.; Mubarack, H.M.; Dhanabalan, R. Antibacterial activity of tannins from the leaves of Solanum trilobatum Linn. Indian J. Sci. Technol. 2009, 2, 41–43. [Google Scholar] [CrossRef]
- Ogawa, S.; Yazaki, Y. Tannins from Acacia mearnsii De Wild. Bark: Tannin Determination and Biological Activities. Molecules 2018, 23, 837. [Google Scholar] [CrossRef] [PubMed]
- Latté, K.P.; Kolodziej, H. Antifungal effects of hydrolysable tannins and related compounds on dermatophytes, mould fungi and yeasts. Z. Naturforsch. C. J. Biosci. 2000, 55, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Lei, M.; Andargie, M.; Zeng, J.; Li, J. Antifungal activity and mechanism of action of tannic acid against Penicillium digitatum. Physiol. Mol. Plant P. 2019, 107, 46–50. [Google Scholar] [CrossRef]
- Anttila, A.-K.; Pirttilä, A.M.; Häggman, H.; Harju, A.; Venäläinen, M.; Haapala, A.; Holmbom, B.; Julkunen-Tiitto, R. Condensed conifer tannins as antifungal agents in liquid culture. Holzforschung 2013, 67, 825–832. [Google Scholar] [CrossRef]
- Morey, A.T.; de Souza, F.C.; Santos, J.P.; Pereira, C.A.; Cardoso, J.D.; de Almeida, R.S.C.; Costa, M.A.; de Mello, J.C.P.; Nakamura, C.V.; Pinge-Filho, P.; et al. Antifungal activity of condensed tannins from Stryphnodendron adstringens: Effect on Candida tropicalis growth and adhesion properties. Curr. Pharm. Biotechnol. 2016, 17, 365–375. [Google Scholar] [CrossRef]
- Laks, P.E. Condensed tannins as a source of novel biocides. In Chemistry and Significance of Condensed Tannins; Springer: Berlin/Heidelberg, Germany, 1989; pp. 503–515. [Google Scholar]
- Hart, J.H.; Hillis, W.E. Inhibition of wood-rotting fungi by ellagitannins in the heartwood of Quercus alba. Phytopathology 1972, 62, 620–626. [Google Scholar] [CrossRef]
- Özgenç, Ö.; Durmaz, S.; Yıldız, Ü.C.; Erişir, E. A Comparison between Some Wood Bark Extracts: Antifungal Activity. Kastamonu Üniv. Orman Fakültesi Dergisi 2017, 502–508. [Google Scholar] [CrossRef]
- Sen, S.; Tascioglu, C.; Tırak, K. Fixation, leachability, and decay resistance of wood treated with some commercial extracts and wood preservative salts. Int. Biodeter. Biodegr. 2009, 63, 135–141. [Google Scholar] [CrossRef]
- Tascioglu, C.; Yalcin, M.; Sen, S.; Akcay, C. Antifungal properties of some plant extracts used as wood preservatives. Int. Biodeter. Biodegr. 2013, 85, 23–28. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Okuda, K. Chemically modified tannin and tannin-copper complexes as wood preservatives. Holzforsch. Int. J. Biol. Chem. Phys. Technol. Wood 1998, 52, 596–602. [Google Scholar] [CrossRef]
- Silveira, A.G.; Santini, E.J.; Kulczynski, S.M.; Trevisan, R.; Wastowski, A.D.; Gatto, D.A. Tannic extract potential as natural wood preservative of Acacia mearnsii. Anais Acad. Bras. Ciências 2017, 89, 3031–3038. [Google Scholar] [CrossRef]
- Mansour, M.M.A.; Salem, M.Z.M. Evaluation of wood treated with some natural extracts and Paraloid B-72 against the fungus Trichoderma harzianum: Wood elemental composition, in-vitro and application evidence. Int. Biodeter. Biodegr. 2015, 100, 62–69. [Google Scholar] [CrossRef]
- Tomak, E.D.; Gonultas, O. The wood preservative potentials of valonia, chestnut, tara and sulphited oak tannins. J. Wood Chem. Technol. 2018, 38, 183–197. [Google Scholar] [CrossRef]
- Laks, P.E.; McKaig, P.A.; Hemingway, R.W. Flavonoid biocides: Wood preservatives based on condensed tannins. Holzforsch. Int. J. Biol. Chem. Phys. Technol. Wood 1988, 42, 299–306. [Google Scholar] [CrossRef]
- Ramírez, M.G.L.; Ruiz, H.G.O.; Arzate, F.N.; Gallegos, M.A.C.; Enriquez, S.G. Evaluation of fungi toxic activity of tannins and a tannin-copper complex from the mesocarp of Cocos nucifera Linn. Wood Fiber Sci. 2012, 44, 357–364. [Google Scholar]
- Bernardis, A.C.; Popoff, O. Durability of Pinus elliottii wood impregnated with Quebracho Colorado (Schinopsis balansae) bio-protectives extracts and CCA. Maderas-Cienc. Tecnol. 2009, 11, 107–115. [Google Scholar] [CrossRef][Green Version]
- Thevenon, M.-F.; Tondi, G.; Pizzi, A. High performance tannin resin-boron wood preservatives for outdoor end-uses. Eur. J. Wood Wood Prod. 2009, 67, 89. [Google Scholar] [CrossRef]
- Tondi, G.; Wieland, S.; Lemenager, N.; Petutschnigg, A.; Pizzi, A.; Thevenon, M.-F. Efficacy of Tannins in Fixing Boron in Wood: Fungal and termite resistance. BioResources 2012, 7, 1238–1252. [Google Scholar]
- Efhamisisi, D.; Thevenon, M.-F.; Hamzeh, Y.; Pizzi, A.; Karimi, A.; Pourtahmasi, K. Tannin-boron complex as a preservative for 3-ply beech plywoods designed for humid conditions. Holzforschung 2017, 71, 249–258. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Mansour, M.M.A.; Elansary, H.O. Evaluation of the effect of inner and outer bark extracts of Sugar Maple (Acer saccharum var. saccharum) in combination with citric acid against the growth of three common molds. J. Wood Chem. Technol. 2019, 39, 136–147. [Google Scholar] [CrossRef]
- Valette, N.; Perrot, T.; Sormani, R.; Gelhaye, E.; Morel-Rouhier, M. Antifungal activities of wood extractives. Fungal Biol. Rev. 2017, 31, 113–123. [Google Scholar] [CrossRef]
- Taylor, A.M.; Gartner, B.L.; Morrell, J.J. Heartwood formation and natural durability-a review. Wood Fiber Sci. 2002, 4, 587–611. [Google Scholar]
- Sablík, P.; Giagli, K.; Pařil, P.; Baar, J.; Rademacher, P. Impact of extractive chemical compounds from durable wood species on fungal decay after impregnation of nondurable wood species. Eur. J. Wood Wood Prod. 2016, 74, 231–236. [Google Scholar] [CrossRef]
- Kirker, G.T.; Blodgett, A.B.; Arango, R.A.; Lebow, P.K.; Clausen, C.A. The role of extractives in naturally durable wood species. Int. Biodeter. Biodegr. 2013, 82, 53–58. [Google Scholar] [CrossRef]
- Brocco, V.F.; Paes, J.B.; Costa, L.G.d.; Brazolin, S.; Arantes, M.D.C. Potential of teak heartwood extracts as a natural wood preservative. J. Clean. Prod. 2017, 142, 2093–2099. [Google Scholar] [CrossRef]
- Kokutse, A.D.; Stokes, A.; Baillères, H.; Kokou, K.; Baudasse, C. Decay resistance of Togolese teak (Tectona grandis Lf) heartwood and relationship with colour. Trees 2006, 20, 219–223. [Google Scholar] [CrossRef]
- Anda, R.R.; Koch, G.; Richter, H.-G.; Talavera, F.J.F.; Guzmán, J.A.S.; Satyanarayana, K.G. Formation of heartwood, chemical composition of extractives and natural durability of plantation-grown teak wood from Mexico. Holzforschung 2019, 73, 547–557. [Google Scholar] [CrossRef]
- Windeisen, E.; Klassen, A.; Wegener, G. On the chemical characterisation of plantation teakwood from Panama. Holz als Roh-und Werkstoff 2003, 61, 416–418. [Google Scholar] [CrossRef]
- Haupt, M.; Leithoff, H.; Meier, D.; Puls, J.; Richter, H.G.; Faix, O. Heartwood extractives and natural durability of plantation-grown teakwood (Tectona grandis L.)—A case study. Holz Roh Werkst. 2003, 61, 473–474. [Google Scholar] [CrossRef]
- Thulasidas, P.K.; Bhat, K.M. Chemical extractive compounds determining the brown-rot decay resistance of teak wood. Holz als Roh-und Werkstoff 2007, 65, 121–124. [Google Scholar] [CrossRef]
- Füchtner, S.; Brock-Nannestad, T.; Smeds, A.; Fredriksson, M.; Pilgård, A.; Thygesen, L.G. Hydrophobic and Hydrophilic Extractives in Norway Spruce and Kurile Larch and Their Role in Brown-Rot Degradation. Front. Plant Sci. 2020, 11, 855. [Google Scholar] [CrossRef]
- Anouhe, J.-B.S.; Niamké, F.B.; Faustin, M.; Virieux, D.; Pirat, J.-L.; Adima, A.A.; Kati-Coulibaly, S.; Amusant, N. The role of extractives in the natural durability of the heartwood of Dicorynia guianensis Amsh: New insights in antioxydant and antifungal properties. Ann. For. Sci. 2018, 75, 15. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.-X.; Lin, J.-G.; Liu, J.; Jiang, M.-S.; Chu, L.-X. Chemical composition and antifungal activity of extracts from the xylem of Cinnamomum camphora. BioResources 2014, 9, 2560–2571. [Google Scholar] [CrossRef]
- Maoz, M.; Karchesy, J.J.; Morrell, J.J. Ability of natural extracts to limit mold growth on Douglas-fir sapwood. BioResources 2012, 7, 5415–5421. [Google Scholar] [CrossRef][Green Version]
- Salem, M.Z.; Zidan, Y.E.; El Hadidi, N.M.; Mansour, M.M.; Elgat, W.A.A. Evaluation of usage three natural extracts applied to three commercial wood species against five common molds. Int. Biodeter. Biodegr. 2016, 110, 206–226. [Google Scholar] [CrossRef]
- Fernández-Costas, C.; Palanti, S.; Charpentier, J.-P.; Sanromán, M.Á.; Moldes, D. A sustainable treatment for wood preservation: Enzymatic grafting of wood extractives. ACS Sustain. Chem. Eng. 2017, 5, 7557–7567. [Google Scholar] [CrossRef]
- Mohammed, M.J.; Al-Bayati, F.A. Isolation, identification and purification of caffeine from Coffea arabica L. and Camellia sinensis L.: A combination antibacterial study. IJGP 2009, 3. [Google Scholar] [CrossRef]
- Raut, J.S.; Chauhan, N.M.; Shinde, R.B.; Karuppayil, S.M. Inhibition of planktonic and biofilm growth of Candida albicans reveals novel antifungal activity of caffeine. J. Med. Plant Res. 2013, 7, 777–782. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Qian, W.-J.; Li, N.-N.; Hao, X.-Y.; Wang, L.; Xiao, B.; Wang, X.-C.; Yang, Y.-J. Metabolic changes of caffeine in tea plant (Camellia sinensis (L.) O. Kuntze) as defense response to Colletotrichum fructicola. J. Agric. Food Chem. 2016, 64, 6685–6693. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.S.; Ohlan, D. In vitro studies on antifungal activity of tea (Camellia sinensis) and coffee (Coffea arabica) against wood-rotting fungi. J. Basic Microbiol. 1997, 37, 159–165. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, L.; Zhang, Z. Antifungal activity of caffeine against fungal pathogens of tea plant. J. Nanjing Agric. Univ. 2010, 2, 63–67. [Google Scholar]
- Barbero-López, A.; Monzó-Beltrán, J.; Virjamo, V.; Akkanen, J.; Haapala, A. Revalorization of coffee silverskin as a potential feedstock for antifungal chemicals in wood preservation. Int. Biodeter. Biodegr. 2020, 152, 105011. [Google Scholar] [CrossRef]
- Kwaśniewska-Sip, P.; Cofta, G.; Nowak, P.B. Resistance of fungal growth on Scots pine treated with caffeine. Int. Biodeter. Biodegr. 2018, 132, 178–184. [Google Scholar] [CrossRef]
- Broda, M.; Mazela, B.; Frankowski, M. Durability of wood treated with aatmos and caffeine-towards the long-term carbon storage. Maderas-Cienc. Tecnol. 2018, 20, 455–468. [Google Scholar] [CrossRef]
- Ratajczak, I.; Woźniak, M.; Kwaśniewska-Sip, P.; Szentner, K.; Cofta, G.; Mazela, B. Chemical characterization of wood treated with a formulation based on propolis, caffeine and organosilanes. Eur. J. Wood Prod. 2018, 76, 775–781. [Google Scholar] [CrossRef]
- Goktas, O.; Mammadov, R.; Duru, M.E.; Ozen, E.; Colak, A.M. Application of extracts from the poisonous plant, Nerium Oleander L., as a wood preservative. Afr. J. Biotechnol. 2007, 6, 2000–2003. [Google Scholar]
- Ozen, E. A Study about Poisonous Plant (Geophytes) Extracts as a Wood Preservative to Wood Decay Fungi. Ph.D. Thesis, Institute of Natural Science, Mugla University, Mugla, Turkey, 2005. [Google Scholar]
- Yildiz, Ü.C.; Kiliç, C.; Gürgen, A.; Yildiz, S. Possibility of using lichen and mistletoe extracts as potential natural wood preservative. Maderas-Cienc. Tecnol. 2020, 22. [Google Scholar] [CrossRef]
- Barbero-López, A.; Chibily, S.; Tomppo, L.; Salami, A.; Ancin-Murguzur, F.J.; Venäläinen, M.; Lappalainen, R.; Haapala, A. Pyrolysis distillates from tree bark and fibre hemp inhibit the growth of wood-decaying fungi. Ind. Crops Prod. 2019, 129, 604–610. [Google Scholar] [CrossRef]
- Sunarta, S.; Darmadji, P.; Uehara, T.; Katoh, S. Production and characterization of palm fruit shell bio-oil for wood preservation. Forest Prod. J. 2011, 61, 180–184. [Google Scholar] [CrossRef]
- Al-Huqail, A.A.; Behiry, S.I.; Salem, M.Z.M.; Ali, H.M.; Siddiqui, M.H.; Salem, A.Z. Antifungal, antibacterial, and antioxidant activities of Acacia saligna (Labill.) HL Wendl. flower extract: HPLC analysis of phenolic and flavonoid compounds. Molecules 2019, 24, 700. [Google Scholar] [CrossRef] [PubMed]
- EL-Hefny, M.; Salem, M.Z.M.; Behiry, S.I.; Ali, H.M. The potential antibacterial and antifungal activities of wood treated with Withania somnifera fruit extract, and the phenolic, caffeine, and flavonoid composition of the extract according to HPLC. Processes 2020, 8, 113. [Google Scholar] [CrossRef]
- Saidulu, C.; Venkateshwar, C.; Rao, S.G.; Vardhan, T.A. In vitro Antimicrobial Activity of Withania somnifera Leaf and Root Extracts grown in Heavy. Int. J. Adv. Pharm. Biol. Chem. 2014, 872–879. [Google Scholar]
- Khan, Z.S.; Nasreen, S. Phytochemical analysis, antifungal activity and mode of action of methanol extracts from plants against pathogens. J. Agric. Technol. 2010, 6, 793–805. [Google Scholar]
- Bi, Z.; Yang, F.; Lei, Y.; Morrell, J.J.; Yan, L. Identification of antifungal compounds in konjac flying powder and assessment against wood decay fungi. Ind. Crops Prod. 2019, 140, 111650. [Google Scholar] [CrossRef]
- Tripathi, S.; Rawat, K.; Dhyani, S.; Pant, H. Potential of Lantana camara Linn. weed against wood destroying fungi. Indian For. 2009, 135, 403. [Google Scholar]
- Hosseinihashemi, S.K.; HosseinAshrafi, S.K.; Goldeh, A.J.; Salem, M.Z.M. Antifungal and antioxidant activities of heartwood, bark, and leaf extracts of Robinia pseudoacacia. BioResources 2016, 11, 1634–1646. [Google Scholar] [CrossRef]
- Mazela, B.; Bartkowiak, M.; Ratajczak, I. Animal protein impact on fungicidal properties of treatment formulations. Wood Res. 2007, 52, 13–22. [Google Scholar]
- Conner, A.H. Wood: Adhesives. Encyclopedia of Materials: Science and Technology; Elsevier Science, Ltd.: Amsterdam, The Netherlands; New York, NY, USA, 2001. [Google Scholar]
- Adhikari, B.B.; Chae, M.; Bressler, D.C. Utilization of slaughterhouse waste in value-added applications: Recent advances in the development of wood adhesives. Polymers 2018, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Cai, Y.; Ren, L.; Yang, H. Preparation of Modified Beeswax and Its Influence on the Surface Properties of Compressed Poplar Wood. Materials 2016, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Németh, R.; Tsalagkas, D.; Bak, M. Effect of soil contact on the modulus of elasticity of beeswax-impregnated wood. BioResources 2015, 10, 1574–1586. [Google Scholar] [CrossRef]
- Simone-Finstrom, M.; Spivak, M. Propolis and bee health: The natural history and significance of resin use by honey bees. Apidologie 2010, 41, 295–311. [Google Scholar] [CrossRef]
- Zabaiou, N.; Fouache, A.; Trousson, A.; Baron, S.; Zellagui, A.; Lahouel, M.; Lobaccaro, J.-M.A. Biological properties of propolis extracts: Something new from an ancient product. Chem. Phys. Lipids 2017, 207, 214–222. [Google Scholar] [CrossRef]
- Sforcin, J.M. Biological properties and therapeutic applications of propolis. Phytother. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef]
- Kujumgiev, A.; Tsvetkova, I.; Serkedjieva, Y.; Bankova, V.; Christov, R.; Popov, S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J. Ethnopharmacol. 1999, 64, 235–240. [Google Scholar] [CrossRef]
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Park, Y.K. Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evid. Based Complement. Altern. Med. 2013, 2013, 697390. [Google Scholar] [CrossRef]
- Bankova, V.S.; de Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Gong, S.; Luo, L.; Gong, W.; Gao, Y.; Xie, M. Multivariate analyses of element concentrations revealed the groupings of propolis from different regions in China. Food Chem. 2012, 134, 583–588. [Google Scholar] [CrossRef]
- Machado, C.S.; Mokochinski, J.B.; de Lira, T.O.; de Oliveira, F.d.C.E.; Cardoso, M.V.; Ferreira, R.G.; Sawaya, A.C.H.F.; Ferreira, A.G.; Pessoa, C.; Cuesta-Rubio, O.; et al. Comparative study of chemical composition and biological activity of yellow, green, brown, and red Brazilian propolis. Evid. Based Complementary Altern. Med. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Luo, L.; Chen, B.; Fu, Y. Recent development of chemical components in propolis. Front. Biol. China 2009, 4, 385. [Google Scholar] [CrossRef]
- Daffalla, K.A.; Mahmoud, A.S. Propolis as a natural remedy. J. Int. Oral Health 2016, 8, 646. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M.; Trusheva, B. New emerging fields of application of propolis. Maced. J. Chem. Chem. Eng. 2016, 35, 1–11. [Google Scholar] [CrossRef]
- Popova, M.; Giannopoulou, E.; Skalicka-Woźniak, K.; Graikou, K.; Widelski, J.; Bankova, V.; Kalofonos, H.; Sivolapenko, G.; Gaweł-Bęben, K.; Antosiewicz, B.; et al. Characterization and biological evaluation of propolis from Poland. Molecules 2017, 22, 1159. [Google Scholar] [CrossRef] [PubMed]
- Salas, A.L.; Alberto, M.R.; Zampini, I.C.; Cuello, A.S.; Maldonado, L.; Ríos, J.L.; Schmeda-Hirschmann, G.; Isla, M.I. Biological activities of polyphenols-enriched propolis from Argentina arid regions. Phytomedicine 2016, 23, 27–31. [Google Scholar] [CrossRef]
- Budija, F.; Kricej, B.; Petric, M. Possibilities of use of propolis for wood finishing. Wood Res. 2008, 53, 91–101. [Google Scholar]
- Quiroga, E.N.; Sampietro, D.A.; Soberón, J.R.; Sgariglia, M.A.; Vattuone, M.A. Propolis from the northwest of Argentina as a source of antifungal principles. J. Appl. Microbiol. 2006, 101, 103–110. [Google Scholar] [CrossRef]
- Popova, M.; Silici, S.; Kaftanoglu, O.; Bankova, V. Antibacterial activity of Turkish propolis and its qualitative and quantitative chemical composition. Phytomedicine 2005, 12, 221–228. [Google Scholar] [CrossRef]
- González-Búrquez, M.d.J.; González-Díaz, F.R.; García-Tovar, C.G.; Carrillo-Miranda, L.; Soto-Zárate, C.I.; Canales-Martínez, M.M.; Penieres-Carrillo, J.G.; Crúz-Sánchez, T.A.; Fonseca-Coronado, S. Comparison between In Vitro Antiviral Effect of Mexican Propolis and Three Commercial Flavonoids against Canine Distemper Virus. Evid. Based Complement. Alternat. Med. 2018, 2018. [Google Scholar] [CrossRef]
- Imhof, M.; Lipovac, M.; Kurz, C.; Barta, J.; Verhoeven, H.C.; Huber, J.C. Propolis solution for the treatment of chronic vaginitis. Int. J. Gynaecol. Obstet. 2005, 89, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.J.; Shin, H.M.; Perumalsamy, H.; Wang, X.; Ahn, Y.-J. Antiviral effects and possible mechanisms of action of constituents from Brazilian propolis and related compounds. J. Apic. Res. 2019, 0, 1–13. [Google Scholar] [CrossRef]
- Meneses, E.A.; Durango, D.L.; García, C.M. Antifungal activity against postharvest fungi by extracts from Colombian propolis. Quim. Nova 2009, 32, 2011–2017. [Google Scholar] [CrossRef]
- Jolly, V.G. Propolis Varnish for Violins. Bee World 1978, 59, 158–161. [Google Scholar] [CrossRef]
- Woźniak, M.; Ratajczak, I.; Lis, B.; Krystofiak, T. Hydrophobic Properties of Wood Treated with Propolis-Silane Formulations. Wood Res. 2018, 63, 517–524. [Google Scholar]
- Budija, F.; Humar, M.; Kricej, B.; Petric, M. Propolis for wood finishing IRG/WP/08-30464. In Proceedings of the IRG 39, International Research Group on Wood Protection, Istanbul, Turkey, 25–29 May 2008. [Google Scholar]
- Akçay, Ç.; Birinci, E.; Birinci, C.; Kolaylı, S. Durability of Wood Treated with Propolis. BioResources 2020, 15, 1547–1562. [Google Scholar]
- Abanikannda, J.O.; Adetogun, A.C.; Mukhtar, R.B. Evaluation of Honey Bee Propolis as Wood Preservative Using Weight Loss. Sci. World J. 2020, 15, 45–47. [Google Scholar]
- Woźniak, M.; Kwaśniewska-Sip, P.; Waśkiewicz, A.; Cofta, G.; Ratajczak, I. The Possibility of Propolis Extract Application in Wood Protection. Forests 2020, 11, 465. [Google Scholar] [CrossRef]
- Casado-Sanz, M.M.; Silva-Castro, I.; Ponce-Herrero, L.; Martín-Ramos, P.; Martín-Gil, J.; Acuña-Rello, L. White-rot fungi control on Populus spp. Wood by pressure treatments with silver nanoparticles, chitosan oligomers and propolis. Forests 2019, 10, 885. [Google Scholar] [CrossRef]
- Xu, X.; Pu, R.; Li, Y.; Wu, Z.; Li, C.; Miao, X.; Yang, W. Chemical Compositions of Propolis from China and the United States and their Antimicrobial Activities Against Penicillium notatum. Molecules 2019, 24, 3576. [Google Scholar] [CrossRef]
- Kumar, M.N.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Song, Z.; Li, G.; Guan, F.; Liu, W. Application of chitin/chitosan and their derivatives in the papermaking industry. Polymers 2018, 10, 389. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Avelelas, F.; Horta, A.; Pinto, L.F.V.; Cotrim Marques, S.; Marques Nunes, P.; Pedrosa, R.; Leandro, S.M. Antifungal and Antioxidant Properties of Chitosan Polymers Obtained from Nontraditional Polybius henslowii Sources. Mar. Drugs 2019, 17, 239. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef] [PubMed]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbo 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L.V. Molecular mechanisms of chitosan interactions with fungi and plants. Int. J. Mol. Sci. 2019, 20, 332. [Google Scholar] [CrossRef]
- Palma-Guerrero, J.; Lopez-Jimenez, J.A.; Pérez-Berná, A.J.; Huang, I.-C.; Jansson, H.-B.; Salinas, J.; Villalaín, J.; Read, N.D.; Lopez-Llorca, L.V. Membrane fluidity determines sensitivity of filamentous fungi to chitosan. Mol. Microbiol. 2010, 75, 1021–1032. [Google Scholar] [CrossRef]
- Vesentini, D.; Steward, D.; Singh, A.P.; Ball, R.; Daniel, G.; Franich, R. Chitosan-mediated changes in cell wall composition, morphology and ultrastructure in two wood-inhabiting fungi. Mycol. Res. 2007, 111, 875–890. [Google Scholar] [CrossRef]
- Singh, T.; Vesentini, D.; Singh, A.P.; Daniel, G. Effect of chitosan on physiological, morphological, and ultrastructural characteristics of wood-degrading fungi. Int. Biodeter. Biodegr. 2008, 62, 116–124. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Pina, C.; Tavaria, F.K.; Pintado, M.M. Evaluation and insights into chitosan antimicrobial activity against anaerobic oral pathogens. Anaerobe 2012, 18, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Xing, K.; Xing, Y.; Liu, Y.; Zhang, Y.; Shen, X.; Li, X.; Miao, X.; Feng, Z.; Peng, X.; Qin, S. Fungicidal effect of chitosan via inducing membrane disturbance against Ceratocystis fimbriata. Carbohydr. Polym. 2018, 192, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Torr, K.M.; Chittenden, C.; Franich, R.A.; Kreber, B. Advances in understanding bioactivity of chitosan and chitosan oligomers against selected wood-inhabiting fungi. Holzforschung 2005, 59, 559–567. [Google Scholar] [CrossRef]
- Galván Márquez, I.; Akuaku, J.; Cruz, I.; Cheetham, J.; Golshani, A.; Smith, M.L. Disruption of protein synthesis as antifungal mode of action by chitosan. Int. J. Food Microbiol. 2013, 164, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Moya, F.; Lopez-Llorca, L.V. Omics for Investigating Chitosan as an Antifungal and Gene Modulator. J. Fungi 2016, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Garba, B.; Ren, Y.; Yao, M.; Xia, X.; Li, M.; Wang, Y. Antifungal activity of chitosan against Aspergillus ochraceus and its possible mechanisms of action. Int. J. Biol. Macromol. 2020. [Google Scholar] [CrossRef]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in plant protection. Mar. Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Recent Advances of Chitosan Applications in Plants. Polymers 2018, 10, 118. [Google Scholar] [CrossRef]
- Munhuweyi, K.; Lennox, C.L.; Meitz-Hopkins, J.C.; Caleb, O.J.; Sigge, G.O.; Opara, U.L. Investigating the effects of crab shell chitosan on fungal mycelial growth and postharvest quality attributes of pomegranate whole fruit and arils. Sci. Hortic. 2017, 220, 78–89. [Google Scholar] [CrossRef]
- Qin, Y.; Li, P.; Guo, Z. Cationic chitosan derivatives as potential antifungals: A review of structural optimization and applications. Carbohydr. Polym. 2020, 236, 116002. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, P.; Benhamou, N.; Bussières, G.; Dessureault, M. Differential effect of chitosan on root rot fungal pathogens in forest nurseries. Can. J. Bot. 2000, 77, 1460–1468. [Google Scholar] [CrossRef]
- Bell, A.A.; Hubbard, J.C.; Liu, L.; Davis, R.M.; Subbarao, K.V. Effects of chitin and chitosan on the incidence and severity of Fusarium yellows of celery. Plant Dis. 1998, 82, 322–328. [Google Scholar] [CrossRef] [PubMed]
- El Ghaouth, A.; Arul, J.; Grenier, J.; Asselin, A. Effect of chitosan and other polyions on chitin deacetylase inRhizopus stolonifer. Exp. Mycol. 1992, 16, 173–177. [Google Scholar] [CrossRef]
- Eikenes, M.; Alfredsen, G.; Christensen, B.E.; Militz, H.; Solheim, H. Comparison of chitosans with different molecular weights as possible wood preservatives. J. Wood Sci. 2005, 51, 387–394. [Google Scholar] [CrossRef]
- Chittenden, C.; Singh, T. In vitro evaluation of combination of Trichoderma harzianum and chitosan for the control of sapstain fungi. Biol. Control 2009, 50, 262–266. [Google Scholar] [CrossRef]
- Alfredsen, G.; Eikenes, M.; Militz, H.; Solheim, H. Screening of chitosan against wood-deteriorating fungi. Scand. J. For. Res. 2004, 19, 4–13. [Google Scholar] [CrossRef]
- Kobayashi, T.; Furukawa, I. Wood-preserving effectiveness of chitosan-metal salts against wood decaying fungi. J. Anti Bact. Antifung. Agents 1995, 23, 343–348. [Google Scholar]
- Furukawa, I.; Yamamoto, S. Improvement of wood quality with chitin and chitosan.(II). Assessment of the fungicidal effect of chitosan-treated wood on wood decaying fungi and microorganisms in the soil. Res. Bull. Tottori Univ. For. 1990, 49–58. [Google Scholar]
- Schmidt, O.; Müller, J.; Moreth, U. Potential Protection Effect of Chitosan against Wood Fungi; Holz-Zentralbl: Leinfelden-Echterdingen, Germany, 1995. [Google Scholar]
- Gorgij, R.; Tarmian, A.; Karimi, A.N. Effect of chitosan on the mold resistance of wood and its surface properties. Int. J. Lignocellul. Prod. 2014, 1, 39–49. [Google Scholar] [CrossRef]
- Larnøy, E.; Dantz, S.; Eikenes, M.; Militz, H. Screening of properties of modified chitosan-treated wood. Wood Mater. Sci. Eng. 2006, 1, 59–68. [Google Scholar] [CrossRef]
- El-Gamal, R.; Nikolaivits, E.; Zervakis, G.I.; Abdel-Maksoud, G.; Topakas, E.; Christakopoulos, P. The use of chitosan in protecting wooden artifacts from damage by mold fungi. Electron. J. Biotechnol. 2016, 24, 70–78. [Google Scholar] [CrossRef]
- Papadopoulos, A.N.; Foti, D.; Kyzas, G.Z. Sorption behavior of water vapor of wood treated by chitosan polymer. Eur. J. Wood Wood Prod. 2020, 1–9. [Google Scholar] [CrossRef]
- Velmurugan, N.; Kumar, G.G.; Han, S.S.; Nahm, K.S.; Lee, Y.S. Synthesis and characterization of potential fungicidal silver nano-sized particles and chitosan membrane containing silver particles. Iran. Polym. J. 2009, 18, 383–392. [Google Scholar]
- Sun, F.; Bao, B.; Ma, L.; Chen, A.; Duan, X. Mould-resistance of bamboo treated with the compound of chitosan-copper complex and organic fungicides. J. Wood Sci. 2012, 58, 51–56. [Google Scholar] [CrossRef]
- Ding, X.; Richter, D.L.; Matuana, L.M.; Heiden, P.A. Efficient one-pot synthesis and loading of self-assembled amphiphilic chitosan nanoparticles for low-leaching wood preservation. Carbohydr. Polym. 2011, 86, 58–64. [Google Scholar] [CrossRef]
| Type of Fungi | Degraded Wood Type and Components | Effect on Wood |
|---|---|---|
| Wood-decaying fungi | ||
| brown-rot (Basidiomycota) | mainly softwoods; degradation of hemicelluloses and cellulose, demethylation of lignin | wood shrinkage and cracking into cubical pieces, brown colouration due to the presence of lignin remained, reduction of wood mechanical properties |
| white-rot (Basidiomycota) | mainly hardwoods but also softwoods; degradation of lignin and hemicelluloses, but also cellulose | fibre-like appearance and white colouration of wood due to the presence of lighter-coloured cellulose remains, wood becomes soft and spongy or stringy, its strength properties decrease along with the decay progress |
| soft-rot (Ascomycota, fungi imperfecti) | hemicelluloses and cellulose, less extensively lignin | formation of cavities inside the cell wall, discolouration and cracking pattern similar to brown-rot, deterioration of wood strength properties |
| Mould | ||
| mould (Zygomycota or Ascomycetes) | easily available sugars, not structural polymers | superficial discolouration of wood, minor degradation of the wood surface |
| Blue stain | ||
| blue stain (Ascomycota and Deuteromycota) | protein content of the parenchyma cells, easily available sugars, not structural polymers | dark discolouration of sapwood by dark-coloured hyphae, degradation of pit membranes leading to increased water permeability |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broda, M. Natural Compounds for Wood Protection against Fungi—A Review. Molecules 2020, 25, 3538. https://doi.org/10.3390/molecules25153538
Broda M. Natural Compounds for Wood Protection against Fungi—A Review. Molecules. 2020; 25(15):3538. https://doi.org/10.3390/molecules25153538
Chicago/Turabian StyleBroda, Magdalena. 2020. "Natural Compounds for Wood Protection against Fungi—A Review" Molecules 25, no. 15: 3538. https://doi.org/10.3390/molecules25153538
APA StyleBroda, M. (2020). Natural Compounds for Wood Protection against Fungi—A Review. Molecules, 25(15), 3538. https://doi.org/10.3390/molecules25153538






